Abstract
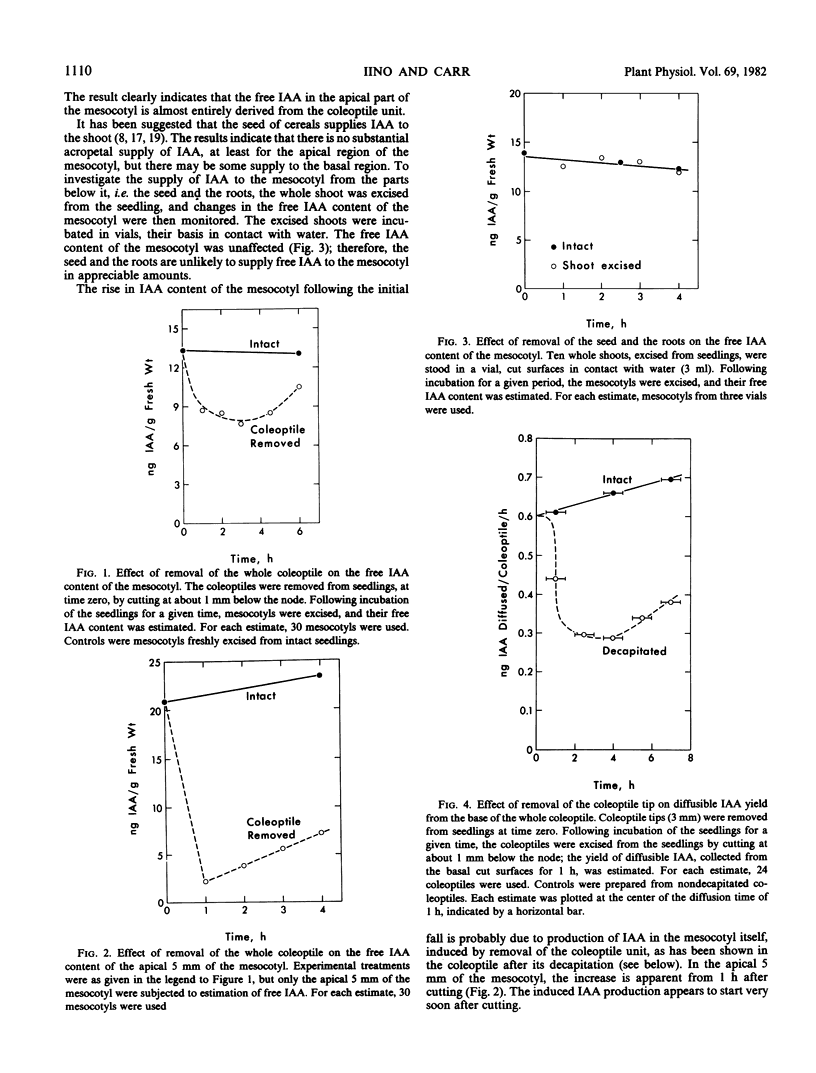
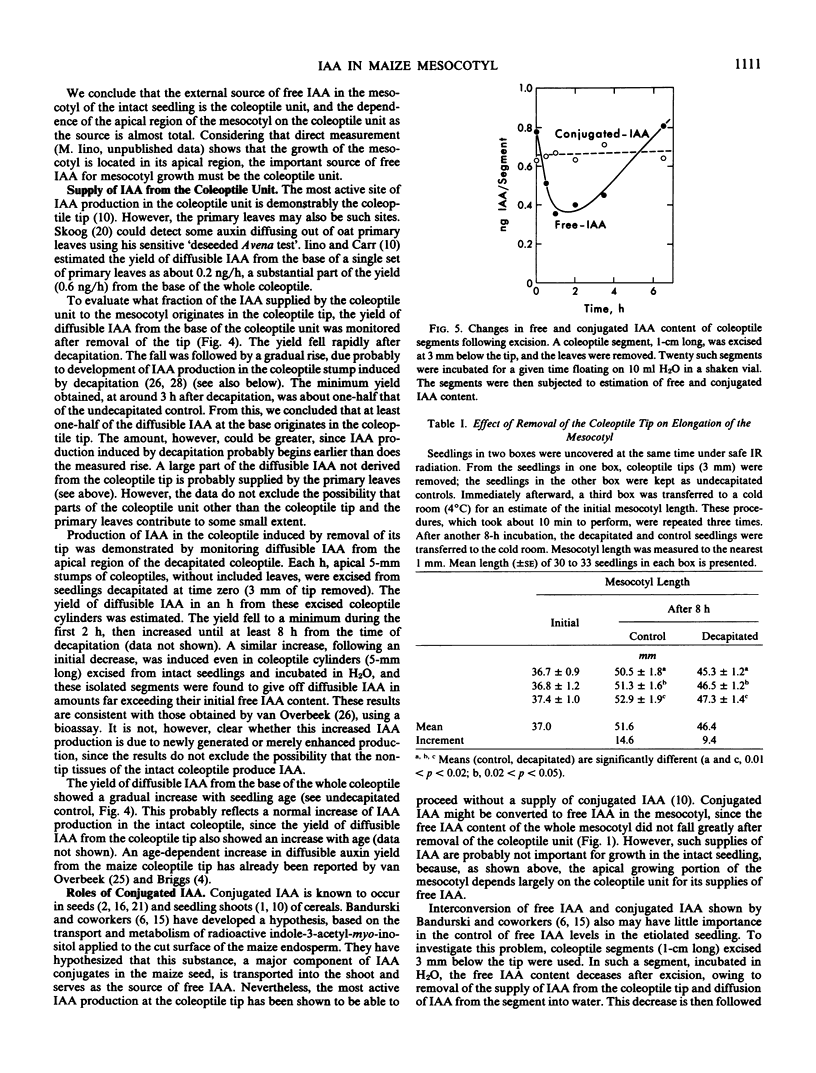
Sources of free indole-3-acetic acid (IAA) for the mesocotyl of intact etiolized maize ((Zea mays L.) seedlings are evaluated. The coleoptile unit, which includes the primary leaves and the coleoptilar node, is the main source of free IAA for the mesocotyl. The seed and the roots are not immediate sources of IAA supply. Dependence of the apical growing region of the mesocotyl on the coleoptile unit as a source of free IAA is almost total. One-half or more of the supply of IAA comes from the coleoptile tip, the rest mainly from the primary leaves. Removal of the coleoptile tip results in inhibition of mesocotyl elongation. The hypothesis that growth of the mesocotyl is regulated by auxin supplied by the coleoptile is supported. Conjugated forms of IAA appear to play little part in regulating the levels of free IAA in the shoot.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bandurski R. S., Schulze A. Concentration of Indole-3-acetic Acid and Its Derivatives in Plants. Plant Physiol. 1977 Aug;60(2):211–213. doi: 10.1104/pp.60.2.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bandurski R. S., Schulze A. Concentrations of Indole-3-acetic Acid and Its Esters in Avena and Zea. Plant Physiol. 1974 Sep;54(3):257–262. doi: 10.1104/pp.54.3.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epstein E., Cohen J. D., Bandurski R. S. Concentration and Metabolic Turnover of Indoles in Germinating Kernels of Zea mays L. Plant Physiol. 1980 Mar;65(3):415–421. doi: 10.1104/pp.65.3.415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall P. L., Bandurski R. S. Movement of Indole-3-acetic Acid and Tryptophan-derived Indole-3-acetic Acid from the Endosperm to the Shoot of Zea mays L. Plant Physiol. 1978 Mar;61(3):425–429. doi: 10.1104/pp.61.3.425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iino M., Yu R. S., Carr D. J. Improved Procedure for the Estimation of Nanogram Quantities of Indole-3-acetic Acid in Plant Extracts using the Indolo-alpha-pyrone Fluorescence Method. Plant Physiol. 1980 Dec;66(6):1099–1105. doi: 10.1104/pp.66.6.1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nowacki J., Bandurski R. S. Myo-Inositol Esters of Indole-3-acetic Acid as Seed Auxin Precursors of Zea mays L. Plant Physiol. 1980 Mar;65(3):422–427. doi: 10.1104/pp.65.3.422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Overbeek J. V. AUXIN PRODUCTION IN SEEDLINGS OF DWARF MAIZE. Plant Physiol. 1938 Jul;13(3):587–598. doi: 10.1104/pp.13.3.587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Percival F. W., Bandurski R. S. Esters of indole-3-acetic Acid from Avena seeds. Plant Physiol. 1976 Jul;58(1):60–67. doi: 10.1104/pp.58.1.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueda M., Bandurski R. S. A Quantitative Estimation of Alkali-labile Indole-3-Acetic Acid Compounds in Dormant and Germinating Maize Kernels. Plant Physiol. 1969 Aug;44(8):1175–1181. doi: 10.1104/pp.44.8.1175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanderhoef L. N., Briggs W. R. Red Light-inhibited Mesocotyl Elongation in Maize Seedlings: I. The Auxin Hypothesis. Plant Physiol. 1978 Apr;61(4):534–537. doi: 10.1104/pp.61.4.534. [DOI] [PMC free article] [PubMed] [Google Scholar]



