The diagnostic accuracy of MR elastography in the prediction of esophageal varices is comparable to that of dynamic contrast material–enhanced (DCE) MR imaging, while combined assessment with MR elastography and DCE MR imaging can increase diagnostic sensitivity.
Abstract
Purpose
To determine the diagnostic performance of magnetic resonance (MR) elastography in comparison to spleen length and dynamic contrast material–enhanced (DCE) MR imaging in association with esophageal varices in patients with liver cirrhosis by using endoscopy as the reference standard.
Materials and Methods
This retrospective study received institutional review board approval, and informed consent was waived. One hundred thirty-nine patients with liver cirrhosis who underwent liver DCE MR imaging, including MR elastography, were included. Hepatic stiffness (HS) and spleen stiffness (SS) values assessed with MR elastography, as well as spleen length, were correlated with the presence of esophageal varices and high-risk varices by using Spearman correlation analysis. The diagnostic performance of MR elastography was compared with that of DCE MR imaging and combined assessment of MR elastography and DCE MR imaging by using receiver operating characteristic analysis. MR elastography reproducibility was assessed prospectively, with informed consent, in another 15 patients by using intraclass correlation coefficients.
Results
There were significant positive linear correlations between HS, SS, and spleen length and the grade of esophageal varices (r = 0.46, r = 0.48, and r = 0.36, respectively; all P < .0001). HS and SS values (>4.81 kPa and >7.60 kPa, respectively) showed better performance than did spleen length in the association with esophageal varices (P = .0306 and P = .0064, respectively). Diagnostic performance of HS and SS in predicting high-risk varices was comparable to that of DCE MR imaging (P = .1282 and P = .1371, respectively). When MR elastography and DCE MR imaging were combined, sensitivity improved significantly (P = .0004). MR elastography was highly reproducible (intraclass correlation coefficient > 0.9).
Conclusion
HS and SS are associated with esophageal varices and showed better performance than did spleen length in assessing the presence of esophageal varices. MR elastography is comparable to DCE MR imaging in predicting the presence of esophageal varices and high-risk varices, but, when assessed in combination, sensitivity is higher.
© RSNA, 2014
Introduction
Liver cirrhosis is defined pathologically as fibrosis and inflammation of the liver, which is mainly caused by chronic hepatitis B virus infection, chronic hepatitis C virus infection, nonalcoholic steatohepatitis, nonalcoholic fatty liver disease, and chronic alcohol abuse (1–4). It is known to lead to metabolic hepatic failure, as well as portal hypertension. The population of patients with cirrhosis has been observed to be growing, along with increased incidence of hepatitis C virus infections and increased detection of nonalcoholic steatohepatitis or nonalcoholic fatty liver disease (5,6).
One of the major complications of portal hypertension is the development of esophageal varices, which occur in approximately 30%–70% of patients with cirrhosis and have been shown to be correlated with the severity of liver disease (7–9). Considering that the mortality rate of variceal bleeding remains high (10–14), screening endoscopy for esophageal varices is recommended for all patients with established cirrhosis (14,15). Unfortunately, endoscopy is invasive, uncomfortable, expensive, and time consuming and frequently requires sedation. Therefore, there is a clinical demand for a noninvasive yet sensitive method to assess esophageal varices, particularly high-risk varices.
In this context, there have been several attempts to find noninvasive parameters that may help identify patients with esophageal varices or suggest the risk of variceal bleeding in patients with cirrhosis, such as spleen length, portal vein diameter, Child-Pugh score, platelet count, prothrombin time, or a combination of multiple indexes, as well as ultrasonographic (US) elastography (16–22). However, none of these variables has been validated satisfactorily in an independent series of cirrhotic patients (23). A recent meta-analysis of 14 studies in which 10 panels of indirect blood markers were examined in patients with chronic hepatitis C demonstrated that these markers cannot reliably differentiate stages of fibrosis in individual patients (24). In the past several years, elastography performed with US and magnetic resonance (MR) imaging has been introduced as a noninvasive technology for the diagnosis and monitoring of liver stiffness. In addition, hepatic stiffness (HS) and spleen stiffness (SS) measured with MR elastography have been suggested as potential parameters to identify the presence of esophageal varices (25,26). To our knowledge, however, no studies have been conducted to compare the usefulness of HS and SS in assessing the association with esophageal varices in patients with cirrhosis. Thus, the purpose of our study was to determine the diagnostic performance of MR elastography and compare it with spleen length and dynamic contrast material–enhanced (DCE) MR imaging in predicting the presence of esophageal varices and high-risk varices in patients with liver cirrhosis by using endoscopy as the reference standard.
Materials and Methods
This retrospective study was approved by the institutional review board of Seoul National University Hospital, with waiver of informed consent. The reproducibility of MR elastography was assessed with a prospective design and was approved by a separate institutional review board with written informed consent.
Patients
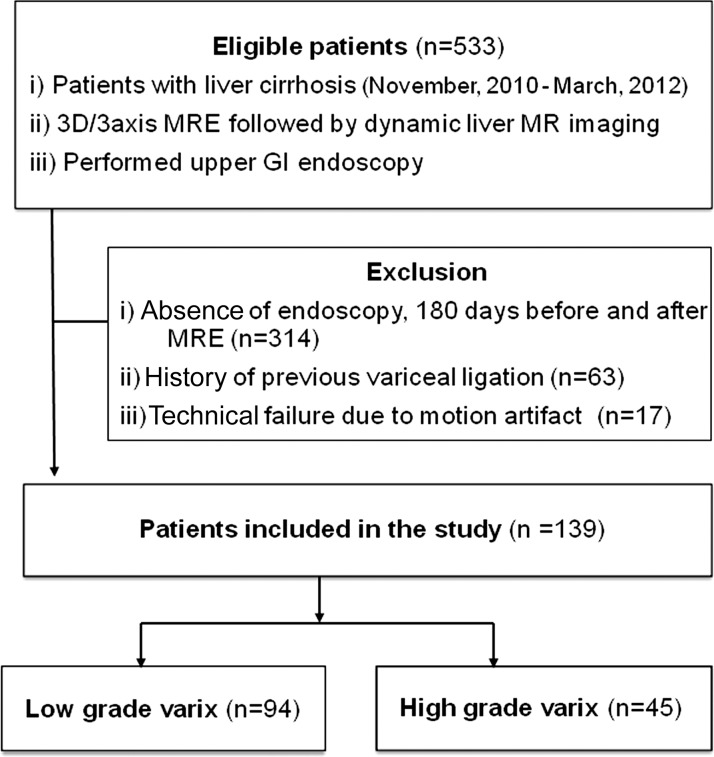
Between November 2010 and March 2012, 533 consecutive patients with liver cirrhosis based on imaging findings or clinical and/or laboratory data were referred to our radiology department for liver MR imaging, in which three-dimensional (3D) MR elastography was performed as a part of routine liver MR imaging. Among them, our study population met the following inclusion criteria: (a) patients who underwent esophagogastroduodenoscopy (EGD) for variceal screening within 180 days before or after MR imaging and (b) no history of esophageal variceal ligation. Three hundred fourteen patients who had not undergone EGD within 180 days were excluded (27,28). Another 63 patients who had undergone endoscopic esophageal variceal ligation therapy prior to MR imaging were excluded, as prior treatment may have caused a change in lesion characteristics. Seventeen patients were also excluded for suboptimal image quality owing to failure to generate a satisfactory mechanical wave through the abdomen for MR elastography. When data from more than one MR examination were available (n = 7), the MR data obtained closest to the time of EGD were analyzed. The final cohort (Fig 1) for our study consisted of 139 patients (mean age ± standard deviation, 57.3 years ± 10.5; range, 18–80 years; 102 men [mean age, 55.7 years ± 9.8; range, 18–76 years] and 37 women [mean age, 62.1 years ± 11.1, range, 31–80 years]). The diagnosis of cirrhosis was assigned on the basis of liver pathology findings (n = 69) or a combination of typical clinical findings (symptoms and stigmata of cirrhosis and its complications), radiologic findings (morphologic changes of the liver, splenomegaly, ascites, and collateral vessels), and the results of laboratory examinations, including Child-Pugh classification (n = 70). The causes of liver cirrhosis were hepatitis B (n = 84), hepatitis C (n = 27), both hepatitis B and C (n = 1), chronic alcohol abuse (n = 13), primary biliary cirrhosis (n = 3), recurrent pyogenic cholangitis (n = 1), autoimmune hepatitis (n = 1), or an unknown cause (n = 9).
Figure 1:
Flowchart shows inclusion and exclusion criteria and selection process of the study cohort. GI = gastrointestinal, MRE = MR elastography.
EGD was performed by one of five gastroenterologists. All operators used the following classification of esophageal varices (13): 0, no varices; 1, varices run straight; 2, varices show a beaded appearance; and 3, varices run oblique and are tortuous, with a tumorlike appearance (Fig 2). Patients were divided into two groups—a low-risk group with no or small varices (grade 0 or 1) and a high-risk group with large varices (grade 2 or 3)—on the basis of the probability of developing esophageal variceal bleeding (29). The mean interval between MR elastography and endoscopy was 23.0 days (range, 0 to 168 days).
Figure 2:
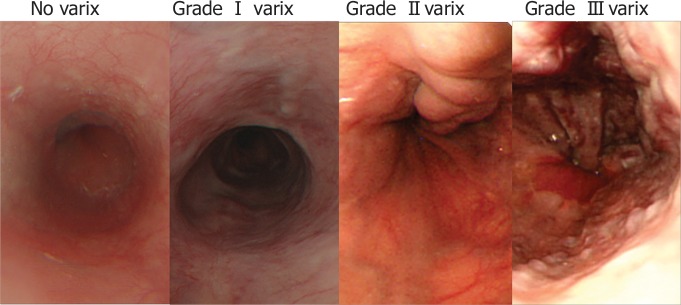
Representative endoscopic images demonstrate esophageal varices.
Clinical follow-up was performed to assess whether bleeding had occurred by reviewing electronic medical records. In the high-risk group, 15 patients had undergone prophylactic variceal ligation after acquisition of MR images and MR elastography images; thus, they were not included in the follow-up group. Another 15 patients (age range, 54–81 years; seven men and eight women) with liver cirrhosis were recruited separately to evaluate the repeatability of liver and spleen stiffness measurements assessed with MR elastography from December 2012 to January 2013.
MR Imaging and MR Elastography Protocols
All MR images were obtained with a 1.5-T whole-body MR imaging unit (Signa HDx; GE Healthcare, Milwaukee, Wis) by using an eight-channel torso phased-array coil centered over the liver. MR elastography was performed at the end of the examination after obtaining standard precontrast liver MR images, prior to gadoxetic acid injection. Imaging was conducted with the patient in the supine position, with an acoustic pressure-activated driver placed against the body wall adjacent to the liver. Low-frequency longitudinal mechanical waves of 60 Hz were transmitted into the right lobe of the liver by a passive driver (synchronized with the imaging sequence), which was placed against the right chest wall over the liver, with the center of the driver at the level of the xiphisternum (30,31). Thirty-two axial sections were obtained for each MR elastography examination from the dome to the tip of the liver. Details of the MR protocol are given in Appendix E1 (online). Two authors (R.L.E. and K.J.G.) and the Mayo Clinic hold intellectual property for the MR elastography technique.
MR Elastography Analysis
One reader (S.U.S., with 3 years of experience in liver MR imaging) who was blinded to the patients’ clinical and biochemical data, as well as the grade of esophageal varices, measured the spleen length and stiffness of the liver and spleen. The splenic length was obtained by multiplying the number of sections where the spleen was visualized by the thickness of the sections (32–34). The reader referenced conventional MR images and placed three circular or oval-shaped regions of interest in the right lobe of the liver and adjusted them geographically according to adjacent anatomic landmarks, by using three different sections (mean, 6384.1 mm2 ± 2919.4; range, 1577.7–11 092.1 mm2). Bile ducts, large vessels within the liver and fissures, artifacts from motion (including pulsation artifacts from the heart and aorta), areas with poor signal-to-noise ratio, the region just below the driver, the left lobe of the liver, and regions without adequate magnitude signal or wave amplitude were avoided (26,35,36). Regions of interest were also placed in the spleen (mean, 3583.8 mm2 ± 1335.2; range, 1839–6845.7 mm2) at three levels where the spleen showed a large area, by avoiding boundaries and large vessels. Overall stiffness of the heterogeneous liver and spleen was calculated by averaging the mean stiffness values (in kilopascals) recorded from each section. To assess reproducibility, MR elastography was performed two times in the same session in a separate group of 15 patients that were not included in the retrospective analysis. The same reader performed the region of interest measurement of liver and spleen stiffness by applying the same technique described earlier. Two sessions of measurements were conducted with a 2-week separation. Mean liver stiffness and maximum liver stiffness values within three sections in each session were calculated for further statistical analyses.
In addition, a large amount of ascites at the perihepatic space was assessed, along with whether the liver or spleen had iron depositions. Iron accumulation was defined as either the presence of definite low signal intensity on T2*-weighted MR images or reduced signal intensity on in-phase images as compared with opposed-phase images.
Analysis of Esophageal Varices by Using DCE MR Imaging
Two abdominal radiologists (J.H.Y. and M.H.Y, each with 6 years of experience), who were blinded to the patients’ physical findings, laboratory values, previous imaging results, and endoscopic results, independently interpreted DCE MR images by using the portal phase and 3-minute delayed phase images to detect the presence of esophageal varices and high-risk esophageal varices. In the first session, they were blinded to the stiffness values of the liver and spleen, and in the second session, they were provided with the stiffness values of the liver and spleen. Prior to the interpretation session, radiologists underwent a training session by using 10 cases with various grades of esophageal varices. The cases in the training session were not included in the interpretation session. On the basis of a prior study by Kim et al (37), each radiologist determined the presence of esophageal varices by using a four-point confidence scale (1 = definitely absent, 2 = probably absent, 3 = probably present, and 4 = definitely present), as well as the approximate size, by measuring the diameter of the largest observed varix. Sensitivity for the detection of varices was determined by using the number of patients with varices assigned a score of 3 or 4. A quantitative cutoff for high-risk varices was chosen as those larger than 2 mm in diameter as seen on MR images, on the basis of previous investigation (37).
In addition, one reader (S.U.S.) reviewed all DCE MR images and contrast-enhanced CT scans (mean interval between MR imaging and CT, 8.2 days ± 12.7; range, 0–66 days) to evaluate collateral venous channels for false-positive cases.
Statistical Analysis
Correlation analyses were performed between esophageal varices and MR elastography values and splenic length. To determine the diagnostic accuracy for the prediction of the presence of varices or high-risk varices and for the association with unprotected variceal bleeding, the mean area under the receiver operating characteristic (ROC) curve (AUC), sensitivity, and specificity were calculated. To evaluate the added value of MR elastography, the diagnostic accuracy of a combined technique by using MR elastography and DCE MR imaging was compared with that of DCE MR imaging alone. The reproducibility of liver and spleen stiffness was also evaluated. All statistical analyses, except ROC curves, were performed by using the SPSS software package (SPSS, version 19.0; SPSS, Chicago, Ill). Results from ROC curves were obtained by using MedCalc software (MedCalc Software, Mariakerke, Belgium). A P value less than .05 was considered to indicate a significant difference. Details of the statistical analyses are provided in Appendix E1 (online).
Results
Patient Characteristics
Among 139 patients, 78 (56.1%) had esophageal varices (33 patients, grade 1; 40 patients, grade 2; and five patients, grade 3; Table 1). In the high-risk group, 15 patients had undergone prophylactic variceal ligation after acquisition of MR images and MR elastography images and thus were not included in the follow-up group to determine whether bleeding had occurred. Another 11 patients experienced unprotected variceal bleeding during the follow-up period. Age, sex, disease origin, and interval between MR elastography and EGD did not show any statistical differences between the two groups. There were 64 patients with iron deposition in the liver and 41 patients with iron deposition in the spleen.
Table 1.
Patient Characteristics in High- and Low-Risk Groups
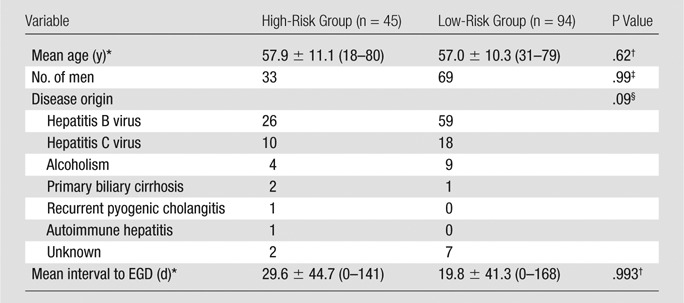
Data are means ± standard deviations. Numbers in parentheses are ranges.
Calculated with the independent sample t test.
Calculated with the Fisher exact test.
Calculated with the Pearson χ2 test.
Correlation of MR Elastography and Spleen Length with Esophageal Varix Grade
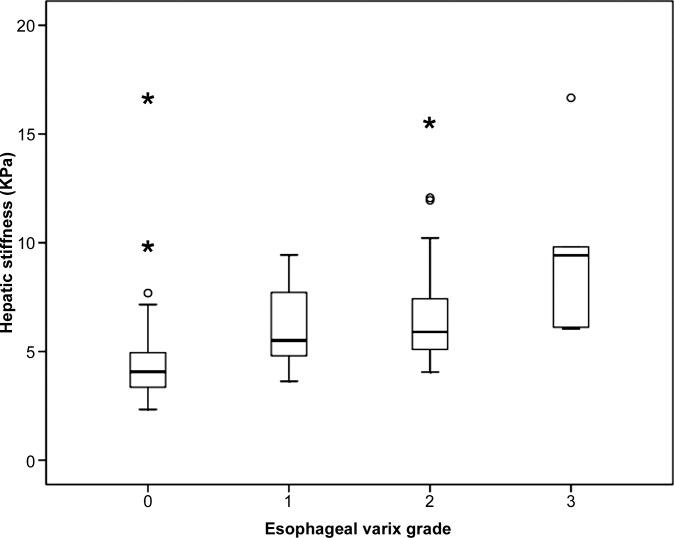
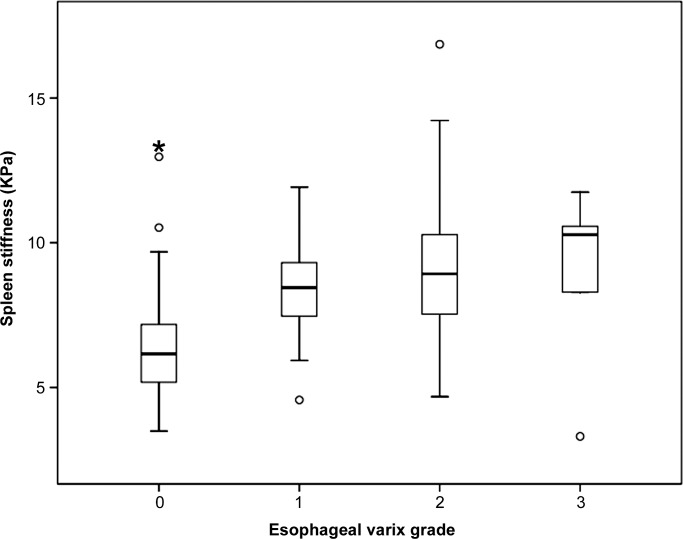
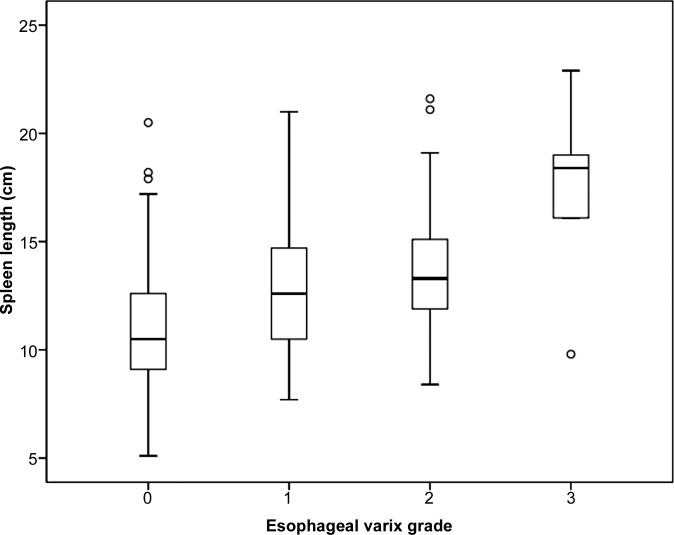
Mean values of HS and SS measured with MR elastography were significantly lower in the group without esophageal varices than in the group with varices of any grade (4.5 kPa vs 6.6 kPa and 6.4 kPa vs 8.8 kPa, respectively; both P < .0001). HS and SS values measured with MR elastography and spleen length were significantly lower in the low-risk group than in the high-risk group (5.1 kPa vs 7.1 kPa [P < .0001] and 7.1 kPa vs 9.1 kPa [P < .0001], respectively; 11.9 cm vs 13.9 cm [P = .0014], respectively). Positive linear correlations were observed between MR elastography values and the grade of esophageal varices (r = 0.46, r = 0.48, and r = 0.36, respectively; all P < .0001). As esophageal variceal grade increased, HS, SS, and spleen length also increased (Fig 3). Representative images are displayed in Figure 4.
Figure 3a:
Box and whisker plots are shown for (a) HS, (b) SS, and (c) splenic length according to the grade of esophageal varices.
Figure 4:
MR images (left) and MR elastograms (right) serve as an example of a magnitude image (left) and shear stiffness map (elastogram, right) of the liver and spleen in patients with four different grades of esophageal varices proven at endoscopy. MR images were obtained in patients with esophageal varices of grade 0, grade 1, grade 2, and grade 3. Elastograms show progressively increasing liver and spleen stiffness in the four patients, a finding that corresponds to increases in the grade of esophageal varices.
Figure 3b:
Box and whisker plots are shown for (a) HS, (b) SS, and (c) splenic length according to the grade of esophageal varices.
Figure 3c:
Box and whisker plots are shown for (a) HS, (b) SS, and (c) splenic length according to the grade of esophageal varices.
Independent Predictive Factors for Esophageal Varices and Variceal Bleeding
Multivariate logistic regression analysis showed that HS and SS were significant independent factors associated with the presence of varices (P = .0035 and P < .0001, respectively) and high-grade varices (P = .0158 and P = .0026). For association with variceal bleeding, only HS was a significant independent predictor (P = .0038).
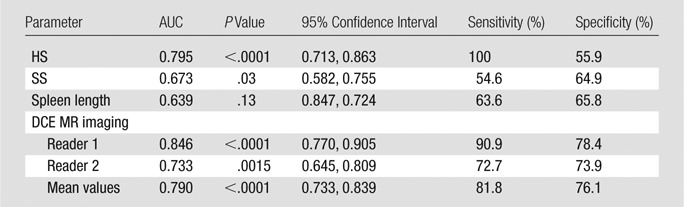
Diagnostic Performance of MR Elastography in Identifying Varices and Assessing the Risk of Variceal Bleeding as Compared with DCE MR Imaging
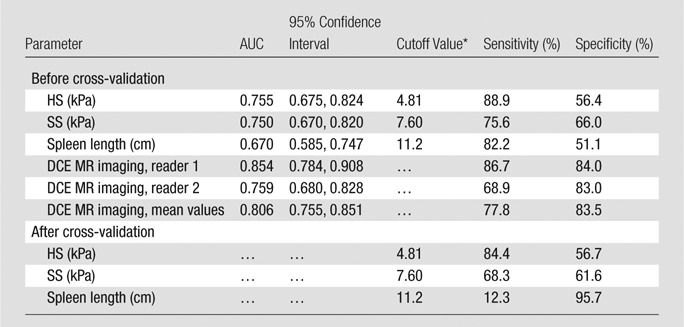
In pairwise comparisons of ROC curves, DCE MR imaging, HS, and SS allowed better assessment of the presence of varices compared with spleen length (P = .0021, P = .0001, and P = .0001, respectively; Table 2, Fig 5). However, there were no significant differences between DCE MR imaging and HS or SS (P = .55 and P = .84, respectively) or between HS and SS (P = .77).
Table 2.
Diagnostic Performance of MR Elastography in Assessing the Presence of Varices
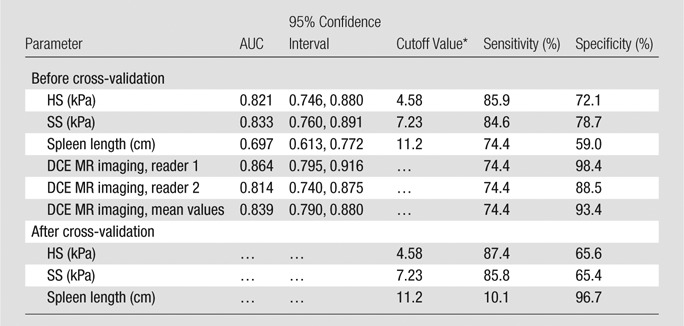
Best cutoff values were chosen by using ROC analysis.
Figure 5a:
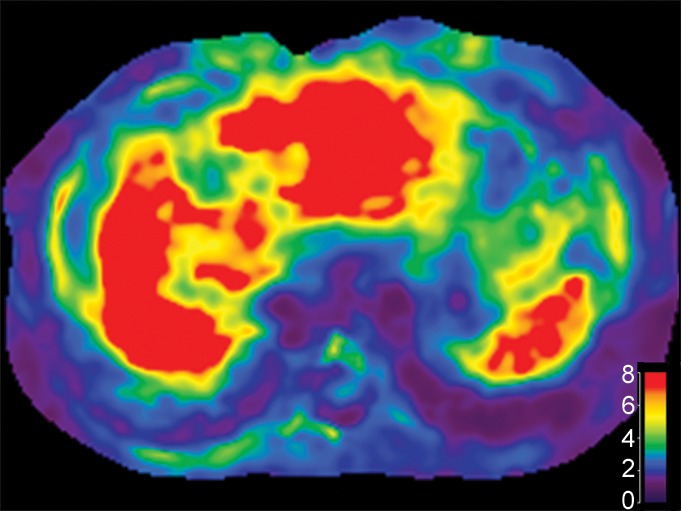
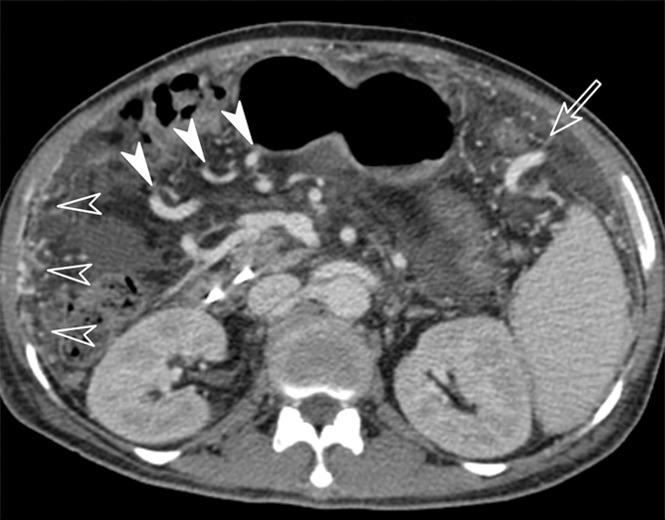
Images obtained in a 61-year-old man with hepatitis B virus–associated liver cirrhosis. (a) MR elastogram shows the mean stiffness of the liver to be 9.3 kPa and mean stiffness of the spleen to be 8.6 kPa. (b) Endoscopic image demonstrates a grade 1 esophageal varix. (c, d) Axial CT scans obtained in the portal venous phase demonstrate that the patient has multiple collateral veins, such as omental (open arrowheads on c), perisplenic (open arrow in c), mesenteric (solid arrowheads on c and d), and retroperitoneal (solid arrow in d) varices.
Figure 5b:
Images obtained in a 61-year-old man with hepatitis B virus–associated liver cirrhosis. (a) MR elastogram shows the mean stiffness of the liver to be 9.3 kPa and mean stiffness of the spleen to be 8.6 kPa. (b) Endoscopic image demonstrates a grade 1 esophageal varix. (c, d) Axial CT scans obtained in the portal venous phase demonstrate that the patient has multiple collateral veins, such as omental (open arrowheads on c), perisplenic (open arrow in c), mesenteric (solid arrowheads on c and d), and retroperitoneal (solid arrow in d) varices.
Figure 5c:
Images obtained in a 61-year-old man with hepatitis B virus–associated liver cirrhosis. (a) MR elastogram shows the mean stiffness of the liver to be 9.3 kPa and mean stiffness of the spleen to be 8.6 kPa. (b) Endoscopic image demonstrates a grade 1 esophageal varix. (c, d) Axial CT scans obtained in the portal venous phase demonstrate that the patient has multiple collateral veins, such as omental (open arrowheads on c), perisplenic (open arrow in c), mesenteric (solid arrowheads on c and d), and retroperitoneal (solid arrow in d) varices.
Figure 5d:
Images obtained in a 61-year-old man with hepatitis B virus–associated liver cirrhosis. (a) MR elastogram shows the mean stiffness of the liver to be 9.3 kPa and mean stiffness of the spleen to be 8.6 kPa. (b) Endoscopic image demonstrates a grade 1 esophageal varix. (c, d) Axial CT scans obtained in the portal venous phase demonstrate that the patient has multiple collateral veins, such as omental (open arrowheads on c), perisplenic (open arrow in c), mesenteric (solid arrowheads on c and d), and retroperitoneal (solid arrow in d) varices.
In pairwise comparisons of ROC curves, DCE MR imaging, HS, and SS allowed better demonstration of high-risk varices than did spleen length (P = .0004, P = .046, and P = .049, respectively; Table 3, Fig 6). There were no significant differences between DCE MR imaging and HS or SS (P = .13 and P = .14, respectively) or between HS and SS (P = .93).
Table 3.
Diagnostic Performance of MR Elastography in Assessing High-Risk Varices
Best cutoff values were chosen by using ROC analysis.
Figure 6a:
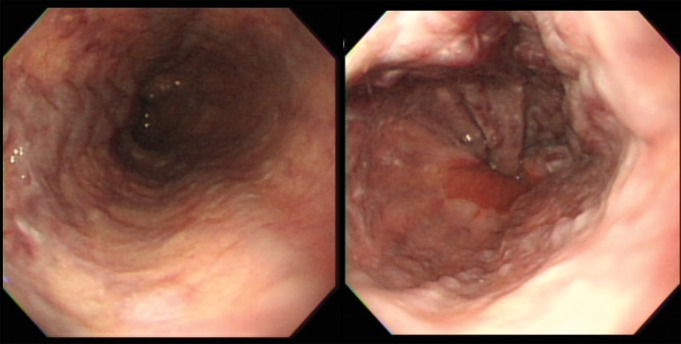
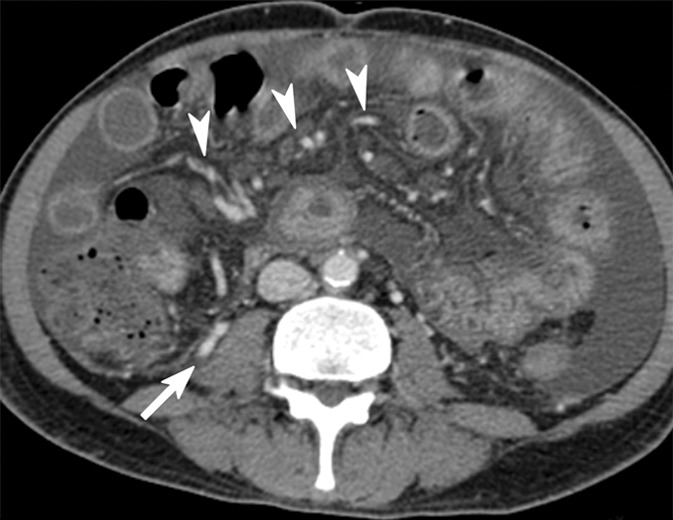
Images obtained in a 67-year-old man with alcoholic liver cirrhosis and grade 3 esophageal varices. (a) Endoscopic images depict the esophageal varices. (b) Axial MR magnitude image obtained with the 3D, three-axis echo-planar sequence is used as the anatomic landmark for analysis of the elastogram. Spleen size is measured as 10.1 cm, which is not well correlated with the grade of esophageal varices. (c) On the MR elastogram, shear stiffness was measured to be 16.7 kPa in the liver and 10.3 kPa in the spleen, which is representative of high-grade esophageal varices.
Figure 6b:

Images obtained in a 67-year-old man with alcoholic liver cirrhosis and grade 3 esophageal varices. (a) Endoscopic images depict the esophageal varices. (b) Axial MR magnitude image obtained with the 3D, three-axis echo-planar sequence is used as the anatomic landmark for analysis of the elastogram. Spleen size is measured as 10.1 cm, which is not well correlated with the grade of esophageal varices. (c) On the MR elastogram, shear stiffness was measured to be 16.7 kPa in the liver and 10.3 kPa in the spleen, which is representative of high-grade esophageal varices.
Figure 6c:
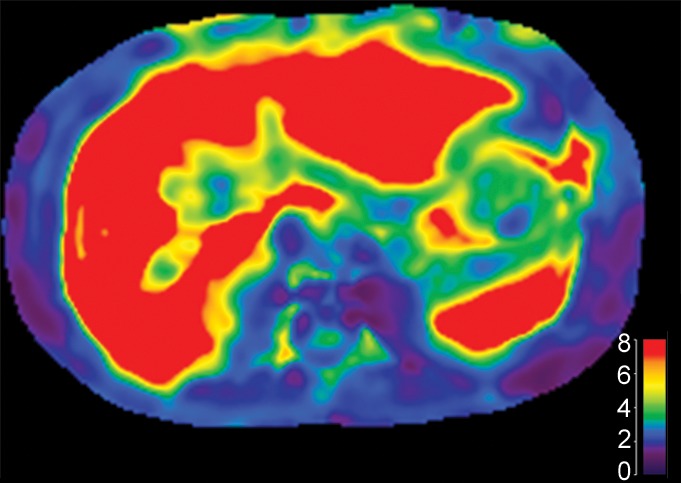
Images obtained in a 67-year-old man with alcoholic liver cirrhosis and grade 3 esophageal varices. (a) Endoscopic images depict the esophageal varices. (b) Axial MR magnitude image obtained with the 3D, three-axis echo-planar sequence is used as the anatomic landmark for analysis of the elastogram. Spleen size is measured as 10.1 cm, which is not well correlated with the grade of esophageal varices. (c) On the MR elastogram, shear stiffness was measured to be 16.7 kPa in the liver and 10.3 kPa in the spleen, which is representative of high-grade esophageal varices.
In our study, MR elastography showed several cases of false-positive diagnoses for esophageal varices (18 for HS and 13 for SS) or high-risk varices (41 for HS and 32 for SS). In all cases of false-positive diagnoses for esophageal varices and high-risk varices, we found dilated collateral veins other than esophageal varices, such as distal splenorenal shunts, retroperitoneal veins, recanalized periumbilical veins, and epigastric veins. Also, in our study, there were 11 false-negative cases that showed low HS values, despite the presence of esophageal varices. Among 11 false-negative cases, seven patients had iron deposition in the liver, which was present on T2*-weighted images or in- and opposed-phase images, which caused a decrease in signal intensity on the elastogram; two patients had a large amount of ascites in the perihepatic space that may have possibly interrupted the wave transmission to the liver.
In the high-risk group without prophylactic variceal ligation, HS was significantly higher in patients with variceal bleeding (n = 11) than in those without variceal bleeding (n = 19) (6.4169 vs 7.9802, P = .016), but SS did not show a significant difference (8.9664 vs 6.6505, P = .77). HS, SS, and DCE MR imaging were factors associated with unprotected variceal bleeding (P < .0001, P = .03, and P < .0001, respectively), whereas spleen length was not (P = .13). The AUC of HS was significantly higher than that of SS (0.795 vs 0.673, P = .01). However, there were no significant differences between DCE MR imaging and HS or SS (P = .92 and P = .09, respectively; Table 4). ROC analysis conducted by using HS as a binary variable with the cutoff value of HS (>4.81 kPa) obtained previously from the ROC curve for high-risk varices demonstrated an AUC of 0.579 (P = .066), sensitivity of 100%, and specificity of 15.8%.
Table 4.
Diagnostic Performance of MR Elastography in Assessing Unprotected Variceal Bleeding
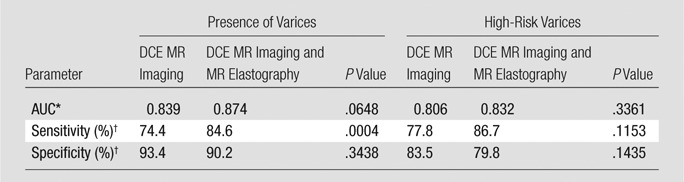
Additional Value of MR Elastography in DCE MR Imaging
When both MR elastography and DCE MR imaging were taken into account and then compared with the result of DCE MR imaging, AUC did not change significantly for the group with varices of any grade (0.874 vs 0.839, P = .06) or the high-risk group (0.832 vs 0.806, P = .34). However, sensitivity increased significantly compared with that of DCE MR imaging alone for the detection of varices of any grade (84.6% vs 74.4%, P = .0004). There were no significant differences in sensitivity for the high-risk group (86.7% vs 77.8%, P = .12) and specificity for the group with varices of any grade (90.2% vs 93.4%, P = .34) and high-risk varices (79.8% vs 83.5%, P = .14; Table 5).
Table 5.
Diagnostic Performance of DCE MR Imaging Compared with Combined DCE MR Imaging and MR Elastography
The differences between the AUC values were compared by using the method described by De Long et al (Appendix E1 [online]).
Sensitivities and specificities were compared by using the McNemar test.
Repeatability of MR Elastography
For both HS and SS, mean stiffness values were more reproducible than maximum stiffness values (0.999 vs 0.997 and 0.981 vs 0.933, respectively). Bland-Altman analysis demonstrated that coefficients of repeatability were 0.142, 0.402, 0.679, and 1.538 for mean HS, maximum HS, mean SS, and maximum SS, respectively.
Discussion
The results of our study indicate that HS and SS values measured on 3D, three-axis MR elastography images obtained by using the spin-echo echo-planar imaging technique correlated well with esophageal varices. In addition, the diagnostic accuracy of MR elastography in the prediction of esophageal varices is comparable to that of DCE MR imaging, while combined assessment of MR elastography and DCE MR images can increase diagnostic sensitivity. These results are in good agreement with those of other studies, which also demonstrated that liver stiffness values measured with transient US elastography were associated with the presence of esophageal varices (38–41). However, there is some debate regarding the correlation between HS and SS in patients with chronic liver diseases when using MR elastography (26,42). In our study, there was no significant difference between HS and SS in assessing the presence of varices or identifying high-risk varices. When a cutoff value of more than 4.81 kPa was used for HS, however, 100% sensitivity for high-risk varices was observed, and there was a significant difference in HS in patients who had esophageal variceal bleeding versus those who did not. Currently, the American Association for the Study of Liver Diseases guidelines suggest that all patients with cirrhosis should undergo regular screening endoscopy (14). If we were to add MR elastography to routine MR imaging performed for cancer surveillance or management of cirrhosis, MR elastography may play a useful adjunctive role by allowing better targeted, more cost-effective application of diagnostic and therapeutic endoscopy in cirrhotic patients.
In our study, MR elastography showed several cases of false-positive diagnoses for esophageal varices (18 for HS and 13 for SS) or high-risk varices (41 for HS and 32 for SS). In those patients, although there were neither varices nor high-risk varices, high stiffness values were noted. Interestingly, we also observed that in all false-positive cases in our study, there were dilated collateral veins other than esophageal varices. In severe liver fibrosis or cirrhosis with portal hypertension, it is not surprising that hypertensive portal blood flow could drain through many other pathways, not only through esophageal varices (41,43,44). Also, in our study, there were 11 false-negative cases that showed low HS values, despite the presence of esophageal varices. Iron deposition and large ascites would also partly explain these false-negative cases. Another possibility, as some other investigators have suggested, is that false-negative results may be due to portal hypertension, which precedes the development of hepatic fibrosis (21,26,45).
The 3D MR elastography sequence we used had been used previously in clinical studies. However, problems still remain with this approach. First, although the spin-echo nature of echo-planar image acquisition makes it more resistant to signal loss than the gradient-echo sequence, because of iron deposition, measured stiffness values can be erroneously low in cases of severe iron deposition in the liver or spleen (26,46,47). This is because iron deposits can increase magnetic susceptibility, which can decrease signal intensity, resulting in poor wave depiction on echo-planar images. In our study, however, many cases showed good signal intensity at MR elastography, even though they had parenchymal iron deposition. Therefore, further studies are warranted to better explore the relationship between the echo-planar MR elastography sequence and iron deposition. Second, there were 17 patients with technical failure of MR elastography image acquisition. Several other previous studies have also demonstrated that patients with ascites, colonic interposition, and excessive subcutaneous or mesenteric fat are likely to have improper propagation of shear waves, even though the technical failure rate of MR elastography is much lower than that of US-based elastography (38,48–51).
There were several limitations in our study. First, given the retrospective nature of our study, the possibility of selection bias exists, with the low percentage of patients with high-risk varices. Second, the time window between EGD and MR elastography examinations was relatively long in some patients (up to 168 days). Third, we tested the reproducibility of MR elastography in another, separate test population (n = 15). However, all stiffness values were measured by one reader, although reader variability would be another weakness of our study. Further studies on the reproducibility of MR elastography are warranted, even though other investigators have previously reported its high reproducibility (45,52–55). Last, although there were 45 patients in the high-grade varices group, 15 of them had undergone prophylactic variceal ligation to prevent variceal bleeding after acquisition of MR images, and 10 patients experienced variceal bleeding. Therefore, the direct relationship between HS and SS and the occurrence of esophageal variceal bleeding was not fully analyzed in our study. In addition, there was a lot of overlap between groups. This suggests that, for individual patients, the technique may be of limited value in grading varices. Despite these limitations, we found that hepatic and splenic stiffness values assessed with MR elastography are associated with esophageal varices and that the diagnostic accuracy of MR elastography is comparable to that of DCE MR imaging, while combined assessment of MR elastography and DCE MR imaging can increase diagnostic sensitivity.
Advances in Knowledge
■ Hepatic and splenic stiffness measurements at three-dimensional MR elastography are associated with esophageal varices and would be more helpful than the morphologic changes of the spleen, which are the traditionally accepted indicators of liver cirrhosis (P = .0001 and P = .0001 for the presence of esophageal varices, respectively; and P = .046 and P = .049 for high-risk varices, respectively).
■ Combined assessment of MR elastography and dynamic contrast material–enhanced (DCE) MR imaging significantly increased the sensitivity for the detection of varices of any grade compared with DCE MR imaging alone (84.6% vs 74.4%, P = .0004).
Implications for Patient Care
■ In the setting of advanced cirrhosis, even though MR elastography cannot replace or eliminate the need for endoscopy, it can play an adjunctive role in detecting esophageal varices, with diagnostic accuracy comparable to that of DCE MR imaging.
■ By adding MR elastography to DCE MR imaging, clinicians may attain better sensitivity for the detection of esophageal varices than that achieved by using DCE MR imaging alone.
APPENDIX
Acknowledgments
Acknowledgment
We thank Chris Woo, BA, for his assistance with editing the English in this article.
Received April 21, 2013; revision requested June 3; revision received November 3; accepted November 29; final version accepted December 26.
From the 2012 RSNA Annual Meeting.
Funding: This research was supported by the National Institutes of Health (grant EB001981).
Disclosures of Conflicts of Interest: S.U.S. No relevant conflicts of interest to disclose. J.M.L. Financial activities related to the present article: none to disclose. Financial activities not related to the present article: author received fees for a board membership and consultancy with Bayer Healthcare, a consultancy with Siemens Healthcare, and lectures for Bayer Healthcare and Supersonic; and author and institution received grants from GE Healthcare, Dongseo Medical, CMS, and Acuzen. Other relationships: none to disclose. M.H.Y. No relevant conflicts of interest to disclose. J.H.Y. No relevant conflicts of interest to disclose. J.K.H. No relevant conflicts of interest to disclose. B.I.C. Financial activities related to the present article: none to disclose. Financial activities not related to the present article: author received a grant from Samsung Electronics. Other relationships: none to disclose. K.J.G. Financial activities related to the present article: none to disclose. Financial activities not related to the present article: author has stock in Resoundant and has intellectual property related to MR elastography. Other relationships: author has a patent and receives licensing royalties through Resoundant and GE Medical Systems. R.L.E. Financial activities related to the present article: none to disclose. Financial activities not related to the present article: author is an equity holder in and chief executive officer of Resoundant. Other relationships: author has a patent and receives licensing royalties for MR elastography.
Abbreviations
- AUC
- area under the ROC curve
- DCE
- dynamic contrast material–enhanced
- EGD
- esophagogastroduodenoscopy
- HS
- hepatic stiffness
- ROC
- receiver operating characteristic
- SS
- spleen stiffness
- 3D
- three-dimensional
References
- 1.Jou JH, Muir AJ. In the clinic. Hepatitis C. Ann Intern Med 2008;148(11):ITC6–ITC1, ITC6–ITC16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Perz JF, Armstrong GL, Farrington LA, Hutin YJ, Bell BP. The contributions of hepatitis B virus and hepatitis C virus infections to cirrhosis and primary liver cancer worldwide. J Hepatol 2006;45(4):529–538. [DOI] [PubMed] [Google Scholar]
- 3.Seeff LB, Hoofnagle JH. Epidemiology of hepatocellular carcinoma in areas of low hepatitis B and hepatitis C endemicity. Oncogene 2006;25(27):3771–3777. [DOI] [PubMed] [Google Scholar]
- 4.Schuppan D, Afdhal NH. Liver cirrhosis. Lancet 2008;371(9615):838–851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vuppalanchi R, Chalasani N. Nonalcoholic fatty liver disease and nonalcoholic steatohepatitis: selected practical issues in their evaluation and management. Hepatology 2009;49(1):306–317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Michitaka K, Nishiguchi S, Aoyagi Y, et al. Etiology of liver cirrhosis in Japan: a nationwide survey. J Gastroenterol 2010;45(1):86–94. [DOI] [PubMed] [Google Scholar]
- 7.Merli M, Nicolini G, Angeloni S, et al. Incidence and natural history of small esophageal varices in cirrhotic patients. J Hepatol 2003;38(3):266–272. [DOI] [PubMed] [Google Scholar]
- 8.Bosch J, Berzigotti A, Garcia-Pagan JC, Abraldes JG. The management of portal hypertension: rational basis, available treatments and future options. J Hepatol 2008;48(Suppl 1):S68–S92. [DOI] [PubMed] [Google Scholar]
- 9.National Guideline Clearinghouse . World Gastroenterology Organisation practice guideline: esophageal varices. Rockville, Md: Agency for Healthcare Research and Quality. http://www.guideline.gov/. Published February 13, 2012. [Google Scholar]
- 10.Liu CH, Hsu SJ, Liang CC, et al. Esophageal varices: noninvasive diagnosis with duplex Doppler US in patients with compensated cirrhosis. Radiology 2008;248(1):132–139. [DOI] [PubMed] [Google Scholar]
- 11.D’Amico G, De Franchis R; Cooperative Study Group. Upper digestive bleeding in cirrhosis. Post-therapeutic outcome and prognostic indicators. Hepatology 2003;38(3):599–612. [DOI] [PubMed] [Google Scholar]
- 12.Chalasani N, Kahi C, Francois F, et al. Improved patient survival after acute variceal bleeding: a multicenter, cohort study. Am J Gastroenterol 2003;98(3):653–659. [DOI] [PubMed] [Google Scholar]
- 13.North Italian Endoscopic Club for the Study and Treatment of Esophageal Varices . Prediction of the first variceal hemorrhage in patients with cirrhosis of the liver and esophageal varices: a prospective multicenter study. N Engl J Med 1988;319(15):983–989. [DOI] [PubMed] [Google Scholar]
- 14.Garcia-Tsao G, Sanyal AJ, Grace ND, Carey W; Practice Guidelines Committee of the American Association for the Study of Liver Diseases; Practice Parameters Committee of the American College of Gastroenterology. Prevention and management of gastroesophageal varices and variceal hemorrhage in cirrhosis. Hepatology 2007;46(3):922–938. [DOI] [PubMed] [Google Scholar]
- 15.de Franchis R. Evolving consensus in portal hypertension. Report of the Baveno IV consensus workshop on methodology of diagnosis and therapy in portal hypertension. J Hepatol 2005;43(1):167–176. [DOI] [PubMed] [Google Scholar]
- 16.Chalasani N, Imperiale TF, Ismail A, et al. Predictors of large esophageal varices in patients with cirrhosis. Am J Gastroenterol 1999;94(11):3285–3291. [DOI] [PubMed] [Google Scholar]
- 17.Barrera F, Riquelme A, Soza A, et al. Platelet count/spleen diameter ratio for non-invasive prediction of high risk esophageal varices in cirrhotic patients. Ann Hepatol 2009;8(4):325–330. [PubMed] [Google Scholar]
- 18.Chang MH, Sohn JH, Kim TY, et al. Non-endoscopic predictors of large esophageal varices in patients with liver cirrhosis [in Korean]. Korean J Gastroenterol 2007;49(6):376–383. [PubMed] [Google Scholar]
- 19.Hong WD, Dong LM, Jiang ZC, Zhu QH, Jin SQ. Prediction of large esophageal varices in cirrhotic patients using classification and regression tree analysis. Clinics (Sao Paulo) 2011;66(1):119–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Thomopoulos KC. Non-invasive prediction of esophageal varices: is it possible? Saudi J Gastroenterol 2011;17(1):1–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zaman A, Hapke R, Flora K, Rosen HR, Benner K. Factors predicting the presence of esophageal or gastric varices in patients with advanced liver disease. Am J Gastroenterol 1999;94(11):3292–3296. [DOI] [PubMed] [Google Scholar]
- 22.Horváth G. New non-invasive tool for assessment of liver fibrosis: transient elastography [in Hungarian]. Orv Hetil 2011;152(22):860–865. [DOI] [PubMed] [Google Scholar]
- 23.Berzigotti A, Gilabert R, Abraldes JG, et al. Noninvasive prediction of clinically significant portal hypertension and esophageal varices in patients with compensated liver cirrhosis. Am J Gastroenterol 2008;103(5):1159–1167. [DOI] [PubMed] [Google Scholar]
- 24.Parkes J, Guha IN, Roderick P, Rosenberg W. Performance of serum marker panels for liver fibrosis in chronic hepatitis C. J Hepatol 2006;44(3):462–474. [DOI] [PubMed] [Google Scholar]
- 25.Talwalkar JA, Yin M, Venkatesh S, et al. Feasibility of in vivo MR elastographic splenic stiffness measurements in the assessment of portal hypertension. AJR Am J Roentgenol 2009;193(1):122–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nedredal GI, Yin M, McKenzie T, et al. Portal hypertension correlates with splenic stiffness as measured with MR elastography. J Magn Reson Imaging 2011;34(1):79–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Berzigotti A, Seijo S, Arena U, et al. Elastography, spleen size, and platelet count identify portal hypertension in patients with compensated cirrhosis. Gastroenterology 2013;144(1):102–111, e1. [DOI] [PubMed] [Google Scholar]
- 28.Morisaka H, Motosugi U, Ichikawa T, et al. MR-based measurements of portal vein flow and liver stiffness for predicting gastroesophageal varices. Magn Reson Med Sci 2013;12(2):77–86. [DOI] [PubMed] [Google Scholar]
- 29.Beppu K, Inokuchi K, Koyanagi N, et al. Prediction of variceal hemorrhage by esophageal endoscopy. Gastrointest Endosc 1981;27(4):213–218. [DOI] [PubMed] [Google Scholar]
- 30.Huwart L, Peeters F, Sinkus R, et al. Liver fibrosis: non-invasive assessment with MR elastography. NMR Biomed 2006;19(2):173–179. [DOI] [PubMed] [Google Scholar]
- 31.Yin M, Talwalkar JA, Glaser KJ, et al. Assessment of hepatic fibrosis with magnetic resonance elastography. Clin Gastroenterol Hepatol 2007;5(10):1207–1213, e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bezerra AS, D’Ippolito G, Faintuch S, Szejnfeld J, Ahmed M. Determination of splenomegaly by CT: is there a place for a single measurement? AJR Am J Roentgenol 2005;184(5):1510–1513. [DOI] [PubMed] [Google Scholar]
- 33.Lamb PM, Lund A, Kanagasabay RR, Martin A, Webb JA, Reznek RH. Spleen size: how well do linear ultrasound measurements correlate with three-dimensional CT volume assessments? Br J Radiol 2002;75(895):573–577. [DOI] [PubMed] [Google Scholar]
- 34.Ying L, Lin X, Xie ZL, Hu YP, Shi KQ. Performance of platelet count/spleen diameter ratio for diagnosis of esophageal varices in cirrhosis: a meta-analysis. Dig Dis Sci 2012;57(6):1672–1681. [DOI] [PubMed] [Google Scholar]
- 35.Rouvière O, Yin M, Dresner MA, et al. MR elastography of the liver: preliminary results. Radiology 2006;240(2):440–448. [DOI] [PubMed] [Google Scholar]
- 36.Wang Y, Ganger DR, Levitsky J, et al. Assessment of chronic hepatitis and fibrosis: comparison of MR elastography and diffusion-weighted imaging. AJR Am J Roentgenol 2011;196(3):553–561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kim H, Choi D, Gwak GY, et al. Evaluation of esophageal varices on liver computed tomography: receiver operating characteristic analyses of the performance of radiologists and endoscopists. J Gastroenterol Hepatol 2009;24(9):1534–1540. [DOI] [PubMed] [Google Scholar]
- 38.Kazemi F, Kettaneh A, N’kontchou G, et al. Liver stiffness measurement selects patients with cirrhosis at risk of bearing large oesophageal varices. J Hepatol 2006;45(2):230–235. [DOI] [PubMed] [Google Scholar]
- 39.Castéra L, Le Bail B, Roudot-Thoraval F, et al. Early detection in routine clinical practice of cirrhosis and oesophageal varices in chronic hepatitis C: comparison of transient elastography (FibroScan) with standard laboratory tests and non-invasive scores. J Hepatol 2009;50(1):59–68. [DOI] [PubMed] [Google Scholar]
- 40.Lim JK, Groszmann RJ. Transient elastography for diagnosis of portal hypertension in liver cirrhosis: is there still a role for hepatic venous pressure gradient measurement? Hepatology 2007;45(5):1087–1090. [DOI] [PubMed] [Google Scholar]
- 41.Vizzutti F, Arena U, Romanelli RG, et al. Liver stiffness measurement predicts severe portal hypertension in patients with HCV-related cirrhosis. Hepatology 2007;45(5):1290–1297. [DOI] [PubMed] [Google Scholar]
- 42.Spina GP, Arcidiacono R, Bosch J, et al. Gastric endoscopic features in portal hypertension: final report of a consensus conference, Milan, Italy, September 19, 1992. J Hepatol 1994;21(3):461–467. [DOI] [PubMed] [Google Scholar]
- 43.Matsuo M, Kanematsu M, Kim T, et al. Esophageal varices: diagnosis with gadolinium-enhanced MR imaging of the liver for patients with chronic liver damage. AJR Am J Roentgenol 2003;180(2):461–466. [DOI] [PubMed] [Google Scholar]
- 44.Cho KC, Patel YD, Wachsberg RH, Seeff J. Varices in portal hypertension: evaluation with CT. RadioGraphics 1995;15(3):609–622. [DOI] [PubMed] [Google Scholar]
- 45.Hines CD, Bley TA, Lindstrom MJ, Reeder SB. Repeatability of magnetic resonance elastography for quantification of hepatic stiffness. J Magn Reson Imaging 2010;31(3):725–731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Taouli B, Ehman RL, Reeder SB. Advanced MRI methods for assessment of chronic liver disease. AJR Am J Roentgenol 2009;193(1):14–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Adhoute X, Foucher J, Laharie D, et al. Diagnosis of liver fibrosis using FibroScan and other noninvasive methods in patients with hemochromatosis: a prospective study. Gastroenterol Clin Biol 2008;32(2):180–187. [DOI] [PubMed] [Google Scholar]
- 48.Kim BK, Han KH, Park JY, et al. A liver stiffness measurement-based, noninvasive prediction model for high-risk esophageal varices in B-viral liver cirrhosis. Am J Gastroenterol 2010;105(6):1382–1390. [DOI] [PubMed] [Google Scholar]
- 49.Sandrin L, Fourquet B, Hasquenoph JM, et al. Transient elastography: a new noninvasive method for assessment of hepatic fibrosis. Ultrasound Med Biol 2003;29(12):1705–1713. [DOI] [PubMed] [Google Scholar]
- 50.Bensamoun SF, Wang L, Robert L, Charleux F, Latrive JP, Ho Ba Tho MC. Measurement of liver stiffness with two imaging techniques: magnetic resonance elastography and ultrasound elastometry. J Magn Reson Imaging 2008;28(5):1287–1292. [DOI] [PubMed] [Google Scholar]
- 51.Huwart L, van Beers BE. MR elastography. Gastroenterol Clin Biol 2008;32(6 Suppl 1):68–72. [DOI] [PubMed] [Google Scholar]
- 52.Yin M, Talwalkar JA, Glaser KJ, et al. Dynamic postprandial hepatic stiffness augmentation assessed with MR elastography in patients with chronic liver disease. AJR Am J Roentgenol 2011;197(1):64–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Shire NJ, Yin M, Chen J, et al. Test-retest repeatability of MR elastography for noninvasive liver fibrosis assessment in hepatitis C. J Magn Reson Imaging 2011;34(4):947–955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Huwart L, Sempoux C, Vicaut E, et al. Magnetic resonance elastography for the noninvasive staging of liver fibrosis. Gastroenterology 2008;135(1):32–40. [DOI] [PubMed] [Google Scholar]
- 55.Kim BH, Lee JM, Lee YJ, et al. MR elastography for noninvasive assessment of hepatic fibrosis: experience from a tertiary center in Asia. J Magn Reson Imaging 2011;34(5):1110–1116. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.