Abstract
Emotions have been proposed to inform risky decision-making through the influence of affective physiological responses on subjective value. The ability to perceive internal body states, or “interoception” may influence this relationship. Here, we examined whether interoception predicts participants' degree of loss aversion, which has been previously linked to choice-related arousal responses. Participants performed both a heartbeat detection task indexing interoception and a risky monetary decision-making task, from which loss aversion, risk attitudes, and choice consistency were parametrically measured. Interoceptive ability correlated selectively with loss aversion, and was unrelated to the other value parameters. This finding suggests that specific and separable component processes underlying valuation are shaped not only by our physiological responses, as shown in previous findings, but also by our interoceptive access to such signals.
Keywords: Emotion, decision-making, interoception, loss aversion
Introduction
Individuals vary widely not only in their physiological reactions to emotional situations, but also in the extent to which they perceive those responses. Several prominent psychological theories have proposed that the perception of one's own physiological emotional responses plays a central role in shaping subjective emotional experience, as well as cognitive and behavioral reactions to external events (e.g. Damasio, 1994; Izard, 2007; Lange & James, 1922; Schachter & Singer, 1962). Consistent with these theories, individuals who show heightened “interoception,” or perception of internal physiological states, report greater subjective intensity of emotional feelings (Barrett, Quigley, Bliss-Moreau, & Aronson, 2004; Critchley, Wiens, Rotshtein, Ohman, & Dolan, 2004; Wiens, Mezzacappa, & Katkin, 2000; Zaki, Davis, & Ochsner, 2012). Heightened interoception is also proposed to play a mechanistic role in anxiety (Paulus & Stein, 2006) and addictive disorders (Koob & Volkow, 2010; Naqvi & Bechara, 2010), in which the highly intense subjective experience of physiological states of fear or craving powerfully influences behavior. Collectively, this work suggests that interoception may critically contribute to subjective emotional experience by increasing the subjective strength of bodily responses. In this manner interoception may also influence a wide range of everyday cognitive and affective evaluations that incorporate perceived physiological information.
A growing body of work has related the physiological arousal response, a component of emotion (Scherer, 2005), to differences in risky decision-making (Bechara, Damasio, Tranel, & Damasio, 1997; Lo & Repin, 2002; van 't Wout, Kahn, Sanfey, & Aleman, 2006). A recent study examining this relationship more specifically found that physiological arousal selectively predicted individual differences in “loss aversion,” or the overweighting of losses relative to equal gains during risky decision-making (Sokol-Hessner et al., 2009). Participants showing greater physiological arousal responses to loss versus win outcomes also exhibited greater aversion to loss behaviorally in their choices. This finding suggests that the physiological response to loss events specifically informs the computations contributing to loss aversion (and not, for example, risk attitudes). Consistent with the notion that heightened interoception might potentiate perceived physiological responses to loss, we hypothesized that these heightened subjective responses might in turn give rise to increased loss averse behavior. In the present study, we take an initial step toward testing this hypothesis by assessing whether individual differences in interoception selectively predict loss aversion in a risky monetary decision-making task.
Methods
In accordance with journal policy, we certify that we report below how we determined our sample size, all data exclusions, all manipulations, and all measures in the study.
Participants
We sought a final sample size of roughly 25 assuming that a possible relationship between loss aversion and interoception might be similar to that observed with physiological responses in a previous study (Sokol-Hessner, et al., 2009). To attain this approximate number, 37 adults recruited from the general population gave informed consent and completed both a heartbeat-detection (HD) task and a monetary decision-making task (see details below). Participants in the HD task were asked to judge whether a set of tones triggered by their actual heartbeats were in or out of sync with their heartbeats. Ten participants were excluded from analysis because on more than 20% of out-of-sync trials, the intervals between their heartbeats were momentarily too brief to allow presentation of tones at the necessary delay of 500ms (a proportion of participants not significantly different [chi square with 1 degree of freedom, p > 0.05] from that reported in an exceptionally large study; Wiens & Palmer, 2001). This short interbeat interval caused the computer to skip heartbeats during tone presentation, creating a potential source of bias in participants' judgments. Of the 27 participants remaining, five participants were also excluded because they exhibited more misses and false alarms than hits and correct rejections on the heartbeat detection task, resulting in negative d′ estimates (see details below). The remaining 22 participants (14 F; age 18 to 36, M = 24.7) were included in the analyses presented here. Participants received $15 for participation. They were endowed with $30 prior to the decision-making task, and paid the actual outcomes of 18 randomly selected trials (10% of all trials).
Heartbeat-Detection Task
Interoception was measured with a commonly-used signal detection task in which participants indicated on each trial whether a sequence of ten tones (800Hz, 100-ms square wave tones; Audacity software; Apple, Cupertino, CA) were perceived as in or out of sync with their heartbeat (Critchley, et al., 2004; Eichler & Katkin, 1994; Katkin, Wiens, & Öhman, 2001; Khalsa et al., 2008; Schneider, Ring, & Katkin, 1998; Wiens, et al., 2000; Wiens & Palmer, 2001). Participants' heartbeats were recorded from chest electrodes using AcqKnowledge software (Biopac Systems, Goleta, CA). The software detected the R-wave, indicating the peak of ventricular depolarization, and triggered tone presentations at delays of 200ms or 500ms (delay was constant within-trial). Tones at a 200 ms delay are typically perceived as in sync with the heartbeat, whereas a 500ms delay is perceived as out of sync (Wiens, et al., 2000). Participants were instructed to attend to their heartbeats without manually feeling for their pulse. Labeled practice trials (two synchronous and two asynchronous) were completed first, followed by 25 trials of each type in the actual task. Interoception was indexed by the difference between participants' normalized hit rate and normalized false alarm rate (z(Hits) - z(False Alarms)), yielding a d-prime (d′) signal-detection performance measure. Five participants had negative d′ values, all between -1 and 0. Though it is possible their response pattern may have resulted from poor ability to perform the task, the negative values also raised the possibility that they did not understand the task and/or the button mappings. Because of the inherent difficulty in interpreting these negative d′ values, we therefore excluded those participants from subsequent analyses unless otherwise indicated.
Choice Task
We measured participants' choice behavior in a risky monetary decision-making task (Sokol-Hessner, Camerer, & Phelps, 2013; Sokol-Hessner, et al., 2009). After endowment (see above), participants were thoroughly instructed, quizzed, and practiced on the task before beginning, as in prior studies (Sokol-Hessner, et al., 2013; Sokol-Hessner, et al., 2009).
Each decision (2s view window, followed by a 2s-or-less response window) was followed 1s later by its outcome (1s), before the next trial began 1-3s later. Choices were made in five blocks of 36 trials, separated by 45s breaks during which participants rated their feelings in the previous block using analog scales.
Participants made 180 choices between a risky gamble (2 options, each with probability of .5) and a guaranteed alternative. 150 choices were between a mixed valence gamble (positive and negative possible outcomes) and $0 guaranteed, and 30 choices were between gain-only gambles (positive and zero possible outcomes) and a smaller positive guaranteed alternative. For the exact monetary amounts, see Table S1 and Figure S1. Monetary amounts were selected to enable separate individual estimates of loss aversion (λ), risk attitudes (ρ), and consistency over choices (μ) using a standard maximum likelihood estimation procedure in MATLAB v7.14 (MathWorks, Natick, MA). The details of estimation, including utility, softmax, and likelihood functions were identical to that used in prior work (Sokol-Hessner, et al., 2013; Sokol-Hessner, et al., 2009).
Results
Participants varied in interoceptive ability (d': range: 0-2.9; M = 0.83, SE = 0.16). Because d′ estimates were non-normally distributed (Shapiro-Wilk test, W = .85 p = .004), we performed a square-root transform (as the log of a d′ of 0 would be infinite), resulting in a normalized distribution of d′ (W = .97, p = .60) with no outliers (all points within two standard deviations; mean sqrt(d′) = 0.79, corresponding to d′ = 0.63). Estimates of loss aversion (λ; M = 1.38, SE = 0.21), risk attitudes (ρ, M = 0.91, SE = 0.07), and consistency over choices (μ, M = 2.51, SE = 0.76) were consistent with previous observations (Sokol-Hessner, et al., 2013; Sokol-Hessner, et al., 2009). Because loss aversion coefficients (λ) are generally positively skewed, a log transformation produces a more normally distributed value (log(λ) M = 0.11, SE = 0.14); corresponding to λ = 1.12). Only risk attitudes (ρ) and consistency (μ) were marginally correlated with each other, r(20) = -.41, p = .06 (all other p's > .49), however, this finding should be interpreted cautiously as μ is non-normally distributed and no correlations were observed in previous studies (Sokol-Hessner, et al., 2013; Sokol-Hessner, et al., 2009).
To examine the connection between interoception and decision-making, we correlated individuals' d′ values with their loss aversion, risk attitudes, and choice consistency. Interoception correlated only with loss aversion (log(λ) and sqrt(d′); r(20) = .57, p = .006; see Figure 1) and not with risk attitudes (ρ and sqrt(d′); r(20) = -.36, p = .10) or choice consistency (μ and sqrt(d′); r(20) = .17, p = .44). Using Fisher's r-to-z transformation, we tested the correlations against one another, and found the correlation with loss aversion was significantly greater than that with risk attitudes (z = 3.16 p = .002), though it was not significantly different from that with choice consistency (z = 1.47 p = .14). The correlations with risk attitudes and with consistency were marginally different (z = 1.69, p = .09).
Figure 1.
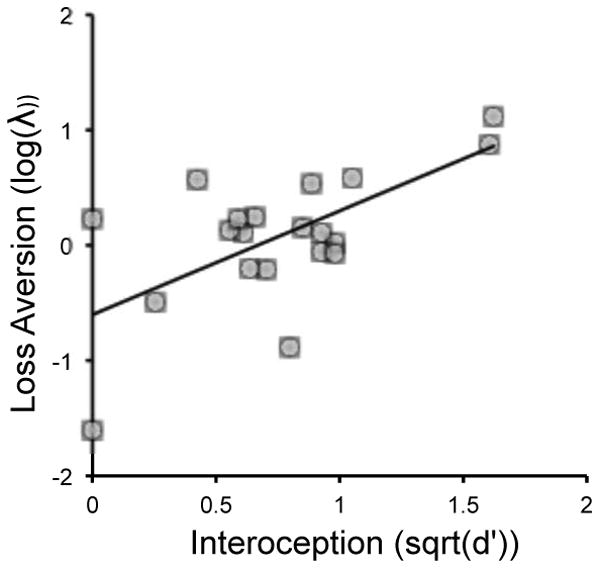
Individuals' interoceptive sensitivity (d′) in a heartbeat detection task was correlated with their loss aversion in a risky monetary choice task (r(20)=.57, p=.006).
The same pattern of selective correlation between loss aversion and interoception holds when we include the five participants excluded based on negative d′ values (median behavioral parameters of λ = 1.90, ρ = 0.88, and μ = 0.44). Though we believe their inclusion to be problematic due to the inherent difficulties in interpreting and analyzing negative d′ values, we replicated the above analyses using the absolute value of d′ (which would reflect participants' interoceptive ability if, for example, they had failed to note the correct response keys). In analyzing all 27 participants, we find that sqrt(|d′|) is significantly correlated with log(λ) (r(25) = .37, p = .05), but not with ρ (r(25) = -.20, p = .31) or μ (r(25) = .16, p = .43), and that the correlation of sqrt(|d′|) with log(λ) is significantly greater than that with ρ (Fisher's r-to-z, z = 2.08, p = .04), though not with μ (z = 0.80, p = .42). There was no significant difference between the correlations with ρ and with μ (z = 1.27, p = .20). Despite replicating the effects observed in participants with positive d′ values, we consider the initial analyses that exclude negative d′ participants to be conservative in that we only include participants demonstrating successful performance of the tasks at a clear and objective level.
Discussion
Here, we show that interoceptive sensitivity to physiological signals selectively predicts aversion to loss in one's choices, but is unrelated to risk attitudes and choice consistency. Individuals who are better able to perceive their bodily states are also most loss averse. This result complements a previous finding that greater physiological responses to losses compared to gains also predicts loss aversion (Sokol-Hessner, et al., 2009), as well as extensive evidence that interoception leads to more intense emotional experiences (Barrett, et al., 2004; Critchley, et al., 2004; Wiens, et al., 2000; Zaki, et al., 2012). Our finding, in the context of this body of research, suggests that heightened interoception may magnify the relative weight placed on losses during decision-making by increasing the subjective intensity of choice-relevant emotional signals.
While previous studies have suggested a general relationship between interoception and decision-making under risk (Dunn et al., 2010; Werner, Jung, Duschek, & Schandry, 2009), we extend these findings by identifying a specific choice process affected by interoception. This specificity is achieved through the use of a quantitative model of monetary decision-making from behavioral economics that enables the precise resolution of distinct component processes underlying an individual's evaluation of risky choices. In teasing apart these separable components of risky decision making, we have revealed that interoception is related to the relative weighting of gains and losses, but not to attitudes toward risk. We note that while these two processes can have similar apparent effects on choices, their mechanisms are very different. Heightened risk aversion causes one to accept fewer gambles because the value of the larger, risky option is discounted – losses and gains are treated no differently. While loss aversion also results in fewer gambles being accepted, this stems instead from the greater weight placed on potential losses relative to gains. Without a properly designed task and model, risk and loss aversion can be behaviorally conflated since their effects on choice are coarsely similar – fewer gambles are accepted. Only by separately quantifying the processes underlying valuation in this monetary decision-making task were we able to identify the specific relationship between interoceptive ability and aversion to loss.
Our study did not directly assess the role of physiological responses in this association between interoception and choice behavior. However, recent work has shown that larger physiological responses to losses versus gains predicts increased loss aversion, suggesting that individuals who experience losses more intensely avoid them more in their choices (Sokol-Hessner, et al., 2009). Our present finding suggests that increased interoception may exert a similar influence upon choice by intensifying the subjective experience of such signals. In other words, there may be two mechanisms that can lead to the heightened subjective experience of loss that contributes to loss aversion: substantially larger physiological responses to losses versus gains, or increased sensitivity to a differential response of average magnitude. As an analogy, one may subjectively experience a light as very bright either because the light is in fact objectively intense, or because one's eyes are very sensitive. Thus, physiological responses and increased interoception, through their similar effects on subjective experience, might both influence decision-making processes that incorporate perceived bodily information.
Although physiological reactivity and interoception could in theory be independent of one another, they might also interact in a number of possible ways. Some previous findings suggest that good interoceptors may have greater physiological responses (Eichler & Katkin, 1994), suggesting a possible feedback loop in which sensitivity to physiological responses subsequently leads to an amplification of the bodily signal itself, which is then perceived even more intensely. Alternatively, interoception and physiological responses may statistically interact (Dunn, Evans, Makarova, White, & Clark, 2012) such that responses drive behavior only for good interoceptors who can accurately perceive those responses. Future research directly assessing the relationship between physiological responses, interoception, and loss aversion may further refine our understanding of the interaction between these variables.
Beyond the limitations discussed above with respect to specifying the interplay between interoception, physiological responses, and choice behavior, our study is also limited by a relatively small sample size (22 or 27, depending on participant exclusion), and the inability of several participants to satisfactorily complete the interoception task (see Methods). Nevertheless, the robustness of our results across analyses and their consistency with prior studies lend validity to the present findings and suggest that they might be replicated in future work.
The neural link between loss aversion and interoception may involve the anterior insula. The insula receives afferent vicerosensory input about the physiological state of the body (Craig, 2009), and is implicated in the interoceptive awareness of such bodily information (Critchley, et al., 2004; Khalsa, Rudrauf, Feinstein, & Tranel, 2009). In addition, both lesion and functional neuroimaging data implicate the insula in the anticipation and avoidance of loss (Palminteri et al., 2012; Samanez-Larkin, Hollon, Carstensen, & Knutson, 2008). These data suggest that the insula may play a critical role in connecting interoceptive information to value-related processes that shape choice. As further evidence, dysregulated insula activity is also proposed to contribute to the etiology of anxiety (Paulus & Stein, 2006), consistent with reports that anxious individuals exhibit both increased interoceptive sensitivity (Critchley, et al., 2004) and excessive avoidance of potentially negative situations (Hartley & Phelps, 2012). Our present finding links these symptoms of anxiety, suggesting that heightened interoception may mechanistically increase loss aversion and in turn motivate avoidance behavior.
Contrary to the centuries-old conventional distinction between thoughts and feelings, contemporary theories view cognition and emotion as inextricably intertwined. Appraisal theories of emotion propose that emotional responses arise through cognitive evaluations of both salient external events and their resulting internal physiological responses (Schachter & Singer, 1962; Scherer, 2005; Smith & Kirby, 2009). Heterogeneity in such appraisal processes is proposed to give rise to individual variability in the subjective feelings and behavioral responses elicited by emotional events. The findings in the present study are compatible with this view, highlighting interoception as a specific appraisal process underlying the subjective evaluation of monetary options, and therefore the decisions we make. More broadly, the link observed in this study between interoception and loss aversion provides further evidence of a naturally integrated role for affective processes in decision-making, demonstrating that not only objective emotional signals, but also our subjective experiences of them, play specific and powerful roles in shaping our choices.
Supplementary Material
Acknowledgments
The authors thank Daanish Chawala for assistance with data collection.
Funding: This work was supported by the National Institutes of Health under Grant AG039283 to EAP.
Contributor Information
Peter Sokol-Hessner, Email: psokolhessner@nyu.edu, Department of Psychology, New York University, Phone: 212-998-8317.
Catherine A Hartley, Email: cah369@nyu.edu, Department of Psychology, New York University, Phone: 212-998-8317.
Jeffrey R. Hamilton, Email: jrh334@nyu.edu, Department of Psychology, New York University, Phone: 212-998-8317.
Elizabeth A. Phelps, Email: liz.phelps@nyu.edu, Department of Psychology and Center for Neural Science, New York University, Nathan Kline Institute, Mailing: 6 Washington Place New York, NY 10003; Phone: (212) 998-8337, Fax: (212) 995-4768.
References
- Barrett LF, Quigley KS, Bliss-Moreau E, Aronson KR. Interoceptive sensitivity and self-reports of emotional experience. JPSP. 2004;87(5):684–697. doi: 10.1037/0022-3514.87.5.684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bechara A, Damasio H, Tranel D, Damasio AR. Deciding advantageously before knowing the advantageous strategy. Science. 1997;275(5304):1293–1295. doi: 10.1126/science.275.5304.1293. [DOI] [PubMed] [Google Scholar]
- Craig ADB. How do you feel - now? The anterior insula and human awareness. Nature Reviews Neuroscience. 2009;10:59–70. doi: 10.1038/nrn2555. [DOI] [PubMed] [Google Scholar]
- Critchley HD, Wiens S, Rotshtein P, Ohman A, Dolan RJ. Neural systems supporting interoceptive awareness. Nat Neurosci. 2004;7(2):189–195. doi: 10.1038/nn1176. [DOI] [PubMed] [Google Scholar]
- Damasio AR. Descartes' Error: Emotion, Reason, and the Human Brain. New York: Grosset/Putnam; 1994. [Google Scholar]
- Dunn B, Evans D, Makarova D, White J, Clark L. Gut feelings and the reaction to perceived inequity: The interplay between bodily responses, regulation, and perception shapes the rejection of unfair offers on the ultimatum game. Cognitive, Affective, & Behavioral Neuroscience. 2012:1–11. doi: 10.3758/s13415-012-0092-z. 10.3758/s13415-012-0092-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn B, Galton H, Morgan R, Evans D, Oliver C, Meyer M, Dalgleish T. Listening to Your Heart: How Interoception Shapes Emotion Experience and Intuitive Decision Making. Psychological Science. 2010;21(12):1835–1844. doi: 10.1177/0956797610389191. 10.1177/0956797610389191. [DOI] [PubMed] [Google Scholar]
- Eichler S, Katkin ES. The relationship between cardiovascular reactivity and heartbeat detection. Psychophysiology. 1994;31(3):229–234. doi: 10.1111/j.1469-8986.1994.tb02211.x. [DOI] [PubMed] [Google Scholar]
- Hartley CA, Phelps EA. Anxiety and Decision-Making. Biological Psychiatry. 2012;72(2):113–118. doi: 10.1016/j.biopsych.2011.12.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izard CE. Basic emotions, natural kinds, emotion schemas, and a new paradigm. Perspectives on Psychological Science. 2007;2(3):260–280. doi: 10.1111/j.1745-6916.2007.00044.x. [DOI] [PubMed] [Google Scholar]
- Katkin ES, Wiens S, Öhman A. Nonconscious Fear Conditioning, Visceral Perception, and the Development of Gut Feelings. Psychological Science. 2001;12(5):366–370. doi: 10.1111/1467-9280.00368. [DOI] [PubMed] [Google Scholar]
- Khalsa SS, Rudrauf D, Damasio AR, Davidson RJ, Lutz A, Tranel D. Interoceptive awareness in experienced meditators. Psychophysiology. 2008;45(4):671–677. doi: 10.1111/j.1469-8986.2008.00666.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khalsa SS, Rudrauf D, Feinstein JS, Tranel D. The pathways of interoceptive awareness. Nat Neurosci. 2009;12:1494–1496. doi: 10.1038/nn.2411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF, Volkow ND. Neurocircuitry of Addiction. Neuropsychopharmacology. 2010;35(1):217–238. doi: 10.1038/npp.2009.110. 10.1038/npp.2009.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lange CG, James W. The Emotions (vol 4) Williams & Wilkins; 1922. [Google Scholar]
- Lo AW, Repin DV. The Psychophysiology of Real-Time Financial Risk Processing. J Cognit Neurosci. 2002;14(3):323–339. doi: 10.1162/089892902317361877. [DOI] [PubMed] [Google Scholar]
- Naqvi NH, Bechara A. The insula and drug addiction: an interoceptive view of pleasure, urges, and decision-making. Brain Structure and Function. 2010;214:435–450. doi: 10.1007/s00429-010-0268-7. 10.1007/s00429-010-0268-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palminteri S, Justo D, Jauffret C, Pavlicek B, Dauta A, Delmaire C, Pessiglione M. Critical Roles for Anterior Insula and Dorsal Striatum in Punishment-Based Avoidance Learning. Neuron. 2012;76(5):998–1009. doi: 10.1016/j.neuron.2012.10.017. 10.1016/j.neuron.2012.10.017. [DOI] [PubMed] [Google Scholar]
- Paulus MP, Stein MB. An Insular View of Anxiety. Biological Psychiatry. 2006;60(4):383–387. doi: 10.1016/j.biopsych.2006.03.042. [DOI] [PubMed] [Google Scholar]
- Samanez-Larkin G, Hollon N, Carstensen L, Knutson B. Individual Differences in Insular Sensitivity During Loss Anticipation Predict Avoidance Learning. Psychological Science. 2008;19(4):320–323. doi: 10.1111/j.1467-9280.2008.02087.x. 10.1111/j.1467-9280.2008.02087.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schachter S, Singer JE. Cognitive, Social and Physiological Determinants of Emotional State. Psychological Review. 1962;69(5):379–399. doi: 10.1037/h0046234. [DOI] [PubMed] [Google Scholar]
- Scherer KR. What are emotions? And how can they be measured? Social Science Information. 2005;44(4):695–729. doi: 10.1177/0539018405058216. [DOI] [Google Scholar]
- Schneider TR, Ring C, Katkin ES. A test of the validity of the method of constant stimuli as an index of heartbeat detection. Psychophysiology. 1998;35:86–89. doi: 10.1111/1469-8986.3510086. [DOI] [PubMed] [Google Scholar]
- Smith CA, Kirby LD. Putting appraisal in context: Toward a relational model of appraisal and emotion. Cognition & Emotion. 2009;23(7):1352–1372. doi: 10.1080/02699930902860386. [DOI] [Google Scholar]
- Sokol-Hessner P, Camerer CF, Phelps EA. Emotion Regulation Reduces Loss Aversion and Decreases Amygdala Responses to Losses. Social Cognitive and Affective Neuroscience. 2013;8:341–350. doi: 10.1093/scan/nss002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sokol-Hessner P, Hsu M, Curley NG, Delgado MR, Camerer CF, Phelps EA. Thinking like a trader selectively reduces individuals' loss aversion. PNAS. 2009;106(13):5035–5040. doi: 10.1073/pnas.0806761106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van 't Wout M, Kahn R, Sanfey AG, Aleman A. Affective state and decision-making in the Ultimatum Game. Exp Brain Res. 2006;169(4):564–568. doi: 10.1007/s00221-006-0346-5. [DOI] [PubMed] [Google Scholar]
- Werner N, Jung K, Duschek S, Schandry R. Enhanced cardiac perception is associated with benefits in decision-making. Psychophysiology. 2009;46(6):1123–1129. doi: 10.1111/j.1469-8986.2009.00855.x. 10.1111/j.1469-8986.2009.00855.x. [DOI] [PubMed] [Google Scholar]
- Wiens S, Mezzacappa E, Katkin E. Heartbeat detection and the experience of emotions. Cognition & Emotion. 2000;14(3):417–427. doi: 10.1080/026999300378905. 10.1080/026999300378905. [DOI] [Google Scholar]
- Wiens S, Palmer SN. Quadratic trend analysis and heartbeat detection. Biological Psychology. 2001;58(01):159–175. 00110–7. doi: 10.1016/S0301-0511. [DOI] [PubMed] [Google Scholar]
- Zaki J, Davis JI, Ochsner KN. Overlapping activity in anterior insula during interoception and emotional experience. NeuroImage. 2012;62(1):493–499. doi: 10.1016/j.neuroimage.2012.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


