The results of this study show that measures of longer duration of diabetes or biochemical severity correlated primarily with brain atrophy, but not with white matter lesion volume, which is the major MR imaging marker of small vessel ischemic disease.
Abstract
Purpose
To investigate the association of characteristics of type 2 diabetes mellitus (duration and biochemical severity of diabetes) to brain structure measured on magnetic resonance (MR) images, specifically testing whether more severity in metrics of diabetes is inversely correlated with brain volumes and positively correlated with ischemic lesion volumes.
Materials and Methods
This study protocol was approved by the institutional review board of each center and participants provided written informed consent. Baseline severity of diabetes was evaluated by testing fasting plasma glucose levels, hemoglobin A1c levels, and duration of diabetes. MR imaging was performed with fluid-attenuated inversion recovery, proton-density, T2-weighted, and T1-weighted sequences, which were postprocessed with an automated computer algorithm that classified brain tissue as gray or white matter and as normal or ischemic. Separate linear regression models adjusted for potential confounding factors were used to investigate the relationship of the diabetes measures to MR imaging outcomes in 614 participants (mean age, 62 years; mean duration of type 2 diabetes mellitus, 9.9 years).
Results
The mean volumes of total gray matter (463.9 cm3) and total white matter (463.6 cm3) were similar. The mean volume of abnormal tissue was 2.5 cm3, mostly in the white matter (81% white matter, 5% gray matter, 14% deep gray and white matter). Longer duration of diabetes and higher fasting plasma glucose level were associated with lower normal (β = −0.431 and −0.053, respectively; P < .01) and total gray matter volumes (β = −0.428 and −0.053, respectively; P < .01). Fasting plasma glucose was also inversely correlated with ischemic lesion volume (β = −0.006; P < .04). Hemoglobin A1c level was not associated with any MR imaging measure.
Conclusion
Longer duration of diabetes is associated with brain volume loss, particularly in the gray matter, possibly reflecting direct neurologic insult; biochemical measures of glycemia were less consistently related to MR imaging changes. Contrary to common clinical belief, in this sample of patients with type 2 diabetes mellitus, there was no association of diabetes characteristics with small vessel ischemic disease in the brain.
© RSNA, 2014
Clinical trial registration no. NCT00182910
Introduction
The advent of brain magnetic resonance (MR) imaging made possible the in vivo detection of leukoaraiosis or small vessel ischemic disease (SVID) of the brain. This pathologic entity is primarily reflected as hyperintensities in the white matter and basal ganglia on T2 images (1,2). These lesions are most strongly associated with age, hypertension, and various markers of micro- and macrovascular disease (3,4). At histologic examination, the lesions consist of nonnecrotic demyelination, postulated to be ischemic in origin (5). Some of these lesions that appear hyperintense on T2 images are also hypointense on T1-weighted images and are often classified as infarcts with presumed necrosis, a subset of which are small lacunae. Clinically, the number and extent of these lesions are inversely correlated with cognition and various physical functions, such as balance and gait (3,6). SVID is now recognized as a major factor in cognitive decline and dementia in the elderly.
Given the long-known and presumably etiologic relationship between diabetes and vascular disease, diabetes has been hypothesized to be a risk factor for SVID (1,7). Such a relationship might explain the association of diabetes and cognitive decline, although it also has been speculated that advanced glycemic end-products may contribute to the “plaque and tangle” pathologic substrates of Alzheimer disease in patients with diabetes (7,8). Indeed, a higher prevalence of Alzheimer disease in diabetic versus nondiabetic subjects has been shown in several but not all epidemiologic studies (9,10). MR imaging findings in patients with diabetes include global and regional brain atrophy, and less consistently, leukoaraiosis or SVID of the brain (11–13). Authors of most studies compared subjects with diabetes to those without diabetes, but did not address the question of whether measures of disease severity are associated with indicators of brain pathology detected on MR images.
The Action to Control Cardiovascular Risk in Diabetes (ACCORD) trial provided the opportunity to examine this question. ACCORD is a randomized controlled trial of 10 251 individuals with type 2 diabetes that was designed to determine whether therapeutic strategies targeting euglycemia and normotension and/or normal lipid profile can reduce the rate of cardiovascular events more than standard therapy can (14). The ACCORD Memory in Diabetes (MIND) substudy of the trial was designed to determine whether these interventions reduce cognitive decline (measured with neuropsychological tests) and structural brain changes (measured with MR imaging) (15).
By using ACCORD-MIND baseline data, we investigated the association of characteristics of diabetes mellitus (duration and biochemical severity of diabetes) to brain structure measured at MR imaging, specifically testing whether more severity in metrics of diabetes is inversely correlated with brain volumes and positively correlated with ischemic lesion volumes.
Materials and Methods
Study Sample and Participant Characteristics
The design of the ACCORD trial was described elsewhere (15). Study participants were aged 45–79 years and had type 2 diabetes, high hemoglobin A1c (HbA1c) concentrations (> 7.5%, > 58 mmol/mol), and a high risk for cardiovascular disease events suggested by a clinical history of cardiovascular disease, atherosclerosis, albuminuria, left ventricular hypertrophy, or at least two additional risk factors for cardiovascular disease. Key exclusion criteria were frequent or recent serious hypoglycemic events, unwillingness to monitor glucose at home or inject insulin, body mass index greater than 45 kg/m2, serum creatinine level greater than 1.5 mg/dL (133 μmol/L), or other serious illness. For the MIND/MR imaging substudy, participants younger than 55 years old were excluded. Participants were eligible for the MR imaging study if they did not have any standard exclusion criteria for this examination. Four of the six clinical coordinating networks participated in the MR imaging substudy. The study protocol was approved by the institutional review board of each center, and participants provided written informed consent.
Participants who were fluent in English or Spanish and who had been randomized into the ACCORD trial were invited to participate in the MIND substudy between 2003 and 2005. Baseline images were acquired within 45 days of randomization. A total of 2977 subjects agreed to participate in the MIND substudy (15) (Appendix E1 [online]), with 691 (23%) recruited into the MR imaging component. Of these, 59 (9%) either did not attend their MR imaging examination or were found to have MR imaging exclusion criteria, resulting in 632 participants with a baseline MR imaging examination, 18 (3%) of which had missing or incomplete data and were not analyzed (16).
MR Imaging
A common MR imaging protocol was used at the four participating MR imaging field centers (University of Minnesota, Minneapolis, Minn; Wake Forest Medical School, Winston-Salem, NC; Columbia University, New York, NY; and Case Western Reserve University, Cleveland, Ohio). All MR imagers operated at 1.5 T, and MR imaging sequences included a three-dimensional fast spoiled gradient-echo T1-weighted (repetition time msec/echo time msec 21/8; flip angle, 30°), two-dimensional axial fast spin-echo fluid-attenuated inversion recovery (8000/100; inversion time, 2000 msec), and proton-density T2-weighted (3200/27; inversion time, 120 msec) sequences. Voxel size for the T1 sequence was 1.5 × 0.9 × 0.9 mm and 3.0 × 0.9 × 0.9 mm for the remaining sequences. In general, the T1 sequence was used to study brain morphology, including normal tissue volumes, and the fast spin-echo sequences were used to study neuropathology reflected by MR signal intensity changes.
The MR imaging reading center was responsible for the imaging protocol, image analysis, and MR imaging quality control. The latter was based on the American College of Radiology MR imaging quality control program, which incorporates the monthly analysis of imaging examinations of an American College of Radiology–National Electrical Manufacturers Association quality control phantom (http://www.acr.org/accreditation/mri.aspx). MR imaging quality control phantom results confirmed that all imagers met or exceeded American College of Radiology quality performance guidelines throughout the study.
Image Analysis
The image analysis methodology has been described previously (17) and includes use of an automated computer program that classifies all supratentorial brain tissue into either normal or abnormal gray or white matter and assigns the tissue type to 92 anatomic regions of interest in the cerebrum. These regions of interest were organized in an anatomically hierarchical system that was combined into four anatomic regions of interest for this analysis: total brain, total gray matter, total white matter, and deep gray and white structures (operationally defined as caudate nucleus, putamen, globus pallidus, internal capsule, and thalamus). aTissue in each region of interest was further classified as normal or abnormal. The MR imaging outcomes were total brain; normal, abnormal, and total gray matter; normal, abnormal, and total white matter; and abnormal deep gray and white tissue volumes.
T1-weighted volumetric MR images were first preprocessed according to a standardized protocol for alignment, removal of extracerebral tissue, and segmentation of brain parenchyma into gray matter, white matter, and cerebrospinal fluid. According to additional preprocessing steps, including histogram standardization and coregistration, the lesion segmentation component of the algorithm was applied on the basis of local signal features extracted from coregistered multiparametric MR imaging sequences (ie, T1, proton-density, T2, and fast spin-echo fluid-attenuated inversion recovery). A support vector machine classifier was first trained on expert-defined SVID lesions in 45 ACCORD-MIND patients, and was then used to classify SVID on new images. For algorithm training purposes, SVID was operationally defined as a nonmass lesion having fast spin-echo fluid-attenuated inversion recovery signal intensity greater than that of normal gray matter in a vascular distribution. Regional volumetric measurements were obtained via an automated computer-based template warping method that summed the number of respective voxels in each anatomic region.
Measures of Glycemic Status
Three measures of diabetes exposure were examined. The HbA1c level, a measure of intermediate-term diabetes control, was measured by using an automated high-performance liquid chromatograph (Tosoh G7; Tosoh Bioscience, Worcestershire, United Kingdom); fasting plasma glucose (FPG) level, an indicator of short-term control, was measured enzymatically with an autoanalyzer (Hitachi 917; Hitachi, Tokyo, Japan). Both assays were measured in the central laboratory, which has national glycohemoglobin standardization program level I certification for traceability to the ACCORD trial. Diabetes duration was quantified as time from reported diagnosis of diabetes to randomization in the ACCORD trial.
The following factors associated with both MR brain lesions and diabetes were entered into statistical models as covariates: age, sex, education level, baseline history of cardiovascular disease, smoking, hyperlipidemia (use of lipid medications at screening), and hypertension (use of blood pressure medications at screening).
Statistical Methods
All statistical analyses were conducted at the ACCORD-MIND Coordinating Center with the use of software (S-Plus version 8.0; Insightful, Seattle, Wash; or SAS software 9.1; SAS Institute, Cary, NC). Frequencies and means were used to summarize baseline characteristics. Separate linear regression models were used to investigate the relationship of each of the three diabetes variables (duration, HbA1c, FPG) to the MR imaging outcomes at baseline. Three models controlling for potential confounders were selected a priori on the basis of a rationale that these covariates potentially were confounded with the diabetes variable: Model 1 was adjusted for age and intracranial volume; model 2 additionally included prior cardiovascular disease, smoking history, history of hyperlipidemia, sex, education, and an indicator of MR imaging site; model 3 added hypertension to model 2. Thus, this series of models allowed for inspection of the effect of the potentially confounding variables on the relationship between the diabetes variables and MR imaging outcomes. Hypothesis tests for each diabetes severity variable were performed by using a 0.05 type I error level; whereas no hypothesis tests were considered for potentially confounding covariates. Partial Pearson correlations were calculated after removal of the effect of intracranial volume and age, the same covariates included in model 1. Corresponding P values were very similar to those of the model 1 regression coefficients.
To investigate for a possible nonlinear relationship of diabetes duration to total brain volume, diabetes duration time was divided into quartiles and models 1 and 3 were re-estimated. Pairwise comparisons between groups defined by the quartiles of diabetes duration were performed by using the Fisher least significant difference procedure, which required that the overall F test for the effect be significant before making any pairwise comparisons.
Results
A total of 614 participants underwent baseline MR imaging studies that were successfully processed and used in this analysis. Key baseline characteristics were similar for those in the MR imaging study and the remaining ACCORD-MIND participants (Table 1). Slightly less than 45% of the MR imaging participants were women, approximately 70% were white, 80% were hypertensive, 45% had never smoked, and 26% had a history of cardiovascular disease.
Table 1.
Baseline Characteristics
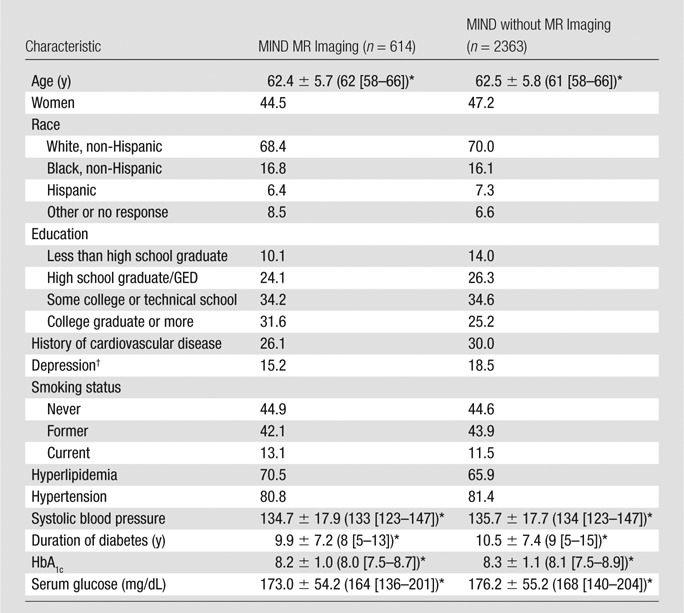
Note.—Unless otherwise indicated, data are percentages. GED = general educational development test.
For continuous variables, data are mean ± standard deviation, with median in parentheses and interquartile range in brackets.
Defined as a patient health questionnaire score of ≥ 10.
At baseline, the mean volumes of total gray (463.8 cm3) and total white matter (463.6 cm3) were similar (Table 2). There was very little abnormal gray matter (0.15 mL; < 0.02% of total gray matter), with an order of magnitude more abnormal white matter (2.02 mL; 0.4% of total white matter). The volume of abnormal deep gray and white tissue was 0.33 mL. Of the total abnormal tissue, approximately 81% was in the white matter, 14% in the deep gray and white matter, and 5% in the gray matter.
Table 2.
Baseline MR Imaging Measures
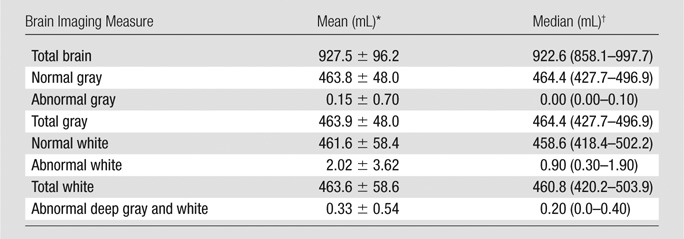
Data are means ± standard deviation.
Data are medians, with interquartile range in parentheses.
Longer duration of diabetes was associated with decreased volumes of total and normal gray matter (Table 3). In model 3, which was controlled for all covariates, a 10-year difference in diabetes duration was predictive of a 4.28 cm3 difference in total gray matter volume, as reflected by the β coefficient. A longer duration of diabetes was also associated with a larger volume of abnormal tissue in the white matter and deep gray and white matter, adjusted for age and intracranial volume. After adjustments were made for health indexes and demographics, the difference in the white matter relationship was no longer statistically significant.
Table 3.
Baseline Results
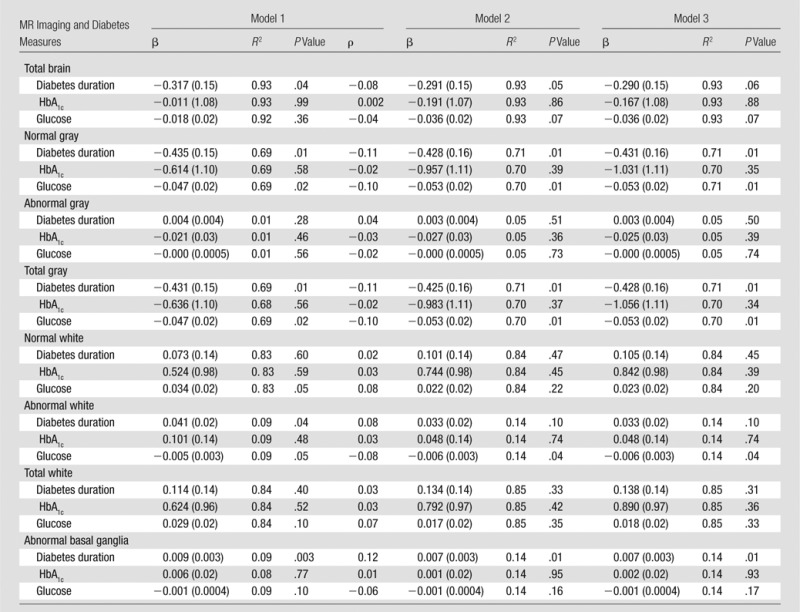
Notes.—Model 1 contains diabetes measure of interest, age, and intracranial volume. Model 2 contains factors in model 1 plus history of cardiovascular disease, smoking history, hyperlipidemia, sex, education, and MR imaging machine. Model 3 controlled for all factors in model 2 and added baseline hypertension.
When we controlled for all covariates (model 3), baseline FPG was inversely related to brain volumes. Specifically, increased FPG was significantly associated with smaller volumes for total gray and normal gray and also abnormal white matter. For total gray and normal gray matter, respectively, a 50-unit difference in blood glucose levels was predictive of a 2.65 cm3 volume difference in total gray, 3.00 cm3 in normal gray, and 0.3 mL in abnormal white matter. In comparison with the other measures, baseline HbA1c levels were not significantly associated with any MR imaging measure in any of the models.
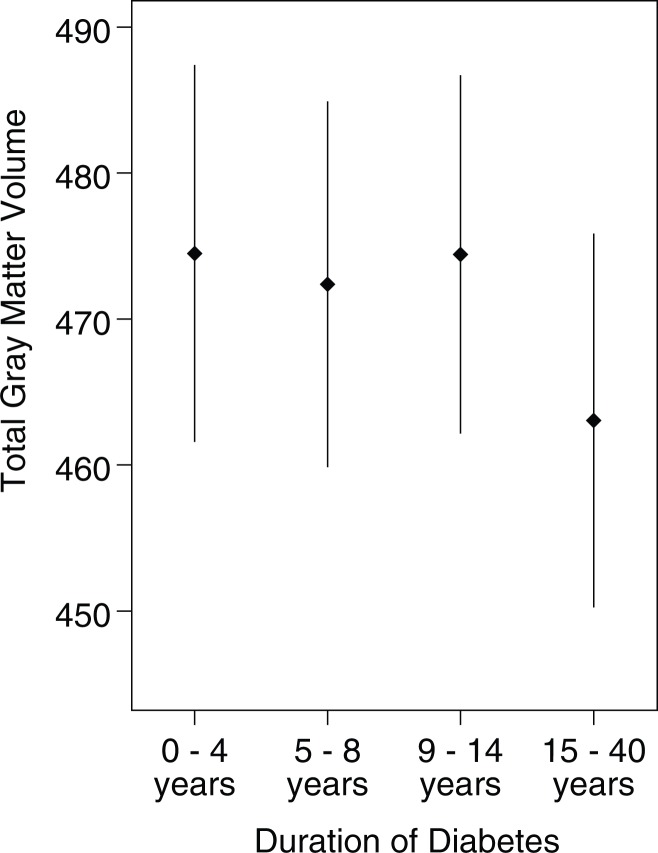
In our investigation of the relationship between quartiles of duration of diabetes and total gray matter volume, controlling for the covariates in model 3, we identified a statistically significant relationship between the grouped quartile variable and total gray matter volume (Figure; least square means quartile 1 = 474.5, quartile 2 = 472.4, quartile 3 = 474.4, quartile 4 = 463.0; P = .003). Those participants with duration of 15 years or more (quartile 4) had significantly less total gray matter volume, on average, than did those with 0–4 years duration of diabetes (P = .002 for pairwise comparison). Differences among the first, second, and third quartiles were not statistically significant. These results were consistent when models 1 or 3 were used.
Graph shows least squares means for total gray matter volume by duration of diabetes quartiles (at baseline).
Discussion
In this study population of 614 patients with type 2 diabetes mellitus, there were significant relationships between measures of diabetes severity and brain structure. Specifically, longer disease duration and increased FPG were inversely correlated with total gray matter and normal gray matter volume. Duration of diabetes also was associated with increased abnormal tissue in the deep gray and white matter, while increased FPG was weakly associated with less abnormal white matter tissue volume. HbA1c level was not significantly correlated with any of our MR imaging brain structure measures. In summary, measures of greater diabetes duration or biochemical severity correlated primarily with brain atrophy, but not with white matter lesion volume, the major MR imaging marker of SVID. Therefore, our study results provided additional support for the position that, despite common clinical perception, diabetes is not directly related to SVID. Age, hypertension and, to a lesser extent, smoking remain the strongest predictors of SVID (1). Our data, however, do not preclude associations between white matter lesions and other consequences of diabetes, such as nephropathy or comorbid conditions such as hypertension.
Our quantitative results showing an incremental loss of approximately 4.28 mL of gray matter tissue for every 10 years of diabetes duration are directly related to neuroanatomy and can be compared with results of previous reports, such as the age-related gray matter volume loss of 2.4 mL per year reported by the Baltimore Longitudinal Study of Aging, in which similar quantitative morphometric methodology was used in a slightly older cohort of normal subjects (18). Stated another way, our results suggested that, for every 10 years of diabetes duration, the brain of a patient with diabetes looks approximately two years older than that of a nondiabetic person, in terms of gray matter volume.
These findings confirm and extend the results in the current literature suggesting that diabetes is associated most strongly and consistently with brain atrophy, more specifically gray matter atrophy, but not with the extent of white matter pathology. As previously noted, most study results show evidence of brain atrophy in diabetic groups compared with nondiabetic groups (11). Although this finding has been reported most frequently in patients with type 2 diabetes mellitus, it has also been found in studies of type 1 diabetes (19). On the other hand, results of a few reports showed a statistically significant relationship between diabetes and white matter lesions, but results of a meta-analysis by van Harten et al (20) did not support such a relationship. Our study included three major diabetes predictors (FPG, HbA1c, diabetes duration) of brain integrity in a diabetic cohort. Authors of most previous diabetes studies analyzed only fasting glucose levels and/or treatment history as diabetes predictors, although a few recent studies (7,11,21) incorporated HbA1c levels and diabetes duration in their analyses. Although Saczynski et al (11) found a significant association between duration of disease, ischemic tissue volume, and presence of infarcts, authors of other studies have not analyzed the independent contribution of these variables to MR imaging brain structure measures.
Although our results generally were consistent with those of previous reports, there are important methodologic differences that extend the contribution of our results beyond those of prior literature. We incorporated higher-spatial-resolution three-dimensional images that allowed more precise volumetric analysis and fast spin-echo fluid-attenuated inversion recovery sequences that increased the sensitivity to SVID (7). For image analyses, we used a different method than those used in previous studies: a method of computer analysis yielding quantitative volumetric data (17). Authors of most previous studies used human observers who applied semiquantitative scales to visual observations. Such rating systems are subjective, have limited dynamic range, and are not directly comparable (22). Authors of recent studies from the Age, Gene, and Environment Susceptibility study and Utrecht Diabetic Encephalopathy Study groups have used computer-based protocols for image analysis, and in preliminary reports (11,23,24) they show results similar to ours. Automated computer analysis provides reduced variability and more refined quantitative results that mitigate the shortcomings of subjective rating scales, and it is becoming the standard methodology in brain MR imaging epidemiologic and clinical trials (25,26).
There were several weaknesses in this study. The study population included only patients with diabetes, so we could not compare our findings to those of a nondiabetic population. Measures of short-term glycemic events such as hypo- or hyperglycemia have not been incorporated in our model. We did not specifically identify necrotic infarcts, reflected as T1 hypointensity on images, which limits comparisons to previous studies of this MR imaging marker of vascular disease. Finally, our goal was to define brain integrity at the time treatment began and not to address the relationship among these brain structural changes, and treatment, cognition, which are topics to be addressed in the clinical trial results.
It is difficult to explain the finding that increased FPG was associated with less abnormal white matter volume. The inclusion and exclusion criteria allowed acceptance into the study of only those participants with a recent HbA1c level greater than 7.5% (> 0.58 mmol/mol) and excluded those with a history of frequent or serious hypoglycemic events. These criteria could have limited the range of FPG levels at baseline. Single time point measurements of glycemia probably do not reflect the full dynamics of diabetes such as insulin use and levels and durations of hypo- and hyperglycemic events that could affect brain morphology, which is a topic for future investigation.
In the diabetic population of the ACCORD-MIND substudy, duration of diabetes and FPG are associated with brain atrophy, specifically that of gray matter, but are not associated with greater ischemic lesion volumes. Our findings raise the possibility that cognitive changes arising in patients with diabetes might not be strongly related to vascular dementia but to neurodegenerative disorders, such as Alzheimer disease.
Advances in Knowledge
■ Longer duration of diabetes was associated with lower gray matter volumes (ρ = −0.11), possibly reflecting direct neurologic insult; higher fasting plasma glucose levels showed similar associations with lower brain volumes (ρ = −0.10).
■ No measure of diabetes severity was associated with increased ischemic lesion volume; fasting plasma glucose was inversely correlated with ischemic lesion volume.
■ Contrary to common clinical belief, type 2 diabetes mellitus may not be directly associated with small vessel ischemic disease.
Implications for Patient Care
■ Diabetes duration correlated primarily with brain atrophy, which may have implications for future decline in cognitive function.
■ In this sample of late middle-aged persons with type 2 diabetes, variability in diabetes characteristics was not directly related to white matter lesions.
APPENDIX
PODCAST
Acknowledgments
Acknowledgments
ACCORD-MIND was funded through an intra-agency agreement between NIA and NHLBI (AG-0002) and the NIA Intramural Research Program.
Received July 15, 2013; revision requested September 12; revision received November 27; accepted December 20; final version accepted January 31, 2014.
Funding: This research was supported by the National Institutes of Health (grants N01-HC-95178, N01-HC-95179, N01-HC-95180, N01-HC-95181, N01-HC-95182, N01-HC-95183, and N01-HC-95184).
Clinical trial registration no. NCT00182910
Disclosures of Conflicts of Interest: R.N.B. Financial activities related to the present article: none to disclose. Financial activities not related to the present article: board membership for RadDx, patents planned, pending, or issued with the University of Pennsylvania. M.B. No relevant conflicts of interest to disclose. C.D. No relevant conflicts of interest to disclose. R.M.L. No relevant conflicts of interest to disclose. A.M. No relevant conflicts of interest to disclose. K.H. No relevant conflicts of interest to disclose. J.L. No relevant conflicts of interest to disclose. M.E.M. No relevant conflicts of interest to disclose. Other relationships: none to disclose. J.W. No relevant conflicts of interest to disclose. L.J.L. No relevant conflicts of interest to disclose.
Abbreviations:
- ACCORD
- Action to Control Cardiovascular Risk in Diabetes
- FPG
- fasting plasma glucose
- HbA1c
- hemoglobin A1c
- MIND
- memory in diabetes
- SVID
- small vessel ischemic disease
References
- 1.Yue NC, Arnold AM, Longstreth WT, Jr, et al. Sulcal, ventricular, and white matter changes at MR imaging in the aging brain: data from the cardiovascular health study. Radiology 1997;202(1):33–39. [DOI] [PubMed] [Google Scholar]
- 2.de Leeuw FE, de Groot JC, Achten E, et al. Prevalence of cerebral white matter lesions in elderly people: a population based magnetic resonance imaging study. The Rotterdam Scan Study. J Neurol Neurosurg Psychiatry 2001;70(1):9–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Longstreth WT, Jr, Manolio TA, Arnold A, et al. Clinical correlates of white matter findings on cranial magnetic resonance imaging of 3301 elderly people. The Cardiovascular Health Study. Stroke 1996;27(8):1274–1282. [DOI] [PubMed] [Google Scholar]
- 4.Bots ML, van Swieten JC, Breteler MM, et al. Cerebral white matter lesions and atherosclerosis in the Rotterdam Study. Lancet 1993;341(8855):1232–1237. [DOI] [PubMed] [Google Scholar]
- 5.Pantoni L. Pathophysiology of age-related cerebral white matter changes. Cerebrovasc Dis 2002;13(Suppl 2):7–10. [DOI] [PubMed] [Google Scholar]
- 6.De Groot JC, De Leeuw FE, Oudkerk M, et al. Periventricular cerebral white matter lesions predict rate of cognitive decline. Ann Neurol 2002;52(3):335–341. [DOI] [PubMed] [Google Scholar]
- 7.Manschot SM, Brands AM, van der Grond J, et al. Brain magnetic resonance imaging correlates of impaired cognition in patients with type 2 diabetes. Diabetes 2006;55(4):1106–1113. [DOI] [PubMed] [Google Scholar]
- 8.Luchsinger JA, Tang MX, Stern Y, Shea S, Mayeux R. Diabetes mellitus and risk of Alzheimer’s disease and dementia with stroke in a multiethnic cohort. Am J Epidemiol 2001;154(7):635–641. [DOI] [PubMed] [Google Scholar]
- 9.MacKnight C, Rockwood K, Awalt E, McDowell I. Diabetes mellitus and the risk of dementia, Alzheimer’s disease and vascular cognitive impairment in the Canadian Study of Health and Aging. Dement Geriatr Cogn Disord 2002;14(2):77–83. [DOI] [PubMed] [Google Scholar]
- 10.Yoshitake T, Kiyohara Y, Kato I, et al. Incidence and risk factors of vascular dementia and Alzheimer’s disease in a defined elderly Japanese population: the Hisayama Study. Neurology 1995;45(6):1161–1168. [DOI] [PubMed] [Google Scholar]
- 11.Saczynski JS, Siggurdsson S, Jonsson PV, et al. Glycemic status and brain injury in older individuals: the age gene/environment susceptibility-Reykjavik study. Diabetes Care 2009;32(9):1608–1613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schmidt R, Launer LJ, Nilsson LG, et al. Magnetic resonance imaging of the brain in diabetes: the Cardiovascular Determinants of Dementia (CASCADE) Study. Diabetes 2004;53(3):687–692. [DOI] [PubMed] [Google Scholar]
- 13.Korf ES, van Straaten EC, de Leeuw FE, et al. Diabetes mellitus, hypertension and medial temporal lobe atrophy: the LADIS study. Diabet Med 2007;24(2):166–171. [DOI] [PubMed] [Google Scholar]
- 14.Action to Control Cardiovascular Risk in Diabetes Study Group , Gerstein HC, Miller ME, et al. Effects of intensive glucose lowering in type 2 diabetes. N Engl J Med 2008;358(24):2545–2559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Williamson JD, Miller ME, Bryan RN, et al. The Action to Control Cardiovascular Risk in Diabetes Memory in Diabetes Study (ACCORD-MIND): rationale, design, and methods. Am J Cardiol 2007;99(12A):112i–122i. [DOI] [PubMed] [Google Scholar]
- 16.Launer LJ, Miller ME, Williamson JD, et al. Effects of intensive glucose lowering on brain structure and function in people with type 2 diabetes (ACCORD MIND): a randomised open-label substudy. Lancet Neurol 2011;10(11):969–977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lao Z, Shen D, Liu D, et al. Computer-assisted segmentation of white matter lesions in 3D MR images using support vector machine. Acad Radiol 2008;15(3):300–313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Resnick SM, Pham DL, Kraut MA, Zonderman AB, Davatzikos C. Longitudinal magnetic resonance imaging studies of older adults: a shrinking brain. J Neurosci 2003;23(8):3295–3301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Musen G. Cognition and brain imaging in type 1 diabetes. Curr Diab Rep 2008;8(2):132–137. [DOI] [PubMed] [Google Scholar]
- 20.van Harten B, de Leeuw FE, Weinstein HC, Scheltens P, Biessels GJ. Brain imaging in patients with diabetes: a systematic review. Diabetes Care 2006;29(11):2539–2548. [DOI] [PubMed] [Google Scholar]
- 21.van Harten B, Oosterman J, Muslimovic D, van Loon BJ, Scheltens P, Weinstein HC. Cognitive impairment and MRI correlates in the elderly patients with type 2 diabetes mellitus. Age Ageing 2007;36(2):164–170. [DOI] [PubMed] [Google Scholar]
- 22.Kapeller P, Barber R, Vermeulen RJ, et al. Visual rating of age-related white matter changes on magnetic resonance imaging: scale comparison, interrater agreement, and correlations with quantitative measurements. Stroke 2003;34(2):441–445. [DOI] [PubMed] [Google Scholar]
- 23.Jongen C, van der Grond J, Kappelle LJ, et al. Automated measurement of brain and white matter lesion volume in type 2 diabetes mellitus. Diabetologia 2007;50(7):1509–1516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tiehuis AM, van der Graaf Y, Visseren FL, et al. Diabetes increases atrophy and vascular lesions on brain MRI in patients with symptomatic arterial disease. Stroke 2008;39(5):1600–1603. [DOI] [PubMed] [Google Scholar]
- 25.Prins ND, van Straaten EC, van Dijk EJ, et al. Measuring progression of cerebral white matter lesions on MRI: visual rating and volumetrics. Neurology 2004;62(9):1533–1539. [DOI] [PubMed] [Google Scholar]
- 26.Dyrby TB, Rostrup E, Baaré WF, et al. Segmentation of age-related white matter changes in a clinical multi-center study. Neuroimage 2008;41(2):335–345. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.