In their groundbreaking article in this month’s Radiology, Sheth et al provide an exciting new alternative for improving biopsy guidance based on optical fluorescence.
Abstract
Summary
Sheth et al present a fascinating glimpse into the future of imaging-guided biopsies. Increasingly, such biopsies are being performed by fusing two or more modalities, such as computed tomography (CT) and ultrasonography (US). To this list should be added optical fluorescence guidance, which will complement all the advances hitherto developed for conventional imaging guidance. Similar progress is being made in the fields of endoscopy and surgery, and it is becoming clearer that optical fluorescence may light the path for many medical procedures in the “NIR,” or near-infrared, future.
The Setting
The percutaneous biopsy has long been one of the most valuable diagnostic procedures offered by radiologists. Whereas endoscopists and surgeons use direct vision to guide biopsies, radiologists use US, CT, and magnetic resonance (MR) imaging to guide needles into hard-to-reach lesions. As a real-time method, US is ideal for demonstrating that the biopsy needle is within the target, but not all lesions are amenable to US guidance (eg, those in the chest or bone). When using CT or MR imaging, even slight patient motion between the image acquisition and the placement of the biopsy needle may lead to uncertainty regarding the sample location. It is often only the pathologist, several days later, who determines whether the biopsy sample acquired was adequate for diagnosis. In their groundbreaking article in this month’s Radiology, Sheth et al provide an exciting new alternative for improving biopsy guidance based on optical fluorescence (1).
The Science
Fluorescence requires an excitation light, which, in this case, is provided by a laser, with a camera to detect the emission from the fluorophore. The fluorophore—in this case indocyanine green (ICG)—is excited by the laser, and the emission light is received by the camera with a filter in front of its lens (to filter out the excitation light). The investigators developed a tiny optical endoscope, replete with laser and video camera, that fits coaxially into the sheath of a biopsy needle. In their experiments, mice with tumors implanted in their livers were injected with ICG, which binds albumin and leaks into vascular parts of a tumor. Excitation in the near-infrared range results in near-infrared fluorescence, a part of the visual spectrum with the longest light penetration in tissue (2). ICG is a U.S. Food and Drug Administration (FDA)–approved optical agent that has been used clinically in ophthalmology and for hepatic function tests for more than 30 years, and, thus, there is a large amount of safety data on it (3).
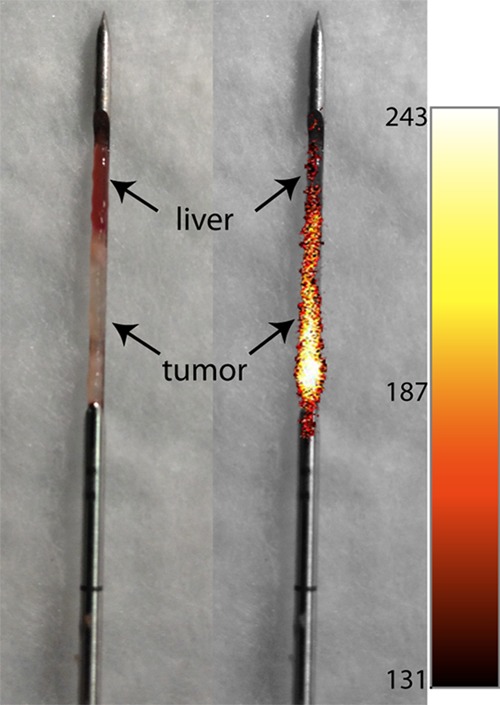
In the experiment of Sheth et al, tumors implanted in the liver were readily apparent with the near-infrared needle endoscope, and biopsy samples obtained from the tumors showed a sharp demarcation between the tumor (fluorescent) and normal (nonfluorescent) parts of the biopsy core, as shown in Figure 6b in their article and reproduced here (Fig 1), thus verifying the proper sampling of the lesion. Although ICG is somewhat nonspecific, it will accumulate in angiogenic tumors, such as those used in this study. Thus, ICG-enhanced biopsies could be useful in guiding biopsy needles to the lesion and also in confirming that the lesion has been sampled properly by looking for traces of the optical probe in the specimen.
The Practice
This development is not only exciting but highly practical. The needle endoscope is simply a miniaturization of a conventional endoscope and therefore should not face substantial regulatory barriers (4). ICG is already approved for clinical use and could be used by physicians off-label, at least in the short term, while clinical data accumulate to justify FDA approval for this indication. Thus, the method described could very well be used in humans very shortly, if it hasn’t already. One disadvantage is that ICG enhancement is relatively weak and nonspecific. It may be possible to use higher–quantum yield fluorophores, thus enabling deeper penetration of light (5). These brighter fluorophores could be coupled to more highly specific targeting ligands that would help the radiologist sample specific parts of the tumor, rather than just the vascular parts. For instance, to determine if a tumor expressed MET (a receptor associated with an aggressive phenotype), an optical MET probe could be injected into the patient. The radiologist would sample the area that “lit up” by using a needle endoscope, thus confirming the presence of MET and suggesting that the patient could benefit from the use of MET inhibitors. Moreover, the sensitivity of the needle endoscope could be increased by combining all the light fibers of the endoscope into one detector, analogous to a photomultiplier tube on a Geiger counter. In that mode, an audible count rate that reflects photon flux could guide the operator to the right location in real time and confirm proper sampling. This might require far less light than an optical image and could potentially expand the range of optical fluorescence guidance up to several centimeters within tissue. Conventional imaging would still be required to get the needle close to the lesion, but the final few centimeters could be guided optically.
Footnotes
See also Sheth et al.
Disclosures of Conflicts of Interest: : P.L.C. No relevant conflicts of interest to disclose.
References
- 1.Sheth RA, Heidari P, Esfahani SA, Wood BJ, Mahmood U. Interventional optical molecular imaging guidance during percutaneous biopsy. Radiology 2014;271(3):770–777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Moody ED, Viskari PJ, Colyer CL. Non-covalent labeling of human serum albumin with indocyanine green: a study by capillary electrophoresis with diode laser-induced fluorescence detection. J Chromatogr B Biomed Sci Appl 1999;729(1-2):55–64. [DOI] [PubMed] [Google Scholar]
- 3.Alford R, Simpson HM, Duberman J, et al. Toxicity of organic fluorophores used in molecular imaging: literature review. Mol Imaging 2009;8(6):341–354. [PubMed] [Google Scholar]
- 4.Ishizawa T, Fukushima N, Shibahara J, et al. Real-time identification of liver cancers by using indocyanine green fluorescent imaging. Cancer 2009;115(11):2491–2504. [DOI] [PubMed] [Google Scholar]
- 5.Liu H, Xu CT, Dumlupinar G, Jensen OB, Andersen PE, Andersson-Engels S. Deep tissue optical imaging of upconverting nanoparticles enabled by exploiting higher intrinsic quantum yield through use of millisecond single pulse excitation with high peak power. Nanoscale 2013;5(20):10034–10040. [DOI] [PubMed] [Google Scholar]


