Saturation recovery single-shot acquisition and saturation pulse prepared heart rate independent inversion recovery have superior accuracy, inferior precision, and similar reproducibility compared with modified Look-Locker inversion recovery and shortened modified Look-Locker inversion recovery.
Abstract
Purpose
To compare accuracy, precision, and reproducibility of four commonly used myocardial T1 mapping sequences: modified Look-Locker inversion recovery (MOLLI), shortened MOLLI (ShMOLLI), saturation recovery single-shot acquisition (SASHA), and saturation pulse prepared heart rate independent inversion recovery (SAPPHIRE).
Materials and Methods
This HIPAA-compliant study was approved by the institutional review board. All subjects provided written informed consent. Accuracy, precision, and reproducibility of the four T1 mapping sequences were first compared in phantom experiments. In vivo analysis was performed in seven healthy subjects (mean age ± standard deviation, 38 years ± 19; four men, three women) who were imaged twice on two separate days. In vivo reproducibility of native T1 mapping and extracellular volume (ECV) were measured. Differences between the sequences were assessed by using Kruskal-Wallis and Wilcoxon rank sum tests (phantom data) and mixed-effect models (in vivo data).
Results
T1 mapping accuracy in phantoms was lower with ShMOLLI (62 msec) and MOLLI (44 msec) than with SASHA (13 msec; P < .05) and SAPPHIRE (12 msec; P < .05). MOLLI had similar precision to ShMOLLI (4.0 msec vs 5.6 msec; P = .07) but higher precision than SAPPHIRE (6.8 msec; P = .002) and SASHA (8.7 msec; P < .001). All sequences had similar reproducibility in phantoms (P = .1). The four sequences had similar in vivo reproducibility for native T1 mapping (∼25–50 msec; P > .05) and ECV quantification (∼0.01–0.02; P > .05).
Conclusion
SASHA and SAPPHIRE yield higher accuracy, lower precision, and similar reproducibility compared with MOLLI and ShMOLLI for T1 measurement. Different sequences yield different ECV values; however, all sequences have similar reproducibility for ECV quantification.
© RSNA, 2014
Introduction
Quantitative myocardial T1 mapping is a cardiovascular magnetic resonance (MR) technique that provides in vivo tissue characterization (1,2). Alterations of native myocardial T1 times have been observed in the presence of a variety of pathologic conditions (3). Furthermore, native and postcontrast administration T1 mapping can be performed to measure the extracellular volume fraction (ECV) (4), which has important prognostic value (5,6) and shows promise for the detection of diffuse myocardial fibrosis (7,8).
Several T1 mapping techniques have been proposed by using different acquisition schemes to sample the T1 recovery signal (1,9–20). Multiple images with different T1-weighting are generally acquired and used to provide quantitative T1 estimates by using a model of the T1 recovery signal (1,9–20). Despite the promise of these T1 mapping techniques to improve diagnosis, prognosis, and monitoring response to therapy in a variety of cardiomyopathies, there is no current standard approach or recommendation for clinical cardiac MR protocols. T1 measurements can be altered by several factors, such as the acquisition scheme, magnetization transfer, flow, T2 effect, and motion (3,10,21–24). Therefore, the characterization of each approach in term of accuracy, precision, and reproducibility is crucial to reach a consensus (3).
Although these techniques have been individually evaluated, no comparison has yet been performed across inversion recovery, saturation recovery, and combined saturation and inversion recovery sequences. The purpose of this study was to compare accuracy, precision, and reproducibility of four commonly used myocardial T1 mapping sequences: modified Look-Locker inversion recovery (MOLLI), shortened MOLLI (ShMOLLI), saturation recovery single-shot acquisition (SASHA), and saturation pulse prepared heart rate independent inversion recovery (SAPPHIRE).
Materials and Methods
S.W., W.J.M, and R.N. are inventors of a pending U.S. patent entitled “Methods for scar imaging in patients with arrhythmia,” which described the SAPPHIRE imaging sequence for imaging of scar and fibrosis. All subjects were imaged by using a 1.5-T MR imager (Achieva; Philips Healthcare, Best, the Netherlands) and a 32-channel cardiac phased array receiver coil. In this Health Insurance Portability and Accountability Act–compliant study, the imaging protocol was approved by our institutional review board, and informed consent was obtained from all participants. Detailed methods are provided in Appendix E1 (online).
Phantom Study
A phantom that contained 14 vials (nickel chloride doped agarose) with different T1 and T2 values was used for the comparison of the four T1 mapping sequences. Reference T1 and T2 measurements were first obtained by using spin-echo acquisitions. Each of the four T1 mapping sequences was then acquired 10 times by using an electrocardiogram-triggered single shot acquisition with a balanced steady-state free precession readout (repetition time msec/echo time msec, 3.1/1.5; field of view, 360 × 337 mm2; voxel size, 1.9 mm2 ± 2.5; section thickness, 8 mm; number of phase-encoding lines, 70; linear ordering, 10 linear ramp-up pulses; sensitivity encoding factor, 2; MOLLI and ShMOLLI flip angle, 35°; SASHA and SAPPHIRE flip angle, 70°).
In Vivo Study
Seven healthy adults (mean age, 38 years ± 19 [standard deviation]; four men, three women) were recruited for in vivo comparison of the four sequences. Figure E1 (online) shows the study design of the in vivo study. Each subject participated in two cardiac MR examinations on two separate days (mean interval, 53 days ± 24). On the first day, precontrast imaging was performed twice (examinations 1 and 2) and was followed by two postcontrast MR examinations approximately 15–20 minutes and approximately 30–35 minutes after injection of 0.1 mmol/kg of gadobenate dimeglumine (MultiHance; Bracco Diagnostic, Princeton, NJ). A blood sample was drawn from each subject before the first cardiac MR examination to measure hematocrit. Each subject was removed from the imager after the first precontrast imaging session to simulate a new examination. Each sequence was acquired within an end-expiration breath-hold by using an electrocardiogram-triggered single-shot acquisition with a balanced steady-state free precession readout (3.1/1.5; field of view, 360 × 337 mm2; acquisition matrix, 188 × 135; voxel size, 1.9 × 2.5 mm2; section thickness, 8 mm; number of phase-encoding lines, 70; linear ordering, 10 linear ramp-up pulses; sensitivity encoding factor, 2; flip angle, 70°; bandwidth, 1085 Hz/pixel). Three sections were acquired in the left ventricular short axis orientation with each T1 mapping sequence. On the second day, a single native T1 mapping session (examination 3) was set up to acquire three sections in a similar short-axis orientation with the same four T1 mapping sequences.
Data Analysis
Accuracy, precision, and reproducibility of the four T1 mapping sequences were analyzed in the phantom study. Reproducibility of native T1 mapping and ECV measurement was evaluated in vivo in healthy subjects. Native T1 mapping reproducibility was measured from the two precontrast examinations that were acquired within the same MR examination (ie, two precontrast examinations on day 1) and across two separate MR examinations (ie, second precontrast examination on day 1 vs examination on day 2) ECV reproducibility was assessed between two ECVs derived from the second precontrast examination and the first postcontrast examination, and the second precontrast examination and the second postcontrast examination.
Statistical Analysis
The accuracy of the four sequences measured in the phantom study was compared by using a Kruskal-Wallis test, and a P value less than .05 indicated statistical significance. When the Kruskal-Wallis test found statistical significance, Wilcoxon rank sum tests were performed for each pair of sequences. The Wilcoxon rank sum test was considered to be statistically significant if it had a P value less than .05. The same methodologic parameter was used to analyze the signed accuracy bias, the precision, and the reproducibility of the four sequences in the phantom study.
The in vivo reproducibility of native T1 measurement obtained with the four sequences was compared by using a mixed-effect model. A similar test was used to compare the in vivo reproducibility of ECV measurements. P values less than .05 indicated statistical significance for each effect. Bonferroni correction was used for pair-wise comparison of sequences, which resulted in a statistical significance threshold of P < .008.
Results
Table E1 (online) summarizes the T1 and T2 measurements of the four sequences obtained in the phantom study. Results are reported for each of the 14 vials. Substantial variations were observed in T1 estimates measured from the four sequences, especially in the presence of elevated T1 times or low T2 times. Accuracy and signed accuracy bias of each sequence are reported in Table E2 (online). Excellent accuracy was achieved by using SASHA and SAPPHIRE for all T1 and T2 ranges. MOLLI and ShMOLLI showed less accuracy than SASHA (P = .02 and P = .04, respectively) and SAPPHIRE (P = .025 and P = .045, respectively), with substantial T1 underestimation for large T1 times (>1000 msec) or lower T2 times (<100 msec). Statistical differences were found among the four sequences in term of precision (P = .001) (Table E3 [online]). MOLLI had higher precision than both SASHA (P < .001) and SAPPHIRE (P = .002). In addition, there was a trend for MOLLI to be more precise than ShMOLLI (P = .07) and for ShMOLLI to be more precise than SASHA (P = .07). There were no statistical differences in term of precision between ShMOLLI and SAPPHIRE (P = .11) or between SAPPHIRE and SASHA (P = .22). The reproducibility analysis over all vials revealed no statistical difference among the four sequences (P = .1).
Figure 1 shows examples of in vivo T1 maps obtained in a 53-year-old patient with all four T1 mapping sequences. Myocardium T1 obtained from MOLLI and ShMOLLI appears much lower than with SASHA and SAPPHIRE. However, greater variability is observed in SASHA and SAPPHIRE T1 maps.
Figure 1:
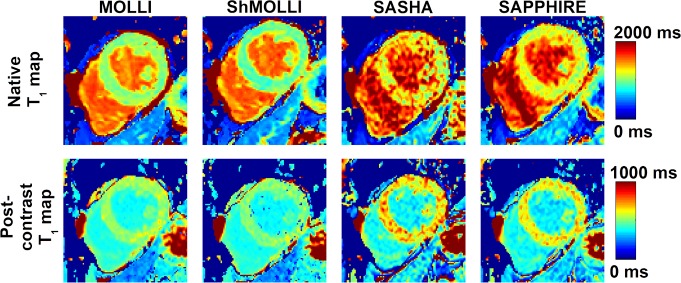
Images show example T1 maps acquired with all four T1 mapping sequences (top row) before and (bottom row) after administration of contrast material in a 53-year-old man. Myocardial T1 values over the left ventricle obtained with MOLLI (native, 1012 msec ± 60; postcontrast administration, 527 msec ± 30) and ShMOLLI (native, 924 msec ± 70; postcontrast administration, 501 msec ± 33) were lower than those obtained with SASHA (native, 1254 msec ± 191; postcontrast administration, 659 msec ± 81) and SAPPHIRE (native, 1160 msec ± 95; postcontrast administration, 625 msec ± 55). MOLLI and ShMOLLI provided improved precontrast and postcontrast map quality with less variability.
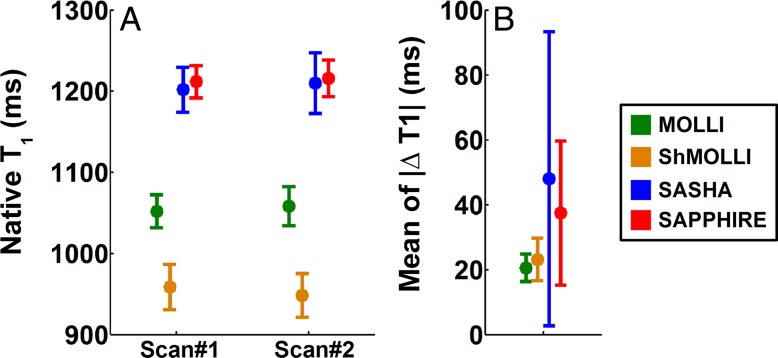
Summary measurements of native myocardial T1 data obtained in healthy subjects during the same MR examination are reported in Figure 2. There was a statistically significant difference among the four sequences in native T1 time (P < .001). SASHA and SAPPHIRE provided similar native T1 times of approximately 1200 msec (P = .83). Lower native T1 times were obtained with ShMOLLI and MOLLI compared with SASHA (P < .001) and SAPPHIRE (P < .001). Reproducibility of native T1 mapping within the same MR examination is reported in Figure 2B. There was a statistically significant difference among the four sequences (P = .03). MOLLI and ShMOLLI had similar reproducibility (P = .91). SAPPHIRE and SASHA had similar reproducibility (P = .39). There was a trend for MOLLI and ShMOLLI to be more reproducible than SAPPHIRE (P = .06 and P = .07, respectively) and SASHA (P = .01 and P = .02, respectively).
Figure 2:
Box and whisker plots show, A, native myocardial T1 measurements and, B, reproducibility within the same MR examination in healthy subjects by using MOLLI, ShMOLLI, SASHA, and SAPPHIRE. There were no statistically significant differences in native T1 times obtained with SASHA (examination [scan] 1, 1202 msec ± 56; examination 2, 1210 msec ± 76) and SAPPHIRE (examination 1, 1212 msec ± 40; examination 2, 1216 msec ± 46) (P = .83). Remaining paired sequence comparisons were statistically significant (P < .001). Lower native T1 times were obtained with ShMOLLI (examination 1, ∼959 msec ± 56; examination 2, 948 msec ± 54) and MOLLI (examination 1, ∼1052 msec ± 41; examination 2, 1058 msec ± 48) compared with SASHA (P < .001) and SAPPHIRE (P < .001). There were statistically significant differences among the four sequences in term of reproducibility (P = .03). MOLLI and ShMOLLI had similar reproducibility (21 msec ± 9 vs 23 msec ± 13, respectively; P = .91). SAPPHIRE and SASHA had similar reproducibility (37 msec ± 44 vs 48 msec ± 91; P = .39). MOLLI and ShMOLLI trended toward being more reproducible than SAPPHIRE (P = .06 and P = .07, respectively) or SASHA (P = .01 and P = .02, respectively). △T1 = change in T1.
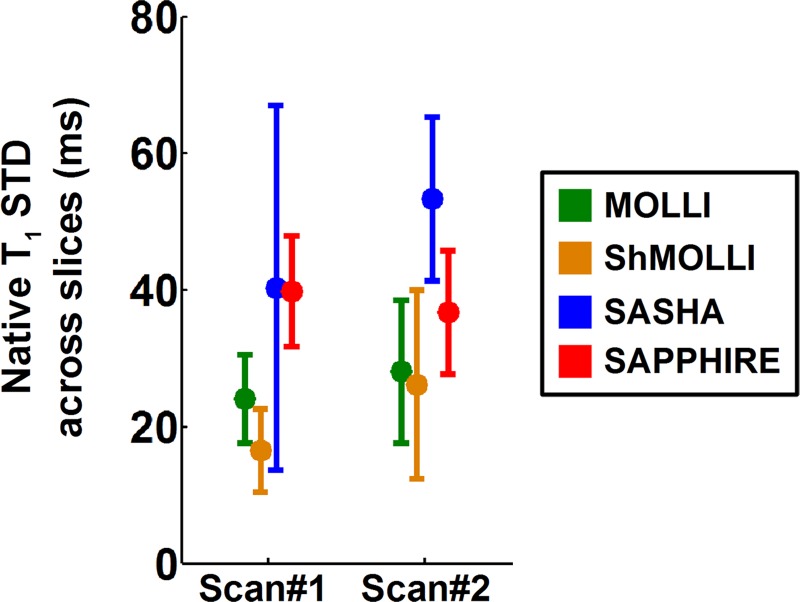
Figure 3 shows the variation of myocardial T1 measurements across sections. There was no section effect on native T1 time measurements, which was revealed by the mixed-effect model (P = .16).
Figure 3:
Box and whisker plot shows native myocardial T1 variation across sections (slices) calculated from four T1 mapping sequences in healthy subjects. Shown are standard deviations over sections of the T1 mean of a region of interest over the septum. Over the two precontrast examinations of day 1, the T1 variation across sections was lower with MOLLI (examination [scan] 1, 24 msec ± 13; examination 2, 28 msec ± 21) and ShMOLLI (examination 1, 17 msec ± 12; examination 2, 26 msec ± 28) than SAPPHIRE (examination 1, 40 msec ± 16; examination 2, 37 msec ± 18) and SASHA (examination 1, 40 msec ± 53; examination 2, 53 msec ± 24). Mixed-effect model did not reveal any statistically significant section effect (P = .16).
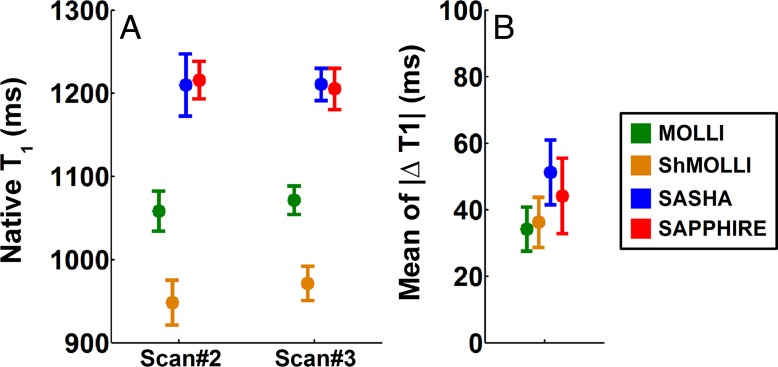
Figure 4 shows the reproducibility of native T1 mapping from two separate MR examinations. Heart rate in the healthy subjects was 68 beats/min ± 9 on day 1 and 65 beats/min ± 9 on day 2, with an intrasubject variation of 8 beats/min ± 3 between the 2 days. The four sequences provided similar reproducibility (P = .11).
Figure 4:
Box and whisker plots show, A, native myocardial T1 measurements and, B, reproducibility between two separate MR examinations in healthy subjects by using the four sequences of MOLLI, ShMOLLI, SASHA, and SAPPHIRE. Shown are the second native T1-weighted examination on day 1 (examination [scan] 2) versus native T1-weighted examination on day 2 (examination 3). Reproducibility is reported as the absolute difference between the two precontrast myocardial T1-weighted examinations. MOLLI and ShMOLLI provided similar reproducibility with an average absolute T1 variation of 34 msec ± 13 and 36 msec ± 15, respectively. Although SASHA and SAPPHIRE provided slightly lower reproducibility (51 msec ± 19 and 44 msec ± 23, respectively), there were no statistical differences among the sequences (P = .11). △T1 = change in T1.
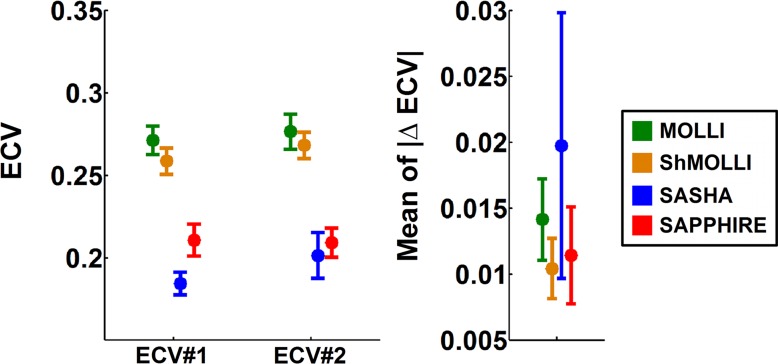
ECV measurements and reproducibility are shown in Figure 5. ECV values measured with MOLLI (ECV, ∼0.27) and ShMOLLI (ECV, ∼0.26) were higher than with SASHA (ECV, ∼0.18; P < .001) and SAPPHIRE (ECV, ∼0.20; P < .001). There was no statistically significant difference in term of ECV reproducibility among the four sequences (P = .11).
Figure 5:
Box and whisker plots show, A, ECV measurements and, B, ECV reproducibility in healthy subjects by using the four T1 mapping sequences. ECV reproducibility was measured as the absolute difference between two ECVs, which were calculated from the second precontrast examination and the first postcontrast examination (ECV #1) and the second precontrast examination and the second postcontrast examination (ECV #2). Variations were observed in ECV measurements among the four T1 mapping sequences (P < .001). Similar ECV measurements were obtained with MOLLI and ShMOLLI (∼0.27 vs ∼0.26; P = .13). Lower ECV values were obtained with SAPPHIRE (∼0.20) and SASHA (∼0.18) compared with MOLLI or ShMOLLI (P < .001). The difference between SAPPHIRE and SASHA was statistically significant (P = .002). Reproducibility of ECV measurements ranged from 0.01 to 0.02 for all sequences. There was no statistically significant difference for ECV reproducibility among the four sequences (P = .11).
Discussion
In this study, we compared four T1 mapping sequences and assessed their accuracy, precision, and reproducibility. The phantom study revealed that SASHA and SAPPHIRE have superior accuracy, inferior precision, and similar reproducibility to MOLLI and ShMOLLI. Reproducibility results were confirmed in vivo in healthy subjects where all sequences had similar reproducibility for native myocardial T1 mapping. Different sequences yield different in vivo ECV values but reproducibility of ECV measurements is similar with all sequences.
We found that MOLLI and ShMOLLI led to an underestimation of myocardial T1 values for both the phantom and the healthy subject study. These underestimations were found to be more prominent with higher T1 values. These data are consistent with previous studies (21–23,25,26), which identified several factors affecting MOLLI measurements, such as T2-dependence, magnetization transfer effect, and dependence on the inversion efficiency. SASHA and SAPPHIRE yielded excellent accuracy for all range of studied T1 values. These results are also in good agreement with previous work (12,24), which showed that SASHA is not T2-dependent, is insensitive to inversion efficiency (21), and has low sensitivity to magnetization transfer (23). Since phantom and in vivo T1 estimates are in good agreement between SASHA and SAPPHIRE, this validates that the T2-dependence and the magnetization transfer have little effect on SAPPHIRE. This is a major finding which demonstrates that SAPPHIRE estimate true T1 times and not apparent T1 times. Myocardial ECV measurements obtained in normal subjects were in good agreement with previous studies that reported values of 0.25 ± 0.03 for MOLLI (27) and 0.27 ± 0.03 for ShMOLLI (28). ShMOLLI and MOLLI had a statistically significant difference in ECV measurements compared with SASHA and SAPPHIRE. This implies that standardized ECV and thresholds need to be defined independently for each sequence. The ECV reproducibility of the four sequences was similar in healthy subjects. The ECV accuracy was not studied in this study because it is difficult to obtain in vivo reference ECV measurements. However, bias in T1 estimates obtained with the ShMOLLI and MOLLI approach are nonlinear (ie, they affect more T1 values that are higher) and are thus expected to cause ECV measurements bias.
There are several limitations in this study. The sample size of the in vivo study was small, which may have limited ability to detect subtle differences between the sequences. A parametric procedure was used for in vivo analysis based on an assumed normal distribution of our data. However, this assumption had limitations that could have affected our in vivo findings. A flip angle of 70° was used for in vivo imaging with MOLLI and ShMOLLI, and could have slightly degraded the reproducibility of these two sequences. Different regions of interest were drawn for each T1 map, which could have introduced different partial volume contributions. Fatigue effects could have affected the in vivo study since the sequence order was not randomized. In vivo T1 times were only measured in the septum. Therefore, comparison of reproducibility in the septum may not be reflective of other regions. Our in vivo data were not motion corrected because no commercial image registration technique was available for our imager. Despite specific care taken to ensure that the delineated myocardial area was contained within the myocardium in all T1-weighted images, motion-induced artifacts could have led to some bias in T1 estimates. The effect of contrast agent and contrast dose was not evaluated in this study. Finally, this study was performed in healthy adults. Patients will demonstrate a wide range of pathologic myocardial T1 and ECV values, and greater variability in contrast agent excretion rates, heart rates, and motion artifact compared with healthy volunteers, which may affect the results.
Advances in Knowledge
■ Saturation recovery single-shot acquisition (SASHA) and saturation pulse prepared heart rate independent inversion recovery (SAPPHIRE) sequences yield consistent high accuracy of T1, but modified Look-Locker inversion recovery (MOLLI) and shortened modified Look-Locker inversion recovery (ShMOLLI) are biased by several confounding factors (P < .05).
■ MOLLI and ShMOLLI have higher precision for T1 mapping than SASHA and SAPPHIRE (P < .05).
■ All four T1 mapping sequences have similar reproducibility for T1 mapping (∼25–50 msec; P > .05).
■ The four T1 mapping sequences yielded different in vivo ECV values (P < .001); however, they all have similar reproducibility (∼0.01–0.02; P = .11).
Implications for Patient Care
■ SAPPHIRE and SASHA can be used for accurate myocardial T1 assessment.
■ MOLLI and ShMOLLI provide higher precision for myocardial T1 assessment.
APPENDIX AND TABLES
SUPPLEMENTAL FIGURE
Acknowledgments
Acknowledgments
We thank Kraig V. Kissinger, Beth Goddu, and Sophie Berg for subject recruitment and imaging.
Received February 5, 2014; revision requested February 14; revision received February 28; accepted March 10; final version accepted March 10.
Current address: Department of Computer Assisted Clinical Medicine, University Medical Center Mannheim, Heidelberg University, Mannheim, Germany.
Funding: This research was supported by the National Institutes of Health (grant R01EB008743-01A2). P.K. is an employee of the National Institutes of Health.
Disclosures of Conflicts of Interest: S.R. No relevant conflicts of interest to disclose. S.W. Financial activities related to the present article: author has a patent pending for methods for scar imaging in patients with arrhythmia. Financial activities not related to the present article: none to disclose. Other relationships: none to disclose. M.F. No relevant conflicts of interest to disclose. K.C. Financial activities related to the present article: none to disclose. Financial activities not related to the present article: author has a patent pending for variable flip angle readout for improved magnetization prepared imaging. Other relationships: none to disclose. K.K. No relevant conflicts of interest to disclose. L.H.N. No relevant conflicts of interest to disclose. P.K. No relevant conflicts of interest to disclose. W.J.M. Financial activities related to the present article: author has a patent pending for methods for scar imaging in patients with arrhythmia. Financial activities not related to the present article: none to disclose. Other relationships: none to disclose. R.B.T. Financial activities related to the present article: none to disclose. Financial activities not related to the present article: author receives nonfinancial support from Siemens Medical Solutions and has a patent pending for variable flip angle readout for improved magnetization prepared imaging. Other relationships: none to disclose. R.N. Financial activities related to the present article: author has a patent pending for methods for scar imaging in patients with arrhythmia. Financial activities not related to the present article: author receives patent royalties from Phillips Healthcare. Other relationships: none to disclose.
Abbreviations:
- ECV
- extracellular volume fraction
- MOLLI
- modified Look-Locker inversion recovery
- SAPPHIRE
- saturation pulse prepared heart rate independent inversion recovery
- SASHA
- saturation recovery single-shot acquisition
- ShMOLLI
- shortened modified Look-Locker inversion recovery
References
- 1.Messroghli DR, Radjenovic A, Kozerke S, Higgins DM, Sivananthan MU, Ridgway JP. Modified Look-Locker inversion recovery (MOLLI) for high-resolution T1 mapping of the heart. Magn Reson Med 2004;52(1):141–146. [DOI] [PubMed] [Google Scholar]
- 2.Messroghli DR, Niendorf T, Schulz-Menger J, Dietz R, Friedrich MG. T1 mapping in patients with acute myocardial infarction. J Cardiovasc Magn Reson 2003;5(2):353–359. [DOI] [PubMed] [Google Scholar]
- 3.Moon JC, Messroghli DR, Kellman P, et al. Myocardial T1 mapping and extracellular volume quantification: a Society for Cardiovascular Magnetic Resonance (SCMR) and CMR Working Group of the European Society of Cardiology consensus statement. J Cardiovasc Magn Reson 2013;15(1):92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Arheden H, Saeed M, Higgins CB, et al. Measurement of the distribution volume of gadopentetate dimeglumine at echo-planar MR imaging to quantify myocardial infarction: comparison with 99mTc-DTPA autoradiography in rats. Radiology 1999;211(3):698–708. [DOI] [PubMed] [Google Scholar]
- 5.Wong TC, Piehler K, Meier CG, et al. Association between extracellular matrix expansion quantified by cardiovascular magnetic resonance and short-term mortality. Circulation 2012;126(10):1206–1216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wong TC, Piehler KM, Kang IA, et al. Myocardial extracellular volume fraction quantified by cardiovascular magnetic resonance is increased in diabetes and associated with mortality and incident heart failure admission. Eur Heart J 2014;35(10):657–664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Iles L, Pfluger H, Phrommintikul A, et al. Evaluation of diffuse myocardial fibrosis in heart failure with cardiac magnetic resonance contrast-enhanced T1 mapping. J Am Coll Cardiol 2008;52(19):1574–1580. [DOI] [PubMed] [Google Scholar]
- 8.Mewton N, Liu CY, Croisille P, Bluemke D, Lima JA. Assessment of myocardial fibrosis with cardiovascular magnetic resonance. J Am Coll Cardiol 2011;57(8):891–903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Look DC, Locker DR. Time saving in measurement of NMR and EPR relaxation times. Rev Sci Instrum 1970;41(2):250–251. [Google Scholar]
- 10.Piechnik SK, Ferreira VM, Dall’Armellina E, et al. Shortened Modified Look-Locker Inversion recovery (ShMOLLI) for clinical myocardial T1-mapping at 1.5 and 3 T within a 9 heartbeat breathhold. J Cardiovasc Magn Reson 2010;12:69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chow K, Flewitt JA, Green JD, Pagano JJ, Friedrich MG, Thompson RB. Saturation recovery single-shot acquisition (SASHA) for myocardial T1 mapping. Magn Reson Med 2013 Jul 23. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 12.Weingärtner S, Akçakaya M, Basha T, et al. Combined saturation/inversion recovery sequences for improved evaluation of scar and diffuse fibrosis in patients with arrhythmia or heart rate variability. Magn Reson Med 2013 May 6. [Epub ahead of print] [DOI] [PubMed]
- 13.Clique H, Cheng HL, Marie PY, Felblinger J, Beaumont M. 3D myocardial T(1) mapping at 3T using variable flip angle method: Pilot study. Magn Reson Med 2013 Feb 25. [Epub ahead of print] [DOI] [PubMed]
- 14.Coniglio A, Di Renzi P, Vilches Freixas G, et al. Multiple 3D inversion recovery imaging for volume T1 mapping of the heart. Magn Reson Med 2013;69(1):163–170. [DOI] [PubMed] [Google Scholar]
- 15.Coolen BF, Geelen T, Paulis LE, Nauerth A, Nicolay K, Strijkers GJ. Three-dimensional T1 mapping of the mouse heart using variable flip angle steady-state MR imaging. NMR Biomed 2011;24(2):154–162. [DOI] [PubMed] [Google Scholar]
- 16.Fitts M, Breton E, Kholmovski EG, et al. Arrhythmia insensitive rapid cardiac T1 mapping pulse sequence. Magn Reson Med 2013;70(5):1274–1282. [DOI] [PubMed] [Google Scholar]
- 17.Flacke SJ, Fischer SE, Lorenz CH. Measurement of the gadopentetate dimeglumine partition coefficient in human myocardium in vivo: normal distribution and elevation in acute and chronic infarction. Radiology 2001;218(3):703–710. [DOI] [PubMed] [Google Scholar]
- 18.Slavin GS, Stainsby JA. True T1 mapping with SMART1Map (saturation method using adaptive recovery times for cardiac T1 mapping): a comparison with MOLLI. J Cardiovasc Magn Reson 2013;15(Suppl 1):P3. [Google Scholar]
- 19.Song T, Stainsby JA, Ho VB, Hood MN, Slavin GS. Flexible cardiac T1 mapping using a modified Look-Locker acquisition with saturation recovery. Magn Reson Med 2012;67(3):622–627. [DOI] [PubMed] [Google Scholar]
- 20.Higgins DM, Ridgway JP, Radjenovic A, Sivananthan UM, Smith MA. T1 measurement using a short acquisition period for quantitative cardiac applications. Med Phys 2005;32(6):1738–1746. [DOI] [PubMed] [Google Scholar]
- 21.Chow K, Flewitt J, Pagano JJ, Green JD, Friedrich MG, Thompson RB. T2-dependent errors in MOLLI T1 values: simulations, phantoms, and in-vivo studies. J Cardiovasc Magn Reson 2012;14(Suppl 1):P281. [Google Scholar]
- 22.Chow K, Flewitt JA, Pagano JJ, Green JD, Friedrich MG, Thompson RB. MOLLI T1 values have systematic T2 and inversion efficiency dependent errors [abstr]. In: Proceedings of the Twentieth Meeting of the International Society for Magnetic Resonance in Medicine. Berkeley, Calif: International Society for Magnetic Resonance in Medicine, 2012; 3288. [Google Scholar]
- 23.Robson MD, Piechnik SK, Tunnicliffe EM, Neubauer S. T1 measurements in the human myocardium: The effects of magnetization transfer on the SASHA and MOLLI sequences. Magn Reson Med 2013 Jul 15. [Epub ahead of print] [DOI] [PubMed]
- 24.Kellman P, Hansen MS. T1-mapping in the heart: accuracy and precision. J Cardiovasc Magn Reson 2014;16(1):2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kellman P, Herzka DA, Hansen MS. Adiabatic inversion pulses for myocardial T1 mapping. Magn Reson Med 2013 May 30. [Epub ahead of print] [DOI] [PMC free article] [PubMed]
- 26.Cooper MA, Nguyen TD, Spincemaille P, Prince MR, Weinsaft JW, Wang Y. Flip angle profile correction for T₁ and T₂ quantification with look-locker inversion recovery 2D steady-state free precession imaging. Magn Reson Med 2012;68(5):1579–1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kellman P, Wilson JR, Xue H, et al. Extracellular volume fraction mapping in the myocardium, part 2: initial clinical experience. J Cardiovasc Magn Reson 2012;14:64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fontana M, White SK, Banypersad SM, et al. Comparison of T1 mapping techniques for ECV quantification. Histological validation and reproducibility of ShMOLLI versus multibreath-hold T1 quantification equilibrium contrast CMR. J Cardiovasc Magn Reson 2012;14:88. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.