Abstract
Objective
To compare long-term functional outcome trajectories of individuals with traumatic brain injury (TBI) who survive with those who expire more than 5 years postinjury, using individual growth curve analysis.
Design
Secondary analysis of data from a multicenter longitudinal cohort study.
Setting
Acute inpatient rehabilitation facilities that are current or former TBI Model Systems.
Participants
Individuals 16 years and older with a primary diagnosis of TBI.
Main Outcome Measures
Glasgow Outcome Scale–Extended; Disability Rating Scale.
Results
Individuals in the TBI Model Systems who expire several years after injury demonstrate worse functional status at baseline and a steeper rate of decline over time as measured by both the Glasgow Outcome Scale–Extended and the Disability Rating Scale. There was significant variability in each growth parameter (P < .05) for both instruments. A reduced model was built for each outcome, including all covariates that related significantly to the growth parameters. An interactive tool was created for each outcome to generate individual-level trajectories based on various combinations of covariate values.
Conclusion
Individuals with TBI who die several years after injury demonstrate functional trajectories that differ markedly from those of survivors. Opportunities should be sought for health management interventions to improve health and longevity after TBI.
Keywords: disability, global outcome, longitudinal outcomes, mortality, traumatic brain injury
TRAUMATIC BRAIN INJURY (TBI) is a major health problem associated with significant mortality and morbidity.1 A wealth of research demonstrates increased mortality relative to the general population, even among individuals who survive the injury itself.2–6 Results from a large prospective case-control study indicated that the risk of death among individuals with TBI compared with controls remained up to 7 times greater for at least 13 years after hospital admission.7
Although there is a perception that mortality after TBI has become less common in recent years thanks to improvements in field triage and critical care, the data do not support this notion.8 A recent meta-analysis of case series studies on severe TBI and acute mortality found that although the overall mortality rate in TBI decreased significantly between 1970 and 1990 due to advances in imaging technology and intensive care, there has been no significant decline in acute TBI–related mortality since 1990.9 Anothermeta-analysis of observational outcome studies of severe TBI conducted between 1980 and 201110 similarly revealed no major reduction in mortality or other unfavorable outcomes. Mortality among individuals who survive a moderate-to-severe TBI has also remained constant. Brooks and colleagues11 evaluated mortality rates of individuals with moderate-to-severe TBI admitted to a TBI Model Systems (TBIMS) facility between 1989 and 2011 who survived at least 1 year postinjury and found no reduction in mortality over this time period.
The factors leading to mortality among individuals who survive TBI are poorly understood. Several studies have evaluated causes of death among individuals with a history of TBI often using data extracted from death certificates. Compared with community controls, one study found that the rates of death among individuals with TBI were significantly higher for circulatory, respiratory, digestive, mental/behavioral, and external causes.7 In another study comparing individuals with TBI with the general population, the likelihood of death was higher among individuals with TBI for all cause of death categories, especially seizures, aspiration pneumonia, sepsis, accidental poisonings, and falls.4 Across nearly all age groups, individuals with TBI were more likely to die of a variety of potentially treatable conditions.2 Premature death among individuals who survive a TBI is likely a result of multifactorial processes that may include poor health management, exacerbation of preexisting conditions, and consequences of the injury or other injuries.12
Little is known about the functional trajectories that precede death among TBI survivors who experience a shortened overall life span. If there is a functional decline, it is not known whether it is abrupt or gradual. Several studies have demonstrated that functioning may vary in the years postinjury and trajectories may include periods of both improvement and decline over time.12 Further research is needed to understand the relationship between mortality and individual trajectories of overall functioning.
The goal of this study was to evaluate whether the long-term functional ability trajectories of individuals with TBI who go on to die during the course of the study are distinguishable from those who survive by evaluating death as a covariate while controlling for baseline characteristics using 2 of the most commonly used TBI outcome measures: the Glasgow Outcome Scale–Extended (GOS-E) and the Disability Rating Scale (DRS). To this end, if trajectories of nonsurvivors are distinguishable from those of their surviving counterparts, this may alert clinicians of the need to implement preventive interventions when their own patients begin to display trajectories similar to those of nonsurvivors.
METHODS
Data source and participants
The TBIMS National Database (NDB) contains findings from a multicenter, longitudinal study of TBI outcomes funded by the US Department of Education, National Institute on Disability and Rehabilitation Research. Further information about the database, measures, and study protocols can be found at www.tbindsc.org. Individuals who are enrolled in the TBIMS NDB have sustained a TBI as defined by at least one of the following characteristics: Glasgow Coma Scale score of less than 13 (not because of intubation, sedation, or intoxication) on emergency admission, loss of consciousness for more than 30 minutes (not because of sedation or intoxication), posttraumatic amnesia for more than 24 hours, or trauma-related intracranial abnormality on neuroimaging. All TBIMS NDB participants are 16 years or older at the time of injury, receive medical care in a TBIMS-affiliated trauma center within 72 hours of injury, are transferred to an affiliated inpatient TBI rehabilitation program, and provide informed consent to participate or consent by legal proxy. Participants complete a standard assessment protocol during inpatient rehabilitation and are followed prospectively (1, 2, and 5 years postinjury and every 5 years thereafter) with a standard follow-up assessment protocol.
Study design
This cohort study intended to describe the longitudinal characteristics of participants within the TBIMS based on their scores on the GOS-E and the DRS, using individual growth curve (IGC) analysis. The IGC, also known as mixed-effects modeling, is a special case of hierarchical linear modeling. The IGC analysis is well suited to the current longitudinal study because outcomes can be directly related to time while taking into account correlations between data points resulting from repeated measures. More importantly, however, IGC analysis allows for modeling outcome at the individual level by establishing relationships between change and the factors that influence it. A benefit of IGC analysis that is pertinent to this study is the ability to gain meaningful information about change in outcome even when data are collected at staggered time points: that is, across individuals, data need not be recorded at equivalent time points. Additional benefits (and limitations) of conducting an IGC analysis are outlined in Kozlowski et al.13
The dates that define each cohort ranged from the date the outcome measure was introduced into the database through September 2012. The first data on the DRS were collected in January 1989, whereas the start date of the GOS-E was July 2000. Prior to July 2000, information on TBIMS participants was captured using the 5-point Glasgow Outcome Scale (GOS). The more psychometrically sound GOS-E replaced the GOS in July 2000; individuals who had previously been assessed with the GOS were evaluated at subsequent follow-ups with the GOS-E. Consequently, for many individuals, their first GOS-E assessment did not occur at their first study follow-up but instead some time afterward. To control for this variability in time at initial GOS-E measure, age at first GOS-E assessment was used as a statistical control.
An essential requirement of conducting an IGC analysis is that each individual must have at least 3 temporal measures. Those who die soon after injury (before 3 measures can be recorded) would not have enough information with which to form temporal trajectories. Therefore, only those who participated in at least 3 follow-up assessments were included in these analyses, with study results not reflective of those who die soon after injury. For the GOS-E, 3870 of 4178 individuals had sufficient data for inclusion in longitudinal analysis whereas 4233 of 7817 individuals were eligible for longitudinal analysis based on the DRS. Of the 3870 individuals with sufficient GOS-E records, 159 were reported as deceased, and, of the 4233 individuals with sufficient DRS records, 229 were deceased. Demographic information is presented in Table 1 for the samples included in the GOS-E and DRS analyses who did and did not survive. The demographic makeup of survivors and nonsurvivors is similar, although nonsurvivors tend to be older. Note that for GOS-E analyses, age at injury, and age at first GOS-E assessment are highly collinear (r = 0.91) and therefore only age at first GOS-E assessment was used in the GOS-E analysis, as discussed earlier.
TABLE 1.
Descriptive statistics for covariates (survivors, nonsurvivors, and total for the GOS-E and the DRS)
| GOS-E | DRS | |||||
|---|---|---|---|---|---|---|
| Covariate | Survivor (n = 3711) |
Nonsurvivor (n = 159) |
Total (N = 3870) |
Survivor (n = 4004) |
Nonsurvivor (n = 229) |
Total (N = 4233) |
| Age at injury DRS measure, mean (SD) | 35.7 (19.5) | 54.0 (18.7) | 36.4 (16.7) | 35.4 (16.0) | 53.5 (19.5) | 36.4 (16.7) |
| Age at first legitimate GOS-E assessment, mean (SD) | 43.7 (16.1) | 43.8 (14.5) | 43.7 (16.0) | N/A | N/A | N/A |
| RLOS, mean (SD) | 27.4 (24.4) | 27.7 (18.3) | 27.4 (24.2) | 28.1 (24.8) | 33.5 (25.5) | 28.4 (24.9) |
| Admission Motor FIM, mean (SD) | 37.1 (19.4) | 36.2 (18.1) | 37.1 (19.4) | 37.2 (19.5) | 36.3 (18.04) | 37.1 (19.4) |
| Admission Cognitive FIM, mean (SD) | 15.9 (8.0) | 15.3 (7.4) | 15.9 (7.9) | 15.7 (7.9) | 14.5 (7.2) | 15.6 (7.8) |
| Gender, % | ||||||
| Male | 72 | 76 | 72 | 72 | 77 | 72 |
| Female | 28 | 24 | 28 | 28 | 23 | 28 |
| Race, % | ||||||
| White | 72 | 70 | 72 | 70 | 65 | 70 |
| Nonwhite | 28 | 30 | 28 | 30 | 35 | 30 |
Abbreviations: DRS, Disability Rating Scale; FIM, Functional Independence Measure; GOS-E, Glasgow Outcome Scale–Extended; N/A, not available; RLOS, rehabilitation length of stay.
Variables
Outcome measures
The GOS-E is a measure of overall disability that is widely used in TBI outcome studies.14 The GOS-E overcomes some of the measurement limitations of the original GOS15–17 using an 8-point scale that includes the following levels: dead (level 1), vegetative state, lower severe disability, upper severe disability, lower moderate disability, upper moderate disability, lower good recovery, and upper good recovery (level 8). Since death was a covariate of interest in this study, level 1 was removed from the measure. In other words, if the GOS-E was coded as “1” in the NDB upon notification of a participant’s death, that data point would not be included in this study. In the TBIMS, the GOS-E is first administered at the 1-year postinjury follow-up evaluation and at each subsequent follow-up.
The DRS was designed to measure general functional changes throughout the course of recovery from moderate-severe TBI. The DRS includes 8 items that assess skills such as eye opening, communication, motor response, feeding, toileting, grooming, level of functioning, and employability. Scores on the DRS range from 0 (no disability) to 30 (death), but since death was a covariate in this study, we again removed scores of 30 from outcome assessment and included only DRS scores ranging from 0 to 29 (extreme vegetative state). In the TBIMS, the DRS is administered at inpatient rehabilitation admission and discharge, as well as interval follow-ups. For this study only DRS data collected at follow-up were used for 2 reasons: first, we wanted to align temporal measures recorded on the DRS with those captured by the GOS-E. More importantly, previous modeling18,19 showed that change from admission to discharge greatly overshadows that which occurs during follow-up. Thus, to better understand how change may be related to death among individuals who survive past acute rehabilitation, it was necessary to remove the overpowering influence of change from the rehabilitation process.
Covariate selection
Covariates were selected a priori based on previous literature, suggesting associations between covariates and outcome or change in outcome over time. Age,3,20–24 sex,22,25 education,20,26 race,23 rehabilitation length of stay (RLOS),27 and Functional Independence Measure (FIM) performance at inpatient rehabilitation admission20,23 are all associated with functional outcome and recovery and therefore were considered as candidate covariates. The FIM is an 18-item measure of functional independence28; the current study uses data collected at rehabilitation admission on both the 13-item Motor FIM and 5-item Cognitive FIM subscales. Each item in these subscales is scored from 1 (total assistance) to 7 (complete independence), yielding a score range of 13 to 91 for the Motor FIM subscale and 5 to 35 for the Cognitive FIM subscale. Race is entered in the TBIMS NDB on the basis of self-report and is coded here as white or other. Sex is coded as male or female, and RLOS is the total number of days between inpatient rehabilitation admission and discharge. Age at injury was a continuous variable. Education was measured in years and coded categorically as greater than high school or high school or less (12 years of education). The covariate of particular interest in the current study was “living status,” which distinguished individuals who were alive at the time of third measurement point from those who were deceased. As described in our previous article on the GOS-E,19 education could not be included as a covariate in these models because extensive missing data for this variable resulted in a reduction in the sample size of 24%. Therefore, the covariates included in the GOS-E models are living status, age at first GOS-E assessment, Cognitive FIM at rehabilitation admission, Motor FIM at rehabilitation admission, race, sex, and RLOS. The covariates included in DRS models are living status, Cognitive FIM at rehabilitation admission, Motor FIM at rehabilitation admission, age at injury, race, sex, education, and RLOS.
Data analysis
All analyses were conducted using SAS (version 9.3). We used IGC analysis to provide a descriptive account of individual-level trajectories for participants in the TBIMS NDB. In the current study, we investigated temporal trajectories at the individual level for the GOS-E and the DRS conditioned upon the a priori selected covariates discussed earlier, where statistically significant variances across growth parameters (for both models) warranted covariate inclusion. In addition, because individual-level change can be readily observed, an interactive tool that generates longitudinal trajectories based on different covariate values was created (https://www.tbindsc.org/Researchers.aspx). For additional information on the modeling/statistical approaches adopted in this study, see the works by Pretz and Dams-O’Connor,18 Kozlowski et al,13 and Pretz et al.19
This study is an extension of the works of Pretz and Dams-O’Connor18 and Pretz et al29 in that we focus on how trajectories for those known to be deceased differ from those living. We compared trajectories of deceased and living TBIMS participants for each outcome by constructing a model that (in addition to the covariates used in the previous studies) included living status where 1 = “alive” and 0 = “deceased.” It is important to recognize that not only do covariates act as individual-level descriptors they can also be seen as factors upon which trajectories are conditioned, that is, the covariates serve as statistical controls. After introducing living status into the modeling process, the data for each outcome were reanalyzed, and we used type III sums of squares (with a cutoff P value of .05) to identify which covariates should be retained, resulting in a reduced model. Although model diagnostics for each outcome were performed, no data alterations were deemed necessary because of the robustness of the modeling approach and the descriptive focus of the study.
RESULTS
Glasgow outcome scale–extended
As was seen in the prior study that used these data,18 comparison of Akaike information criterion values indicated a quadratic model was the best descriptor of change in the GOS-E over time; hence, the growth parameters of interest were the intercept, linear change, and quadratic change. The estimates of the growth parameters and the associations between the covariates and growth parameters for the GOS-E are provided in Table 2.
TABLE 2.
Estimates of the growth parameters and the relationships between the growth parameters and covariates for the GOS-E
| Growth parameter/covariate |
Estimate | P | Lower 95% confidence limit |
Upper 95% confidence limit |
|---|---|---|---|---|
| Intercepta | 5.9962 | <.0001 | 5.9383 | 6.0541 |
| Linear changea | 0.09993 | <.0001 | 0.08481 | 0.1150 |
| Quadratic changea | − 0.00505 | <.0001 | − 0.00637 | − 0.00373 |
| Intercept/living status | − 0.6523 | <.0001 | − 0.8988 | − 0.4057 |
| Linear change/living status | − 0.1073 | <.0001 | − 0.1486 | − 0.06593 |
| Intercept/age at first legitimate GOS-E assessment | − 0.01224 | <.0001 | − 0.01520 | − 0.00928 |
| Linear change/age at first legitimate GOS-E assessment | − 0.00289 | <.0001 | − 0.00381 | − 0.00197 |
| Quadratic change/age at first legitimate GOS-E assessment | 0.000139 | .0019 | 0.000052 | 0.000227 |
| Intercept/Cognitive FIM at admission | 0.02648 | <.0001 | 0.01951 | 0.03344 |
| Intercept/Motor FIM at admission | 0.01696 | <.0001 | 0.01374 | 0.02019 |
| Linear change/Motor FIM at admission | − 0.00141 | <.0001 | − 0.00209 | − 0.00074 |
| Quadratic change/Motor FIM at admission | 0.000067 | .0108 | 0.000015 | 0.000118 |
| Intercept/race | − 0.6245 | <.0001 | − 0.7173 | − 0.5316 |
| Intercept/RLOS | − 0.01274 | <.0001 | − 0.01478 | − 0.01070 |
Abbreviations: FIM, Functional Independence Measure; GOS-E, Glasgow Outcome Scale–Extended; RLOS, rehabilitation length of stay.
Growth parameter.
Living status was directly linked with individual-level change measured by the GOS-E as indicated by Table 2. In particular, living status was related to the intercept and linear change. Although Table 2 displays estimates of the growth parameters as well as estimates of the relationships between growth parameters and covariates, this table is unable to comprehensively illustrate the differences in projected outcome as measured by the GOS-E for survivors and nonsurvivors. To better illustrate this finding, Figure 1 shows the functional trajectory of survivors compared with those who were deceased, using the remaining covariates as controls. In Figure 1, trajectories for the living versus the deceased are presented for individuals who were white, aged 26 years when their first GOS-E score is recorded, had an RLOS of 30 days, and had Cognitive and Motor FIM scores at admission to rehabilitation of 11 and 33, respectively.
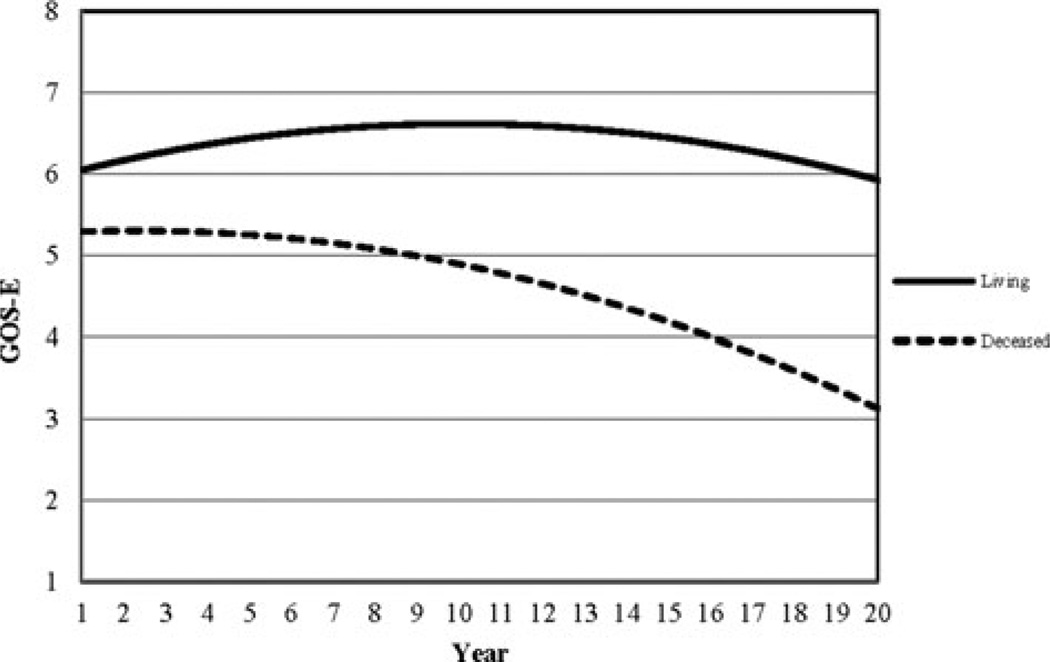
Figure 1.
Sample trajectories of GOS-E scores for individuals who were living (solid line) and deceased (hashed line). GOS-E indicates Glasgow Outcome Scale–Extended.
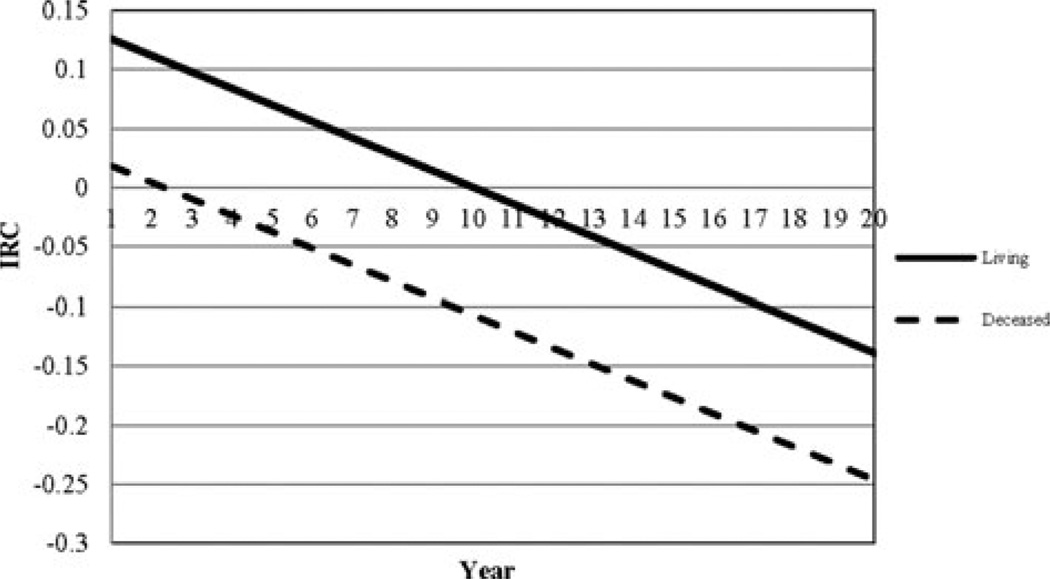
The trajectory for those who were deceased is markedly different from those who were living as seen in Figure 1. Those who were deceased at follow-up begin with a first GOS-E score nearly a point below those living. The trajectory for the deceased displayed an almost constant decline compared with a trajectory of slight improvement, followed by delayed decline beginning near year 10 for the living. Amore detailed description of how trajectories change over time is given by the corresponding instantaneous rate of change plot (see Figure 2), which provides the rate of change in outcome for any point in time. According to Figure 2, the rate of decline for the deceased was twice that of survivors after year 10.
Figure 2.
Instantaneous rate of change plots of GOS-E scores for individuals who were living (solid line) and deceased (hashed line). GOS-E indicates Glasgow Outcome Scale–Extended; IRC, instantaneous rate of change.
This example illustrates trajectories for only one of numerous subsamples of individuals in the NDB that can be created with the interactive tool: readers can enter any plausible combination of covariate values and view the resulting trajectories for survivors and nonsurvivors with those characteristics. Thus, the interactive tool can be used to create customized trajectory comparisons of survivors and nonsurvivors based on specific individual characteristics as defined by a particular combination of covariates. Regardless of the covariate combination selected, a common theme was observed: trajectories for the deceased begin lower and decline more rapidly than those for survivors despite variation in the individual-level trajectories. The interactive tool can be found on the TBIMS National Data and Statistical Center Web site at https://www.tbindsc.org/Researchers.aspx.
Disability rating scale
The initial IGC analysis of the DRS using the NDB19,29 investigated a time span ranging from rehabilitation admission to the most recent follow-up measure (up to 20 years after injury for some individuals) and was best described by a negative exponential function. As the focus of this study was long-term postrehabilitation change, we revisited the modeling process to identify the best-fitting model for describing temporal change on the DRS from 1 year postinjury and beyond. Comparison of Akaike information criterion values suggested a quadratic model best related time to outcome for the timeline of interest. Table 3 displays the growth parameter estimates along with estimates of the relationships between the covariates and growth parameters for the DRS.
TABLE 3.
Estimates of the growth parameters and the relationships between the growth parameters and covariates for the DRS
| Growth parameter/covariate |
Estimate | P | Lower 95% confidence limit |
Upper 95% confidence limit |
|---|---|---|---|---|
| Intercepta | 2.6754 | <.0001 | 2.5429 | 2.8079 |
| Linear changea | − 0.1406 | <.0001 | − 0.1640 | − 0.1173 |
| Quadratic changea | 0.01034 | <.0001 | 0.008796 | 0.01189 |
| Intercept/age at injury | 0.02009 | <.0001 | 0.01465 | 0.02553 |
| Linear change/age at injury | 0.004732 | <.0001 | 0.004031 | 0.005433 |
| Intercept/Cognitive FIM at admission | − 0.05328 | <.0001 | − 0.06673 | − 0.03984 |
| Intercept/Motor FIM at admission | − 0.02856 | <.0001 | − 0.03496 | − 0.02215 |
| Linear change/Motor FIM at admission | 0.001982 | .0030 | 0.000675 | 0.003289 |
| Quadratic change/Motor FIM at admission | − 0.00018 | <.0001 | − 0.00027 | − 0.00010 |
| Intercept/race | 0.6421 | <.0001 | 0.4670 | 0.8172 |
| Intercept/RLOS | 0.03305 | <.0001 | 0.02858 | 0.03752 |
| Linear change/RLOS | − 0.00325 | <.0001 | − 0.00428 | − 0.00223 |
| Quadratic change/RLOS | 0.000105 | .0020 | 0.000038 | 0.000172 |
| Intercept/education | − 0.8824 | <.0001 | − 1.0504 | − 0.7143 |
| Intercept/living status | 0.8195 | .0003 | 0.3786 | 1.2604 |
| Linear change/living status | 0.2973 | <.0001 | 0.1904 | 0.4041 |
| Quadratic change/living status | − 0.01292 | .0008 | − 0.02043 | − 0.00541 |
Abbreviations: DRS, Disability Rating Scale; FIM, Functional Independence Measure; RLOS, rehabilitation length of stay.
Growth parameter.
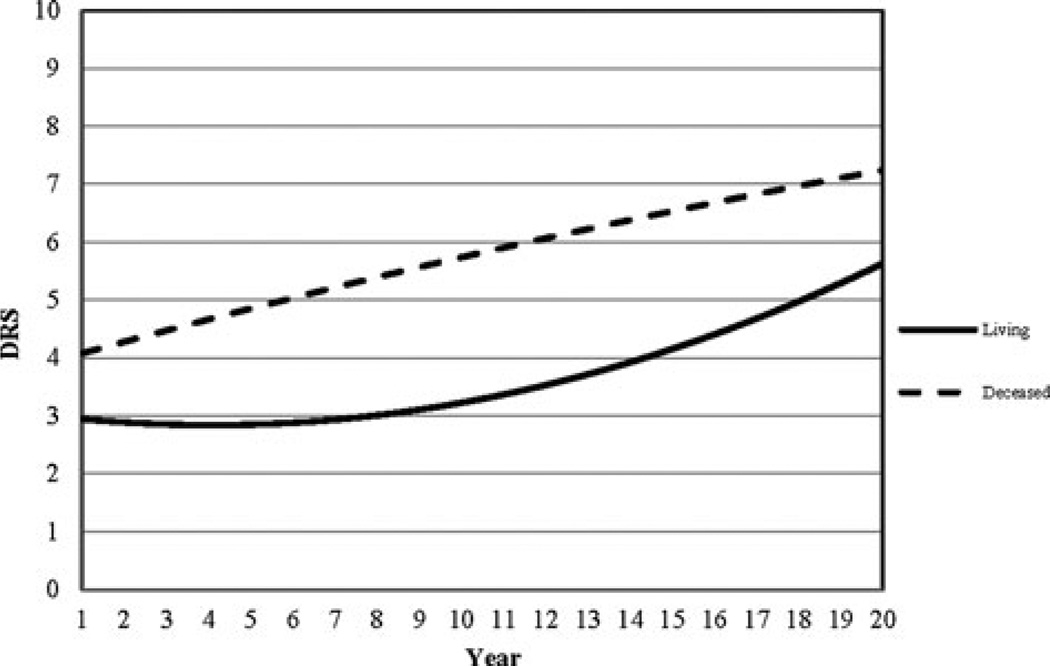
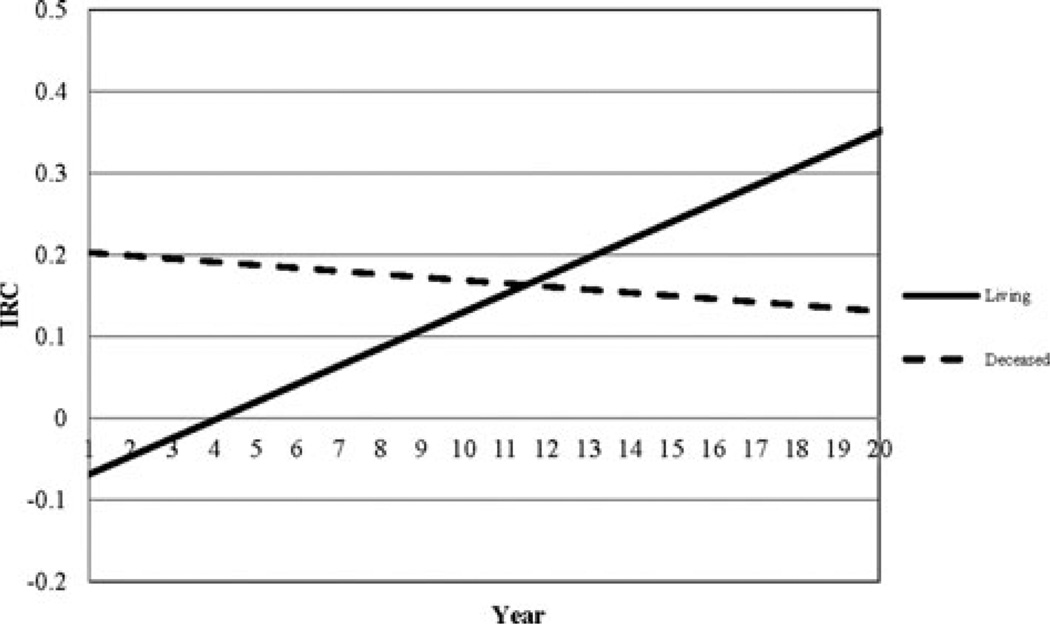
Living status was related to the intercept, linear, and quadratic change of the DRS-based trajectories as demonstrated in Table 3. Since these results are easier to understand visually, Figure 3 illustrates how trajectories of nonsurvivors differ from those of survivors. The example in Figure 3 shows trajectories of functional outcome as measured by the DRS for individuals who were white, 34 years old when they sustained a TBI, had cognitive and Motor FIM admission scores of 15 and 40, respectively, had more than high school education, and had a RLOS of 39 days. Individuals in this subsample who were living had an initial DRS score of 3 (at 1 year postinjury) and typically maintained that score until year 8. After year 8, DRS scores increased in a parabolic fashion in this subsample, indicating a decline in functional status such that by year 20, the projected DRS score was 5.5. In contrast, those who died began with a DRS score of 4, indicating a greater level of disability at baseline than survivors and that score increased steadily over time resulting in a projected DRS score just above 7 by year 20. There was a notable gap of at least 2 points on the DRS between the living and deceased by about 10 years postinjury and for those who died demonstrating worse functional status. The instantaneous rate of change plot for this subsample (see Figure 4) shows that the change trajectory for the living was minimized (minimum DRS score) at year 4 and that the rate of decline increases beyond this point. For nonsurvivors, the instantaneous rate of change plot confirms that decline was relatively steady with no minimum or maximum rate of change evident over time. The interactive tool can be found on the TBIMS National Data and Statistical Center Web site at https://www.tbindsc.org/Researchers.aspx.
Figure 3.
Sample trajectories of DRS scores for individuals who were living (solid line) and deceased (hashed line). DRS indicates Disability Rating Scale.
Figure 4.
IRC plots of DRS scores for individuals who were living (solid line) and deceased (hashed line). DRS indicates Disability Rating Scale; IRC, instantaneous rate of change.
In addition to establishing the associations between the growth parameters and covariates, we also assessed the amount of variability explained by themodel covariates. In the case of the GOS-E, 28.4% of the variability in the intercepts, 17.5% of the variability in the linear term, and 23.8% of the variability in the quadratic term were explained by the model covariates. For the DRS, 26.1% of the variance in the intercepts was explained by the model covariates whereas 8% and 6.2% of the variance was explained in the linear and quadratic terms, respectively. These results indicate that in future studies, additional covariates may be used to explain yet more variability in the growth parameters.
DISCUSSION
Results from this study demonstrated that among individuals who survived several years (at least 5 years, in most cases) after sustaining a moderate-severe TBI, those who died during the course of the study experienced a different trajectory of functional status compared with those who survived. In particular, those who died demonstrated worse functional status at baseline and demonstrated a steeper rate of functional decline over time. The survivors tended to experience a subtle improvement in the years following injury, followed by a delayed and more gradual decline. These findings may suggest that the rate of functional decline, as measured by brief and readily available assessment instruments, could be a telling marker of health risk that can be used in primary care or rehabilitation settings to signal when more intensive health management may be needed.
Several studies have demonstrated that individuals with TBI who survive the injury event tend to have shortened life expectancy.2–7 While it is generally accepted that someone who has sustained a moderate-to-severe TBI is likely to die sooner than a demographically matched control, very few studies have investigated individual differences in survival among those who live many years after TBI. Consequently, little is known about the risk factors that may unfold over time prior to premature mortality or about the potential protective factors associated with survivorship. Premature mortality is likely a consequence of a multitude of factors that evolve over time, with healthcare access and usage likely playing a role. Some studies indicate that people with disabilities underutilize primary and preventive healthcare,30,31 whereas others find higher costs and greater numbers of medical encounters among individuals with disabilities.32,33 Frequent doctor visits could reflect either excellent health management or extreme sickness; infrequent visits could indicate either excellent health (low need for medical care) or poor health that is not being properly managed. Although research on barriers to post-TBI healthcare access is scarce, Powell and colleagues34 reported that unmet service needs in TBI survivors result from physical mobility impairments, worsening cognitive symptoms in the year immediately postinjury, and/or impaired awareness of cognitive changes. Forty seven percent of individuals with TBI reported at least 1 barrier to service access 1 year after hospital discharge.35 Given that TBI-related cognitive, physical, and emotional symptoms may pose unique barriers to healthcare utilization and health maintenance, undertreated poor health is most concerning, particularly when considered in light of research that suggests individuals who survived a moderate-to-severe TBI were more likely than the general population to die of conditions that are potentially treatable.2
As TBI is increasingly recognized as a chronic health condition,12,36,37 questions arise regarding medical management needs of survivors as they age. A chronic disease management approach that includes proactive preventive healthcare, health maintenance interventions, and supported self-management may be warranted, at least for some individuals with TBI. The results of this study suggest that global outcome status may be an important indicator that a TBI survivor is experiencing a decline in functioning that may suggest or reflect a risk for health decline. A decline as indexed by a global outcome measure may warrant clinical follow-up to investigate what aspects of health, disability, or functioning underlie this decline. Fortunately, functional status assessments such as the GOS-E and the DRS can be implemented quickly and inexpensively in a variety of settings.
Limitations
When interpreting the results of this study, it is critical to consider individual-level trajectories as direct products of the covariate/growth parameter associations; they do not necessarily represent explicit pathways taken by a particular individual or group of individuals. As such, each trajectory created by the interactive tool is a mathematical projection of how we would expect individual-level change to occur for individuals who share similar values on the selected covariates based on the longitudinal information contained within the TBIMS NDB. Accordingly, if one were to enter implausible or unlikely combinations of covariate values into the interactive tool (eg, 22 years of age and 2 years of education), the resulting trajectories would be invalid since the NDB likely does not contain data from individuals with that set of characteristics.
The GOS-E and DRS functional outcome trajectories that were presented, as well as those generated by the interactive tool, were based on current TBIMS NDB participants who met the inclusion/exclusion criteria of the parent TBIMS study and of this study. Therefore, it may not be accurate to generalize those trajectories to individuals with TBI who are dissimilar to those included in the TBIMS. In addition, the results may not fully represent more recent TBIMS NDB participants who have not yet completed the necessary 3 assessment points. The current study was primarily descriptive, and neither the results of this study nor the interactive tools were intended to be used to make statistical inferences about individual TBI survivors outside of the TBIMS NDB. As in all studies, we encourage reconfirmation of these findings 5 to 10 years from now when additional follow-up data are available.
Finally, there were some measurement limitations to the outcome measures used in this study. Both the GOS-E and the DRS are global outcome measures that are relatively insensitive to subtle variations, particularly among higher-functioning individuals. While the DRS and the GOS-E are attractive options to use as screeners in primary care settings, given their brevity and ease of administration, these measures may not detect subtle functional decline. For the purposes of IGC, both the GOS-E and the DRS are limited in that they are not continuous, and continuous outcomes are preferred when using IGC analysis. This limitation is not unique to this study, as most rehabilitation outcome measures, including these, are quasi continuous at best. However, simulation studies have demonstrated that quasi-continuous outcomes adequately approximate their continuous counterparts and produce valid results.38
CONCLUSIONS
Individuals with TBI may be at risk for a variety of secondary health problems, high disease comorbidity, and shortened life span. The results of this study demonstrate clear differences between survivors and nonsurvivors on measures of global functional outcome. Individuals who died had lower functional status at baseline and steeper decline over time. These findings suggest that there may be opportunities for health management or other interventions to improve life quality and longevity after TBI. Further research and surveillance work is needed to determine the precise mechanisms contributing to health decline to inform the development of interventions to prevent or slow the trajectory of functional decline among adult TBI survivors.
Acknowledgments
This work was funded by the Traumatic Brain InjuryModel Systems (TBIMS) National Data and Statistical Center (National Institute on Disability and Rehabilitation Research [NIDRR] grant no. H133A110006), the New York TBIMS (NIDRR grant no. H133A070033), and Indiana University TBIMS (NIDRR grant no. H133A120035).
We certify that no party having a direct interest in the results of the research supporting this article has or will confer a benefit on us or on any organization with which we are associated and we certify that all financial and material support for this research are clearly identified in the article.
Footnotes
The authors declare no conflicts of interest.
REFERENCES
- 1.Faul M, Xu L, Wald MW, Coronado VG. Traumatic Brain Injury in the United States: Emergency Department Visits, Hospitalizations, and Deaths 2002–2006. Atlanta, GA: Centers for Disease Control and Prevention, National Center for Injury Prevention and Control; 2010. [Google Scholar]
- 2.Harrison-Felix C, Kolakowsky-Hayner SA, Hammond FM, et al. Mortality after surviving traumatic brain injury: risks based on age groups. J Head Trauma Rehabil. 2012;27(6):E45–E56. doi: 10.1097/HTR.0b013e31827340ba. [DOI] [PubMed] [Google Scholar]
- 3.Harrison-Felix C, Kreider SE, Arango-Lasprilla JC, et al. Life expectancy following rehabilitation: a NIDRR Traumatic Brain Injury Model Systems study. J Head Trauma Rehabil. 2012;27(6):E69–E80. doi: 10.1097/HTR.0b013e3182738010. [DOI] [PubMed] [Google Scholar]
- 4.Ventura T, Harrison-Felix C, Carlson N, et al. Mortality after discharge from acute care hospitalization with traumatic brain injury: a population-based study. Arch Phys Med Rehabil. 2010;91(1):20–29. doi: 10.1016/j.apmr.2009.08.151. [DOI] [PubMed] [Google Scholar]
- 5.Harrison-Felix CL, Whiteneck GG, Jha A, DeVivo MJ, Hammond FM, Hart DM. Mortality over four decades after traumatic brain injury rehabilitation: a retrospective cohort study. Arch Phys Med Rehabil. 2009;90(9):1506–1513. doi: 10.1016/j.apmr.2009.03.015. [DOI] [PubMed] [Google Scholar]
- 6.Strauss D, Shavelle RM, DeVivo MJ, Harrison-Felix C, Whiteneck GG. Life expectancy after traumatic brain injury. Neuro Rehabilitation. 2004;19(3):257–258. [PubMed] [Google Scholar]
- 7.McMillan TM, Teasdale GM, Weir CJ, Stewart E. Death after head injury: the 13 year outcome of a case control study. J Neurol Neurosurg Psychiatry. 2011;82(8):931–935. doi: 10.1136/jnnp.2010.222232. [DOI] [PubMed] [Google Scholar]
- 8.Roozenbeek B, Maas AI, Menon DK. Changing patterns in the epidemiology of traumatic brain injury. Nat Rev Neurol. 2013;9(4):231–236. doi: 10.1038/nrneurol.2013.22. [DOI] [PubMed] [Google Scholar]
- 9.Stein SC, Georgoff P, Meghan S, Mizra K, Sonnad SS. 150 years of treating severe traumatic brain injury: a systematic review of progress in mortality. J Neurotrauma. 2010;27(7):1343–1353. doi: 10.1089/neu.2009.1206. [DOI] [PubMed] [Google Scholar]
- 10.Rosenfeld JV, Maas AI, Bragge P, Morganti-Kossmann MC, Manley GT, Gruen RL. Early management of severe traumatic brain injury. Lancet. 2012;380(9847):1088–1098. doi: 10.1016/S0140-6736(12)60864-2. [DOI] [PubMed] [Google Scholar]
- 11.Brooks JC, Strauss DJ, Shavelle RM, Paculdo DR, Hammond FM, Harrison-Felix CL. Long-term disability and survival in traumatic brain injury: results from the National Institute on Disability and Rehabilitation Research Model Systems. Arch Phys Med Rehabil. 2013;94(11):2203–2209. doi: 10.1016/j.apmr.2013.07.005. [DOI] [PubMed] [Google Scholar]
- 12.Corrigan JD, Hammond FM. Traumatic brain injury as a chronic health condition. Arch Phys Med Rehabil. 2013;94(6):1199–1201. doi: 10.1016/j.apmr.2013.01.023. [DOI] [PubMed] [Google Scholar]
- 13.Kozlowski AJ, Pretz CR, Dams-O’Connor K, Kreider S, Whiteneck G. An introduction to applying individual growth curve models to evaluate change in rehabilitation: a National Institute on Disability and Rehabilitation Research Traumatic Brain Injury Model Systems report. Arch Phys Med Rehabil. 2013;94(3):589–596. doi: 10.1016/j.apmr.2012.08.199. [DOI] [PubMed] [Google Scholar]
- 14.Teasdale GM, Pettigrew LE, Wilson JT, Murray G, Jennett B. Analyzing outcome of treatment of severe head injury: are view and update on advancing the use of the Glasgow Outcome Scale. J Neurotrauma. 1998;15(8):587–597. doi: 10.1089/neu.1998.15.587. [DOI] [PubMed] [Google Scholar]
- 15.Jennett B, Bond M. Assessment of outcome after severe brain damage. Lancet. 1975;1(7905):480–484. doi: 10.1016/s0140-6736(75)92830-5. [DOI] [PubMed] [Google Scholar]
- 16.Shukla D, Devi BI, Agrawal A. Outcome measures for traumatic brain injury. Clin Neurol Neurosurg. 2011;113(6):435–441. doi: 10.1016/j.clineuro.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 17.Wilson JT, Pettigrew LE, Teasdale GM. Structured interviews for the Glasgow Outcome Scale and the Extended Glasgow Outcome Scale: guidelines for their use. J Neurotrauma. 1998;15(8):573–585. doi: 10.1089/neu.1998.15.573. [DOI] [PubMed] [Google Scholar]
- 18.Pretz CR, Dams-O’Connor K. Longitudinal description of the Glasgow Outcome Scale-Extended for individuals in the Traumatic Brain Injury Model Systems National Database: a national institute on Disability and Rehabilitation Research Traumatic Brain Injury Model Systems study. Arch Phys Med Rehabil. 2013;94(12):2486–2493. doi: 10.1016/j.apmr.2013.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pretz CR, Kozlowski AJ, Dams-O’Connor K, et al. Descriptive modeling of longitudinal outcome measures in traumatic brain injury: a National Institute on Disability and Rehabilitation Research Traumatic Brain Injury Model Systems study. Arch Phys Med Rehabil. 2013;94(3):579–588. doi: 10.1016/j.apmr.2012.08.197. [DOI] [PubMed] [Google Scholar]
- 20.Bush BA, Novack TA, Malec JF, Stringer AY, Millis SR, Madan A. Validation of a model for evaluating outcome after traumatic brain injury. Arch Phys Med Rehabil. 2003;84(12):1803–1807. doi: 10.1016/s0003-9993(03)00367-8. [DOI] [PubMed] [Google Scholar]
- 21.Brown AW, Malec JF, McClelland RL, Diehl NN, Englander J, Cifu DX. Clinical elements that predict outcome after traumatic brain injury: a prospective multicenter recursive partitioning (decision-tree) analysis. J Neurotrauma. 2005;22(10):1040–1051. doi: 10.1089/neu.2005.22.1040. [DOI] [PubMed] [Google Scholar]
- 22.Graham JE, Radice-Neumann DM, Reistetter TA, Hammond FM, Dijkers M, Granger CV. Influence of sex and age on inpatient rehabilitation outcomes among older adults with traumatic brain injury. Arch Phys Med Rehabil. 2010;91(1):43–50. doi: 10.1016/j.apmr.2009.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hammond FM, Hart T, Bushnik T, Corrigan JD, Sasser H. Change and predictors of change in communication, cognition, and social function between 1 and 5 years after traumatic brain injury. J Head Trauma Rehabil. 2004;19(4):314–328. doi: 10.1097/00001199-200407000-00006. [DOI] [PubMed] [Google Scholar]
- 24.Kolakowsky-Hayner SA, Hammond FM, Wright J, et al. Ageing and traumatic brain injury: age, decline in function and level of assistance over the first ten years postinjury. Brain Inj. 2012;26(11):1328–1337. doi: 10.3109/02699052.2012.706353. [DOI] [PubMed] [Google Scholar]
- 25.Ratcliff JJ, Greenspan AI, Goldstein FC, et al. Gender and traumatic brain injury: do the sexes fare differently? Brain Inj. 2007;21(10):1023–1030. doi: 10.1080/02699050701633072. [DOI] [PubMed] [Google Scholar]
- 26.Connelly J, Chell S, Tennant A, Rigby AS, Airey CM. Modelling 5-year functional outcome in a major traumatic injury survivor cohort. Disabil Rehabil. 2006;28(10):629–636. doi: 10.1080/09638280500276513. [DOI] [PubMed] [Google Scholar]
- 27.Arango-Lasprilla JC, Ketchum JM, Cifu D, et al. Predictors of extended rehabilitation length of stay after traumatic brain injury. Arch Phys Med Rehabil. 2010;91(10):1495–1504. doi: 10.1016/j.apmr.2010.07.010. [DOI] [PubMed] [Google Scholar]
- 28.Granger CV, Deutsch A, Russell C, Black T, Ottenbacher KJ. Modifications of the FIM instrument under the inpatient rehabilitation facility prospective payment system. Am J Phys Med Rehabil. 2007;86(11):883–892. doi: 10.1097/PHM.0b013e318152058a. [DOI] [PubMed] [Google Scholar]
- 29.Pretz CR, Malec JF, Hammond FM. Longitudinal description of the disability rating scale for individuals in the National Institute on Disability and Rehabilitation Research Traumatic Brain Injury Model Systems National Database. Arch Phys Med Rehabil. 2013;94(12):2478–2485. doi: 10.1016/j.apmr.2013.06.019. [DOI] [PubMed] [Google Scholar]
- 30.Hall A, Wood D, Hou T, Zhang J. Patterns in primary health care utilization among individuals with intellectual and developmental disabilities in Florida. Intellect Dev Disabil. 2007;45(5):310–322. doi: 10.1352/0047-6765(2007)45[310:PIPHCU]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 31.Swaine JG, Parish SL, Luken K, Son E, Dickens P. Test of an intervention to improve knowledge of women with intellectual disabilities about cervical and breast cancer screening [published online ahead of print June 24, 2013] J Intellect Disabil Res. doi: 10.1111/jir.12062. [DOI] [PubMed] [Google Scholar]
- 32.Chan L, Beaver S, Maclehose RF, Jha A, Maciejewski M, Doctor JN. Disability and health care costs in the Medicare population. Arch Phys Med Rehabil. 2002;83(9):1196–1201. doi: 10.1053/apmr.2002.34811. [DOI] [PubMed] [Google Scholar]
- 33.Adler M. The Disabled: Their Health Care and Health Insurance. Washington, DC: Office of Disability, Aging and Long-Term Care Policy, with the US Department of Health and Human Services; 1990. [Google Scholar]
- 34.Powell JM, Machamer JE, Temkin NR, Dikmen SS. Self-report of extent of recovery and barriers to recovery after traumatic brain injury: a longitudinal study. Arch Phys Med Rehabil. 2001;82(8):1025–1030. doi: 10.1053/apmr.2001.25082. [DOI] [PubMed] [Google Scholar]
- 35.Pickelsimer EE, Selassie AW, Sample PL, W Heinemann A, Gu JK, Veldheer LC. Unmet service needs of persons with traumatic brain injury. J Head Trauma Rehabil. 2007;22(1):1–13. doi: 10.1097/00001199-200701000-00001. [DOI] [PubMed] [Google Scholar]
- 36.Masel BE, DeWitt DS. Traumatic brain injury: a disease process, not an event. J Neurotrauma. 2010;27(8):1529–1540. doi: 10.1089/neu.2010.1358. [DOI] [PubMed] [Google Scholar]
- 37.Institute of Medicine Committee on Gulf War Health. Gulf War and Health: Volume 7. Long-term Consequences of Traumatic Brain Injury. Washington, DC: National Academies Press; 2009. [PubMed] [Google Scholar]
- 38.Norman G. Likert scales, levels of measurement and the “laws” of statistics. Adv Health Sci Educ Theory Pract. 2010;15(5):625–632. doi: 10.1007/s10459-010-9222-y. [DOI] [PubMed] [Google Scholar]