The results of this study suggest that the combination of high-spatial-resolution MR imaging of the proximal femur and finite element analysis provides information about bone quality that is not available with dual-energy x-ray absorptiometry and may have value as an additional tool for detection of skeletal fragility and assessment of fracture risk in vivo.
Abstract
Purpose
To determine the feasibility of using finite element analysis applied to 3-T magnetic resonance (MR) images of proximal femur microarchitecture for detection of lower bone strength in subjects with fragility fractures compared with control subjects without fractures.
Materials and Methods
This prospective study was institutional review board approved and HIPAA compliant. Written informed consent was obtained. Postmenopausal women with (n = 22) and without (n = 22) fragility fractures were matched for age and body mass index. All subjects underwent standard dual-energy x-ray absorptiometry. Images of proximal femur microarchitecture were obtained by using a high-spatial-resolution three-dimensional fast low-angle shot sequence at 3 T. Finite element analysis was applied to compute elastic modulus as a measure of strength in the femoral head and neck, Ward triangle, greater trochanter, and intertrochanteric region. The Mann-Whitney test was used to compare bone mineral density T scores and elastic moduli between the groups. The relationship (R2) between elastic moduli and bone mineral density T scores was assessed.
Results
Patients with fractures showed lower elastic modulus than did control subjects in all proximal femur regions (femoral head, 8.51–8.73 GPa vs 9.32–9.67 GPa; P = .04; femoral neck, 3.11–3.72 GPa vs 4.39–4.82 GPa; P = .04; Ward triangle, 1.85–2.21 GPa vs 3.98–4.13 GPa; P = .04; intertrochanteric region, 1.62–2.18 GPa vs 3.86–4.47 GPa; P = .006–.007; greater trochanter, 0.65–1.21 GPa vs 1.96–2.62 GPa; P = .01–.02), but no differences in bone mineral density T scores. There were weak relationships between elastic moduli and bone mineral density T scores in patients with fractures (R2 = 0.25–0.31, P = .02–.04), but not in control subjects.
Conclusion
Finite element analysis applied to high-spatial-resolution 3-T MR images of proximal femur microarchitecture can allow detection of lower elastic modulus, a marker of bone strength, in subjects with fragility fractures compared with control subjects. MR assessment of proximal femur strength may provide information about bone quality that is not provided by dual-energy x-ray absorptiometry.
© RSNA, 2014
Introduction
Osteoporosis is a “systemic skeletal disease characterized by low bone mass and microarchitectural deterioration of bone tissue, with a consequent increase in bone fragility and susceptibility to fracture” (1,2). In the United States, 2 million fragility fractures occur per year, resulting in $17 billion in direct annual costs for fracture care (3). The standard-of-care test for diagnosis of osteoporosis, dual-energy x-ray absorptiometry (DXA) for estimation of areal bone mineral density (BMD) in the proximal femur and spine (4), is useful because BMD reflects bone mass and correlates with a higher risk of all types of fragility fractures (eg, spine, distal radius, hip) (5). In addition, mechanical testing of proximal femur specimens has shown that DXA results can explain up to 74% of the variance in bone strength (6–8). However, DXA estimation of BMD still has limitations as a clinical tool to assess bone strength in vivo.
First, because DXA is a low-resolution, two-dimensional projection imaging technique, it cannot account accurately for bone size, geometry, or microarchitecture, which are critical contributors to bone strength independent of BMD. Results of cadaveric studies (6–8) have shown that the addition of structural parameters to BMD improves the prediction of bone strength and failure load, with r2 values as high as 0.94. The importance of bone microarchitecture is also highlighted by its inclusion in the World Health Organization definition of osteoporosis (1,2). Despite the strong correlation of BMD with bone strength in specimen studies, DXA poorly discriminates between subjects without and those with fragility fractures because there is a large overlap in the BMD values of subjects in these two groups (9,10). Furthermore, most women who experience hip fractures do not meet the BMD criterion for a diagnosis of osteoporosis (BMD T score <−2.5) and are classified as having slightly low (BMD T score >−2.5) or normal BMD (BMD T score >−1.0) (11,12). Because an important goal of an imaging test for osteoporosis is to determine which individuals with bone fragility are at high risk for fracture, a better bone strength assessment technique would allow detection and treatment of such patients with a bone-strengthening agent before they experience fracture.
During the past 15 years, one of the most clinically important developments in bone assessment technology has been the arrival of in vivo three-dimensional bone microarchitecture imaging methods, including high-spatial-resolution peripheral quantitative (HR-pQ) computed tomography (CT) (13–15) and high-spatial-resolution magnetic resonance (MR) imaging (16–18). Previously, studies of bone microarchitecture were performed invasively with iliac crest biopsy (19,20). HR-pQ CT and high-spatial-resolution MR imaging have allowed noninvasive study of the microstructural determinants of fracture risk in vivo (21–23). However, HR-pQ CT and high-spatial-resolution MR imaging have been performed only in the distal extremities (14,15,22,24,25) and not in the hip, which is one of the most important fracture sites and a standard BMD assessment site. This is because HR-pQ CT scanners have small imaging bores that can fit only the ankle or wrist and because signal-to-noise ratio limitations arise when MR imaging of deeper anatomy such as the hip is performed.
In parallel with the development of these imaging technologies, the field of bone biomechanics has progressed beyond measurement of structural parameters of bone as a result of the application of a longstanding mechanical engineering method, finite element analysis (FEA), to images of bone microarchitecture (26–28). By using a three-dimensional imaging dataset as input, FEA can account for the geometry, shape, and microarchitecture of bone and provides output metrics of bone mechanical competence or strength (eg, elastic modulus). Because osteoporosis is a disease of low bone strength, the ability to quantify markers of bone strength noninvasively in vivo may improve the detection of patients with skeletal fragility who are at risk for fracture (29–32).
The purpose of this study was to determine the feasibility of applying FEA to in vivo high-spatial-resolution 3-T MR images of proximal femur trabecular microarchitecture for detection of lower elastic modulus, a marker of bone strength, in subjects with clinically defined osteoporosis compared with control subjects. We chose to evaluate the proximal femur because it is a standard site for BMD assessment and an important site of fragility fracture, rich in trabecular bone. As secondary objectives, we also compared differences in BMD T scores between groups, and we examined whether bone strength measurement with MR imaging provides information about bone quality that is not provided by DXA by assessing the relationships between elastic moduli computed with MR imaging and BMD T scores.
Materials and Methods
Subject Recruitment
This prospective study, which was conducted from November 13, 2012 to May 9, 2013, had institutional review board approval and complied with Health Insurance Portability and Accountability Act guidelines. We obtained written informed consent from all subjects. We used a similar case-control study design as that used by other groups (13,33,34). From the osteoporosis center at our institution, we recruited 22 consecutive postmenopausal women with clinical osteoporosis. Osteoporosis was defined as the presence of a fragility fracture that was radiographically confirmed (low-energy fracture due to a fall from a standing height; median time since fracture, 45.5 months; interquartile range (IQR), 66 months; 25th percentile, 12 months; and 75th percentile, 78 months), rather than confirmed by using the DXA criterion, because DXA has low sensitivity and specificity for the detection of bone fragility in patients at risk for fracture (9,10,12). There were 13 subjects with one fracture, seven subjects with two fractures, and two subjects with three fractures. The sites of the fractures were the spine (n = 16), distal radius (n = 8), proximal humerus (n = 3), foot or ankle (n = 3), proximal femur (n = 2), and sacrum (n = 1). We also recruited 22 postmenopausal women without fractures as control subjects. The characteristics of the patients with fractures and control subjects are listed in Table 1. There were no significant differences between fracture and control groups with regard to age, height, weight, body mass index, and bisphosphonate use. Because most subjects with a history of fragility fracture were using bisphosphonates, we felt that it was also necessary to include subjects in the control group who were being treated with a bisphosphonate. We excluded from the fracture group and the control group any subjects with a history of confounding factors that also affect bone strength such as endocrinologic, metabolic, nutritional, or gastrointestinal disorders that may affect the skeleton; corticosteroid use; Paget disease; multiple myeloma; or metastatic bone disease.
Table 1.
Summary of Demographic Characteristics of the Patients with Fractures and Control Subjects

Note.—Unless otherwise indicated, data are medians, with IQR in parentheses. To assess differences between groups, we used the Mann-Whitney test and χ2 test (bisphosphonate use only).
DXA Scanning
All subjects underwent DXA scanning performed with a densitometer (QDR 2000; Hologic, Waltham, Mass) by using established methods to compute BMD T scores for the femoral neck, total hip, and the lumbar spine (35).
MR Imaging
Within 30 days of the DXA scan, we imaged the same hip (nonfractured hip in the two subjects with proximal femur fracture) of all subjects in feet-first supine position with a 3-T MR imager (Skyra; Siemens, Erlangen, Germany) by using a 26-element radiofrequency coil setup (three rows of six elements from the Siemens commercial flexible array coil, two rows of four elements from the Siemens commercial spine coil) to detect the MR signal (36). Data from individual coil elements were combined by using the sum-of-squares method. Multichannel arrays are known to provide higher signal-to-noise ratios, which can be used to decrease image voxel size (ie, increase spatial resolution of the image) and improve image quality (37,38). We acquired high-spatial-resolution images of bone microarchitecture of the entire proximal femur in a slightly oblique coronal plane parallel to the femoral neck by using a three-dimensional fast low-angle shot sequence (repetition time msec/echo time msec, 31/4.92; flip angle, 25°; matrix, 512 × 512; field of view, 120 mm; in-plane voxel size, 0.234 × 0.234 mm; section thickness, 1.5 mm; number of coronal sections, 60; acquisition time, 25 minutes 30 seconds; bandwidth, 200 Hz/pixel) similar to that used in prior studies performed at peripheral skeletal sites (39,40).
Selection of Volumes of Interest and Generation of Bone Volume Fraction Maps
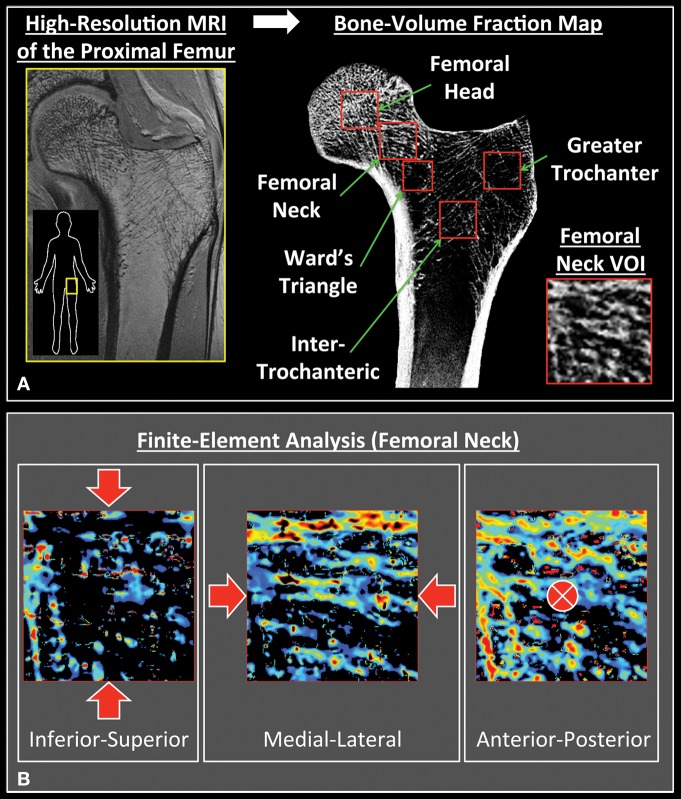
Figure 1 illustrates the workflow for computing elastic moduli from high-spatial-resolution in vivo MR images of the proximal femur. First, the gray-scale values of the images were linearly scaled to include the range of 0%–100%, with minimum and maximum values of pure marrow and bone intensity in the femur, respectively. This approach allowed us to account for both partial volume effects and red marrow, which may have different signal intensity than does fatty marrow. We referred to the resulting three-dimensional array representing the fractional occupancy of bone at each voxel location as the bone-volume fraction map. Next, a radiologist (G.C., with 3 years of experience as a musculoskeletal radiologist and 8 years of experience in bone microarchitecture imaging and analysis techniques) selected five trabecular VOIs in the proximal femur: the head, the intertrochanteric region, the neck, the greater trochanter, and Ward triangle (Fig 1). All VOIs were 10 mm3, with the exception of the VOI in the Ward triangle, which was slightly smaller (8 mm3) to fit in the proximal femur and include only trabecular bone. These VOI sizes were chosen to obtain sufficient coverage in the sites of interest while accommodating subjects with smaller proximal femurs.
Figure 1:
Image analysis workflow. A, After a bone-volume fraction map was generated from high-spatial-resolution MR images of the proximal femur, five volumes of interest (VOIs) were selected. All were 10 mm3, with the exception of Ward triangle, which was selected as 8 mm3 to fit in the trabecular bone compartment. B, FEA was performed on each VOI by simulating compressive loading along three principle axes.
Performance of FEA
We performed FEA in the linear elastic regime to compute the elastic moduli for each VOI (41–44). In brief, each voxel in the bone volume fraction map was converted into a hexahedral finite element with dimensions corresponding to the voxel size. Assuming an empirically determined isotropic tissue modulus of 15 GPa and a Poisson ratio of 0.3 for bone (45), we set the Young modulus of each element to be linearly proportional to the bone volume fraction value such that the Young modulus in gigapascals was 15 times the bone volume fraction for all elements (42,46,47). Compressive uniaxial loading was simulated in the linear elastic regime to compute the elastic moduli separately along the superoinferior (ESI), medial-lateral (EML), and anteroposterior (EAP) directions by applying 1% strain on the opposite faces of the cuboid VOIs (42,48). These three axes approximately corresponded to the directions of primary external force during a sideways fall, backward fall, and standing, respectively. The nodes on the four faces of the VOI lateral to the compressive direction were constrained to lateral displacements to mimic in situ boundary conditions. The finite element system was solved by minimizing the total strain energy of the linear system (42,47). Finally, the elastic moduli were estimated as the ratio of resulting stress along the compression direction to the applied strain. The computation time for each simulation was approximately 4 seconds.
Statistical Analysis
We performed the statistical analyses by using standard software (SPSS; v.20 IBM, Somers, NY). Because the data were not normally distributed by the Shapiro-Wilk test, we assessed differences between groups by using the nonparametric Mann-Whitney test. To assess the relationship between elastic moduli and BMD T score, we used the nonparametric Spearman test. The results were reported as median values with IQRs. A P value less than .05 was considered to indicate a statistically significant difference.
Results
Comparison of Proximal Femur Elastic Moduli
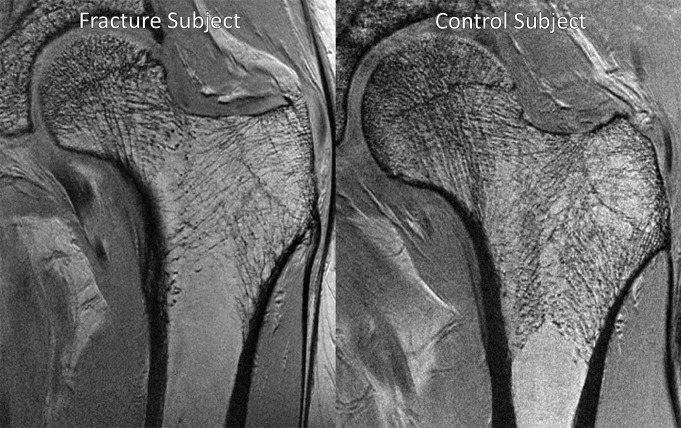
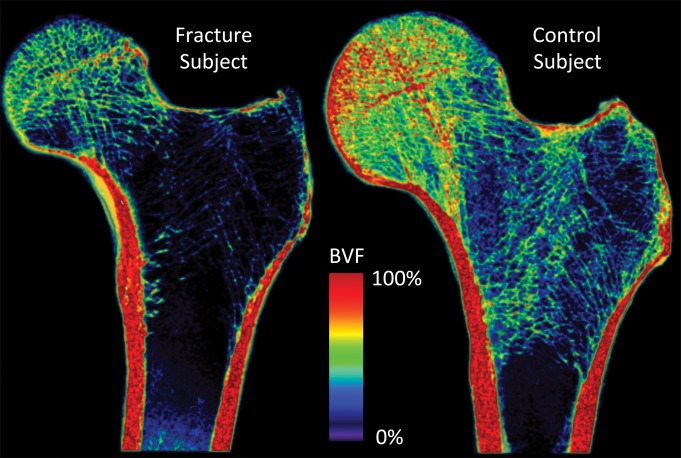
Figures 2 and 3 show representative high-spatial-resolution 3-T MR images of proximal femur microarchitecture and a volume-rendered bone volume fraction map in a patient with fragility fracture and a control subject. Individual trabeculae can be seen on the images, which demonstrate microarchitectural deterioration in the patient with fracture compared with the control subject.
Figure 2:
Representative coronal high-spatial-resolution 3-T MR images of proximal femur microarchitecture in a subject with osteoporotic fracture (left panel) and a control subject (right panel). Trabeculae are hypointense linear foci. There is deterioration in trabecular microarchitecture in the fracture subject compared with the control subject.
Figure 3:
Volume-rendered bone volume fraction maps (BVF) in a subject with osteoporotic fracture (left panel) and a control subject (right panel). The subject with fracture shows lower regional bone volume and deterioration in trabecular microarchitecture compared with the control subject.
Compared with the control subjects, the patients with fractures demonstrated lower elastic moduli in all five regions of the proximal femur (Table 2). In the femoral head, the elastic modulus was 8.7% (−0.81 divided by 9.32) to 9.7% (−0.94 divided by 9.67) lower in patients with fractures than in control subjects (ESI and EAP, both P = .04; EML, P = .06). In the femoral neck, the elastic modulus was 22.8% (−1.1 divided by 4.82) to 29.2% (−1.28 divided by 4.39) lower in patients with fractures than in control subjects (ESI and EAP, both P = .04; EML, P = .05). In the Ward triangle, the elastic modulus was 46.5% (−1.92 divided by 4.13) to 53.5% (−2.13 divided by 3.98) lower in patients with fractures than in control subjects (ESI and EAP, both P = .04; EML, P = .08). In the intertrochanteric region, the elastic modulus was 51.2% (−2.29 divided by 4.47) to 58.8% (−2.31 divided by 3.93) lower in patients with fractures than in control subjects (ESI and EAP, both P = .006; EML, P = .007). Finally, in the greater trochanter, the elastic modulus was 53.8% (−1.41 divided by 2.62) to 66.8% (−1.31 divided by 1.96) lower in patients with fractures than in control subjects (ESI and EAP, both P = .01; EML, P = .02).
Table 2.
Comparison of Proximal Femur Elastic Modulus in Patients with Fracture and Control Subjects
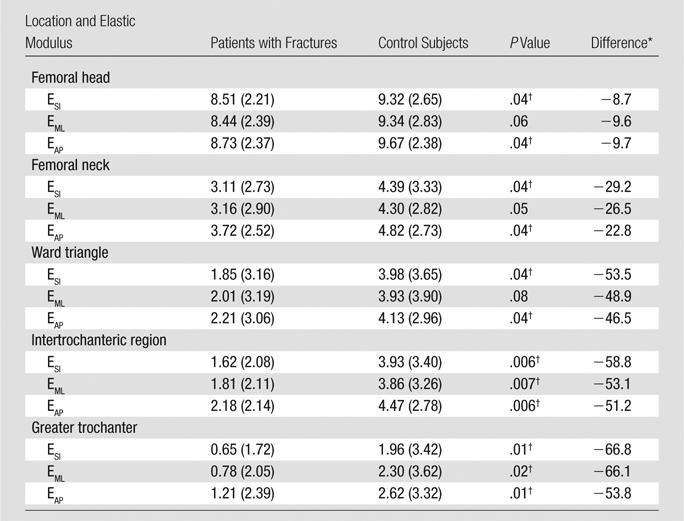
Note.—Unless otherwise indicated, data are median gigapascals, with IQR in parentheses
Data are percentage of difference between results of patients with fractures and those of control subjects.
Denotes statistically significant difference.
Comparison of BMD T Scores
There were no significant differences in BMD T scores between patients with fractures and control subjects (P > .25 for all, Table 3). In patients with fractures and control subjects, the median femoral neck BMD T scores were −2.4 (IQR, 0.5) and −2.5 (IQR, 0.7), respectively. The median total hip BMD T scores in patients with fractures and control subjects were −2.1 (IQR, 0.5) and −2.3 (IQR, 0.8), respectively. Finally, the median spine BMD T scores in patients with fractures and control subjects were −2.8 (IQR, 1.6) and −2.5 (IQR, 1.2), respectively.
Table 3.
Comparison of BMD T Scores in Patients with Fractures and Control Subjects

Note.—Unless otherwise indicated, data are median BMD T scores, with IQR in parentheses.
Relationship between Elastic Moduli and BMD T Scores
To determine whether the information provided by high-spatial-resolution MR imaging overlapped with that provided by DXA, we assessed the relationship between elastic moduli and BMD T scores. In the control group, there was no significant correlation identified between the elastic modulus (measured at any location in the proximal femur) and BMD T score (measured at the total hip, femoral neck, or lumbar spine) (P > .21 for all, Table 4). In the fracture group, weak correlations were identified between femoral neck BMD T score and the elastic modulus measured in the (a) femoral neck (EAP, P < .05, R2 = 0.28), (b) intertrochanteric region (EAP, P < .05, R2 = 0.25), and (c) greater trochanter (ESI, P < .05, R2 = 0.27; EML, P < .05, R2 = 0.27; EAP, P < .05, R2 = 0.31) (Table 5).
Table 4.
Relationship between Proximal Femur Elastic Modulus and BMD T Score in Control Subjects

Table 5.
Relationship between Elastic Modulus and BMD T Score in Patients with Fractures
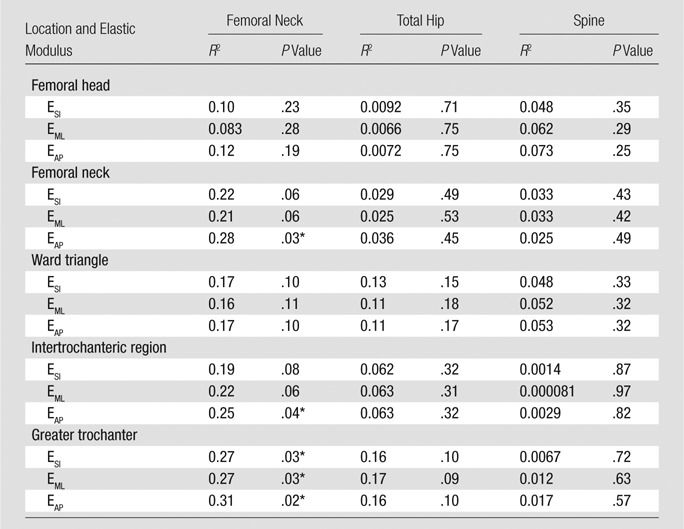
Denotes statistically significant difference.
Discussion
We have applied FEA to 3-T MR images of proximal femur microarchitecture and have shown the feasibility of detecting decreased elastic modulus, a marker of bone strength, in subjects with clinically defined osteoporosis compared with matched control subjects without fracture with similar BMD T scores. The mechanical properties of proximal femur microarchitecture in human subjects in vivo have not been well established in the literature. In addition, we found that there was only a weak correlation between proximal femur elastic moduli and BMD T scores in subjects with fracture. Overall, the results suggest that high-spatial-resolution MR imaging of proximal femur microarchitecture combined with FEA provides information about bone quality that is not completely provided by DXA and may have value in addition to DXA as a means to detect skeletal fragility and assess fracture risk in vivo. Because osteoporosis is a disease of compromised bone strength, the ability of FEA to noninvasively provide an additional, quantitative marker of bone strength in vivo without the use of ionizing radiation in a critically important osteoporotic fracture location such as the hip could have important future implications for both osteoporosis research and clinical care. In addition to its use as a tool to study the effects of microachitectural adaptations on proximal femur strength (in patients with disease or after intervention), this method also could be adapted as an additional clinical care tool to assess patients’ risk of fracture and to determine whether therapy should be initiated (23).
Although there have been many imaging studies of bone microarchitecture in the distal tibia and distal radius, only one group has published research articles (49,50) describing the feasibility of imaging proximal femur structure and microarchitecture in vivo. The high-spatial-resolution MR imaging of the femur was performed with a 3-T imager used for routine clinical imaging. The main reason why in vivo MR imaging of proximal femur microarchitecture has been challenging is because the proximal femur is deeper in location (5–8 cm from the skin surface and/or radiofrequency coil placed on the patient) compared with the distal radius or distal tibia (< 2 cm from the skin surface and/or radiofrequency coil). Because signal-to-noise ratio decreases rapidly as the distance between the anatomic structure of interest and radiofrequency coil increases (37), the signal-to-noise ratio and resolution achievable at the proximal femur are lower than those achievable at the distal radius or tibia. To overcome these signal-to-noise ratio limitations, we used a 26-element receive coil to perform MR imaging of the hip (compared with a standard four- to eight–element receive setup typically used for MR imaging of the hip). The use of a greater number of receive elements to provide higher signal-to-noise ratio has been demonstrated previously in the knee (51,52), brain (38,53), and heart (54). The 26-element receive coil setup allowed depiction of individual trabeculae in the proximal femur. There was no noticeable motion artifact on the images, despite the relatively long imaging time; this is probably because the hip is much less mobile than the distal extremity (distal radius or distal tibia).
Because osteoporosis is a disease of compromised bone strength, FEA is appealing because it provides metrics of bone strength as output. Parameters such as bone volume divided by total volume and BMD, which essentially reflect bone mass, may be easier to compute, but unlike elastic modulus derived from FEA, they do not account for differences in shape, geometry, and spatial arrangement and distribution of bone tissue and trabeculae. These factors have all been shown to critically influence bone mechanical strength (19,55–58). For example, when a given amount of bone is arranged as thick, disconnected trabeculae, it is less mechanically competent than when arranged as numerous thin, interconnected trabeculae (19,58). As another example, the Euler theorem states that the strength of a column (or a trabecula in our case) is inversely related to the square of its effective length (55). Therefore, a column that is twice as long as another can only sustain 25% of the load that the other column (with an identical diameter) can sustain, even though it has twice the mass. In the future, we hope to compare the performance of FEA results with other microarchitectural parameters for distinguishing patients with fractures and control subjects and for the assessment of fracture risk.
The lack of a difference in BMD T scores between the patients with fractures and control subjects is not necessarily surprising. Previous large population studies have shown that there is a large overlap or even no difference in the BMDs of subjects with and without fragility fractures (10,59). On the basis of the median values, approximately 50% of the subjects with fragility fractures in our study did not even meet the DXA criterion for a diagnosis of osteoporosis (BMD T score <−2.5). This is also consistent with the data from large population studies (12,60) and illustrates a shortcoming of BMD as a marker of bone strength. In ex vivo histomorphometric studies (19,61), the decreased bone strength of subjects with osteoporosis has been attributed to decreased bone volume fraction, trabecular number, thickness, connectivity, and a conversion of trabecular plates to rods. FEA can account for these changes in the spatial distribution and arrangement of bone and allow researchers finally to monitor one of the most important bone properties that is altered in patients with osteoporosis: its mechanical competence.
The FEA solver used in our study has been validated at other skeletal locations (41,42). We extended its application to the proximal femur and demonstrated the ability to detect decreased proximal femur elastic modulus in patients with fractures compared with control subjects with similar BMD T scores. This provides evidence that MR imaging may have added value as a tool to assess poor bone quality in human subjects in vivo. Our imaging was performed with a clinical 3-T MR imager by using a product sequence (three-dimensional fast low-angle shot), and the FEA was performed on a desktop computer; all of these are commercially available. Because clinical CT of the hip (including FEA of hip CT scans) also has been shown to provide information about fracture risk beyond DXA (31,32), in the future, we hope to compare the performance of high-spatial-resolution MR imaging with that of clinical CT. Finally, because each FEA simulation required only 4 seconds and was performed on a desktop computer, this method could be used in real time for clinical applications after the analysis is streamlined. Therefore, this method has the potential to be implemented widely as a research or clinical tool to study osteoporosis. Recent potential applications of FEA have been described for use in the distal extremities to assess the mechanical consequences of microarchitectural changes either in patients with chronic disease (eg, after renal transplantation) (28) or in response to pharmacologic interventions (43,62).
We assessed elastic modulus in different regions in the proximal femur because we hypothesized that if osteoporosis is indeed a “systemic skeletal disease” (per the World Health Organization disease definition [1]), then the elastic moduli in all of these locations should be lower than corresponding elastic moduli in the control group. Our results were consistent with this hypothesis. We also assessed elastic modulus with simulated loading along the three principal axes (superior-inferior, medial-lateral, and anterior-posterior). We felt that this was necessary because we hypothesized that subjects with osteoporosis have decreased mechanical competence in the proximal femur compared with that of control subjects, regardless of how the bones are loaded. Our results were also consistent with this hypothesis; however, there was only a trend toward significance for EML in the femoral head, femoral neck, and Ward triangle. Finally, as an incidental observation, in both the patients with fractures and the control subjects, elastic moduli measured in the femoral neck, Ward triangle, intertrochanteric region, and greater trochanter were all three- to fivefold lower than those measured in the femoral head (P < .05). This may explain why fragility fractures in the femoral neck, Ward triangle, intertrochanteric region, and greater trochanter are much more commonly seen in the clinical or emergency setting than are fractures of the femoral head.
We examined the relationship between the MR imaging and DXA results because a strong correlation between these results would suggest that DXA can provide the same mechanical property information provided by MR imaging (ie, that the examinations provide similar information about bone quality). However, there were no significant relationships between elastic moduli and BMD T scores in the control group, and there were only weak relationships between elastic moduli and BMD T scores in the patients with fractures. These results suggest that the bone strength information provided with MR imaging is different from the bone strength information provided with DXA. This is probably because DXA, as a low-resolution two-dimensional planar projection technique, cannot measure properties of bone, such as its microarchitecture, size, and geometry, which are known critical contributors to bone strength that can be accounted for with FEA.
It is important to discuss the limitations of this study. First, the high-spatial-resolution MR images cannot completely resolve trabeculae that are much smaller than the minimum voxel dimension (0.234 mm), and the anisotropic voxels may result in volume averaging. Nevertheless, individual trabeculae were still visible on the MR images. This is likely because, in the coronal plane, trabeculae are oriented perpendicular or parallel to the imaging plane, which will minimize volume averaging effects. In addition, results of a recent specimen study by Chiba et al (63) have shown that trabecular thickness in the proximal femur ranges from 0.190 mm ± 0.025 to 0.261 mm ± 0.029 and trabecular spacing ranges from 0.665 mm ± 120 to 0.980 mm ± 0.159. Because FEA is based on bone volume fraction maps (as opposed to binarized images), FEA incorporates gray-scale information, and thus, the fractional occupancy of bone in each voxel. Second, the number of subjects in this study was limited to only 22 patients with fractures and 22 control subjects. As a result, these groups may not have been representative of the general population, and we cannot exclude the presence of confounding factors. However, as a proof-of-concept study, we still had sufficient power to detect statistically significant differences between groups. Third, to compute elastic moduli for the selected VOIs, the FEA algorithm used in this study includes assumptions regarding the intrinsic material properties and boundary conditions. The numeric values of tissue modulus and Poisson ratio used for FEA are not known with a high level of certainty for the proximal femur, although authors of extensive prior work (46,64) from several laboratories used these values. Fourth, we did not have DXA and MR imaging results for both hips in our study. Between-hip differences in bone health could exist and represent a confounding factor. However, DXA and MR imaging were performed on the same side in all patients. Furthermore, International Society of Clinical Densitometry guidelines state that either hip may be used for DXA scanning (35). Finally, we did not perform a Bonferroni correction for the statistical tests. The Bonferroni correction can be applied when making multiple comparisons between two groups to decrease the chance of committing a type I error (ie, obtaining a statistically significant result due to chance). Had we applied the Bonferroni correction, we would have set the P value that indicated a significant difference at less than .01 (.05 divided by 5, because we made comparisons between groups in five different locations), and only the results in the intertrochanteric and greater trochanter regions would have been statistically significant. However, the main drawback of performing the Bonferroni correction is the increased chance of committing a type II error (failure to reject the null hypothesis) (65). In addition, the Bonferroni correction has received criticism because the interpretation of a finding should not be dependent on the number of other tests that also were performed (65). Because this was an initial feasibility study to determine the potential utility of our MR imaging test of bone strength, we did not apply the Bonferroni correction.
In conclusion, this proof-of-concept study has demonstrated the feasibility of using high-spatial-resolution 3-T MR imaging combined with FEA to detect decreased elastic modulus in the proximal femur in subjects with clinical osteoporosis compared with control subjects with similar BMD T scores. The results of this study provide evidence that an MR imaging test of proximal femur microarchitecture and mechanical competence provides information about bone quality that is not provided by DXA and may have added value as a tool for detection of skeletal fragility and assessment of fracture risk in vivo.
Advances in Knowledge
■ Finite element analysis applied to high-spatial-resolution 3-T MR images of proximal femur microarchitecture can show lower elastic modulus, a marker of bone strength, in patients with fragility fractures compared with control subjects with similar bone mineral density T scores.
■ There is only a weak relationship between elastic modulus computed with MR imaging and bone mineral density T score computed with dual-energy x-ray absorptiometry, which suggests that the high-spatial-resolution MR imaging test of proximal femur strength provides information about bone quality that is not provided by dual-energy x-ray absorptiometry, the current clinical standard for assessment of fracture risk.
Implications for Patient Care
■ Finite element analysis of high-spatial-resolution 3-T MR images of the proximal femur could be used as a tool to assess the effects of microarchitectural changes (to evaluate disease or response to therapy) on proximal femur strength.
■ MR imaging computation of markers of bone strength could be used as an adjunct clinical care tool for detection of skeletal fragility and assessment of fracture risk, which would help physicians make treatment decisions.
Received August 16, 2013; revision requested October 1; revision received December 20; accepted December 28; final version accepted January 20, 2014.
Funding: This research was supported by the National Institutes of Health (grants K23 AR059748 and K25 AR060283).
Research reported in this publication was supported by the National Institute of Arthritis and Musculoskeletal and Skin Diseases of the National Institutes of Health under Award Numbers K23 AR059748 and K25 AR060283 and the content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Disclosures of Conflicts of Interest: G.C. No relevant conflicts of interest to disclose. S.H. No relevant conflicts of interest to disclose. R.B. No relevant conflicts of interest to disclose. C.M.D. No relevant conflicts of interest to disclose. K.A.E. No relevant conflicts of interest to disclose. J.S.B. No relevant conflicts of interest to disclose. R.R.R. No relevant conflicts of interest to disclose. C.S.R. No relevant conflicts of interest to disclose.
Abbreviations:
- BMD
- bone mineral density
- DXA
- dual-energy x-ray absorptiometry
- EAP
- elastic modulus in the anteroposterior direction
- EMI
- elastic modulus in the medial-lateral direction
- ESI
- elastic modulus in the superoinferior direction
- FEA
- finite element analysis
- HR-pQ
- high-spatial-resolution peripheral quantitative
- IQR
- interquartile range
- VOI
- volume of interest
References
- 1.Consensus development conference : diagnosis, prophylaxis, and treatment of osteoporosis. Am J Med 1993;94(6):646–650. [DOI] [PubMed] [Google Scholar]
- 2.Kanis JA. Diagnosis of osteoporosis and assessment of fracture risk. Lancet 2002;359(9321):1929–1936. [DOI] [PubMed] [Google Scholar]
- 3.Burge R, Dawson-Hughes B, Solomon DH, Wong JB, King A, Tosteson A. Incidence and economic burden of osteoporosis-related fractures in the United States, 2005-2025. J Bone Miner Res 2007;22(3):465–475. [DOI] [PubMed] [Google Scholar]
- 4.Cummings SR, Bates D, Black DM. Clinical use of bone densitometry: scientific review. JAMA 2002;288(15):1889–1897. [DOI] [PubMed] [Google Scholar]
- 5.Marshall D, Johnell O, Wedel H. Meta-analysis of how well measures of bone mineral density predict occurrence of osteoporotic fractures. BMJ 1996;312(7041):1254–1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Boehm HF, Horng A, Notohamiprodjo M, et al. Prediction of the fracture load of whole proximal femur specimens by topological analysis of the mineral distribution in DXA-scan images. Bone 2008;43(5):826–831. [DOI] [PubMed] [Google Scholar]
- 7.Le Corroller T, Halgrin J, Pithioux M, Guenoun D, Chabrand P, Champsaur P. Combination of texture analysis and bone mineral density improves the prediction of fracture load in human femurs. Osteoporos Int 2012;23(1):163–169. [DOI] [PubMed] [Google Scholar]
- 8.Bousson V, Le Bras A, Roqueplan F, et al. Volumetric quantitative computed tomography of the proximal femur: relationships linking geometric and densitometric variables to bone strength. Role for compact bone. Osteoporos Int 2006;17(6):855–864. [DOI] [PubMed] [Google Scholar]
- 9.Stone KL, Seeley DG, Lui LY, et al. BMD at multiple sites and risk of fracture of multiple types: long-term results from the Study of Osteoporotic Fractures. J Bone Miner Res 2003;18(11):1947–1954. [DOI] [PubMed] [Google Scholar]
- 10.Cummings SR. Are patients with hip fractures more osteoporotic? Review of the evidence. Am J Med 1985;78(3):487–494. [DOI] [PubMed] [Google Scholar]
- 11.Wainwright SA, Marshall LM, Ensrud KE, et al. Hip fracture in women without osteoporosis. J Clin Endocrinol Metab 2005;90(5):2787–2793. [DOI] [PubMed] [Google Scholar]
- 12.Schuit SC, van der Klift M, Weel AE, et al. Fracture incidence and association with bone mineral density in elderly men and women: the Rotterdam Study. Bone 2004;34(1):195–202. [DOI] [PubMed] [Google Scholar]
- 13.Boutroy S, Bouxsein ML, Munoz F, Delmas PD. In vivo assessment of trabecular bone microarchitecture by high-resolution peripheral quantitative computed tomography. J Clin Endocrinol Metab 2005;90(12):6508–6515. [DOI] [PubMed] [Google Scholar]
- 14.Kazakia GJ, Hyun B, Burghardt AJ, et al. In vivo determination of bone structure in postmenopausal women: a comparison of HR-pQCT and high-field MR imaging. J Bone Miner Res 2008;23(4):463–474. [DOI] [PubMed] [Google Scholar]
- 15.Cohen A, Dempster DW, Müller R, et al. Assessment of trabecular and cortical architecture and mechanical competence of bone by high-resolution peripheral computed tomography: comparison with transiliac bone biopsy. Osteoporos Int 2010;21(2):263–273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Link TM, Majumdar S, Augat P, et al. In vivo high resolution MRI of the calcaneus: differences in trabecular structure in osteoporosis patients. J Bone Miner Res 1998;13(7):1175–1182. [DOI] [PubMed] [Google Scholar]
- 17.Majumdar S, Link TM, Augat P, et al. Trabecular bone architecture in the distal radius using magnetic resonance imaging in subjects with fractures of the proximal femur. Magnetic Resonance Science Center and Osteoporosis and Arthritis Research Group. Osteoporos Int 1999;10(3):231–239. [DOI] [PubMed] [Google Scholar]
- 18.Wehrli FW, Hwang SN, Ma J, Song HK, Ford JC, Haddad JG. Cancellous bone volume and structure in the forearm: noninvasive assessment with MR microimaging and image processing. Radiology 1998;206(2):347–357. [DOI] [PubMed] [Google Scholar]
- 19.Kleerekoper M, Villanueva AR, Stanciu J, Rao DS, Parfitt AM. The role of three-dimensional trabecular microstructure in the pathogenesis of vertebral compression fractures. Calcif Tissue Int 1985;37(6):594–597. [DOI] [PubMed] [Google Scholar]
- 20.Dempster DW, Shane E, Horbert W, Lindsay R. A simple method for correlative light and scanning electron microscopy of human iliac crest bone biopsies: qualitative observations in normal and osteoporotic subjects. J Bone Miner Res 1986;1(1):15–21. [DOI] [PubMed] [Google Scholar]
- 21.Majumdar S. Magnetic resonance imaging of trabecular bone structure. Top Magn Reson Imaging 2002;13(5):323–334. [DOI] [PubMed] [Google Scholar]
- 22.Wehrli FW. Structural and functional assessment of trabecular and cortical bone by micro magnetic resonance imaging. J Magn Reson Imaging 2007;25(2):390–409. [DOI] [PubMed] [Google Scholar]
- 23.Link TM. Osteoporosis imaging: state of the art and advanced imaging. Radiology 2012;263(1):3–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wehrli FW, Saha PK, Gomberg BR, et al. Role of magnetic resonance for assessing structure and function of trabecular bone. Top Magn Reson Imaging 2002;13(5):335–355. [DOI] [PubMed] [Google Scholar]
- 25.Link TM, Saborowski Kisters K, et al. Changes in calcaneal trabecular bone structure assessed with high-resolution MR imaging in patients with kidney transplantation. Osteoporos Int 2002;13(2):119–129. [DOI] [PubMed] [Google Scholar]
- 26.Liu XS, Cohen A, Shane E, et al. Bone density, geometry, microstructure, and stiffness: Relationships between peripheral and central skeletal sites assessed by DXA, HR-pQCT, and cQCT in premenopausal women. J Bone Miner Res 2010;25(10):2229–2238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Boutroy S, Van Rietbergen B, Sornay-Rendu E, Munoz F, Bouxsein ML, Delmas PD. Finite element analysis based on in vivo HR-pQCT images of the distal radius is associated with wrist fracture in postmenopausal women. J Bone Miner Res 2008;23(3):392–399. [DOI] [PubMed] [Google Scholar]
- 28.Rajapakse CS, Leonard MB, Bhagat YA, Sun W, Magland JF, Wehrli FW. Micro-MR imaging-based computational biomechanics demonstrates reduction in cortical and trabecular bone strength after renal transplantation. Radiology 2012;262(3):912–920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Morgan EF, Bouxsein ML. Use of finite element analysis to assess bone strength. Bonekey Osteovision 2005;2(12):8–19. [Google Scholar]
- 30.Bouxsein ML, Seeman E. Quantifying the material and structural determinants of bone strength. Best Pract Res Clin Rheumatol 2009;23(6):741–753. [DOI] [PubMed] [Google Scholar]
- 31.Keyak JH, Sigurdsson S, Karlsdottir G, et al. Male-female differences in the association between incident hip fracture and proximal femoral strength: a finite element analysis study. Bone 2011;48(6):1239–1245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Keaveny TM, Kopperdahl DL, Melton LJ, 3rd, et al. Age-dependence of femoral strength in white women and men. J Bone Miner Res 2010;25(5):994–1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nishiyama KK, Macdonald HM, Hanley DA, Boyd SK. Women with previous fragility fractures can be classified based on bone microarchitecture and finite element analysis measured with HR-pQCT. Osteoporos Int 2013;24(5):1733–1740. [DOI] [PubMed] [Google Scholar]
- 34.Melton LJ, 3rd, Riggs BL, van Lenthe GH, et al. Contribution of in vivo structural measurements and load/strength ratios to the determination of forearm fracture risk in postmenopausal women. J Bone Miner Res 2007;22(9):1442–1448. [DOI] [PubMed] [Google Scholar]
- 35.Schousboe JT, Shepherd JA, Baim S, et al. International Society for Clinical Densitometry Official Positions - Adult. International Society for Clinical Densitometry website. http://www.iscd.org/official-positions/2013-iscd-official-positions-adult/. Updated August 15, 2013. Accessed October 15, 2013. [Google Scholar]
- 36.Chang G, Deniz CM, Honig S, et al. Feasibility of 3-D MRI of proximal femur microarchitecture at 3 T using 26 receive elements without and with parallel imaging. J Magn Reson Imaging (in press). [DOI] [PMC free article] [PubMed]
- 37.Wright SM, Wald LL. Theory and application of array coils in MR spectroscopy. NMR Biomed 1997;10(8):394–410. [DOI] [PubMed] [Google Scholar]
- 38.Wiggins GC, Triantafyllou C, Potthast A, Reykowski A, Nittka M, Wald LL. 32-channel 3 Tesla receive-only phased-array head coil with soccer-ball element geometry. Magn Reson Med 2006;56(1):216–223. [DOI] [PubMed] [Google Scholar]
- 39.Chang G, Rajapakse CS, Babb JS, Honig SP, Recht MP, Regatte RR. In vivo estimation of bone stiffness at the distal femur and proximal tibia using ultra-high-field 7-Tesla magnetic resonance imaging and micro-finite element analysis. J Bone Miner Metab 2012;30(2):243–251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chang G, Rajapakse CS, Diamond M, et al. Micro-finite element analysis applied to high-resolution MRI reveals improved bone mechanical competence in the distal femur of female pre-professional dancers. Osteoporos Int 2013;24(4):1407–1417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rajapakse CS, Magland J, Zhang XH, et al. Implications of noise and resolution on mechanical properties of trabecular bone estimated by image-based finite-element analysis. J Orthop Res 2009;27(10):1263–1271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rajapakse CS, Magland JF, Wald MJ, et al. Computational biomechanics of the distal tibia from high-resolution MR and micro-CT images. Bone 2010;47(3):556–563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wehrli FW, Rajapakse CS, Magland JF, Snyder PJ. Mechanical implications of estrogen supplementation in early postmenopausal women. J Bone Miner Res 2010;25(6):1406–1414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Liu XS, Zhang XH, Rajapakse CS, et al. Accuracy of high-resolution in vivo micro magnetic resonance imaging for measurements of microstructural and mechanical properties of human distal tibial bone. J Bone Miner Res 2010;25(9):2039–2050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zysset PK, Guo XE, Hoffler CE, Moore KE, Goldstein SA. Elastic modulus and hardness of cortical and trabecular bone lamellae measured by nanoindentation in the human femur. J Biomech 1999;32(10):1005–1012. [DOI] [PubMed] [Google Scholar]
- 46.Guo XE, Goldstein XA. Is trabecular bone tissue different from cortical bone tissue? Forma 1997;12(??):185–196. [Google Scholar]
- 47.Magland JF, Zhang N, Rajapakse CS, Wehrli FW. Computationally-optimized bone mechanical modeling from high-resolution structural images. PLoS ONE 2012;7(4):e35525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Vasilic B, Wehrli FW. A novel local thresholding algorithm for trabecular bone volume fraction mapping in the limited spatial resolution regime of in vivo MRI. IEEE Trans Med Imaging 2005;24(12):1574–1585. [DOI] [PubMed] [Google Scholar]
- 49.Krug R, Banerjee S, Han ET, Newitt DC, Link TM, Majumdar S. Feasibility of in vivo structural analysis of high-resolution magnetic resonance images of the proximal femur. Osteoporos Int 2005;16(11):1307–1314. [DOI] [PubMed] [Google Scholar]
- 50.Carballido-Gamio J, Folkesson J, Karampinos DC, et al. Generation of an atlas of the proximal femur and its application to trabecular bone analysis. Magn Reson Med 2011;66(4):1181–1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Eckstein F, Kunz M, Hudelmaier M, et al. Impact of coil design on the contrast-to-noise ratio, precision, and consistency of quantitative cartilage morphometry at 3 Tesla: a pilot study for the osteoarthritis initiative. Magn Reson Med 2007;57(2):448–454. [DOI] [PubMed] [Google Scholar]
- 52.Chang G, Wiggins GC, Xia D, et al. Comparison of a 28-channel receive array coil and quadrature volume coil for morphologic imaging and T2 mapping of knee cartilage at 7T. J Magn Reson Imaging 2012;35(2):441–448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wiggins GC, Polimeni JR, Potthast A, Schmitt M, Alagappan V, Wald LL. 96-Channel receive-only head coil for 3 Tesla: design optimization and evaluation. Magn Reson Med 2009;62(3):754–762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Schmitt M, Potthast A, Sosnovik DE, et al. A 128-channel receive-only cardiac coil for highly accelerated cardiac MRI at 3 Tesla. Magn Reson Med 2008;59(6):1431–1439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Dempster DW. Bone microarchitecture and strength. Osteoporos Int 2003;14(Suppl 5):S54–S56. [DOI] [PubMed] [Google Scholar]
- 56.Guo XE, Kim CH. Mechanical consequence of trabecular bone loss and its treatment: a three-dimensional model simulation. Bone 2002;30(2):404–411. [DOI] [PubMed] [Google Scholar]
- 57.Mosekilde L. Age-related changes in vertebral trabecular bone architecture—assessed by a new method. Bone 1988;9(4):247–250. [DOI] [PubMed] [Google Scholar]
- 58.Weinstein RS, Hutson MS. Decreased trabecular width and increased trabecular spacing contribute to bone loss with aging. Bone 1987;8(3):137–142. [DOI] [PubMed] [Google Scholar]
- 59.Melton LJ, 3rd, Eddy DM, Johnston CC, Jr. Screening for osteoporosis. Ann Intern Med 1990;112(7):516–528. [DOI] [PubMed] [Google Scholar]
- 60.Cranney A, Jamal SA, Tsang JF, Josse RG, Leslie WD. Low bone mineral density and fracture burden in postmenopausal women. CMAJ 2007;177(6):575–580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Amling M, Pösl M, Ritzel H, et al. Architecture and distribution of cancellous bone yield vertebral fracture clues. A histomorphometric analysis of the complete spinal column from 40 autopsy specimens. Arch Orthop Trauma Surg 1996;115(5):262–269. [DOI] [PubMed] [Google Scholar]
- 62.Burghardt AJ, Kazakia GJ, Sode M, de Papp AE, Link TM, Majumdar S. A longitudinal HR-pQCT study of alendronate treatment in postmenopausal women with low bone density: Relations among density, cortical and trabecular microarchitecture, biomechanics, and bone turnover. J Bone Miner Res 2010;25(12):2558–2571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Chiba K, Burghardt AJ, Osaki M, Majumdar S. Heterogeneity of bone microstructure in the femoral head in patients with osteoporosis: an ex vivo HR-pQCT study. Bone 2013;56(1):139–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Keaveny TM. Biomechanical computed tomography-noninvasive bone strength analysis using clinical computed tomography scans. Ann N Y Acad Sci 2010;1192:57–65. [DOI] [PubMed] [Google Scholar]
- 65.Perneger TV. What’s wrong with Bonferroni adjustments. BMJ 1998;316(7139):1236–1238. [DOI] [PMC free article] [PubMed] [Google Scholar]