Free-breathing T2-prepared myocardial blood oxygen level–dependent MR responses under hypercapnia of 10 mm Hg and adenosine are not different.
Abstract
Purpose
To examine whether controlled and tolerable levels of hypercapnia may be an alternative to adenosine, a routinely used coronary vasodilator, in healthy human subjects and animals.
Materials and Methods
Human studies were approved by the institutional review board and were HIPAA compliant. Eighteen subjects had end-tidal partial pressure of carbon dioxide (PetCO2) increased by 10 mm Hg, and myocardial perfusion was monitored with myocardial blood oxygen level–dependent (BOLD) magnetic resonance (MR) imaging. Animal studies were approved by the institutional animal care and use committee. Anesthetized canines with (n = 7) and without (n = 7) induced stenosis of the left anterior descending artery (LAD) underwent vasodilator challenges with hypercapnia and adenosine. LAD coronary blood flow velocity and free-breathing myocardial BOLD MR responses were measured at each intervention. Appropriate statistical tests were performed to evaluate measured quantitative changes in all parameters of interest in response to changes in partial pressure of carbon dioxide.
Results
Changes in myocardial BOLD MR signal were equivalent to reported changes with adenosine (11.2% ± 10.6 [hypercapnia, 10 mm Hg] vs 12% ± 12.3 [adenosine]; P = .75). In intact canines, there was a sigmoidal relationship between BOLD MR response and PetCO2 with most of the response occurring over a 10 mm Hg span. BOLD MR (17% ± 14 [hypercapnia] vs 14% ± 24 [adenosine]; P = .80) and coronary blood flow velocity (21% ± 16 [hypercapnia] vs 26% ± 27 [adenosine]; P > .99) responses were similar to that of adenosine infusion. BOLD MR signal changes in canines with LAD stenosis during hypercapnia and adenosine infusion were not different (1% ± 4 [hypercapnia] vs 6% ± 4 [adenosine]; P = .12).
Conclusion
Free-breathing T2-prepared myocardial BOLD MR imaging showed that hypercapnia of 10 mm Hg may provide a cardiac hyperemic stimulus similar to adenosine.
© RSNA, 2014
Introduction
Cardiac stress testing is the standard of care for diagnosing ischemic heart disease (1). It is performed in nearly 10 million patients each year in the United States alone. It is conventionally initiated with exercise to induce hyperemia, and it is coupled with imaging to identify stress-induced failure to increase perfusion, or frank hypoperfusion in myocardial territories. The approximately 50% of patients who find it difficult to perform sufficient exercise for an adequate test are administered coronary vasodilators, such as adenosine, which carries a rare but serious risk of heart attack and death (2). A limitation of this approach is that all drugs have complex pharmacologic and pharmacokinetic properties, which makes it difficult to achieve a consistent blood level across patients, and there is a large individual variation in response, even to a given blood level.
It has been suggested (3) that increases in the arterial partial pressure of carbon dioxide (PaCO2), a vasodilator of cerebral vessels known as hypercapnia, may increase coronary blood flow in similar measure (4–7). Hypercapnia as a myocardial vasodilator has some theoretical advantages over pharmacologic agents. It is a natural intrinsic molecule and can be administered noninvasively through the respiratory system. It would only need to be applied for 1 or 2 minutes for the target application of cardiac stress testing, and its effect would terminate in seconds. An increase of 10 mm Hg from resting values (∼40 mm Hg) is well tolerated in a population similar to that which requires cardiac stress testing (8). It has a favorable safety profile with sustained levels over three times that of resting with no adverse effect on organ function (9). The main technical drawback of the use of hypercapnia was difficulty of precise targeting, modulation, and monitoring of Paco2, particularly in spontaneously breathing humans.
Recent advances in computerized control of gas delivery (10,11) have enabled precise and independent control of end-tidal partial pressure of carbon dioxide (PetCO2) and end-tidal partial pressure of oxygen. Importantly, the PetCO2 attained with one of these methods, prospective end-tidal targeting, is equal to PaCO2 (11), which is the independent variable with respect to vasoreactivity (12). A second advance that was used for this study is the refinement of noninvasive magnetic resonance (MR) imaging–based approach for examination of cardiac perfusion (13–15). Myocardial blood oxygen level–dependent (BOLD) MR imaging (14,16) is a noninvasive (ie, free of radiation and contrast agents) and high-resolution imaging method that could provide details of myocardial blood flow based on the changes in oxidative state of hemoglobin (17), which reflects the balance between myocardial oxygen demand and supply.
The first hypothesis of this study was that an increase in PaCO2 of up to 10 mm Hg in healthy humans would result in changes in myocardial blood flow of the same order of magnitude as adenosine. To address this hypothesis, we compared the effects of hypercapnia on myocardial blood flow in healthy volunteers to published values with adenosine. The second hypothesis of this study was that hypercapnia would result in coronary vasodilation and increase myocardial blood flow to the same order of magnitude as adenosine and would generate similar myocardial perfusion patterns on myocardial BOLD MR images in intact canines and those with left anterior descending coronary artery (LAD) stenosis. In addressing both hypotheses, we used myocardial BOLD MR imaging as a surrogate measure of myocardial blood flow. The purpose of this study was to examine whether controlled and tolerable levels of hypercapnia may be an alternative to adenosine, a routinely used coronary vasodilator, in healthy human subjects and animals.
Materials and Methods
For brevity, only a concise version of our methods is outlined here; a detailed description can be found in Appendix E1 (online). This study was supported in part by Thornhill Research, who provided the RespirAct unit for the study, and Siemens Medical Solutions, who provided the imaging sequence. The authors who are not employees of Thornhill Research or Siemens Medical Solutions had control of inclusion of any data and information that may present a conflict of interest for those authors who are employees of these companies. J.A.F., O.S., and M.K. are employees of Thornhill Research, and X.B. is an employee of Siemens Medical Solutions. R.D., S.A.T., and D.L. are co-inventors on a pending patent on the use of carbon dioxide for examination of coronary artery disease (WO2012151583, US 61/482,956).
Human Studies
Healthy volunteers (n = 18) were recruited in accordance with the protocol that was reviewed and approved by the institutional review board. All imaging studies were performed on a 3-T clinical MR imaging system, and the electrocardiogram, pulse oximetry and noninvasive blood pressure of all subjects were continuously monitored. T2-prepared, free-breathing, BOLD MR acquisitions were prescribed along the short axis at the midventricular level at baseline PetCO2 (baseline), baseline plus 5 mm Hg (ie, +5), and baseline plus 10 mm Hg (ie, +10) 1 minute after PetCO2 stabilized.
Canine Studies
Mongrel canines (n = 14) were studied according to the protocols approved by our institution’s committee for animal care and use. Thoracotomies were performed and Doppler probes were implanted distal to the first branch of the LAD to enable measurement of coronary blood flow velocity. In a set of animals (n = 7), an externally actuated occluder was affixed proximal to the Doppler probe for the induction of LAD stenosis.
Before undergoing imaging, animals were fasted, sedated, anesthetized, intubated, and transferred to the imager table, and they were mechanically ventilated initially via a pneumatic anesthesia ventilator. A secondary sequential gas delivery circuit was interposed between the canine and the ventilator to administer only the gas supplied by RespirAct (Fig E1 [online]) during the imaging studies. Anesthesia was maintained with propofol and electrocardiography, oxygen saturation, heart rate, invasive systolic and diastolic blood pressures, and mean arterial blood pressure, and temperature were monitored.
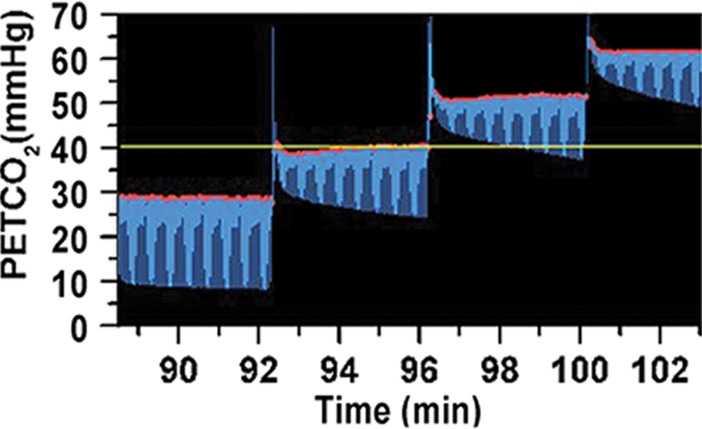
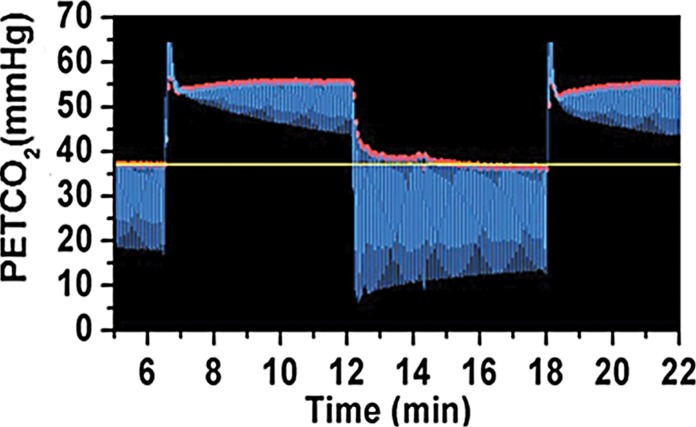
Because the relationship between PaCO2 and changes in coronary flow are unknown, we studied the flow response to a graded range of PetCO2, 30–60 mm Hg (in 10 mm Hg intervals), in seven canines. This PetCO2 range is 10 mm Hg below and 10 mm Hg above our test protocol of target change from 40 mm Hg to 50 mm Hg and would indicate the available dynamic range of coronary blood flow in response to hypercapnia (Fig E2 [online]). Coronary artery blood flow velocities were measured at 40 mm Hg and 50 mm Hg. The protocol used to examine the effect of coronary stenosis on blood flow is summarized in Figure E2 (online).
All canine studies were performed on the same MR imaging system (as human studies), and myocardial BOLD MR acquisitions were prescribed under spontaneous breathing at each targeted PaCO2 level. The order of adenosine and PaCO2 protocols was randomized, and imaging was performed over identical midventricular sections. In the occluder group, BOLD acquisitions were repeated at PetCO2 of approximately 60 mm Hg, 5 minutes after return to baseline PetCO2 of approximately 40 mm Hg, and 3 minutes after starting adenosine infusion (as before).
Image Analysis
Two expert reviewers (H.Y. and R.D., each with more than 4 years of experience in evaluation of cardiac MR images) evaluated the images in consensus. Reviewers were blinded to any additional patient and canine data.
For each experimental condition, the percentage of hyperemic BOLD response was computed as the myocardial BOLD signal intensity acquired under the condition that was normalized by the corresponding mean BOLD intensity value acquired at baseline and multiplied by 100%. From these values, the mean percentage of hyperemic BOLD response was computed for each study group. For the occluder group, the myocardium was divided into six segments and the percentage of hyperemic BOLD response was computed for each segment at PetCO2 of approximately 60 mm Hg and for adenosine infusion. Segmental BOLD responses were compared with contrast agent–enhanced cardiac MR acquisitions (Appendix E1 [online]).
Statistical Analysis
In humans, multiple repeated-measures analysis of variance were performed to test whether the rate-pressure product, and blood pressure (systolic, diastolic, and mean arterial) were different from baseline at the two different hypercapnic states. Repeated-measures analysis of variance was performed on the mean percentage of hyperemic BOLD response measured to determine whether the responses observed at plus 5 mm Hg and plus 10 mm Hg were different from the values reported by Arnold et al (14) for adenosine. All values are reported as mean ± standard deviation, and P value of less than .05 indicated significant difference. Statistical analysis was performed by using statistical software (SPSS, version 21.0; IBM SPSS Statistics, Armonk, NY).
In canines, repeated analysis of variance tests were performed to examine whether the rate-pressure product, heart rate, and blood pressure (systolic, diastolic, and mean arterial) were different between baseline, hypercapnia, and adenosine. Posthoc comparison with Bonferroni correction was used if the null hypothesis was rejected. Correlation analysis was performed between measured values of PaCO2 and PetCO2 at different physiologic states for canines from the ramp group. Percentage of hyperemic BOLD response from the ramp group was fitted to PetCO2 by using a sigmoidal model (3). The reproducibility of percentage of hyperemic BOLD response for a given PetCO2 was evaluated from measurements obtained in canines from repeat myocardial BOLD measurements in the ramp group and was examined with a paired t test. A paired t test was also performed to evaluate whether percentage of hyperemic BOLD response and percentage of hyperemic coronary blood flow velocity response obtained at the highest PetCO2 from the ramp group were different from the percentage of hyperemic responses obtained with adenosine. A two-way repeated-measures analysis of variance was used to test the effects of region (remote and affected) and type of vasodilator (hypercapnia and adenosine) and their interaction on percentage of hyperemic BOLD response. Cohen κ statistic was used to determine the level of concordance between the perfusion defects observed with hypercapnia and adenosine. For this purpose, the segments positive for perfusion defect were identified as those that conformed to mean segmental intensities that were at least 1 standard deviation below the mean intensity of the myocardium; all others were considered to be normal (no perfusion defect).
Results
Healthy Volunteer Studies
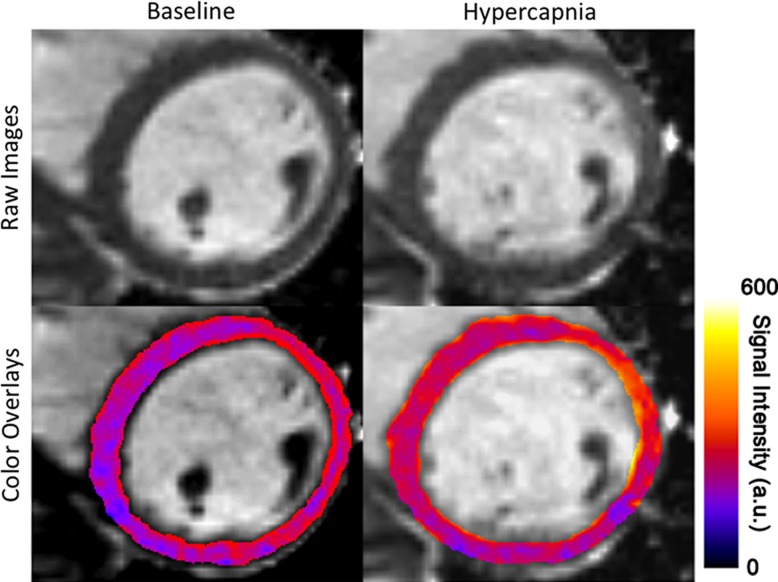
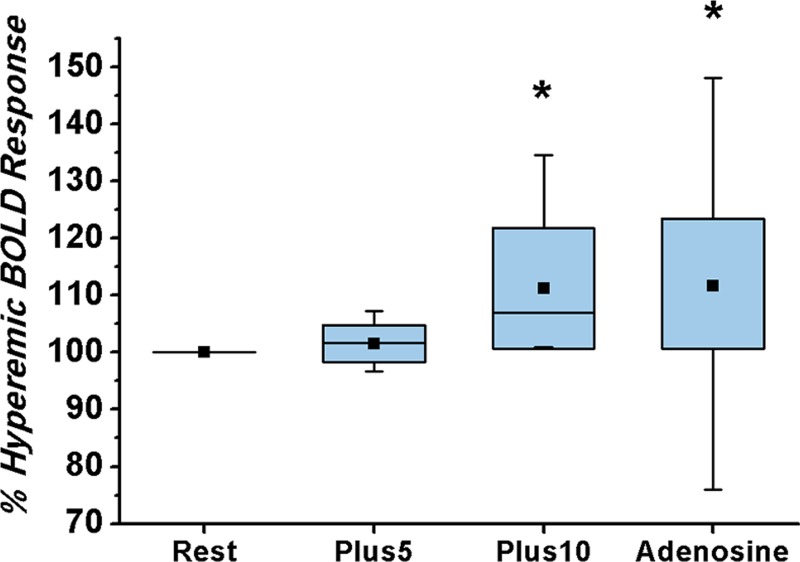
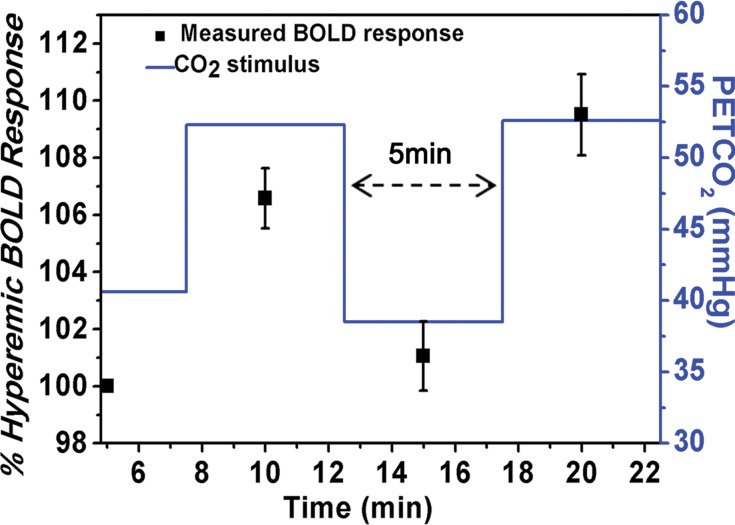
Physiologic parameters recorded from the volunteers at baseline and the two elevated PetCO2 (+5 and +10 mm Hg) are summarized in Table 1. Systolic, diastolic, and mean arterial blood pressure and rate-pressure product did not differ between conditions (baseline, +5, and +10 mm Hg). Myocardial BOLD MR images of a healthy volunteer at baseline and hypercapnia (+10 mm Hg) showed a marked increase in BOLD signal intensity in response to hypercapnia (Fig 1a). The response at plus 5 mm Hg was not greater than that at baseline (102% ± 3 vs 100%, respectively; P = .64). The response at plus 10 mm Hg was greater than the response at baseline (111% ± 10 vs 100%, respectively; P < .01) and at plus 5 mm Hg (111% ± 10 vs 102% ± 3, respectively; P < .01) (Fig 1b). The mean response at plus 10 mm Hg in our volunteers was not different from that reported for adenosine (111% ± 10 vs 112% ± 11, respectively; P = .75) (14).
Table 1.
Physiologic Parameters Measured in Healthy Volunteers during Cardiac MR Studies
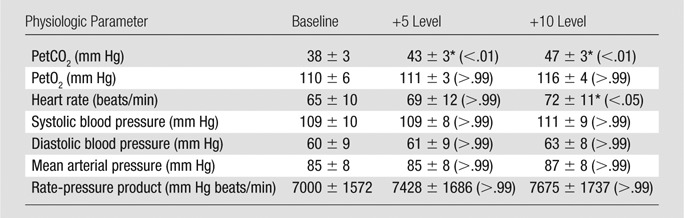
Note.—Data are means and data in parentheses are P values. P values are relative to baseline, and +5 and +10 denote 5 and 10 mm Hg of PetCO2, respectively, above baseline. PetO2 = partial pressure of oxygen in end-tidal (end-exhaled) gas.
Statistically significant.
Figure 1a:
Effect of changing arterial carbon dioxide on myocardial BOLD MR signal intensities in healthy humans. (a) Representative short-axis myocardial BOLD MR images collected from a volunteer at baseline (PetCO2 = 37 mm Hg) and hypercapnia (PetCO2 = 47 mm Hg). BOLD signal intensity increased during hypercapnia. For ease of visualization, color overlays of the left ventricle (with color bar showing BOLD signal intensity in arbitrary units [a.u.]) that correspond to the gray-scale images are shown directly below. (b) Box and whisker plot shows the dependence of percentage of hyperemic BOLD response on PetCO2, and standard dose of adenosine. Percentage of hyperemic response increased at higher PetCO2 values; response at +10 mm Hg (Plus10) did not differ from that of standard adenosine infusion. Percentage of hyperemic BOLD response for adenosine is from reported values in the literature (14). * = significant difference relative to resting (Rest) (P < .05). Top and bottom of boxes indicate upper limit, +1 standard deviation, and lower limit, −1 standard deviation, respectively; error bars (whiskers) are maximum and minimum of data. The mean and median are represented as a point and a band within the box, respectively. For adenosine, the whiskers represent the boundaries of first and 99th percentile of the data.
Figure 1b:
Effect of changing arterial carbon dioxide on myocardial BOLD MR signal intensities in healthy humans. (a) Representative short-axis myocardial BOLD MR images collected from a volunteer at baseline (PetCO2 = 37 mm Hg) and hypercapnia (PetCO2 = 47 mm Hg). BOLD signal intensity increased during hypercapnia. For ease of visualization, color overlays of the left ventricle (with color bar showing BOLD signal intensity in arbitrary units [a.u.]) that correspond to the gray-scale images are shown directly below. (b) Box and whisker plot shows the dependence of percentage of hyperemic BOLD response on PetCO2, and standard dose of adenosine. Percentage of hyperemic response increased at higher PetCO2 values; response at +10 mm Hg (Plus10) did not differ from that of standard adenosine infusion. Percentage of hyperemic BOLD response for adenosine is from reported values in the literature (14). * = significant difference relative to resting (Rest) (P < .05). Top and bottom of boxes indicate upper limit, +1 standard deviation, and lower limit, −1 standard deviation, respectively; error bars (whiskers) are maximum and minimum of data. The mean and median are represented as a point and a band within the box, respectively. For adenosine, the whiskers represent the boundaries of first and 99th percentile of the data.
Canine Studies
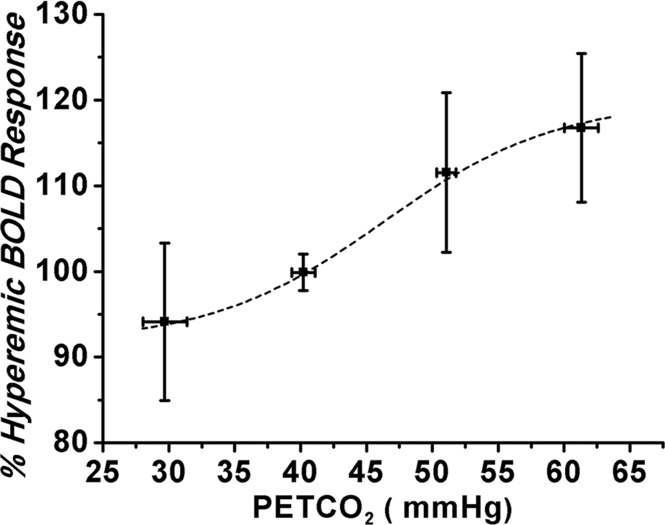
Figure 2 shows examples of raw data for PetCO2 for the ramp group (Fig 2c) and repeat measurement (Fig 2d) and their ensemble BOLD responses (Fig 2a, 2b). Figure 2a shows the flattening out of the slope of BOLD response to changes in PetCO2 above and below the 40–50 mm Hg range. Figure 2b shows a change in BOLD signal in response to hypercapnia that was greater than 6%. Mean percentage of hyperemic BOLD responses between the two hyperemic and baseline states did not differ (first cycle vs second cycle, 7% ± 3 vs 10% ± 4, respectively; P = .74). Mean percentage of hyperemic BOLD responses and percentage of hyperemic coronary blood flow velocity response during peak hypercapnia (mean PetCO2, 56 mm Hg ± 5; Table 1) and adenosine infusion were not statistically different (mean BOLD hypercapnia vs adenosine, 117% ± 14 vs 114% ± 23, respectively; P = .80; coronary blood flow velocity hypercapnia vs adenosine, 120% ± 15 vs 126% ± 22, respectively; P > .99; Fig E3 [online]). Physiologic responses to changes in PaCO2 are summarized in Table 2.
Figure 2a:
Relationship between percentage of hyperemic BOLD response and PetCO2 in canines. (a) Graph shows percentage of hyperemic BOLD response is directly related to PaCO2 (30–60 mm Hg) and shows a sigmoidal relation (data fitted to individual measurements) over the range of PetCO2 values studied. (b) Graph shows when the PetCO2 was modulated between 40 and 50 mm Hg, percentage of hyperemic BOLD response also showed equivalent changes. (c, d) Images show dynamic and precisely targeted changes in PetCO2 administered to the animals during imaging.
Figure 2c:
Relationship between percentage of hyperemic BOLD response and PetCO2 in canines. (a) Graph shows percentage of hyperemic BOLD response is directly related to PaCO2 (30–60 mm Hg) and shows a sigmoidal relation (data fitted to individual measurements) over the range of PetCO2 values studied. (b) Graph shows when the PetCO2 was modulated between 40 and 50 mm Hg, percentage of hyperemic BOLD response also showed equivalent changes. (c, d) Images show dynamic and precisely targeted changes in PetCO2 administered to the animals during imaging.
Figure 2d:
Relationship between percentage of hyperemic BOLD response and PetCO2 in canines. (a) Graph shows percentage of hyperemic BOLD response is directly related to PaCO2 (30–60 mm Hg) and shows a sigmoidal relation (data fitted to individual measurements) over the range of PetCO2 values studied. (b) Graph shows when the PetCO2 was modulated between 40 and 50 mm Hg, percentage of hyperemic BOLD response also showed equivalent changes. (c, d) Images show dynamic and precisely targeted changes in PetCO2 administered to the animals during imaging.
Figure 2b:
Relationship between percentage of hyperemic BOLD response and PetCO2 in canines. (a) Graph shows percentage of hyperemic BOLD response is directly related to PaCO2 (30–60 mm Hg) and shows a sigmoidal relation (data fitted to individual measurements) over the range of PetCO2 values studied. (b) Graph shows when the PetCO2 was modulated between 40 and 50 mm Hg, percentage of hyperemic BOLD response also showed equivalent changes. (c, d) Images show dynamic and precisely targeted changes in PetCO2 administered to the animals during imaging.
Table 2.
Physiologic Parameters Measured in Ramp Group during Cardiac MR Studies

Note—Data are mean values and data in parentheses are P values. P values are relative to baseline. PaO2 = partial pressure of oxygen in arterial blood.
Statistically significant.
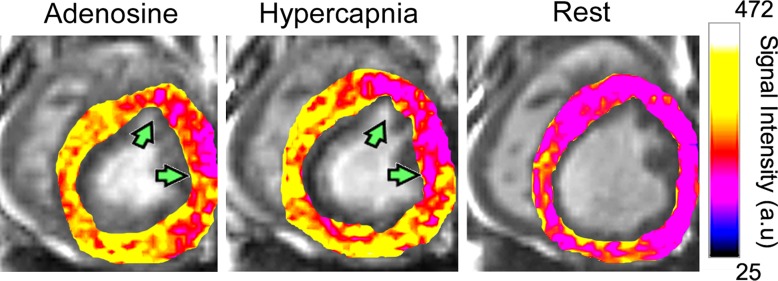
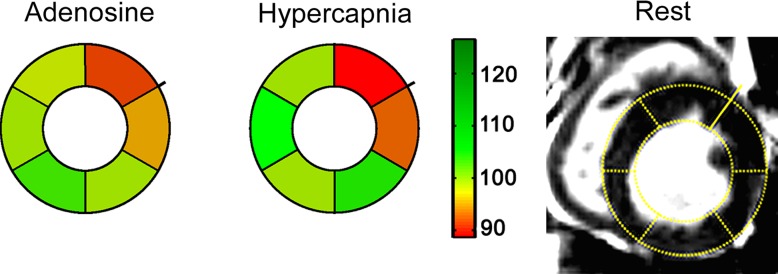
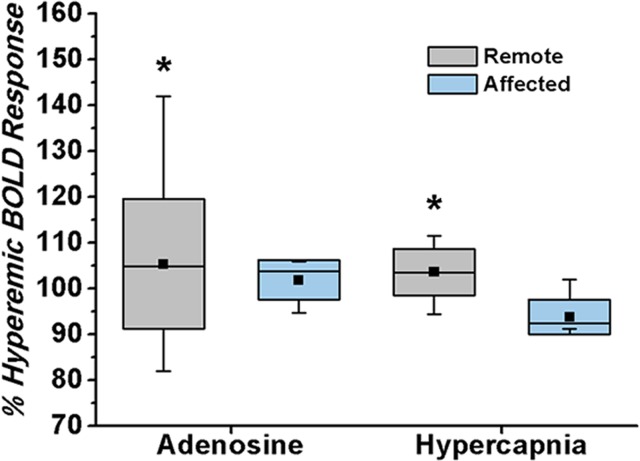
The hypercapnia-induced hyperemic response in healthy canines was visualized as an increase in BOLD signal intensity relative to baseline (Fig E4 [online]). Myocardial BOLD images from the occluder group showed diminished BOLD responses in the affected (ie, LAD) territory, but increased responses in the remote (ie, unaffected) territory in the presence of LAD stenosis (Fig 3). Percentage of hyperemic BOLD response from animals from the occluder group measured under hypercapnia (mean PetCO2, 55 mm Hg ± 3) and adenosine from the affected and remote segments in the presence of LAD stenosis showed no difference in the responses to the two vasodilatory stimuli in canines with patent coronary arteries (hypercapnia vs adenosine, 1% ± 4 vs 6% ± 4, respectively; P = .12); there was, however, a significant difference in percentage of hyperemic BOLD response between remote and affected segments in canines with LAD stenosis (remote vs affected, 105% ± 10 vs 97% ± 5, respectively; P = .01). The concordance between the segments identified as ischemic with adenosine and hypercapnic stimuli was high (κ = 0.75; 95% confidence interval: 0.48, 1.00), which indicated that the region and extent of ischemic and nonischemic territories identified with both forms of stimuli are not different.
Figure 3a:
BOLD response to adenosine versus hypercapnia in the presence of LAD stenosis in canines. (a) Representative color-overlaid BOLD MR images acquired during adenosine infusion, hypercapnia, and rest from a canine with LAD stenosis (arrows). Color bar shows myocardial BOLD MR signal intensity. Images acquired under adenosine infusion and at rest were obtained under normocapnia. (b) Bull’s eye plots constructed from images in panel a; the raw rest image with segmentation details is shown for reference. The point of radial extension on the epicardial contour of the bull’s eye plots and rest BOLD MR image indicates the right-ventricular insertion point. Color base is shown to index the magnitude of the BOLD response between rest and stress. Note the close correspondence in hypoperfused territories between hypercapnia and adenosine (arrows, a) and the normalized BOLD response of myocardial segments at both hyperemic states (adenosine and hypercapnia) relative to resting state in panel b. (c) Box plot shows percentage of hyperemic BOLD response from stenosis (affected) and remote (unaffected) segments in the presence of LAD stenosis in canines. Box plot presentation is the same as before. * = significant difference relative to affected territories (P < .05). Actual values in panel c are as follows: for adenosine, 1.01 ± 0.04 affected segments and 1.06 ± 0.04 unaffected segments; and for hypercapnia, 0.94 ± 0.04 affected segments and 1.04 ± 0.04 unaffected segments.
Figure 3b:
BOLD response to adenosine versus hypercapnia in the presence of LAD stenosis in canines. (a) Representative color-overlaid BOLD MR images acquired during adenosine infusion, hypercapnia, and rest from a canine with LAD stenosis (arrows). Color bar shows myocardial BOLD MR signal intensity. Images acquired under adenosine infusion and at rest were obtained under normocapnia. (b) Bull’s eye plots constructed from images in panel a; the raw rest image with segmentation details is shown for reference. The point of radial extension on the epicardial contour of the bull’s eye plots and rest BOLD MR image indicates the right-ventricular insertion point. Color base is shown to index the magnitude of the BOLD response between rest and stress. Note the close correspondence in hypoperfused territories between hypercapnia and adenosine (arrows, a) and the normalized BOLD response of myocardial segments at both hyperemic states (adenosine and hypercapnia) relative to resting state in panel b. (c) Box plot shows percentage of hyperemic BOLD response from stenosis (affected) and remote (unaffected) segments in the presence of LAD stenosis in canines. Box plot presentation is the same as before. * = significant difference relative to affected territories (P < .05). Actual values in panel c are as follows: for adenosine, 1.01 ± 0.04 affected segments and 1.06 ± 0.04 unaffected segments; and for hypercapnia, 0.94 ± 0.04 affected segments and 1.04 ± 0.04 unaffected segments.
Figure 3c:
BOLD response to adenosine versus hypercapnia in the presence of LAD stenosis in canines. (a) Representative color-overlaid BOLD MR images acquired during adenosine infusion, hypercapnia, and rest from a canine with LAD stenosis (arrows). Color bar shows myocardial BOLD MR signal intensity. Images acquired under adenosine infusion and at rest were obtained under normocapnia. (b) Bull’s eye plots constructed from images in panel a; the raw rest image with segmentation details is shown for reference. The point of radial extension on the epicardial contour of the bull’s eye plots and rest BOLD MR image indicates the right-ventricular insertion point. Color base is shown to index the magnitude of the BOLD response between rest and stress. Note the close correspondence in hypoperfused territories between hypercapnia and adenosine (arrows, a) and the normalized BOLD response of myocardial segments at both hyperemic states (adenosine and hypercapnia) relative to resting state in panel b. (c) Box plot shows percentage of hyperemic BOLD response from stenosis (affected) and remote (unaffected) segments in the presence of LAD stenosis in canines. Box plot presentation is the same as before. * = significant difference relative to affected territories (P < .05). Actual values in panel c are as follows: for adenosine, 1.01 ± 0.04 affected segments and 1.06 ± 0.04 unaffected segments; and for hypercapnia, 0.94 ± 0.04 affected segments and 1.04 ± 0.04 unaffected segments.
Discussion
The key finding of this study is that free-breathing T2-prepared myocardial BOLD MR responses under hypercapnia of 10 mm Hg and adenosine are not different. In particular, we found that in humans, a modest precisely controlled increase in baseline PetCO2 of 10 mm Hg was both tolerable and sufficient to elicit a measurable increase in myocardial BOLD signal intensity. In this pilot study, the extent of increase in myocardial BOLD response was not different from that of adenosine. The controlled studies in canines with and without coronary artery stenosis yielded additional important insights. First, canine studies showed a sigmoidal relationship between PaCO2 and BOLD response. These data also indicated that the mean increase in PaCO2 above baseline by 11 mm Hg encompassed a substantial portion of this dynamic range and resulted in an increase in myocardial blood flow similar to that elicited in response to a standard clinical dose of adenosine. Notably, the reproducible BOLD responses that were observed after repeat stimulation between baseline and equivalent hypercapnic states indicated that the response did not diminish or potentiate with repetition. This implied that hypercapnic stimulation may be reversible and repeatable and would therefore allow repeat testing at a single session. In addition, in canines with coronary artery stenosis, hypercapnia and adenosine resulted in similar BOLD responses. While we found the heart rate to increase during hypercapnia, we also found a concomitant decrease in blood pressure, and neither caused significant changes in the rate-pressure product, a metric that is commonly used to assess cardiac work (Tables 1, 2). Therefore, within the scope of this work (ie, in limited subjects, both animals and humans), it appears that increased heart rate associated with hypercapnia may not lead to an increase in oxygen demand. Nonetheless, additional studies are necessary to explore the mechanism of action of carbon dioxide mediation to coronary vasodilation and observed physiologic changes, such as heart rate, in response to hypercapnia. Collectively, these findings warrant more direct investigation as to whether hypercapnia could result in data equivalent to those of pharmacologic vasodilators for cardiac stress testing.
A major limitation of previous studies has been the inadequate control of the PaCO2, the actual physiologic stimulus to the coronary arteries. Tzou et al (18) found that even in the presence of mild hyperoxia (inspired oxygen concentration of 40%), an increase in PetCO2 by 10 mm Hg increased the proximal LAD flow velocity by 37%. However, Momen et al (19) did not detect any increase in coronary blood flow velocity in response to 5% carbon dioxide in oxygen. Their inspired gas was hyperoxic, which may have resulted in coronary vasoconstriction, and it is well known (11,20–22) that a constant inspired carbon dioxide concentration does not have a consistent effect on the PaCO2, and the authors did not measure PaCO2 or report PetCO2. Yokoyama et al (23) administered 7% carbon dioxide via a nonrebreathing mask and managed to increase the PaCO2 from 40.2 mm Hg ± 2.4 to only 43.1 mm Hg ± 2.7, which is a statistically significant increase, but a physiologically trivial one. It is not surprising that they did not find carbon dioxide–attributable coronary vasodilation (24,25). In our study, an increase in PetCO2 of 5 mm Hg was also insufficient to generate a measurable change in myocardial blood flow, but differed from these studies in important ways. First, we attained significantly greater hypercapnic stimulus of 10 mm Hg. Second, in our study, the PaCO2 was known from the PetCO2 (12). This relationship has only been shown to hold for sequential rebreathing and is used by prospective targeting (12,26). Third, in our subjects, normoxia was maintained to avoid vasoconstriction.
The recent study by Beaudin et al (7) is particularly relevant to our study. These investigators applied a euoxic hypercapnic stimulus of 9 mm Hg in humans and measured the effect on coronary sinus blood flow with MR imaging. They found that myocardial blood flow (indexed by coronary sinus flow) increased by 34% ± 4.6, which is similar to that of cerebral blood flow. After accounting for the increase in myocardial blood flow because of the work-induced increase in myocardial oxygen consumption, the increase in myocardial blood flow from vasodilation alone was 17.1% ± 5.7. This study, with a few caveats, is comparable to ours. Beaudin et al implemented a 14-second breath-hold to enable the cardiac MR imaging–based coronary sinus blood flow measures. Breath hold induces further substantial hypercapnia and rapidly developing hypoxia (27), which are both potent coronary artery vasodilators. Although outside of the breath hold, Beaudin et al accurately target PetCO2 and partial pressure of oxygen in end-tidal (end-exhaled) gas, unlike prospective targeting methods, these values do not reliably reflect the arterial values (28). Finally, the flow data were derived as a product of the cross-sectional area of the coronary sinus (measured from MR images) and the through-plane velocity of the blood. Given the size of the coronary sinus and the spatial and temporal resolution of the imaging, there may be differences between the actual size of the coronary sinus and that designated. Nevertheless, both their study and ours support consideration of hypercapnia as a stimulus for cardiac stress test.
Hypercapnia of about 10 mm Hg from baseline is benign because it is within the physiologic range and is commonly experienced in day-to-day living by most people (29,30). With few exceptions (eg, increased intracranial pressure) there is no known risk or contraindication to an increase of PaCO2 of 10 mm Hg. The main effects of hypercapnia are dyspnea and, in some, stimulation of the sympathetic nervous system that results in increase in heart rate and blood pressure. In a study of 400 consecutive patients who underwent a 10 mm Hg increase in Petco2 for cerebral blood flow studies by using BOLD MR imaging, over 90% of subjects tolerated the stimulus (8). Although we expect this number to be lower than 10% in the cardiac setting, there will likely be patients who will not tolerate this procedure. While there are ways to identify such patient population through a short screening test (ie, examination of tolerance to short hypercapnic stimulus as we have done in this study) before they undergo imaging, we do not expect that hypercapnic stimulus will become an exclusive provocative agent for cardiac stress testing in all subjects. Those who cannot tolerate or are contraindicated for hypercapnic stimulus may need to resort to pharmacological stress tests, should that be a suitable option for them. In every case, the sensation of dyspnea resolved within two to four breaths with restoration of normocapnia.
Because BOLD contrast is based on the oxygen saturation of hemoglobin, it reflects the balance between oxygen supply and demand, a feature that is valuable for the assessment of ischemic heart disease. This feature of myocardial BOLD MR imaging differs from standard perfusion imaging methods that provide information only on blood supply but not demand (31). In the healthy myocardium, adenosine infusion has been reported to elicit BOLD responses that are between 10% and 25% at 3 T (14,32), with the individual amplitude of response determined by the specific imaging approach. Among the various imaging approaches, the T2-prepared scheme has been studied by a number of different investigators and has also been validated against microspheres (13). In particular, the T2-prepared steady-state free precession approach at 3 T (14) has been shown to elicit mean hyperemic BOLD response from healthy myocardium between 15% and 17%. In this study we found that the free-breathing BOLD response at 3 T with T2-prepared steady-state free-precession acquisition scheme is 14% ± 24 with adenosine and 17% ± 14 with hypercapnia in canines, which are consistent with previous reports.
Healthy human subjects were exposed to hypercapnia but not adenosine because we considered it necessary to compare available data before performing invasive tests. We chose a 10 mm Hg hypercapnic stimulus for the human studies because considerable experience exists with this dose in an elderly population with cerebrovascular disease (8), who presumably have similar comorbidities (including ventilatory limitations) to those in patients with coronary disease.
While the observed coronary blood flow velocity responses in canines were similar to previous reports, some groups (18,33) showed that coronary blood flow velocity reserves in excess of 2.0 in healthy subjects. These differences likely stem from the differences in coronary blood flow velocity measurement position along the coronary artery (33). Moreover, because our stenosis studies relied on knowing the peak blood flow velocity before hypoperfusion, we were unable to randomize the PaCO2 levels. Given that the LAD constriction likely only introduced hypoperfusion and not ischemia, we do not anticipate the nonrandomization of PetCO2 between 40 mm Hg and 60 mm Hg to have any effect on our findings. However, additional studies are needed to confirm this.
In this study, we only evaluated one level of stenosis. Stenosis was created by reducing the blood flow to baseline levels in LAD under hypercapnia of 60 mm Hg; therefore, the true reduction of coronary diameter was not available to us. This aspect of the study was intended only as a proof of principle to identify redistributions of blood flow and compare the extent to that of adenosine for perspective.
To our knowledge, there are no validation studies in the literature for defining perfusion deficit territories based on signal thresholding of first-pass perfusion MR images. In this study with limited sample size, we observed that visual assessment of perfusion territories that correspond closely to 1 standard deviation decrease in segmental signal intensity relative to remote segments. However, the same analysis with 2 standard deviation criterion tended to underestimate the segments with perfusion defects, which may be explained on the basis of number of factors. Among these, partial volume effects, extent of perfusion territory, and animal-to-animal variation in supply territory of the coronary arteries are some viable possibilities. A more thorough analysis is expected to be necessary to establish a viable criterion, which is outside the scope of this study.
This was an exploratory study. We anticipate that additional studies are necessary before hypercapnia can be considered a suitable stimulus for cardiac stress testing.
Advances in Knowledge
■ In canines and healthy humans, myocardial blood oxygen level–dependent (BOLD) signal intensity changes associated with tolerable levels of hypercapnia (∼10 mm Hg increase in arterial partial pressure of carbon dioxide [PaCO2]) and clinical dose of adenosine were not different (17% ± 14 vs 14% ± 24 for hypercapnia and adenosine, respectively; P = .80).
■ Coronary artery blood flow velocity changes because of intravenous administration of standard (ie, clinical) adenosine dose and hypercapnia (∼10 mm Hg increase in PaCO2) were not different in canines (hypercapnia and adenosine, 21% ± 16 vs 26% ± 27, respectively; P > .99).
-
■ Myocardial BOLD responses observed after repeat stimulation between baseline and equivalent hypercapnic changes led to reproducible BOLD responses in canines (first and second cycle, 7% ± 3 vs 10% ± 4, respectively; P = .74).
■ Myocardial BOLD signal intensity changes in territories affected by coronary artery stenosis were not different between adenosine and hypercapnia (∼10 mm Hg increase in PaCO2) in canines (hypercapnia and adenosine, 1% ± 4 vs 6% ± 4, respectively; P = .12).
Implications for Patient Care
■ Tolerable levels of controlled iso-oxic hypercapnia may potentially be an alternative to pharmacologic stress agents, such as adenosine, in the examination of myocardial perfusion associated with ischemic heart disease.
■ The proposed imaging approach, combined with controlled and tolerable hypercapnia, may pave the way for a future noninvasive cardiac stress examination that is repeatable, rapidly reversible, and free of exogenous contrast material; such an approach may be valuable in the care of patients who are contraindicated for contrast material and/or adenosine but require multiple cardiac stress tests.
APPENDIX
SUPPLEMENTAL FIGURES
Acknowledgments
Acknowledgment
We thank Laura Smith for her help with imaging studies.
Received November 7, 2013; revision requested December 13; revision received January 16, 2014; accepted January 23; final version accepted February 18.
Funding: This research was supported by the National Institutes of Health (grant number HL091989).
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Heart, Lung, and Blood Institute or the National Institutes of Health.
Disclosures of Conflicts of Interest: H.J.Y. disclosed no relevant relationships. R.Y. disclosed no relevant relationships. R.T. disclosed no relevant relationships. I.C. disclosed no relevant relationships. M.K. Activities related to the present article: author is full-time employee of Thornhill Research. Activities not related to the present article: money paid to author for stock options in Thornhill Research. Other relationships: disclosed no relevant relationships. A.K. disclosed no relevant relationships. O.S. Activities related to the present article: author receives consulting fees from Thornhill Research. Activities not related to the present article: disclosed no relevant relationships. Other relationships: disclosed no relevant relationships. B.S. disclosed no relevant relationships. J.T. disclosed no relevant relationships. X.B. Activities related to the present article: author is full-time employee of Siemens Healthcare. Activities not related to the present article: disclosed no relevant relationships. Other relationships: disclosed no relevant relationships. S.A.T. Activities related to the present article: disclosed no relevant relationships. Activities not related to the present article: money paid to author for patent pending to Cedars Sinai Medical Center entitles “Assessment of Coronary Heart Disease with Carbon Dioxide. Other relationships: disclosed no relevant relationships. D.L. disclosed no relevant relationships. A.H.C. disclosed no relevant relationships. J.A.F. Activities related to the present article: author is full-time employee of Thornhill Research. Activities not related to the present article: money paid to author’s institution for patents related to blood gas control through the lungs. Other relationships: disclosed no relevant relationships. R.D. Activities related to the present article: disclosed no relevant relationships. Activities not related to the present article: money paid to author’s institution for patent pending for Vascularix. Other relationships: disclosed no relevant relationships.
Abbreviations:
- BOLD
- blood oxygen level dependent
- LAD
- left anterior descending coronary artery
- PaCO2
- arterial partial pressure of carbon dioxide
- PetCO2
- end-tidal partial pressure of carbon dioxide
References
- 1.Hachamovitch R, Hayes SW, Friedman JD, Cohen I, Berman DS. Comparison of the short-term survival benefit associated with revascularization compared with medical therapy in patients with no prior coronary artery disease undergoing stress myocardial perfusion single photon emission computed tomography. Circulation 2003;107(23):2900–2907. [DOI] [PubMed] [Google Scholar]
- 2.FDA warns of rare but serious risk of heart attack and death with cardiac nuclear stress test drugs Lexiscan (regadenoson) and Adenoscan (adenosine) . http://www.fda.gov/Drugs/DrugSafety/ucm375654.htm. Published 2013. Accessed March 14, 2014.
- 3.Harper AM, Glass HI. Effect of alterations in the arterial carbon dioxide tension on the blood flow through the cerebral cortex at normal and low arterial blood pressures. J Neurol Neurosurg Psychiatry 1965;28(5):449–452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ledingham IM, McBride TI, Parratt JR, Vance JP. The effect of hypercapnia on myocardial blood flow and metabolism. J Physiol (Paris) 1970;210(1):87–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Feigl EO. Coronary physiology. Physiol Rev 1983;63(1):1–205. [DOI] [PubMed] [Google Scholar]
- 6.Guensch DP, Fischer K, Flewitt JA, Friedrich MG. Impact of intermittent apnea on myocardial tissue oxygenation—a study using oxygenation-sensitive cardiovascular magnetic resonance. PLoS ONE 2013;8(1):e53282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Beaudin AE, Brugniaux JV, Vöhringer M, et al. Cerebral and myocardial blood flow responses to hypercapnia and hypoxia in humans. Am J Physiol Heart Circ Physiol 2011;301(4):H1678–H1686. [DOI] [PubMed] [Google Scholar]
- 8.Spano VR, Mandell DM, Poublanc J, et al. CO2 blood oxygen level-dependent MR mapping of cerebrovascular reserve in a clinical population: safety, tolerability, and technical feasibility. Radiology 2013;266(2):592–598. [DOI] [PubMed] [Google Scholar]
- 9.Feihl F, Perret C. Permissive hypercapnia. How permissive should we be? Am J Respir Crit Care Med 1994;150(6 Pt 1):1722–1737. [DOI] [PubMed] [Google Scholar]
- 10.Wise RG, Pattinson KT, Bulte DP, et al. Dynamic forcing of end-tidal carbon dioxide and oxygen applied to functional magnetic resonance imaging. J Cereb Blood Flow Metab 2007;27(8):1521–1532. [DOI] [PubMed] [Google Scholar]
- 11.Prisman E, Slessarev M, Azami T, Nayot D, Milosevic M, Fisher J. Modified oxygen mask to induce target levels of hyperoxia and hypercarbia during radiotherapy: a more effective alternative to carbogen. Int J Radiat Biol 2007;83(7):457–462. [DOI] [PubMed] [Google Scholar]
- 12.Ito S, Mardimae A, Han J, et al. Non-invasive prospective targeting of arterial P(CO2) in subjects at rest. J Physiol 2008;586(Pt 15):3675–3682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fieno DS, Shea SM, Li Y, Harris KR, Finn JP, Li D. Myocardial perfusion imaging based on the blood oxygen level-dependent effect using T2-prepared steady-state free-precession magnetic resonance imaging. Circulation 2004;110(10):1284–1290. [DOI] [PubMed] [Google Scholar]
- 14.Arnold JR, Karamitsos TD, Bhamra-Ariza P, et al. Myocardial oxygenation in coronary artery disease: insights from blood oxygen level-dependent magnetic resonance imaging at 3 tesla. J Am Coll Cardiol 2012;59(22):1954–1964. [DOI] [PubMed] [Google Scholar]
- 15.Tsaftaris SA, Tang R, Zhou X, Li D, Dharmakumar R. Ischemic extent as a biomarker for characterizing severity of coronary artery stenosis with blood oxygen-sensitive MRI. J Magn Reson Imaging 2012;35(6):1338–1348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Karamitsos TD, Leccisotti L, Arnold JR, et al. Relationship between regional myocardial oxygenation and perfusion in patients with coronary artery disease: insights from cardiovascular magnetic resonance and positron emission tomography. Circ Cardiovasc Imaging 2010;3(1):32–40. [DOI] [PubMed] [Google Scholar]
- 17.Pauling L, Coryell CD. The magnetic properties and structure of hemoglobin, oxyhemoglobin and carbonmonoxyhemoglobin. Proc Natl Acad Sci U S A 1936;22(4):210–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tzou WS, Korcarz CE, Aeschlimann SE, Morgan BJ, Skatrud JB, Stein JH. Coronary flow velocity changes in response to hypercapnia: assessment by transthoracic Doppler echocardiography. J Am Soc Echocardiogr 2007;20(4):421–426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Momen A, Mascarenhas V, Gahremanpour A, et al. Coronary blood flow responses to physiological stress in humans. Am J Physiol Heart Circ Physiol 2009;296(3):H854–H861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Baddeley H, Brodrick PM, Taylor NJ, et al. Gas exchange parameters in radiotherapy patients during breathing of 2%, 3.5% and 5% carbogen gas mixtures. Br J Radiol 2000;73(874):1100–1104. [DOI] [PubMed] [Google Scholar]
- 21.Foëx P, Ryder WA. Effect of CO2 on the systemic and coronary circulations and on coronary sinus blood gas tensions. Bull Eur Physiopathol Respir 1979;15(4):625–638. [PubMed] [Google Scholar]
- 22.Mark CI, Slessarev M, Ito S, Han J, Fisher JA, Pike GB. Precise control of end-tidal carbon dioxide and oxygen improves BOLD and ASL cerebrovascular reactivity measures. Magn Reson Med 2010;64(3):749–756. [DOI] [PubMed] [Google Scholar]
- 23.Yokoyama I, Inoue Y, Kinoshita T, Itoh H, Kanno I, Iida H. Heart and brain circulation and CO2 in healthy men. Acta Physiol (Oxf) 2008;193(3):303–308. [DOI] [PubMed] [Google Scholar]
- 24.Case RB, Greenberg H. The response of canine coronary vascular resistance to local alterations in coronary arterial P CO2. Circ Res 1976;39(4):558–566. [DOI] [PubMed] [Google Scholar]
- 25.Powers ER, Bannerman KS, Fitz-James I, Cannon PJ. Effect of elevations of coronary artery partial pressure of carbon dioxide (PCO2) on coronary blood flow. J Am Coll Cardiol 1986;8(5):1175–1181. [DOI] [PubMed] [Google Scholar]
- 26.Fierstra J, Machina M, Battisti-Charbonney A, Duffin J, Fisher JA, Minkovich L. End-inspiratory rebreathing reduces the end-tidal to arterial PCO2 gradient in mechanically ventilated pigs. Intensive Care Med 2011;37(9):1543–1550. [DOI] [PubMed] [Google Scholar]
- 27.Sasse SA, Berry RB, Nguyen TK, Light RW, Mahutte CK. Arterial blood gas changes during breath-holding from functional residual capacity. Chest 1996;110(4):958–964. [DOI] [PubMed] [Google Scholar]
- 28.St Croix CM, Cunningham DA, Kowalchuk JM, et al. Estimation of arterial PCO2 in the elderly. J Appl Physiol (1985) 1995;79(6):2086–2093. [DOI] [PubMed] [Google Scholar]
- 29.Midgren B, Hansson L. Changes in transcutaneous PCO2 with sleep in normal subjects and in patients with chronic respiratory diseases. Eur J Respir Dis 1987;71(5):388–394. [PubMed] [Google Scholar]
- 30.Trulock EP. Arterial blood gases. In: Walker HK, Hall WD, Hurst JW, eds. Clinical methods: the history, physical, and laboratory examinations. 3rd ed. Boston, Mass: Butterworths, 1990. [PubMed] [Google Scholar]
- 31.Karamitsos TD, Dass S, Suttie J, et al. Blunted myocardial oxygenation response during vasodilator stress in patients with hypertrophic cardiomyopathy. J Am Coll Cardiol 2013;61(11):1169–1176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dharmakumar R, Arumana JM, Tang R, Harris K, Zhang Z, Li D. Assessment of regional myocardial oxygenation changes in the presence of coronary artery stenosis with balanced SSFP imaging at 3.0 T: theory and experimental evaluation in canines. J Magn Reson Imaging 2008;27(5):1037–1045. [DOI] [PubMed] [Google Scholar]
- 33.Ashikawa K, Kanatsuka H, Suzuki T, Takishima T. Phasic blood flow velocity pattern in epimyocardial microvessels in the beating canine left ventricle. Circ Res 1986;59(6):704–711. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.