Abstract
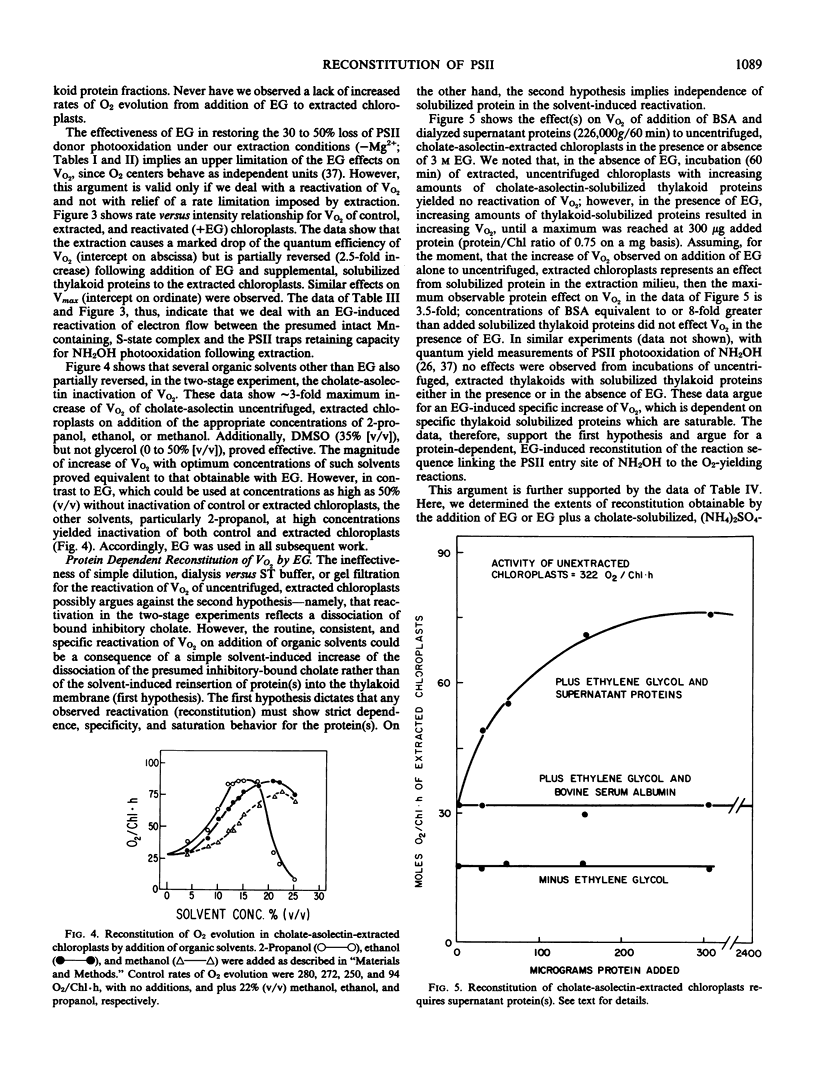
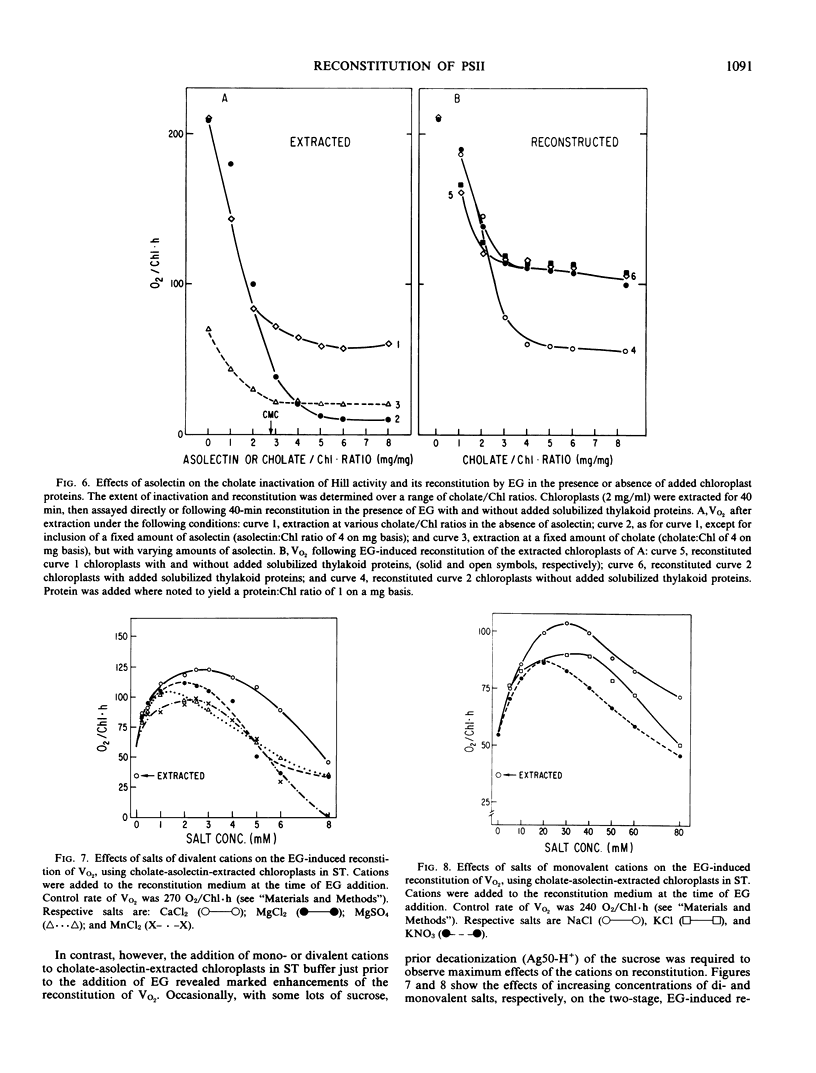
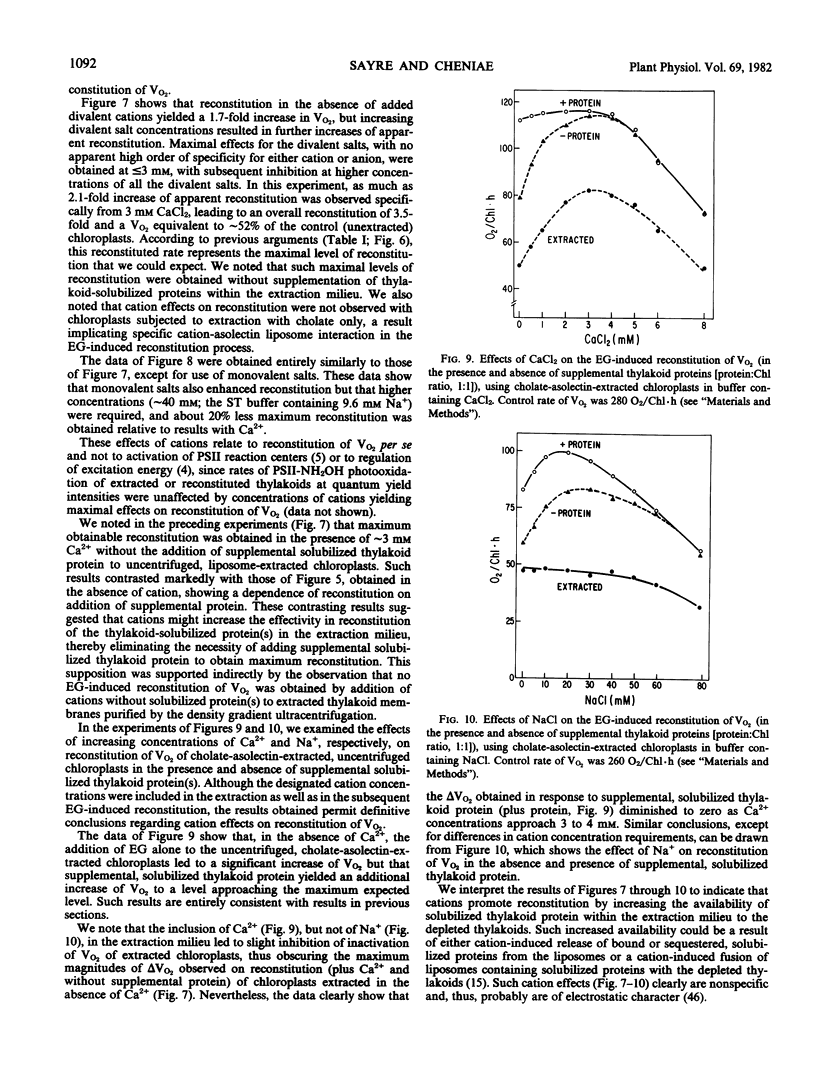
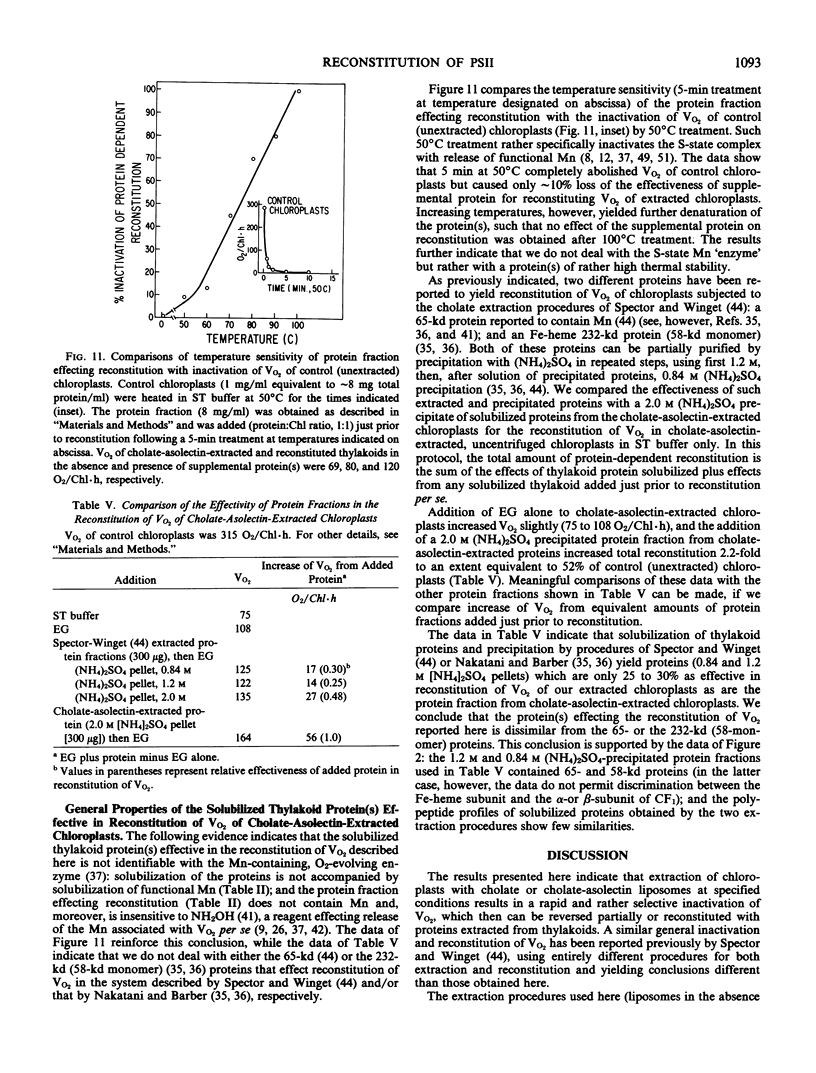
Extraction of spinach (Spinacia oleracea L.) chloroplasts with cholate-asolectin in the absence of Mg2+ results in the rapid and selective inactivation of O2 evolution and a partial (30 to 40%) loss of photosystem II (PSII) donor activity without extraction of thylakoid bound Mn (∼5 to 6 Mn per 400 Chlorophyll). Inclusion of ethylene glycol in the extractions inhibits loss of O2 evolution and results in quantitative and qualitative differences in proteins solubilized but does not significantly inhibit the partial loss of PSII donor activity. Similarly, in two stage experiments (extraction followed by addition of organic solvent and solubilized thylakoid protein), O2 evolution (V and Vmax) of extracted chloroplasts is enhanced approximately 2.5- to 8-fold. However, PSII donor activity remains unaffected. This reversal of cholate inactivation of O2 evolution can be induced by solvents including ethanol, methanol, 2-propanol, and dimethyl sulfoxide. Such enhancements of O2 evolution specifically required cholate-solubilized proteins, which are insensitive to NH2OH and are only moderately heat-labile. NH2OH extraction of chloroplasts prior to cholate-asolectin extraction abolishes reconstitutability of O2 evolution. Thus, the protein(s) affecting reconstitution is unlike those of the O2·Mn enzyme. The specific activity of the protein fraction effecting reconstitution of O2 evolution is greatest in fractions depleted of the reported Mn-containing, 65-kilodalton, and the Fe-heme, 232-kilodalton (58-kilodalton monomer), proteins. Divalent (∼3 millimolar) and monovalent (∼30 millimolar) cations do not affect reconstitution of PSII donor activity but do affect reconstitution of O2 evolution by decreasing the protein(s) concentration required for reconstitution of O2 evolution in nonfractionated, cholate-asolectin extractions. The data indicate a reconstitution of the PSII segment linking the PSII secondary donor(s) to O2-evolving centers.
Full text
PDF

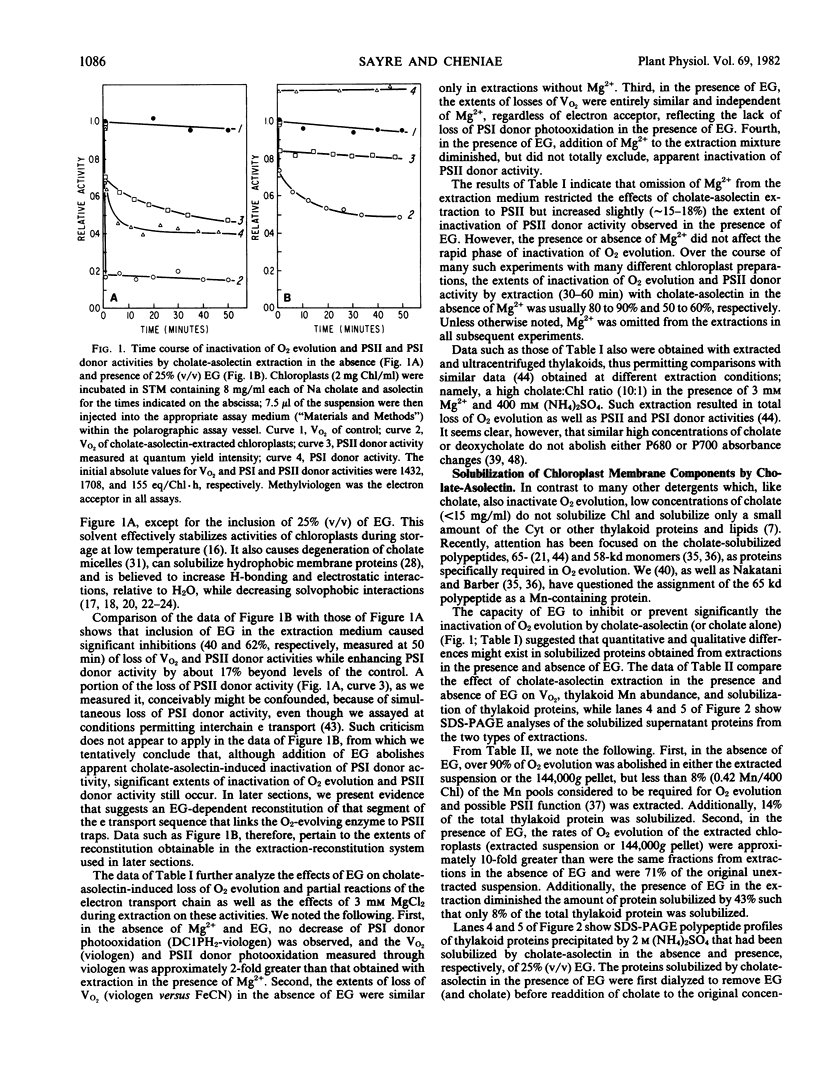
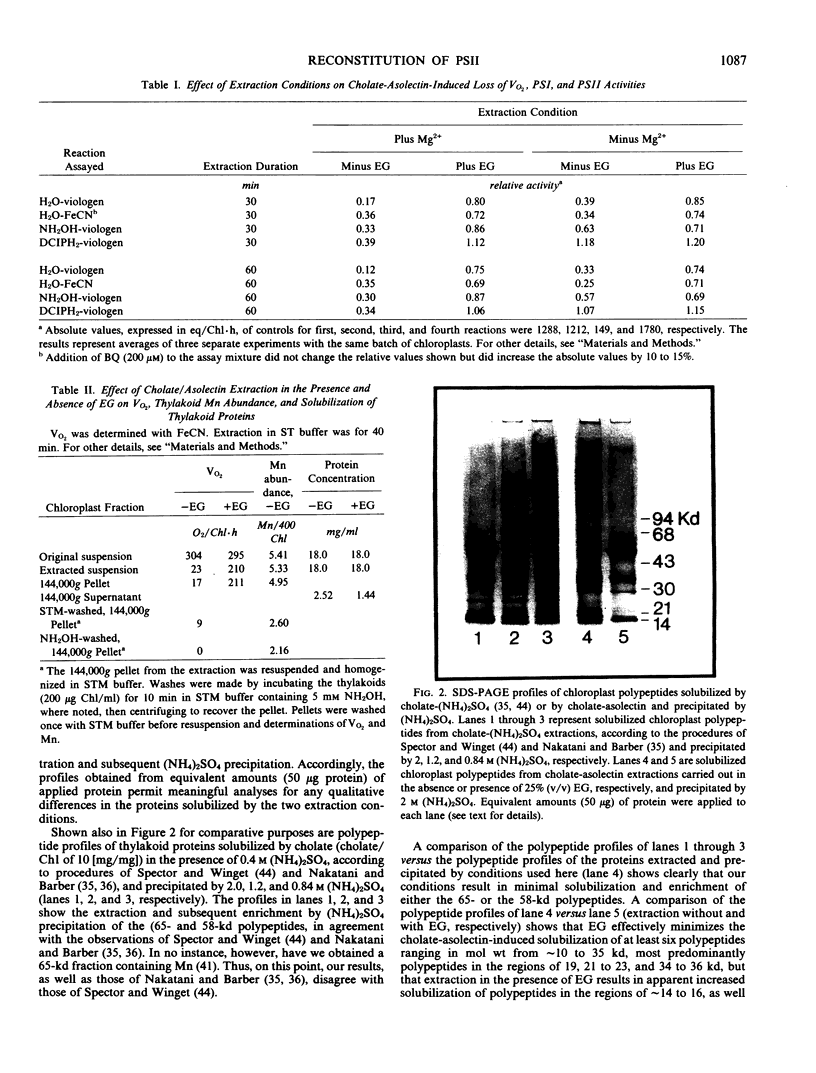
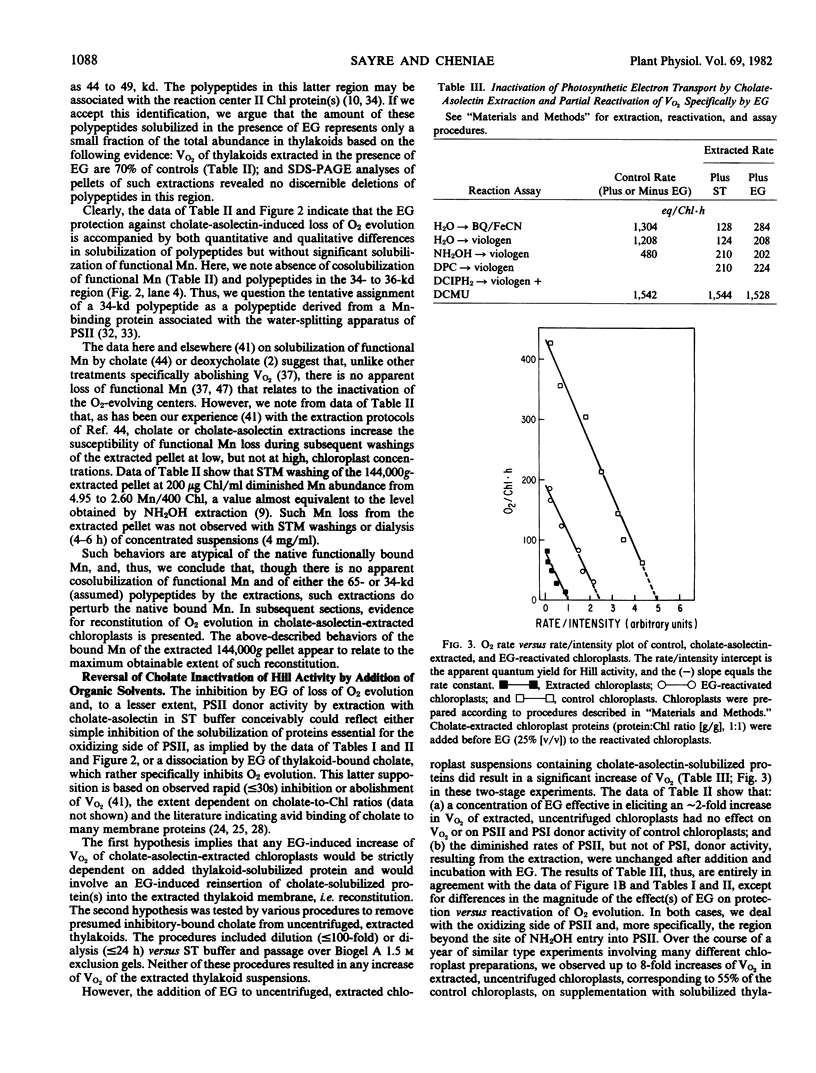



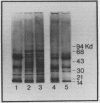
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson W. M., Fisher R. R. Purification and partial characterization of bovine heart mitochondrial pyridine dinucleotide transhydrogenase. Arch Biochem Biophys. 1978 Apr 15;187(1):180–190. doi: 10.1016/0003-9861(78)90021-8. [DOI] [PubMed] [Google Scholar]
- Arnon D. I. COPPER ENZYMES IN ISOLATED CHLOROPLASTS. POLYPHENOLOXIDASE IN BETA VULGARIS. Plant Physiol. 1949 Jan;24(1):1–15. doi: 10.1104/pp.24.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonaventura C., Myers J. Fluorescence and oxygen evolution from Chlorella pyrenoidosa. Biochim Biophys Acta. 1969;189(3):366–383. doi: 10.1016/0005-2728(69)90168-6. [DOI] [PubMed] [Google Scholar]
- Bose S., Arntzen C. J. Reversible inactivation of photosystem II reaction centers in cation-depleted chloroplast membranes. Arch Biochem Biophys. 1978 Jan 30;185(2):567–575. doi: 10.1016/0003-9861(78)90202-3. [DOI] [PubMed] [Google Scholar]
- Bouges-Bocquet B. Kinetic models for the electron donors of photosystem II of photosynthesis. Biochim Biophys Acta. 1980 Dec;594(2-3):85–103. doi: 10.1016/0304-4173(80)90006-3. [DOI] [PubMed] [Google Scholar]
- Carmeli C., Racker E. Partial resolution of the enzymes catalyzing photophosphorylation. XIV. Reconstitution of chlorophyll-deficient vesicles catalyzing phosphate-adenosine triphosphate exchange. J Biol Chem. 1973 Dec 10;248(23):8281–8287. [PubMed] [Google Scholar]
- Cheniae G. M., Martin I. F. Effects of Hydroxylamine on Photosystem II: I. Factors Affecting the Decay of O(2) Evolution. Plant Physiol. 1971 Apr;47(4):568–575. doi: 10.1104/pp.47.4.568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheniae G. M., Martin I. F. Sites of function of manganese within photosystem II. Roles in O2 evolution and system II. Biochim Biophys Acta. 1970 Mar 3;197(2):219–239. doi: 10.1016/0005-2728(70)90033-2. [DOI] [PubMed] [Google Scholar]
- Chua N. H., Bennoun P. Thylakoid membrane polypeptides of Chlamydomonas reinhardtii: wild-type and mutant strains deficient in photosystem II reaction center. Proc Natl Acad Sci U S A. 1975 Jun;72(6):2175–2179. doi: 10.1073/pnas.72.6.2175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cramer W. A., Whitmarsh J., Low P. S. Differential scanning calorimetry of chloroplast membranes: identification of an endothermic transition associated with the water-splitting complex of photosystem II. Biochemistry. 1981 Jan 6;20(1):157–162. doi: 10.1021/bi00504a026. [DOI] [PubMed] [Google Scholar]
- Den Haan G. A., Gorter De Vries H., Duysens L. N. Correlation between flash-induced oxygen evolution and fluorescence yield kinetics in the 0 to 16 mus range in Chlorella pyyrenoidosa during incubation with hydroxylamine. Biochim Biophys Acta. 1976 May 14;430(2):265–281. doi: 10.1016/0005-2728(76)90084-0. [DOI] [PubMed] [Google Scholar]
- Dismukes G. C., Siderer Y. Intermediates of a polynuclear manganese center involved in photosynthetic oxidation of water. Proc Natl Acad Sci U S A. 1981 Jan;78(1):274–278. doi: 10.1073/pnas.78.1.274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eytan G. D., Matheson M. J., Racker E. Incorporation of mitochondrial membrane proteins into liposomes containing acidic phospholipids. J Biol Chem. 1976 Nov 10;251(21):6831–6837. [PubMed] [Google Scholar]
- FASMAN G. D., LINDBLOW C., GROSSMAN L. THE HELICAL CONFORMATIONS OF POLYCYTIDYLIC ACID: STUDIES ON THE FORCES INVOLVED. Biochemistry. 1964 Aug;3:1015–1021. doi: 10.1021/bi00896a002. [DOI] [PubMed] [Google Scholar]
- Farkas D. L., Malkin S. Cold storage of isolated class C chloroplasts: optimal conditions for stabilization of photosynthetic activities. Plant Physiol. 1979 Dec;64(6):942–947. doi: 10.1104/pp.64.6.942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox J. W., Owens D. P., Wong K. P. Structural changes of ribosome by the action of ethylene glycol. Biochemistry. 1978 Apr 18;17(8):1357–1364. doi: 10.1021/bi00601a001. [DOI] [PubMed] [Google Scholar]
- GORDON J. A., JENCKS W. P. The relationship of structure to the effectiveness of denaturing agents for proteins. Biochemistry. 1963 Jan-Feb;2:47–57. doi: 10.1021/bi00901a011. [DOI] [PubMed] [Google Scholar]
- Golbeck J. H., Martin I. F., Fowler C. F. Mechanism of Linolenic Acid-induced Inhibition of Photosynthetic Electron Transport. Plant Physiol. 1980 Apr;65(4):707–713. doi: 10.1104/pp.65.4.707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green G., Mahler H. R. Comparative study of polyribonucleotides in aqueous and glycol solutions. Biochemistry. 1970 Jan 20;9(2):368–387. doi: 10.1021/bi00804a026. [DOI] [PubMed] [Google Scholar]
- Hanlon S., Major E. O. Conformational studies on polyriboadenylic acid in ethylene glycol. Biochemistry. 1968 Dec;7(12):4350–4358. doi: 10.1021/bi00852a030. [DOI] [PubMed] [Google Scholar]
- Helenius A., Simons K. Solubilization of membranes by detergents. Biochim Biophys Acta. 1975 Mar 25;415(1):29–79. doi: 10.1016/0304-4157(75)90016-7. [DOI] [PubMed] [Google Scholar]
- Hofmann A. F., Small D. M. Detergent properties of bile salts: correlation with physiological function. Annu Rev Med. 1967;18:333–376. doi: 10.1146/annurev.me.18.020167.002001. [DOI] [PubMed] [Google Scholar]
- Horton P., Croze E. The relationship between the activity of chloroplast photosystem II and the midpoint oxidation-reduction potential of cytochrome b-559. Biochim Biophys Acta. 1977 Oct 12;462(1):86–101. doi: 10.1016/0005-2728(77)90191-8. [DOI] [PubMed] [Google Scholar]
- Joliot A. Flash induced fluorescence kinetics in chloroplasts in the 20 microseconds-100 s time range in the presence of 3(3,4-dichlorophenyl)-1,1-dimethylurea. Effects of hydroxylamine. Biochim Biophys Acta. 1977 Apr 11;460(1):142–151. doi: 10.1016/0005-2728(77)90160-8. [DOI] [PubMed] [Google Scholar]
- Kagawa Y. Reconstitution of oxidative phosphorylation. Biochim Biophys Acta. 1972 Aug 4;265(3):297–338. [PubMed] [Google Scholar]
- Khoo J. C., Drevon C. A., Steinberg D. Dissociation of the lipid-enzyme complex of hormone-sensitive lipase using high density lipoprotein or apolipoprotein A-I. Biochim Biophys Acta. 1980 Mar 21;617(3):540–544. doi: 10.1016/0005-2760(80)90021-1. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Metz J., Bishop N. I. Identification of a chloroplast membrane polypeptide associated with the oxidizing side of photosystem II by the use of select low-fluorescent mutants of Scenedesmus. Biochem Biophys Res Commun. 1980 May 30;94(2):560–566. doi: 10.1016/0006-291x(80)91268-1. [DOI] [PubMed] [Google Scholar]
- Mullet J. E., Arntzen C. J., Kobayashi Y., Inoue Y. Covalent modification of chloroplast photosystem II polypeptides by p-nitrothiophenol. Biochim Biophys Acta. 1981 Apr 13;635(2):215–224. doi: 10.1016/0005-2728(81)90021-9. [DOI] [PubMed] [Google Scholar]
- Razin S. Reconstruction of biological membranes. Biochim Biophys Acta. 1972 Apr 18;265(2):241–296. [PubMed] [Google Scholar]
- Reinman S., Mathis P., Conjeaud H., Stewart A. Kinetics of reduction of the primary donor of photosystem II. Influence of pH in various preparations. Biochim Biophys Acta. 1981 Apr 13;635(2):429–433. doi: 10.1016/0005-2728(81)90040-2. [DOI] [PubMed] [Google Scholar]
- Sharp R. R., Yocum C. F. Factors influencing hydroxylamine inactivation of photosynthetic water oxidation. Biochim Biophys Acta. 1981 Mar 12;635(1):90–104. doi: 10.1016/0005-2728(81)90010-4. [DOI] [PubMed] [Google Scholar]
- Siggel U., Renger G., Stiehl H. H., Rumberg B. Evidence for electronic and ionic interaction between electron transport chains in chloroplasts. Biochim Biophys Acta. 1972 Feb 28;256(2):328–335. doi: 10.1016/0005-2728(72)90063-1. [DOI] [PubMed] [Google Scholar]
- Spector M., Winget G. D. Purification of a manganese-containing protein involved in photosynthetic oxygen evolution and its use in reconstituting an active membrane. Proc Natl Acad Sci U S A. 1980 Feb;77(2):957–959. doi: 10.1073/pnas.77.2.957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor F. R., Cronan J. E., Jr Cyclopropane fatty acid synthase of Escherichia coli. Stabilization, purification, and interaction with phospholipid vesicles. Biochemistry. 1979 Jul 24;18(15):3292–3300. doi: 10.1021/bi00582a015. [DOI] [PubMed] [Google Scholar]
- Telfer A., Barber J., Jagendorf A. T. Electrostatic control of chloroplast coupling factor binding to thylakoid membranes as indicated by cation effects of electron transport and reconstitution of photophosphorylation. Biochim Biophys Acta. 1980 Jul 8;591(2):331–345. doi: 10.1016/0005-2728(80)90164-4. [DOI] [PubMed] [Google Scholar]
- Warden J. T., Blankenship R. E., Sauer K. A flash photolysis ESR study of photosystem II signal IIvf, the physiological donor to P-680+. Biochim Biophys Acta. 1976 Mar 12;423(3):462–478. doi: 10.1016/0005-2728(76)90201-2. [DOI] [PubMed] [Google Scholar]
- Winget G. D., Kanner N., Racker E. Formation of ATP by the adenosine triphosphatase complex from spinach chloroplasts reconstituted together with bacteriorhodopsin. Biochim Biophys Acta. 1977 Jun 9;460(3):490–499. doi: 10.1016/0005-2728(77)90087-1. [DOI] [PubMed] [Google Scholar]
- Wydrzynski T., Sauer K. Periodic changes in the oxidation state of manganese in photosynthetic oxygen evolution upon illumination with flashes. Biochim Biophys Acta. 1980 Jan 4;589(1):56–70. doi: 10.1016/0005-2728(80)90132-2. [DOI] [PubMed] [Google Scholar]
- van Gorkom H. J. Identification of the reduced primary electron acceptor of photosystem II as a bound semiquinone anion. Biochim Biophys Acta. 1974 Jun 28;347(3):439–442. doi: 10.1016/0005-2728(74)90081-4. [DOI] [PubMed] [Google Scholar]