Abstract
Objective
Dipeptidyl peptidase-4 (DPP4), also known as CD26, is a transmembrane glycoprotein which has a co-stimulatory function in the immune response. DPP4 inhibitors (DPP4i) are oral glucose-lowering drugs for type 2 diabetes mellitus (T2DM). This study evaluated the risk of incident rheumatoid arthritis (RA) and other autoimmune diseases (AD) such as systemic lupus erythematosus, psoriasis, multiple sclerosis, and inflammatory bowel disease, associated with DPP4i in patients with T2DM.
Methods
Using U.S. insurance claims data (2005–2012), we conducted a population-based cohort study that included initiators of combination therapy with DPP4i (DPP4i plus metformin) and non-DPP4i (non-DPP4i plus metformin). RA and other AD were identified with ≥2 diagnoses and ≥1 dispensing for AD-specific immunomodulating drugs or steroids. Composite AD includes RA or other AD. Propensity score (PS)-stratified Cox proportional hazards models compared the risk of AD in DPP4i initiators vs. non-DPP4i, controlling for potential confounders.
Results
After asymmetric trimming on the PS, 73,928 patients with T2DM starting DPP4i combination therapy and 163,062 starting non-DPP4i combination therapy were selected. Risks of incident RA and composite AD were lower in the DPP4i group vs. non-DPP4i with the PS-stratified hazard ratio of 0.66 (95% CI 0.44–0.99) for RA, 0.73 (0.51–1.03) for other AD, and 0.68 (95% CI 0.52–0.89) for composite AD.
Conclusions
In this large cohort of diabetic patients, those initiating DPP4i combination therapy appear to have a decreased risk of incident AD including RA compared to those initiating non-DPP4i combination therapy. These results may suggest possible pharmacologic pathways for prevention or treatment of AD.
Keywords: dipeptidyl peptidase-4 inhibitors, autoimmune disease, rheumatoid arthritis, inflammatory bowel disease, psoriasis, multiple sclerosis, systemic lupus erythematosus, type 2 diabetes
INTRODUCTION
Dipeptidyl peptidase-4 (DPP4) inhibitors, such as sitagliptin, saxagliptin and linagliptin, are oral glucose-lowering drugs that can be used as monotherapy or combination therapy with other oral hypoglycemic agents for type 2 diabetes mellitus (T2DM).[1–4] Sitagliptin was the first DPP4 inhibitor (DPP4i) approved by the U.S. Food and Drug Administration (FDA) for adults with type 2 diabetes in October 2006, followed by saxagliptin FDA-approved in July 2009 and linagliptin in May 2011. These drugs are generally well-tolerated without a specific contraindication.
DPP4i have their hypoglycemic effect by acting through increasing glucagon-like peptide-1 and glucose-dependent insulinotrophic polypeptide, subsequently leading to increases in insulin and C-peptide, decreases in glucagon, and improvements in oral glucose tolerance.[5] However, DPP4 is a transmembrane glycoprotein, also known as CD26, widely expressed in various cell types such as fibroblast, endothelial and epithelial cells, T lymphocytes and macrophages, and thus has many biological functions beyond glucose metabolism, including chemotaxis, signal transduction, as well as T cell activation.[5–8]
While DPP4 has biological functions in pro-inflammatory pathways, the effects of DPP4i on the immune system, particularly in the pathogenesis of autoimmune diseases (AD) are not well-known. On one hand, a number of studies reported decreased levels of DPP4 activity in patients with rheumatoid arthritis (RA),[9, 10] systemic lupus erythematosus,[11] inflammatory bowel disease,[12, 13] and psoriasis.[14] On the other hand, several studies noted up-regulation of DPP4 expression in psoriasis[15, 16] and multiple sclerosis,[17] as well as elevated numbers of CD26-positive T cells in RA[18, 19] and multiple sclerosis.[20] While studies suggested a potential role of DPP4i as a novel therapy for several inflammatory diseases by inhibiting T-cell proliferation and cytokine production,[6, 7, 21–27] a few cases of inflammatory arthritis potentially related to use of DPP4i have been reported.[28]
The objective of this study was to estimate the risk of incident systemic AD including RA, systemic lupus erythematosus, psoriasis, psoriatic arthritis, multiple sclerosis, and inflammatory bowel disease in patients with diabetes starting a DPP4i drug compared to those starting non-DPP4i oral hypoglycemic agents. We hypothesized that patients starting a DPP4i would have a reduced risk of incident RA and other AD compared to those starting non-DPP4i drugs only.
METHODS
Data Source
We conducted a cohort study using the claims data for the period January 1, 2005 to December 31, 2012, from a commercial U.S. health plan which insures primarily working adults and their family members. This database contains longitudinal claims information including medical diagnoses, procedures, hospitalizations, physician visits, and pharmacy dispensing on its approximately 14 million subscribers across the U.S. on a yearly basis. Results for outpatient laboratory tests, including glycated hemoglobin (HbA1c) were available on a subset of beneficiaries. The distribution of race and ethnicity is representative of the U.S. general population.[29] The quality of these data on medical diagnoses, procedures, health care utilization and drug dispensing is also known to be high.[29] Patient informed consent was not required as the dataset was de-identified to protect subject confidentiality. The study protocol was approved by the Institutional Review Board of the Brigham and Women’s Hospital.
Study Cohort
Patients who had at least one dispensing for an oral hypoglycemic agent any time during the study period were first identified. To avoid selecting patients with type 1 DM, we selected patients aged 40 years and older with a visit coded with the International Classification of Diseases, Ninth Revision, Clinical Modification (ICD 9-CM) code, 250.xx, for diabetes mellitus for the study cohort. As DPP4i drugs are more commonly used as a second or third agent for T2DM, two mutually exclusive exposure groups were defined: 1) initiators of DPP4i combination therapy (DPP4i plus metformin) and 2) initiators of non-DPP4i oral combination therapy (metformin plus another non-DPP4i drug). DPP4i drugs include linagliptin, saxagliptin and sitagliptin. Non-DPP4i drugs include metformin, sulfonylureas, thiazolidinediones (TZD), and meglitinides.
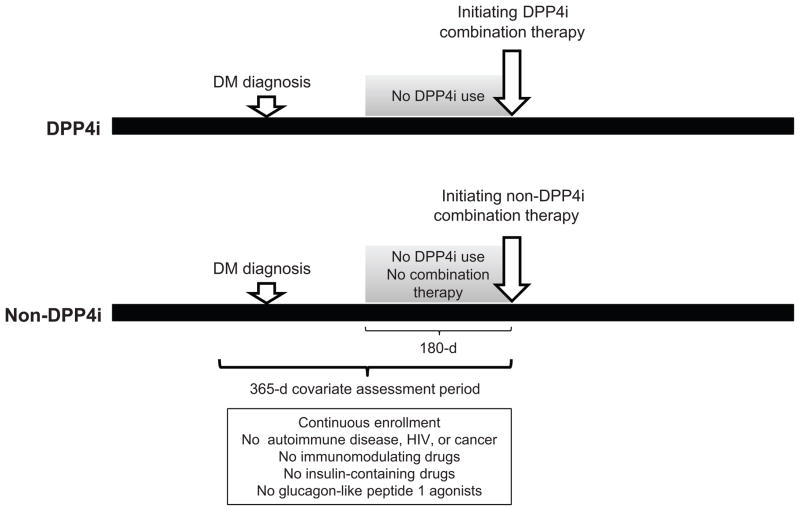
The index date was defined as the earliest date of starting a DPP4i drug with concurrent use of metformin for the DPP4i combination therapy group and the earliest date of adding a second non-DPP4i drug including metformin for the non-DPP4i combination therapy group. Patients were required to have at least 365 days of continuous health plan eligibility before the index date. Therefore, the earliest index date that patients can have is January 1, 2006. Patients with a prior diagnosis of autoimmune disease (RA, systemic lupus erythematosus, psoriasis, psoriatic arthritis, multiple sclerosis, and inflammatory bowel disease), HIV, cancer, and use of insulin-containing drugs, glucagon-like peptide 1 agonists, and immunomodulating drugs including disease-modifying antirheumatic drugs in the 365 days prior to the index date were excluded. Patients were required to be naïve to DPP4i in the 180 days prior to the index date. For the non-DPP4i combination therapy group, patients were required to have at least 180 days without using multiple oral hypoglycemic drugs prior to their index date (Figure 1).
Figure 1. Study design.
Among patients who had at least one visit coded with the International Classification of Diseases, Ninth Revision, Clinical Modification (ICD 9-CM) code, 250.xx, for diabetes mellitus, initiators of oral combination therapy with either DPP4i or non-DPP4i only were identified. The index date was defined as the earliest date of starting a DPP4i drug with concurrent use of metformin for the DPP4i combination therapy group and the earliest date of adding a second non-DPP4i drug including metformin for the non-DPP4i combination therapy group. Patients were required to have at least 365 days of continuous health plan eligibility for covariate assessment before the index date. Patients with a prior diagnosis of autoimmune disease, HIV, cancer, and use of insulin-containing drugs, glucagon-like peptide 1 agonists, and immunomodulating drugs including disease-modifying antirheumatic drugs in the 365 days prior to the index date were excluded. Patients were required to be naïve to DPP4i in the 180 days prior to the index date. For the non-DPP4i combination therapy group, patients were required to have at least 180 days without using multiple oral hypoglycemic drugs prior to their index date.
DM: diabetes mellitus, DPP4i: dipeptidyl peptidase-4 inhibitor.
For the subgroup analysis, 2 additional comparator groups -TZD combination therapy and sulfonylurea combination therapy - were selected. For the comparison between DPP4i and TZD combination therapies, the index date was defined as the earliest date of adding a DPP4i to metformin or a TZD drug plus metformin. For the comparison between DPP4i and sulfonylurea combination therapies, the index date was defined as the earliest date of adding a DPP4i to metformin or a sulfonylurea drug plus metformin. In the subgroup analysis, patients were required to be naïve to both drug categories in the 180 days prior to the index date.
Follow-up began on the day after the index date. In the primary analysis, patients were followed up to the first of any of the following censoring events: discontinuation or switching of study drugs (‘as treated’), occurrence of RA or other AD, loss of health plan eligibility, end of study database, death, or 365 days. Patients were allowed to have gaps of up to 30 days between prescription fill dates in the calculation of continuous therapy. In the case of drug discontinuation or switching, the exposure risk window for each patient treatment episode extended until 30 days after the expiration of the supply of the last fill. Patients were allowed to enter the study cohort one time only.
Study Outcome
Outcomes of interest were a new diagnosis of RA or other AD including systemic lupus erythematosus, psoriasis, psoriatic arthritis, multiple sclerosis, and inflammatory bowel disease, defined with at least two visits, which were at least seven days apart, with a disease-specific diagnosis code and at least one dispensing for disease-specific immunomodulating drugs or steroids (Table S1).[30–36] The date of outcome occurrence was defined as the latest date of either diagnoses or a drug dispensing. The composite outcome, a new diagnosis of RA or other AD, was also assessed.
Covariates
Variables potentially related to development of AD were assessed using data from the 365-day baseline period before the index date. These variables included age, sex, smoking, comorbidities such as obesity, thyroid disease, and other cardiovascular diseases, medications including calcium channel blockers, beta-blockers, anticonvulsants, antipsychotics, procainamide, quinidine, hydralazine, methyldopa, thiazides, systemic steroids, and non-steroidal anti-inflammatory drugs, and health care utilization factors including visits to various specialists (see Table 1). To further quantify patients’ comorbidities at baseline, we also calculated a comorbidity score that combined 20 medical conditions included in both the Charlson Index and the Elixhauser system based on ICD-9.[37] To characterize diabetes treatment intensity, the number of oral hypoglycemic drugs taken at the index date was also determined. Baseline HbA1c levels were available in a subgroup of the study cohort.
Table 1.
Patient characteristics in the 365-day baseline period after asymmetric trimming on propensity score*
| DPP4i group (N=73,928) | Non-DPP4i group (N=163,062) | |
|---|---|---|
| Mean ± SD or percentage | ||
| Demographic | ||
| Age | 55.5 ± 8.4 | 55.4 ± 8.8 |
| Female | 40 | 39 |
| Index year | ||
| 2006 and 2007 | 15 | 37 |
| 2008 | 17 | 17 |
| 2009 | 15 | 14 |
| 2010 | 15 | 12 |
| 2011 | 19 | 10 |
| 2012 | 19 | 9 |
| Comorbidities | ||
| Hypertension | 76 | 73 |
| Cardiovascular disease | 14 | 14 |
| Stroke | 5 | 4 |
| Heart failure | 3 | 3 |
| Dyslipidemia a | 86 | 80 |
| Pulmonary disease | 10 | 10 |
| Kidney disease | 4 | 3 |
| Liver disease | 5 | 4 |
| Thyroid disease | 15 | 12 |
| Smoking | 4 | 5 |
| Obesity | 17 | 15 |
| Combined comorbidity score b | 0.0 ± 0.1 | −0.03 ± 1.2 |
| Diabetes-related | ||
| HbA1c test ordered | 82 | 76 |
| HbA1c level available | 32 | 27 |
| HbA1c level, % c | 8.1 ± 1.8 | 8.1 ± 2.5 |
| No. of oral hypoglycemic drugs at index date | ||
| 2 | 40 | 83 |
| 3 | 45 | 17 |
| ≥ 4 | 14 | 0 |
| Type of oral hypoglycemic drugs at index date | ||
| Metformin | 100 | 100 |
| Sulfonylureas | 43 | 73 |
| Thiazolidinediones | 29 | 42 |
| DPP4i | 100 | 0 |
| Meglitinides | 2 | 2 |
| Medications | ||
| Calcium channel blockers | 19 | 18 |
| Beta blockers | 18 | 19 |
| Thiazides | 31 | 29 |
| Angiotensin converting enzyme inhibitors | 46 | 45 |
| Statins | 62 | 54 |
| Antipsychotics | 1 | 1 |
| Anticonvulsants | 7 | 6 |
| Non-steroidal anti-inflammatory drugs | 21 | 20 |
| Cyclooxygenase-2 inhibitors | 3 | 2 |
| Health care utilization | ||
| No. of physician visits | 7.0 ± 5.7 | 6.4 ± 5.8 |
| Visit to rheumatology | 1 | 1 |
| Visit to neurology | 5 | 5 |
| Visit to gastroenterology | 11 | 10 |
| Visit to endocrinology | 12 | 6 |
| No. of emergency room visits | 0.4 ± 2.4 | 0.3 ± 2.2 |
| Acute hospitalizations | 10 | 12 |
DPP4i: dipeptidyl peptidase-4 inhibitor, SD: standard deviation
Proportion of subjects who had periodontal disease, infectious mononucleosis, alcoholism, and use of procainamide, quinidine, hydralazine, methyldopa, minocycline, isoniazid and lithium were all less than 1%.
Asymmetric trimming with the cut point at the 2.5th percentiles and 97.5th percentiles of the PS distribution in the DPP4i and non-DPP4i group
defined as a diagnosis of hyperlipidemia or use of lipid-lowering drugs
The range of combined comorbidity score is −2 to 26.
In a subgroup of patients with HbA1c levels available
Statistical Analysis
We compared the baseline characteristics between DPP4i and non-DPP4i groups. To control for potential confounders, we a prior decide to use the propensity score (PS) method – PS stratified and PS matched analysis.[38] Multivariable logistic regression including all the baseline covariates listed in Table 1 was used to estimate the PS, defined as the predicted probability of a patient receiving combination therapy with DPP4i versus non-DPP4i. For the PS-stratified analysis, patients were grouped into PS deciles after excluding those in the non-overlapping tails of the PS distribution. We used asymmetric trimming with the cut point at the 2.5th percentiles and 97.5th percentiles of the PS distribution in the exposed and unexposed for each comparison.[39] For PS-matched analysis, we used nearest neighbor matching within a “caliper” of 0.025 on the PS at a fixed ratio of 1:1.[40, 41] In both trimmed and matched cohorts, incidence rates and hazard ratio (HR) of RA and other AD with 95% confidence intervals (CI) were calculated in DPP4i initiators versus non-DPP4i. Kaplan-Meier curves were plotted for the cumulative incidence of each outcome in the PS-matched DPP4i and non-DPP4i cohorts. All these analyses were repeated for the subgroup analyses comparing DPP4i versus TZD initiators, and DPP4i versus sulfonylureas initiators. The proportional hazards assumption was assessed by testing the significance of the interaction term between exposure and time and was not violated except the Cox model for other AD comparing DPP4i versus sulfonylureas.[42] We therefore further stratified analyses of the risk of other AD in DPP4i versus sulfonylureas by follow-up days 0 to 180, and 181 to 365. All analyses were done using SAS 9.2 Statistical Software (SAS Institute Inc., Cary, NC).
RESULTS
Cohort Selection
We identified 1,140,060 patients who had at least one dispensing for a DPP4i or non-DPP4i drug in the study database. After applying the inclusion and exclusion criteria, the cohort included 75,893 diabetic patients who started a combination therapy with a DPP4i drug and 167,260 diabetic patients who started a combination therapy with non-DPP4i drugs only. After the 2.5th and 97.5th asymmetric trimming based on the PS distribution, 73,928 DPP4i and 163,062 non-DPP4i initiators were included. Matching on PS with a 1:1 ratio further selected a total of 47,884 pairs of DPP4i and non-DPP4i initiators (Figure S1).
Patient Characteristics
After asymmetric trimming, the mean age of patients was 55.5 years. 40% of DPP4i group and 39% of non-DPP4i group were female (Tables 1 and S2). Overall, comorbidities, diabetic medications and other drugs, and health care utilization including number of total physician visits and proportions of patients with specialty clinic visits were slightly more common in the DPP4i group. The mean (SD) number of days on metformin in the 365-day baseline period was 207 (130) days for DPP4i and 162 (136) days for non-DPP4i. The mean proportion of days covered by metformin during the 365-day baseline period was 57 (36)% for DPP4i and 44 (37)% for non-DPP4i. 32% of DPP4i and 27% of non-DPP4i had a baseline HbA1c level measured. The mean HbA1c was 8.1% for both groups. In both DPP4i and non-DPP4i groups, most patients started a combination therapy with 2 or 3 oral hypoglycemic agents at the index date. The mean (SD) follow-up was 0.74 (0.86) years for DPP4i and 0.72 (0.91) years for non-DPP4i. The baseline characteristics of the DPP4i and non-DPP4i combination therapy were well-balanced after PS matching (Table S3).
Risk of Autoimmune Diseases
In the PS-trimmed cohorts, there were a total of 179 patients newly diagnosed with RA, 249 with other AD and 424 with composite AD after the initiation of either DPP4i or non-DPP4i combination therapies. The incidence rate was 1.26 per 1,000 person-years for RA and 1.78 per 1,000 person-years for other AD in the DPP4i group, and 1.64 per 1,000 person-years for RA and 2.26 per 1,000 person-years for other AD in the non-DPP4i group. In the PS decile-stratified analysis, the risk of incident RA (HR 0.66, 95% CI 0.44–0.99) and composite AD (HR 0.68, 95% CI 0.52–0.89) within 365 days of follow-up was decreased for DPP4i initiators compared to non-DPP4i (Table 2).
Table 2.
Risk of autoimmune diseases associated with dipeptidyl peptidase-4 inhibitor (DPP4i) versus non-DPP4i within 365 daysa
| DPP4i | Non-DPP4i | ||||||
|---|---|---|---|---|---|---|---|
| PS decile-stratified analysis | |||||||
| N=73,928 | N=163,062 | ||||||
|
| |||||||
| Cases | Person-years | IR b (95% CI) | Cases | Person-years | IR b (95% CI) | HR c (95% CI) | |
|
| |||||||
| RA | 48 | 38135 | 1.26 (0.93–1.67) | 131 | 80020 | 1.64 (1.37–1.94) | 0.66 (0.44–0.99) |
|
| |||||||
| Other AD | 68 | 38120 | 1.78 (1.39–2.26) | 181 | 79997 | 2.26 (1.94–2.62) | 0.73 (0.51–1.03) |
|
| |||||||
| Composite AD | 114 | 38103 | 2.99 (2.47–3.59) | 310 | 79948 | 3.88 (3.46–4.33) | 0.68 (0.52–0.89) |
|
| |||||||
| PS-matched analysis | |||||||
| N=47,884 | N=47,884 | ||||||
|
| |||||||
| Cases | Person-years | IR b (95% CI) | Cases | Person-years | IR b (95% CI) | HR c (95% CI) | |
|
| |||||||
| RA | 28 | 24171 | 1.16 (0.77–1.67) | 40 | 23979 | 1.67 (1.19–2.27) | 0.70 (0.43–1.13) |
|
| |||||||
| Other AD | 38 | 24164 | 1.57 (1.11–2.16) | 46 | 23975 | 1.92 (1.40–2.56) | 0.82 (0.53–1.26) |
|
| |||||||
| Composite AD | 65 | 24154 | 2.69 (2.08–3.43) | 86 | 23959 | 3.59 (2.87–4.43) | 0.75 (0.54–1.04) |
DPP4i: dipeptidyl peptidase-4 inhibitor, IR: incidence rate, HR: hazard ratio, CI: confidence interval, RA: rheumatoid arthritis, AD: autoimmune disease
As treated analysis censoring at discontinuation or switching of study drugs, loss of health plan eligibility, end of study database, death, or 365 days, whichever came first.
Per 1,000 person-years
Non-DPP4i as a referent group
The logistic model for propensity score includes age, sex, comorbidities, smoking, obesity, non-diabetic medications, number of oral diabetic meds, number of primary and specialist visits, and other health care utilization.
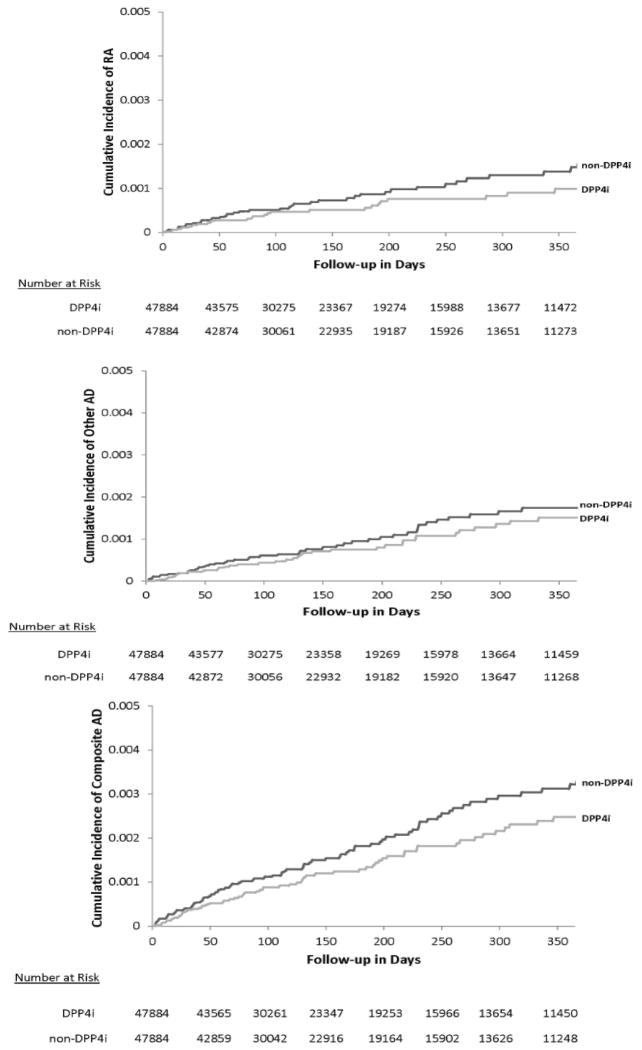
In the PS matched cohorts, the incidence rates of RA or other AD were similar as were HRs for all outcomes with wide confidence intervals due to smaller sample sizes, compared to those in the PS-trimmed cohorts. Figure 2 displays the Kaplan-Meier curves comparing the cumulative incidence of AD between the PS-matched DPP4i and non-DPP4i groups.
Figure 2. Kaplan-Meier curves for cumulative incidence of autoimmune disease: PS-matched analysis.
RA: rheumatoid arthritis, AD: autoimmune disease, DPP4i: dipeptidyl peptidase-4 inhibitor, PS: propensity score DPP4i and nonDPP4i cohorts are propensity score-matched.
Y axis is in percentage.
Subgroup Analysis
Baseline characteristics of the study subgroups after asymmetric trimming also showed slightly more common comorbidities, other medications and health care utilization in the DPP4i group compared to the TZD (Table S4), and sulfonylureas groups (Table S5). In the PS-trimmed subgroups of DPP4i and TZD, and DPP4i and sulfonylureas, overall incidence rates of RA and other AD were low as seen in the main cohorts (Tables 3 and 4). The risk of incident RA was not decreased for DPP4i compared to TZD (HR 1.04, 95% CI 0.51–2.12) or sulfonylureas (HR 0.66, 95% CI 0.38–1.15), while the risk of other AD (HR 0.49, 95% CI 0.30–0.80) and composite AD (HR 0.53, 95% CI 0.36–0.77) remained reduced for DPP4i compared to sulfonylureas. In the subgroup analysis comparing DPP4i to sulfonylureas for other AD which violated the proportionality of hazards, the HR for other AD was 0.42 (95% CI 0.21–0.82) in DPP4i during the first 180 days and 0.90 (95% CI 0.36–2.28) for the follow-up days 181 to 365.
Table 3.
Subgroup analysis 1: Risk of autoimmune diseases associated with dipeptidyl peptidase-4 inhibitor versus thiazolidinediones within 365 daysa
| DPP4i | Thiazolidinediones | ||||||
|---|---|---|---|---|---|---|---|
| PS decile-stratified analysis | |||||||
| N=37,843 | N=22,373 | ||||||
|
| |||||||
| Cases | Person-years | IR b (95% CI) | Cases | Person-years | IR b (95% CI) | HR c (95% CI) | |
|
| |||||||
| RA | 25 | 20524 | 1.22 (0.79–1.80) | 17 | 11713 | 1.45 (0.85–2.32) | 1.04 (0.51–2.12) |
|
| |||||||
| Other AD | 37 | 20515 | 1.80 (1.27–2.49) | 30 | 11706 | 2.56 (1.73–3.66) | 0.60 (0.34–1.07) |
|
| |||||||
| Composite AD | 61 | 20505 | 2.97 (2.28–3.82) | 47 | 11700 | 4.02 (2.95–5.34) | 0.73 (0.47–1.15) |
|
| |||||||
| PS-matched analysis | |||||||
| N=14,248 | N=14,248 | ||||||
|
| |||||||
| Cases | Person-years | IR b (95% CI) | Cases | Person-years | IR b (95% CI) | HR c (95% CI) | |
|
| |||||||
| RA | 14 | 8020 | 1.75 (0.95–2.93) | 13 | 7401 | 1.76 (0.94–3.00) | 1.02 (0.48–2.17) |
|
| |||||||
| Other AD | 14 | 8018 | 1.75 (0.95–2.93) | 23 | 7394 | 3.11 (1.97–4.67) | 0.57 (0.29–1.10) |
|
| |||||||
| Composite AD | 28 | 8013 | 3.49 (2.32–5.05) | 36 | 7390 | 4.87 (3.41–6.74) | 0.73 (0.45–1.20) |
DPP4i: dipeptidyl peptidase-4 inhibitor, IR: incidence rate, HR: hazard ratio, CI: confidence interval, RA: rheumatoid arthritis, AD: autoimmune disease
As treated analysis censoring at discontinuation or switching of study drugs, loss of health plan eligibility, end of study database, death, or 365 days, whichever came first.
Per 1,000 person-years
Non-DPP4i as a referent group
The logistic model for propensity score includes age, sex, comorbidities, smoking, obesity, non-diabetic medications, number of oral diabetic meds, number of primary and specialist visits, and other health care utilization.
Table 4.
Subgroup analysis 2: Risk of autoimmune diseases associated with dipeptidyl peptidase-4 inhibitor versus sulfonylureas within 365 daysa
| DPP4i | Sulfonylureas | ||||||
|---|---|---|---|---|---|---|---|
| PS decile-stratified analysis | |||||||
| N=30,586 | N=45,221 | ||||||
|
| |||||||
| Cases | Person-years | IR b (95% CI) | Cases | Person-years | IR b (95% CI) | HR c (95% CI) | |
|
| |||||||
| RA | 20 | 16666 | 1.20 (0.73–1.85) | 47 | 23166 | 2.03 (1.49–2.70) | 0.66 (0.38–1.15) |
|
| |||||||
| Other AD | 27 | 16663 | 1.62 (1.07–2.36) | 63 | 23161 | 2.72 (2.09–3.48) | 0.49 (0.30–0.80) |
|
| |||||||
| Composite AD | 45 | 16655 | 2.70 (1.97–3.62) | 110 | 23142 | 4.75 (3.91–5.73) | 0.53 (0.36–0.77) |
|
| |||||||
| PS-matched analysis | |||||||
| N=24,738 | N=24,738 | ||||||
|
| |||||||
| Cases | Person-years | IR b (95% CI) | Cases | Person-years | IR b (95% CI) | HR c (95% CI) | |
|
| |||||||
| RA | 20 | 13276 | 1.51 (0.92–2.33) | 26 | 12557 | 2.07 (1.35–3.03) | 0.74 (0.41–1.33) |
|
| |||||||
| Other AD | 21 | 13276 | 1.58 (0.98–2.42) | 37 | 12554 | 2.95 (2.08–4.06) | 0.54 d (0.32–0.92) |
|
| |||||||
| Composite AD | 39 | 13268 | 2.94 (2.09–4.02) | 63 | 12543 | 5.02 (3.86–6.43) | 0.59 (0.40–0.88) |
DPP4i: dipeptidyl peptidase-4 inhibitor, IR: incidence rate, HR: hazard ratio, CI: confidence interval, RA: rheumatoid arthritis, AD: autoimmune disease
As treated analysis censoring at discontinuation or switching of study drugs, loss of health plan eligibility, end of study database, death, or 365 days, whichever came first.
Per 1,000 person-years
Non-DPP4i as a referent group
Proportional hazards assumption was violated.
The logistic model for propensity score includes age, sex, comorbidities, smoking, obesity, non-diabetic medications, number of oral diabetic meds, number of primary and specialist visits, and other health care utilization.
DISCUSSION
To date, the epidemiologic effect of DPP4i on AD has not been examined despite biologic mechanisms and case reports that suggest a possible relationship. In a large population-based cohort of T2DM patients, we found a decreased risk for RA and composite AD among initiators of DPP4i combination therapy compared with initiators of non-DPP4i combination therapy. Subgroup analysis comparing DPP4i to sulfonylureas also showed a decreased risk for other AD and composite AD, although the risk for RA was not significantly reduced. While it is possible that DPP4i does not change a risk of AD but sulfonylureas increase a risk of AD, there is currently no data that suggest such association between sulfonylureas and AD. When comparing DPP4i to TZD combination therapy initiators, no difference in the risk of RA was seen. This might be related to TZD’s immunomodulating or anti-inflammatory action as suggested by the beneficial effects of these agents on disease activity observed in several clinical trials in T2DM patients with RA, psoriatic arthritis or inflammatory bowel disease.[43–46]
This study may have important implications for better understanding the pathogenesis of AD. To date, there are no proven strategies for disease prevention in any of the AD studied. While the current study did not investigate mechanisms of AD pathogenesis, it seems likely that DPP4 (CD26) may play a role in the development of AD. It is known that DPP4 (CD26) is present in various tissues and cells including lymphocytes and monocytes as a transmembrane glycoprotein and is associated with immunoregulatory functions.[5–8] DPP4i inhibits T-cell proliferation and cytokine production,[6, 7, 21–27] both known to be involved in AD pathogenesis. DPP4i is generally well-tolerated: Recent clinical trials of T2DM patients who were at high risk for cardiovascular events showed that DPP4i did not increase the rate of ischemic cardiovascular events.[47, 48] As diabetes is fairly common in patients with preexisting AD,[49–51] it might be worth considering a study that examines a role of DPP4i as a novel treatment of AD in patients with T2DM. This line of study is supported by both animal studies showing partial improvement of inflammatory bowel disease with DPP4i, [7, 52, 53] and improvement of psoriatic skin lesions after the initiating of a DPP4i.[26]
Although it is not known whether any past exposure to DPP4i has an effect on the development of AD, we restricted the stud cohort to ‘new users’ of combination therapy to reduce biases such as survivor bias and time-varying confounding.[54, 55] In addition, to minimize confounding by indication inherent in observational studies, we used rigorous pharmacoepidemiologic approaches in the study design and analysis including active comparator, and PS stratified and matched analyses. Multivariable logistic models for PS estimation incorporated a comprehensive list of potential confounders including age, sex, calendar year, comorbidities, medications, and health care utilization patterns. Nonetheless, residual confounding by indication or by obesity, smoking, family history of AD, and socioeconomic status might be still an issue in this study. However, it is unlikely that physicians who treat patients with T2DM choose oral hypoglycemic drugs based on the future risk of AD in patients with T2DM. Surveillance bias can play a role in diagnosing more or less AD in patients with DPP4i versus non-DPP4i. Prior to PS matching, the DPP4i group had a greater number of physician visits and higher proportions of visits to specialists, which would likely bias the results to the opposite direction (i.e. more AD diagnoses in DPP4i versus non-DPP4i); we then included various health care utilization factors in the PS estimation and achieved balance in these variables between the groups. Furthermore, we conducted a subgroup analysis comparing DPP4i to TZD initiators, as both DPP4i and TZD are relatively newer drugs and frequently used with other oral hypoglycemic drugs, and found similar HRs, albeit with wide confidence intervals including the null, for other and composite AD.
This study has limitations. First, we assessed a number of variables (e.g. smoking, obesity, periodontal disease, infectious mononucleosis, and use of various drugs) potentially related to development of AD using the claims data from the 12 months prior to the index date; however, it is possible that the 12-month baseline period was not long enough to capture all the information on potential confounders and that there was incomplete ascertainment of those variables in the claims data. Second, the requirement of ≥180 days free of DPP4i or non-DPP4i combination therapy may not be long enough to differentiate new users from intermittent users. We assumed that a wash-out period of 180 days would be sufficient for patients who received a DPP4i or non-DPP4i combination therapy on and off. Third, in this study, we mainly relied on diagnosis codes and drug dispensing for outcome ascertainment. A prior validation study using the same data source reported that there was 96% agreement between a claim-based medical diagnosis and the medical record or the patient survey.[56] To further minimize the potential for outcome misclassification, all AD outcomes were defined with at least 2 diagnosis codes and at least 1 dispensing for disease-specific immunomodulating drugs.[30–36] The incidence rates for RA from this study are slightly higher than the incidence rate of RA from the Rochester Epidemiology Project in the U.S.[57]
In conclusion, initiating DPP4i combination therapy appears to be associated with a decreased risk of AD including RA compared to initiating non-DPP4i combination therapy. These results may suggest new mechanistic pathways for preventing or delaying the onset of AD and could lead to a potential new therapeutic approach for patients with preexisting AD. If other studies find DPP4i also effective in prevention of autoimmune disease, future research would be needed to determine the effect and safety of DPP4i in the non-diabetic population.
Supplementary Material
Acknowledgments
Kim is supported by the NIH grant K23 AR059677. Goldfine is supported by the NIH grants R56 DK095451, P50 HL083813, R01 DK088214, U01 HL101422, P30-DK03836, and American Diabetes Association 7-13-CE-17. Schneeweiss is Principal Investigator of the Harvard-Brigham Drug Safety and Risk Management Research Center funded by FDA. His work is partially funded by grants/contracts from PCORI, FDA, and NHLBI. Solomon is supported by the NIH grants K24 AR055989, P60 AR047782, and R01 AR056215.
Footnotes
The abstract of this study was presented as an oral presentation at the American College of Rheumatology 2013 meeting in San Diego, CA on October 29, 2013.
Contributorship Statement
Kim had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. She is the guarantor for the study. All authors conceived and designed the study, analysed and interpreted the data, and critically revised the manuscript for important intellectual content. Kim drafted the paper.
- Kim is supported by the NIH grant K23 AR059677. She received a research grant from Pfizer and tuition support for the Pharmacoepidemiology Program at the Harvard School of Public Health partially funded by the Pharmaceutical Research and Manufacturers of America (PhRMA) foundation.
- Schneeweiss is Principal Investigator of the Harvard-Brigham Drug Safety and Risk Management Research Center funded by FDA. His work is partially funded by grants/contracts from PCORI, FDA, and NHLBI. Schneeweiss is consultant to WHISCON, LLC and to Aetion, Inc. of which he also owns shares. He is principal investigator of investigator-initiated grants to the Brigham and Women’s Hospital from Novartis, and Boehringer-Ingelheim unrelated to the topic of this study.
- Doherty has nothing to disclose.
- Goldfine is supported by the NIH grants R56 DK095451, P50 HL083813, R01 DK088214, U01 HL101422, and P30-DK03836, and American Diabetes Association 7-13-CE-17.
- Glynn received research grants from AstraZeneca and Novartis.
- Solomon is supported by the NIH grants K24 AR055989, P60 AR047782, and R01 AR056215. Solomon receives research grants from Amgen, Lilly, Pfizer, and CORRONA. He serves in unpaid roles on studies sponsored by Pfizer, Novartis, Lilly, and Bristol Myers Squibb. He receives royalties from UpToDate.
Competing interests
Kim receives research support from Pfizer and tuition support for the Pharmacoepidemiology Program at the Harvard School of Public Health partially funded by the Pharmaceutical Research and Manufacturers of America (PhRMA) foundation. Schneeweiss is consultant to WHISCON, LLC and to Aetion, Inc. of which he also owns shares. He is principal investigator of investigator-initiated grants to the Brigham and Women’s Hospital from Novartis, and Boehringer-Ingelheim unrelated to the topic of this study. Doherty has nothing to disclose. Glynn received research grants from AstraZeneca and Novartis. Solomon receives research support from Amgen, Lilly, Pfizer, and CORRONA and serves in unpaid roles on studies sponsored by Pfizer, Novartis, Lilly, and Bristol Myers Squibb. Solomon also receives royalties from UpToDate.
References
- 1.Amori RE, Lau J, Pittas AG. Efficacy and safety of incretin therapy in type 2 diabetes: systematic review and meta-analysis. JAMA. 2007;298:194–206. doi: 10.1001/jama.298.2.194. [DOI] [PubMed] [Google Scholar]
- 2.Karagiannis T, Paschos P, Paletas K, et al. Dipeptidyl peptidase-4 inhibitors for treatment of type 2 diabetes mellitus in the clinical setting: systematic review and meta-analysis. BMJ. 2012:344. doi: 10.1136/bmj.e1369. [DOI] [PubMed] [Google Scholar]
- 3.Aschner P, Kipnes MS, Lunceford JK, et al. Effect of the dipeptidyl peptidase-4 inhibitor sitagliptin as monotherapy on glycemic control in patients with type 2 diabetes. Diabetes Care. 2006;29:2632–7. doi: 10.2337/dc06-0703. [DOI] [PubMed] [Google Scholar]
- 4.Gallwitz B, Rosenstock J, Rauch T, et al. 2-year efficacy and safety of linagliptin compared with glimepiride in patients with type 2 diabetes inadequately controlled on metformin: a randomised, double-blind, non-inferiority trial. Lancet. 2012;380:475–83. doi: 10.1016/S0140-6736(12)60691-6. [DOI] [PubMed] [Google Scholar]
- 5.Drucker DJ, Nauck MA. The incretin system: glucagon-like peptide-1 receptor agonists and dipeptidyl peptidase-4 inhibitors in type 2 diabetes. Lancet. 2006;368:1696–705. doi: 10.1016/S0140-6736(06)69705-5. [DOI] [PubMed] [Google Scholar]
- 6.Ohnuma K, Hosono O, Dang NH, et al. Dipeptidyl peptidase in autoimmune pathophysiology. Adv Clin Chem. 2011:53. doi: 10.1016/b978-0-12-385855-9.00003-5. [DOI] [PubMed] [Google Scholar]
- 7.Yazbeck R, Howarth GS, Abbott CA. Dipeptidyl peptidase inhibitors, an emerging drug class for inflammatory disease? Trends Pharmacol Sci. 2009;30:600–7. doi: 10.1016/j.tips.2009.08.003. [DOI] [PubMed] [Google Scholar]
- 8.Sedo A, Duke-Cohan JS, Balaziova E, et al. Dipeptidyl peptidase IV activity and/or structure homologs: contributing factors in the pathogenesis of rheumatoid arthritis? Arthritis research & therapy. 2005;7:253–69. doi: 10.1186/ar1852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gotoh H, Hagihara M, Nagatsu T, et al. Activities of dipeptidyl peptidase II and dipeptidyl peptidase IV in synovial fluid from patients with rheumatoid arthritis and osteoarthritis. Clin Chem. 1989;35:1016–8. [PubMed] [Google Scholar]
- 10.Kamori M, Hagihara M, Nagatsu T, et al. Activities of dipeptidyl peptidase II, dipeptidyl peptidase IV, prolyl endopeptidase, and collagenase-like peptidase in synovial membrane from patients with rheumatoid arthritis and osteoarthritis. Biochemical medicine and metabolic biology. 1991 Apr;45:154–60. doi: 10.1016/0885-4505(91)90016-e. [DOI] [PubMed] [Google Scholar]
- 11.Wong PT, Wong CK, Tam LS, et al. Decreased expression of T lymphocyte co-stimulatory molecule CD26 on invariant natural killer T cells in systemic lupus erythematosus. Immunological investigations. 2009;38:350–64. doi: 10.1080/08820130902770003. [DOI] [PubMed] [Google Scholar]
- 12.Hildebrandt M, Rose M, Ruter J, et al. Dipeptidyl peptidase IV (DP IV, CD26) in patients with inflammatory bowel disease. Scandinavian journal of gastroenterology. 2001 Oct;36:1067–72. doi: 10.1080/003655201750422675. [DOI] [PubMed] [Google Scholar]
- 13.Moran GW, O’Neill C, Padfield P, et al. Dipeptidyl peptidase-4 expression is reduced in Crohn’s disease. Regulatory peptides. 2012 Aug 20;177:40–5. doi: 10.1016/j.regpep.2012.04.006. [DOI] [PubMed] [Google Scholar]
- 14.Bock O, Kreiselmeyer I, Mrowietz U. Expression of dipeptidyl-peptidase IV (CD26) on CD8+ T cells is significantly decreased in patients with psoriasis vulgaris and atopic dermatitis. Experimental dermatology. 2001 Dec;10:414–9. doi: 10.1034/j.1600-0625.2001.100604.x. [DOI] [PubMed] [Google Scholar]
- 15.van Lingen RG, van de Kerkhof PC, Seyger MM, et al. CD26/dipeptidyl-peptidase IV in psoriatic skin: upregulation and topographical changes. The British journal of dermatology. 2008;158:1264–72. doi: 10.1111/j.1365-2133.2008.08515.x. [DOI] [PubMed] [Google Scholar]
- 16.Novelli M, Savoia P, Fierro MT, et al. Keratinocytes express dipeptidyl-peptidase IV (CD26) in benign and malignant skin diseases. The British journal of dermatology. 1996 Jun;134:1052–6. [PubMed] [Google Scholar]
- 17.Steinbrecher A, Reinhold D, Quigley L, et al. Targeting dipeptidyl peptidase IV (CD26) suppresses autoimmune encephalomyelitis and up-regulates TGF-beta 1 secretion in vivo. Journal of immunology (Baltimore, Md: 1950) 2001 Feb 1;166:2041–8. doi: 10.4049/jimmunol.166.3.2041. [DOI] [PubMed] [Google Scholar]
- 18.Gerli R, Muscat C, Bertotto A, et al. CD26 surface molecule involvement in T cell activation and lymphokine synthesis in rheumatoid and other inflammatory synovitis. Clinical immunology and immunopathology. 1996 Jul;80:31–7. doi: 10.1006/clin.1996.0091. [DOI] [PubMed] [Google Scholar]
- 19.Muscat C, Bertotto A, Agea E, et al. Expression and functional role of 1F7 (CD26) antigen on peripheral blood and synovial fluid T cells in rheumatoid arthritis patients. Clin Exp Immunol. 1994 Nov;98:252–6. doi: 10.1111/j.1365-2249.1994.tb06134.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Khoury SJ, Guttmann CR, Orav EJ, et al. Changes in activated T cells in the blood correlate with disease activity in multiple sclerosis. Archives of neurology. 2000 Aug;57:1183–9. doi: 10.1001/archneur.57.8.1183. [DOI] [PubMed] [Google Scholar]
- 21.Reinhold D, Biton A, Goihl A, et al. Dual inhibition of dipeptidyl peptidase IV and aminopeptidase N suppresses inflammatory immune responses. Ann N Y Acad Sci. 2007;1110:402–9. doi: 10.1196/annals.1423.042. [DOI] [PubMed] [Google Scholar]
- 22.Tanaka S, Murakami T, Horikawa H, et al. Suppression of arthritis by the inhibitors of dipeptidyl peptidase IV. Int J Immunopharmacol. 1997;19:15–24. doi: 10.1016/s0192-0561(97)00004-0. [DOI] [PubMed] [Google Scholar]
- 23.Tanaka S, Murakami T, Nonaka N, et al. Anti-arthritic effects of the novel dipeptidyl peptidase IV inhibitors TMC-2A and TSL-225. Immunopharmacology. 1998;40:21–6. doi: 10.1016/s0162-3109(98)00014-9. [DOI] [PubMed] [Google Scholar]
- 24.Thompson MA, Ohnuma K, Abe M, et al. CD26/dipeptidyl peptidase IV as a novel therapeutic target for cancer and immune disorders. Mini Rev Med Chem. 2007;7:253–73. doi: 10.2174/138955707780059853. [DOI] [PubMed] [Google Scholar]
- 25.Williams YN, Baba H, Hayashi S, et al. Dipeptidyl peptidase IV on activated T cells as a target molecule for therapy of rheumatoid arthritis. Clin Exp Immunol. 2003;131:68–74. doi: 10.1046/j.1365-2249.2003.02020.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nishioka T, Shinohara M, Tanimoto N, et al. Sitagliptin, a dipeptidyl peptidase-IV inhibitor, improves psoriasis. Dermatology (Basel, Switzerland) 2012;224:20–1. doi: 10.1159/000333358. [DOI] [PubMed] [Google Scholar]
- 27.Preller V, Gerber A, Wrenger S, et al. TGF-beta1-mediated control of central nervous system inflammation and autoimmunity through the inhibitory receptor CD26. Journal of immunology (Baltimore, Md: 1950) 2007 Apr 1;178:4632–40. doi: 10.4049/jimmunol.178.7.4632. [DOI] [PubMed] [Google Scholar]
- 28.Crickx E, Marroun I, Veyrie C, et al. DPP4 inhibitor-induced polyarthritis: a report of three cases. Rheumatol Int. 2013 doi: 10.1007/s00296-013-2710-7. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 29.Strom BL. Overview of Automated Databases in Pharmacoepidemiology. In: Strom BL, Kimmel SE, editors. Textbook of Pharmacoepidemiology. Philadelphia: John Wiley & Sons, Ltd; 2006. pp. 167–72. [Google Scholar]
- 30.Kim S, Servi A, Polinski J, et al. Validation of rheumatoid arthritis diagnoses in health care utilization data. Arthritis Res Ther. 2011;13:R32. doi: 10.1186/ar3260. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chibnik LB, Massarotti EM, Costenbader KH. Identification and validation of lupus nephritis cases using administrative data. Lupus. 2010 May;19:741–3. doi: 10.1177/0961203309356289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kristensen SL, Ahlehoff O, Lindhardsen J, et al. Disease activity in inflammatory bowel disease is associated with increased risk of myocardial infarction, stroke and cardiovascular death--a Danish nationwide cohort study. PloS one. 2013;8:e56944. doi: 10.1371/journal.pone.0056944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rezaie A, Quan H, Fedorak RN, et al. Development and validation of an administrative case definition for inflammatory bowel diseases. Canadian journal of gastroenterology = Journal canadien de gastroenterologie. 2012 Oct;26:711–7. doi: 10.1155/2012/278495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Abuabara K, Lee H, Kimball AB. The effect of systemic psoriasis therapies on the incidence of myocardial infarction: a cohort study. Br J Dermatol. 2011 Nov;165:1066–73. doi: 10.1111/j.1365-2133.2011.10525.x. [DOI] [PubMed] [Google Scholar]
- 35.Ahlehoff O, Skov L, Gislason G, et al. Cardiovascular disease event rates in patients with severe psoriasis treated with systemic anti-inflammatory drugs: a Danish real-world cohort study. Journal of internal medicine. 2013 Feb;273:197–204. doi: 10.1111/j.1365-2796.2012.02593.x. [DOI] [PubMed] [Google Scholar]
- 36.Chastek BJ, Oleen-Burkey M, Lopez-Bresnahan MV. Medical chart validation of an algorithm for identifying multiple sclerosis relapse in healthcare claims. Journal of medical economics. 2010;13:618–25. doi: 10.3111/13696998.2010.523670. [DOI] [PubMed] [Google Scholar]
- 37.Gagne JJ, Glynn RJ, Avorn J, et al. A combined comorbidity score predicted mortality in elderly patients better than existing scores. J Clin Epidemiol. 2011;64:749–59. doi: 10.1016/j.jclinepi.2010.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rubin D. Estimating causal effects from large data sets using propensity scores. Ann Intern Med. 1997;127:757–63. doi: 10.7326/0003-4819-127-8_part_2-199710151-00064. [DOI] [PubMed] [Google Scholar]
- 39.Sturmer T, Rothman KJ, Avorn J, et al. Treatment effects in the presence of unmeasured confounding: dealing with observations in the tails of the propensity score distribution--a simulation study. Am J Epidemiol. 2010 Oct 1;172:843–54. doi: 10.1093/aje/kwq198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Austin PC. The performance of different propensity score methods for estimating marginal odds ratios. Statistics in medicine. 2007 Jul 20;26:3078–94. doi: 10.1002/sim.2781. [DOI] [PubMed] [Google Scholar]
- 41.Austin PC. Some methods of propensity-score matching had superior performance to others: results of an empirical investigation and Monte Carlo simulations. Biometrical journal Biometrische Zeitschrift. 2009 Feb;51:171–84. doi: 10.1002/bimj.200810488. [DOI] [PubMed] [Google Scholar]
- 42.Kleinbaum D, Klein M. Evaluating the Proportional Hazards Assumption. In: Gail M, Krickberg K, Samet J, et al., editors. Survival Analysis: A Self-Learning Text. 3. Springer; 2012. [Google Scholar]
- 43.Bongartz T, Coras B, Vogt T, et al. Treatment of active psoriatic arthritis with the PPARgamma ligand pioglitazone: an open-label pilot study. Rheumatology (Oxford, England) 2005 Jan;44:126–9. doi: 10.1093/rheumatology/keh423. [DOI] [PubMed] [Google Scholar]
- 44.Ormseth MJ, Oeser AM, Cunningham A, et al. Peroxisome proliferator-activated receptor gamma agonist effect on rheumatoid arthritis: a randomized controlled trial. Arthritis research & therapy. 2013 Sep 10;15:R110. doi: 10.1186/ar4290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lewis JD, Lichtenstein GR, Deren JJ, et al. Rosiglitazone for active ulcerative colitis: a randomized placebo-controlled trial. Gastroenterology. 2008 Mar;134:688–95. doi: 10.1053/j.gastro.2007.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lund JL, Sturmer T, Porter CQ, et al. Thiazolidinedione use and ulcerative colitis-related flares: an exploratory analysis of administrative data. Inflammatory bowel diseases. 2011 Mar;17:787–94. doi: 10.1002/ibd.21348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Scirica BM, Bhatt DL, Braunwald E, et al. Saxagliptin and Cardiovascular Outcomes in Patients with Type 2 Diabetes Mellitus. The New England journal of medicine. 2013 Sep 2; doi: 10.1056/NEJMoa1307684. [DOI] [PubMed] [Google Scholar]
- 48.White WB, Cannon CP, Heller SR, et al. Alogliptin after Acute Coronary Syndrome in Patients with Type 2 Diabetes. The New England journal of medicine. 2013 Sep 2; doi: 10.1056/NEJMoa1305889. [DOI] [PubMed] [Google Scholar]
- 49.Coto-Segura P, Eiris-Salvado N, Gonzalez-Lara L, et al. Psoriasis, psoriatic arthritis and type 2 diabetes mellitus: a systematic review and meta-analysis. The British journal of dermatology. 2013 Jun 18; doi: 10.1111/bjd.12473. [DOI] [PubMed] [Google Scholar]
- 50.Liao KP, Solomon DH. Traditional cardiovascular risk factors, inflammation and cardiovascular risk in rheumatoid arthritis. Rheumatology (Oxford, England) 2013 Jan;52:45–52. doi: 10.1093/rheumatology/kes243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rostom S, Mengat M, Lahlou R, et al. Metabolic syndrome in rheumatoid arthritis: case control study. BMC musculoskeletal disorders. 2013;14:147. doi: 10.1186/1471-2474-14-147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bank U, Heimburg A, Helmuth M, et al. Triggering endogenous immunosuppressive mechanisms by combined targeting of Dipeptidyl peptidase IV (DPIV/CD26) and Aminopeptidase N (APN/ CD13)-a novel approach for the treatment of inflammatory bowel disease. Int Immunopharmacol. 2006;6:1925–34. doi: 10.1016/j.intimp.2006.09.014. [DOI] [PubMed] [Google Scholar]
- 53.Yazbeck R, Howarth GS, Geier MS, et al. Inhibiting dipeptidyl peptidase activity partially ameliorates colitis in mice. Frontiers in bioscience: a journal and virtual library. 2008;13:6850–8. doi: 10.2741/3193. [DOI] [PubMed] [Google Scholar]
- 54.Johnson ES, Bartman BA, Briesacher BA, et al. The incident user design in comparative effectiveness research. Pharmacoepidemiol Drug Saf. 2013 Jan;22:1–6. doi: 10.1002/pds.3334. [DOI] [PubMed] [Google Scholar]
- 55.Ray WA. Evaluating medication effects outside of clinical trials: new-user designs. American journal of epidemiology. 2003 Nov 1;158:915–20. doi: 10.1093/aje/kwg231. [DOI] [PubMed] [Google Scholar]
- 56.Quam L, Ellis LB, Venus P, et al. Using claims data for epidemiologic research. The concordance of claims-based criteria with the medical record and patient survey for identifying a hypertensive population. Medical care. 1993 Jun;31:498–507. [PubMed] [Google Scholar]
- 57.Myasoedova E, Crowson CS, Kremers HM, et al. Is the incidence of rheumatoid arthritis rising?: results from Olmsted County, Minnesota, 1955–2007. Arthritis Rheum. 2010 Jun;62:1576–82. doi: 10.1002/art.27425. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.