Abstract
Relatively little is known about the human T cell response to HSV-2 in the female genital tract, a major site of heterosexual HSV-2 acquisition, transmission and reactivation. In order to understand the role of local mucosal immunity in HSV-2 infection, T cell lines were expanded from serial cervical cytobrush samples from 30 HSV-2 infected women and examined for reactivity to HSV-2. Approximately 3% of the CD3+ T cells isolated from the cervix were HSV-2 specific and of these, a median of 91.3% were CD4+ while a median of 3.9% were CD8+. HSV-2 specific CD4+ T cells expanded from the cervix were not only more frequent than CD8+ T cells but also exhibited greater breadth in terms of antigenic reactivity. T cells directed at the same HSV-2 protein were often detected in serial cervical cytobrush samples and in blood. Thus, broad and persistent mucosal T cell responses to HSV-2 were detected in the female genital tract of HSV-2+ women suggesting that these cells are resident at the site of HSV-2 infection. Understanding the role of these T cells at this biologically relevant site will be central to the elucidation of adaptive immune mechanisms involved in controlling HSV-2 disease.
Keywords: Human, genital, herpes, endocervix, T cells, mucosa
Introduction
HSV-2 is the major cause of genital herpes, one of the most common sexually transmitted infections worldwide and a significant risk factor of HIV acquisition (1). In the US, 17% of people are seropositive for HSV-2 and 58% are seropositive for HSV-1 (2). Elsewhere, the seroprevalence rate is much higher, reaching 95% in some populations of HIV-infected persons and female sex workers [reviewed in (3)]. The lack of success of HSV-2 vaccines (4-6) and the observation that HSV-2 reactivation can occur in persons taking standard or high doses of antiviral therapy (7) underscore the urgency of defining mechanisms of resistance in order to design strategies to prevent primary HSV-2 infection and reduce or eliminate reactivation of latent HSV-2.
Clinical and experimental data indicate an important role of the adaptive immune response, especially T cell responses, in controlling genital herpes infections. Persons with defects in cellular immunity have more severe HSV-2 infections (8-10). The observations that HSV-2 clearance from genital herpes lesions correlates temporally with the infiltration of CD8 T cells (11), functional HSV-2 specific CD4+ and CD8+ T cells populate the site of HSV-2 reactivation(12-14) and that functional CD8+ T cells persist in genital skin contiguous to sensory neuronal nerve endings (15-17) suggest a role of the peripheral mucosal adaptive immune response in controlling HSV reactivation. Mathematical modeling predicts prolonged duration and increased severity of HSV-2 shedding episodes is strongly associated with a low density of CD8+ T cells within genital skin (18). That the virus has devised numerous strategies to evade host innate and adaptive mechanisms of resistance to primary and recurrent HSV infection further emphasizes the importance of host immune mechanisms.
While numerous human studies characterize the T cell response to HSV-2 in blood, skin and eye [reviewed in (19)], few studies address the local mucosal cellular immune response directed at HSV-2 in the female genital tract (20). In order to more comprehensively analyze the cervical resident T cell response to HSV-2, T cells isolated and expanded from serial cytobrush samples were obtained from 30 HSV-2+ women and analyzed with respect to phenotype, antigenic reactivity and persistence. Cervical cytobrush sampling is a relatively non-invasive procedure for obtaining mucosal T cells from the female genital tract and short-term polyclonal expansion of these cells allowed a more detailed characterization of cervically-derived T cells to HSV-2.
Results
Clinical and demographic characteristics of study participants
The study group consisted of 32 female participants in total including 2 who were seronegative to HSV-1 and HSV-2 (HSVneg) and 30 who were seropositive to HSV-2 (HSV-2+): of these 30 HSV-2+ participants, 9 were coinfected with HSV-1 and HSV-2 (HSV-1+/2+) while 21 were seropositive to HSV-2 only (HSV-1-/2+) (Table 1). The average age of HSV+ participants was 35 years (range 19-73 yrs) and the HSVneg participants were 26 and 51 yrs old. Most HSV-2+ participants were white (84%) and had been infected with HSV-2 a median of 8.3 years (range 0.2-37.9 yrs) (Table 1).
Table 1. Demographic and Clinical Characteristics of Study Participants.
| HSV -2+ | HSV neg | |
|---|---|---|
|
| ||
| No. of participants | 30 | 2 |
| Median Age: years (range) | 35 (19-73) | 39 (26, 51) |
| HSV serostatus: n (%) | ||
| HSV-1-/2+ | 21 (70%) | N/A |
| HSV-1+/2+ | 9 (30%) | N/A |
| Total HSV-2+ | 30 (100%) | N/A |
| Race | 25 White, 5 Black | 2 white |
| Years infected with HSV-2: median (range) | 8.3 (0.2-37.9) | N/A |
HSV-specific lymphoproliferative (LP) responses in cervical lymphocytes
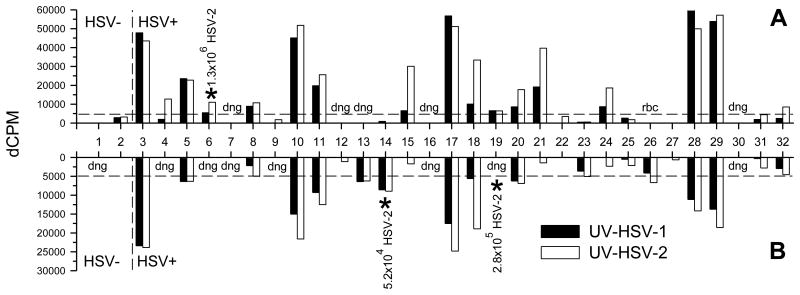
All study participants (2 HSVneg, 30 HSV-2+) provided 2 cervical cytobrush samples spaced approximately one month apart. A median of 0.2×106 mononuclear cells (range 0.08×106 to 1.8×106 cells) per cytobrush were obtained ex vivo. Of the 64 cytobrush samples, 1 was discarded due to red blood cell contamination and T cells could not be expanded from 13 of the samples; cells could not be expanded from either of 2 cytobrushes from 3 HSV-2+ participants (Participants 7, 16 and 30) (Figure 1). The remaining 51 cervical cytobrush samples (48 from HSV-2+ participants, 3 from HSVneg participants) where cell growth was evident after 18-24 days in culture were tested for HSV-specific T cell activity by LP assays. Of the 48 samples from the HSV-2+ participants where cell growth was evident, 31 or 65% proliferated in response to HSV-2; 26 of these also proliferated in response to HSV-1 (Figure 1). Considering only those subjects with samples containing HSV-2 specific LP responses, of the subjects who were seropositive for HSV-1 and HSV-2 (n=5), 1 (20%) had no LP responses to HSV-1 while 4 (80%) had LP responses to both HSV-1 and HSV-2. Similarly, of the subjects who were seropositive for HSV-2 only (n=16), 4 (25%) had no LP response to HSV-1 while 12 (75%) had LP responses to both HSV-1 and HSV-2; these results suggest that HSV serostatus did not influence the ability to detect HSV-1 specific LP responses in the cervical T cell lines. From 10 of the 30 HSV-2+ participants, cervical T cell lines were positive for LP responses to HSV-2 from both cytobrush samples while cervical T cell lines from 17 HSV-2+ participants had positive LP responses in only 1 cytobrush sample (Figure 1). While T cells expanded from 3 of the 4 cytobrush samples obtained from the 2 HSVneg participants, none were positive for LP responses to HSV-1 or HSV-2 (Figure 1) although LP responses to PHA were positive in the 3 samples (data not shown). LP responses to HSV-1 ranged from 1-61,827 dCPM (median 5,491 dCPM), responses to HSV-2 ranged from 1-57,088 dCPM (median 6,696 dCPM) and PHA responses ranged from 0-180,774 dCPM (median 28,890 dCPM): cells expanded from one sample (Participant 15, second cytobrush sample) did not proliferate in response to PHA. An additional cytobrush sample was obtained from 5 HSV-2+ participants approximately 6 months after the first sample; T cells expanded in all 5 samples and all had positive LP responses to HSV-1, HSV-2 and PHA (data not shown).
Figure 1. HSV-specific LP responses in cervical cytobrush samples.
Cervical cytobrush samples were obtained from 2 HSVneg (HSV-) participants and 30 HSV-2+ participants at study entry (A) and at one month (B). Cervical T cell lines expanded from these samples were tested for HSV-specific LP responses using UV-HSV-1 or UV-HSV-2 as the stimulatory antigen. Dashed line represents threshold for positivity (>5,000 dCPM). HSV-2 DNA was detected in 3 samples (*) and the total amount of virus detected in the sample is displayed. dng, did not grow; rbc, red blood cell contamination.
HSV-2 DNA in cervical samples
CVL fluid was collected from both clinic visits from each participant and tested for the presence of HSV-2 DNA by quantitative real-time PCR. Of the 60 CVL collected from the 30 HSV-2+ participants, 3 samples (5%) from 3 different participants tested positive for HSV-2 DNA (Figure 1). From the 3 visits where CVL were positive, low levels of HSV-2 specific LP were detected in the corresponding cytobrush samples from 2 of the participants (Participant 6, visit 1; Participant 14, visit 2) while T cells did not grow from the corresponding cytobrush sample from the third participant (Participant 19, visit 2) (Figure 1). It was interesting to note that HSV-2 DNA was not detected in any CVL sample when a corresponding cytobrush sample possessed a high level of HSV-2 specific LP. As expected, no HSV-2 DNA was detected from the 4 CVL collected from the HSVneg participants (Figure 1).
HSV-2 proteins recognized by cervical T cell lines
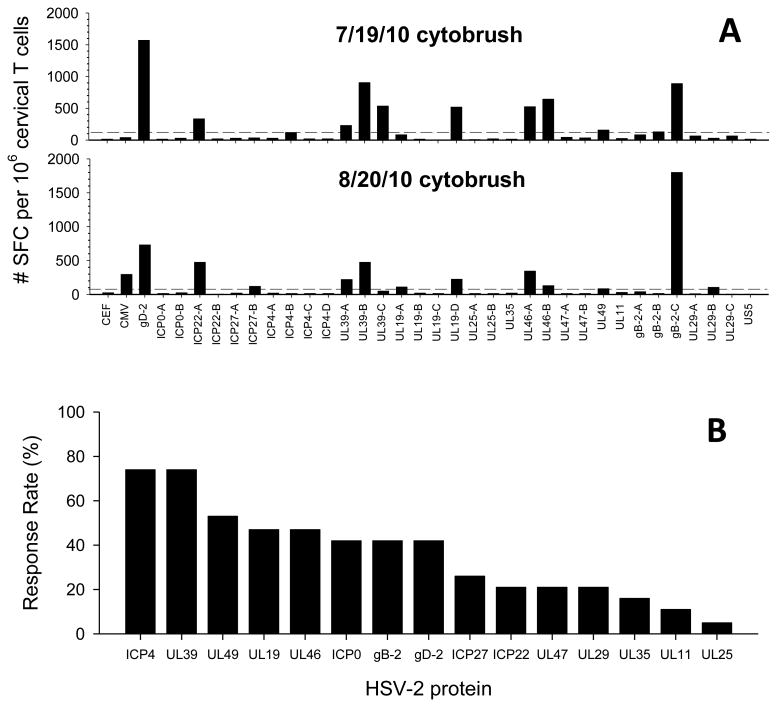
Cervical T cell lines that tested positive for HSV-specific LP responses were subsequently tested by IFN-γ ELISPOT for reactivity to a panel of 34 HSV-peptide pools representing 16 HSV-2 proteins. Figure 2A displays 2 representative IFN-γ ELISPOT assays from 2 cytobrush samples obtained one month apart from HSV-2 Participant 17. Multiple HSV-2 peptide pools were recognized by the cervical T cell lines from each date (11 pools from 7/19/10 and 12 pools from 8/20/10) and multiple sub-pools from an individual HSV-2 protein were also recognized (UL39, UL19 and UL46). Additionally, IFN-γ ELISPOT responses to 8 of the HSV-2 peptide pools were detected in both cytobrush samples (Figure 2A). In total, 33 cytobrush samples from 19 HSV-2 participants were analyzed by IFN-γ ELISPOT: 5 of these participants had 3 cytobrush samples screened, 4 of these participants had 2 cytobrush samples screened while 10 had 1 cytobrush sample screened (Table 2). Positive IFN-γ ELISPOT responses to more than one sub-pool of an individual HSV-2 protein are grouped together. The median number of HSV-2 proteins recognized in individual cytobrush samples was 4 (range 1-11) (Table 2). The HSV-2 proteins most frequently recognized by cervical T cell lines (positive in one, two or three cytobrush samples) were ICP4 (74%), UL39 (74%), UL49 (53%), UL46 (47%) and UL19 (47%), ICP0 (42%), gD-2 (42%), and gB-2 (42%), (Table 2 and Figure 2B). This underestimates the total number of HSV-2 epitopes recognized because in many cases, more than 1 sub-pool from an individual HSV-2 protein was positive for a given cervical T cell line: 11 cervical T cell lines recognized more than 1 sub-pool of UL39 (5 recognized 2 sub-pools and 6 recognized 3 sub-pools) while 5 cervical T cell lines recognized both sub-pools of UL46 (Figure 2A and data not shown). Interestingly, T cells directed at the same HSV-2 protein were often detected in serial cytobrush samples: 8 of the 9 participants who had 2-3 cytobrush samples positive for HSV-LP responses had T cell responses directed at the same HSV-2 protein in more than 1 cytobrush sample; approximately ½ of the IFN-γ ELISPOT responses to a given HSV-2 protein were detected in more than one cytobrush sample from the same participant (Table 2 and Figure 2A).
Figure 2. Antigenic diversity of HSV-2 specific T cells in cervical T cell lines.
(A) Cervical T cell lines that were positive by HSV-LP from HSV-2+ Participant 17 obtained on 7/19/10 (top) and 8/20/10 (bottom) were stimulated with HSV-2, CEF or CMV peptide pools and tested for reactivity by IFN-γ ELISPOT; Y-axis is net spot forming cells (SFC) per million cervical T cells (SFC in DMSO control wells were subtracted). Dashed lines represent the threshold for positivity (4X median of DMSO control wells and ≥55 SFCs per million cervical T cells). (B) Cervical T cell lines from HSV-2+ participants that were positive by HSV-LP were screened for reactivity using the 34 peptide pools representing 16 HSV-2 proteins by IFN-γ ELISPOT. The frequency of detecting HSV-2 protein specific T cell responses in one or both cervical cytobrush samples from 19 HSV-2+ participants is displayed: HSV-2 proteins are stratified based on their hierarchy in % response rate.
Table 2. Antigenic reactivity of cervical T cell lines.
| ID | Study Day | HSV-2 protein | Proteins + per cytobrush | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| glycoprotein | immediate-early | capsid | tegument | other | |||||||||||||
| gD-2 | gB-2 | ICP0 | ICP22 | ICP27 | ICP4 | UL19 | UL25 | UL35 | UL46 | UL47 | UL49 | UL11 | UL39 | UL29 | |||
| 3 | 4/5/2010 | + | + | + | 3 | ||||||||||||
| 6/11/2010 | + | + | + | + | + | 5 | |||||||||||
| 2/8/2011 | + | + | + | + | + | + | + | 7 | |||||||||
| 4 | 4/20/2010 | + | + | + | 3 | ||||||||||||
| 5 | 4/21/2010 | + | + | 2 | |||||||||||||
| 7/12/2010 | + | + | + | + | 4 | ||||||||||||
| 6 | 4/26/2010 | + | + | 2 | |||||||||||||
| 8 | 5/17/2010 | + | + | + | 3 | ||||||||||||
| 10 | 6/1/2010 | + | + | + | 3 | ||||||||||||
| 7/1/2010 | + | + | + | + | + | 5 | |||||||||||
| 5/10/2011 | + | + | 2 | ||||||||||||||
| 11 | 6/7/2010 | + | + | 2 | |||||||||||||
| 7/14/2010 | + | + | + | 4 | |||||||||||||
| 13 | 8/4/2010 | + | + | + | + | 4 | |||||||||||
| 14 | 8/9/2010 | + | + | + | + | + | + | + | + | + | 9 | ||||||
| 15 | 7/1/2010 | + | + | + | 3 | ||||||||||||
| 17 | 7/19/2010 | + | + | + | + | + | + | + | 8 | ||||||||
| 8/20/2010 | + | + | + | + | + | + | + | + | 9 | ||||||||
| 2/8/2011 | + | + | + | + | + | 5 | |||||||||||
| 18 | 7/20/2010 | + | + | + | + | + | + | + | + | 7 | |||||||
| 8/19/2010 | + | + | + | + | + | 5 | |||||||||||
| 19 | 7/22/2010 | + | + | + | + | 4 | |||||||||||
| 20 | 7/2/2010 | + | + | + | + | 4 | |||||||||||
| 8/23/2010 | + | + | + | 3 | |||||||||||||
| 2/15/2011 | + | + | 2 | ||||||||||||||
| 21 | 7/27/2010 | + | + | 2 | |||||||||||||
| 23 | 9/2/2010 | + | + | 2 | |||||||||||||
| 26 | 9/9/2010 | + | + | + | + | 4 | |||||||||||
| 28 | 8/12/2010 | + | 1 | ||||||||||||||
| 9/14/2010 | + | + | + | + | + | + | 6 | ||||||||||
| 29 | 8/19/2010 | + | + | + | + | + | 5 | ||||||||||
| 9/20/2010 | + | + | + | + | + | + | + | + | + | + | 10 | ||||||
| 3/22/2011 | + | + | + | + | + | + | + | + | + | + | + | 11 | |||||
| #pos/subject (n=19) | 8 | 8 | 8 | 4 | 5 | 14 | 9 | 1 | 3 | 9 | 4 | 10 | 2 | 14 | 4 | 4 | |
Cervical T cell lines from HSV-2+ participants with positive HSV-2 specific lymphoproliferative responses were screened by IFN-γ ELISPOT using 34 pools of overlapping peptides representing 16 HSV-2 proteins that are classified as glycoproteins, immediate-early, capsid, tegument or other proteins. Positive IFN-γ ELISPOT responses to sub-pools are grouped together. +, positive IFN-γ ELISPOT response; shaded cells, T cells directed at the same HSV-2 protein detected in more than one cytobrush sample from the same participant.
HSV antigenic specificity of cervical T cells versus blood-derived T cells
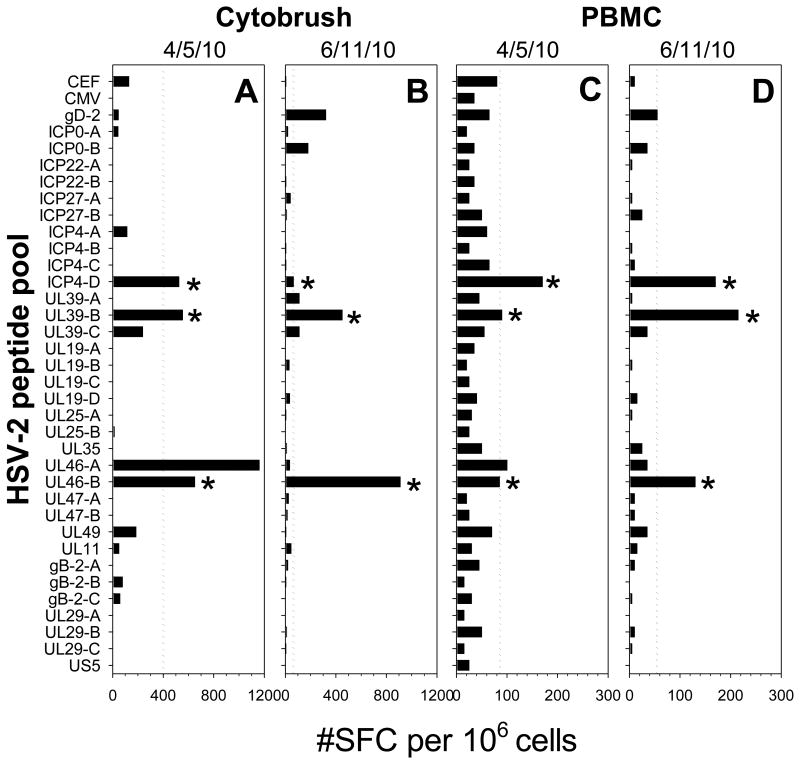
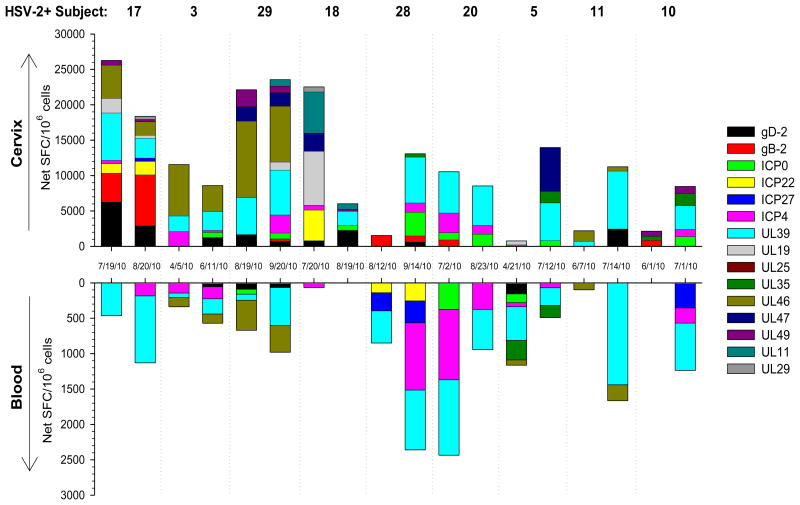
To compare the breadth of the HSV-2 specific T cell response between the cervix and blood, serial cytobrush samples and corresponding PBMC were tested for reactivity to the HSV-2 peptide pools using IFN-γ ELISPOT. Figure 3 displays data from a representative HSV-2+ participant: positive IFN-γ ELISPOT responses were measured to multiple HSV-2 peptide pools and positive responses to the ICP4-D, UL39-B and UL46-A sub-pools were present in all 4 samples (Figure 3). Additionally, positive IFN-γ ELISPOT responses to UL46-A were measured in both compartments on 4/5/10 (Figure 3A and C) while positive IFN-γ ELISPOT responses to gD-2 were detected in both compartments on 6/11/10 (Figure 3B and D). Figure 4 displays the cumulative IFN-γ ELISPOT response to HSV-2 in cervically-derived (top) and blood-derived (bottom) T cells from 9 HSV-2+ participants who had 2 cytobrush samples positive for HSV-specific T cell activity; only those proteins with positive IFN-γ ELISPOT responses are included and positive responses to sub-pools are grouped together. In general, cervically-derived T cell responses were broader in terms of antigenic reactivity compared to those measured in the corresponding PBMC samples. In all but 3 cases (4/5/10 sample from Participant 3, 8/12/10 sample from Participant 28, and 4/21/10 sample from Participant 5), cervically-derived T cells were reactive to more HSV-2 proteins than the corresponding PBMC-derived T cells (Figure 4): a median of 5 HSV-2 proteins (range 1-10) were recognized by these cervically-derived T cell lines compared to a median of 3 HSV-2 proteins (range 0-6) recognized by PBMC-derived T cells, although this was not statistically significant (P>0.05). Similar antigenic reactivity patterns were observed between serial cytobrush samples from the same participant and often IFN-γ ELISPOT responses were positive in both compartments. UL39 was the most commonly recognized HSV-2 protein: T cells directed at UL39 were detected in all cytobrush samples from these 9 participants and in at least one PBMC sample from 8 of the participants (Figure 4). The frequency of the cumulative T cell response was at least an order of magnitude greater in the cervix compared to the blood suggesting that T cells are enriched in this mucosal compartment.
Figure 3. IFN-γ T cell response to HSV-2 peptide pools in blood and genital tract of HSV-2+ women.
Cervical T cell lines (A and B) and PBMC (C and D) from HSV-2+ Participant 3 obtained on 4/5/10 (A and C) or 6/11/10 (B and D) were screened by IFN-γ ELISPOT for reactivity to the HSV-2 peptide pools. X-axis is net spot forming cells (SFC) per million cervical T cells (A and B) or PBMC (C and D) (SFC in DMSO control wells were subtracted). Dashed lines represent the threshold for positivity as described in Figure 2A. *, individual HSV-2 peptide pool responses detected in all 4 samples from the 2 different compartments.
Figure 4. Cumulative IFN-γ T cell response to HSV-2 in blood and genital tract of HSV-2+ women.
IFN-γ ELISPOT responses to HSV-2 peptide pools were measured in serial cervical T cell lines and PBMC from 9 HSV-2+ women. Each bar represents an individual woman's response in the cervix or blood on the date displayed; the SFC from background wells (DMSO) is subtracted from each HSV-2 peptide pool that is considered positive and only data from those pools that are positive are displayed.
Phenotype of HSV-2 specific T cells derived from the cervix
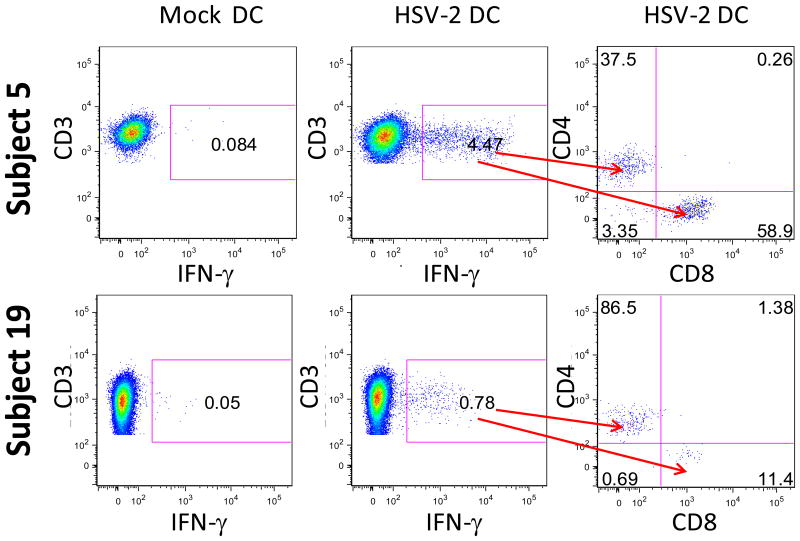
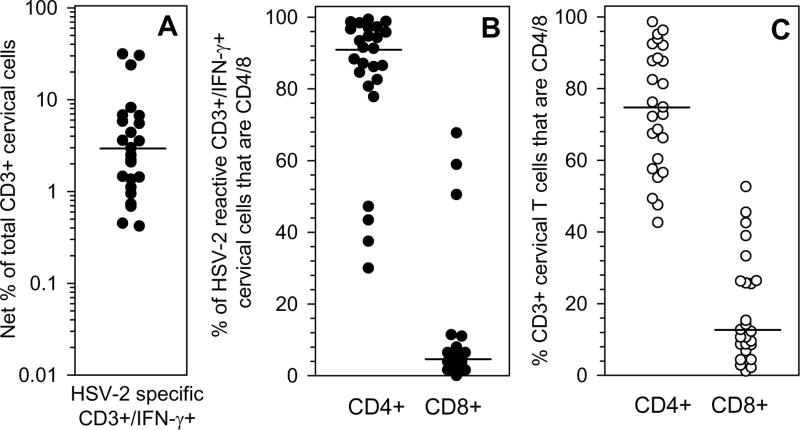
Cervical T cell lines that tested positive for HSV-2 specific LP responses were subsequently stimulated with autologous DC that were mock infected or infected with HSV-2 and the expression of CD3, CD4, CD8 and IFN-γ were measured by intracellular cytokine staining (ICS) and flow cytometry. Figure 5 displays data from 2 representative HSV-2+ participants, Participants 5 and 19. Low background staining (<0.10%) was observed to mock DC in both participants (left panels); in contrast, 4.47% of the CD3+ cells from Participant 5 and 0.78% of CD3+ cells from Participant 19 co-expressed IFN-γ in response to exposure to HSV-2 (Figure 5, center panels). In Participant 5, 37.5% of the CD3+/IFN-γ+ cells were CD4+ and 58.9% were CD8+; in Participant 19, 86.5% of the CD3+/IFN-γ+ cells were CD4+ and 11.4% were CD8+ (Figure 5, right panels). In total, HSV-2 specific CD3+/IFN-γ+ cells were detectable in 25 of the cervical T cell lines that possessed HSV-2 specific LP responses (Figure 6); the frequency of HSV-2 specific CD3+/IFN-γ+ from 11 of the cervical T cell lines that possessed HSV-2 specific LP responses were below the level of detection in our ICS/flow cytometry assay and are not included in Figure 6. The median frequency of HSV-2 specific CD3+/IFN-γ+ T cells detected in the cervical T cell lines was 2.95% (range 0.45%-31.39%; background subtracted) (Figure 6A). The HSV-2 specific CD3+/IFN-γ+ population was predominantly comprised of CD4+ T cells: a median of 91.30% (range 30%-99.40%) of this population was CD4+ while a median of 3.90% was CD8+ (range 0%-67.70%) (P=0.0012) (Figure 6B). Not only was the viral-specific CD3+ T cell response comprised primarily of CD4+ T cells, but the composition of the total T cell population was also predominantly CD4+: a median of 74.8% of the total T cells expanded from the cervical cytobrushes was CD4+ (range 42.7%-98.6%) while a median of 12.8% was CD8+ (range 1.2%-52.6%) (P=0.00044) (Figure 6C). The proportion of total CD4+ T cells that were HSV-2 specific was 2.63% (median; range 0.24-30.14%) while the proportion of total CD8+ T cells that were HSV-2 specific was 0.79% (median; range 0-8.79%) (data not shown).
Figure 5. Phenotype of cervically-derived T cells reactive to HSV-2.
Cervical T cell lines expanded from 2 HSV-2+ participants (Participant 5 and Participant 19) were stimulated with mock DC or HSV-2 DC and the production of IFN-γ from CD3+ cells was determined. Far right graphs display the percentages of CD4+ and CD8+ T cells within the HSV-2 reactive CD3+/IFN-γ+ population.
Figure 6. Frequency of CD3, CD4 and CD8 T cell responses to HSV-2 in T cells derived from the cervix.
Cervical T cell lines were incubated with mock DC or HSV-2 DC and the expression of CD3, CD4, CD8 and IFN-γ was determined by ICS and flow cytometry. (A) The proportion of cervically-derived CD3+ T cells that were HSV-2 specific was calculated by subtracting %CD3+IFN-γ+ cells with mock DC from %CD3+/IFN-γ+ cells with HSV-2 DC. (B) The percentages of CD4+ and CD8+ T cells within the HSV-2 reactive CD3+/IFN-γ+ fraction from (A) is displayed. (C) The proportion of total cervically-derived CD3+ T cells that were CD4+ or CD8+ is displayed. In each graph, median frequencies are displayed by the dashed line.
Phenotype of cervical T cells reactive to HSV-2 peptide pools
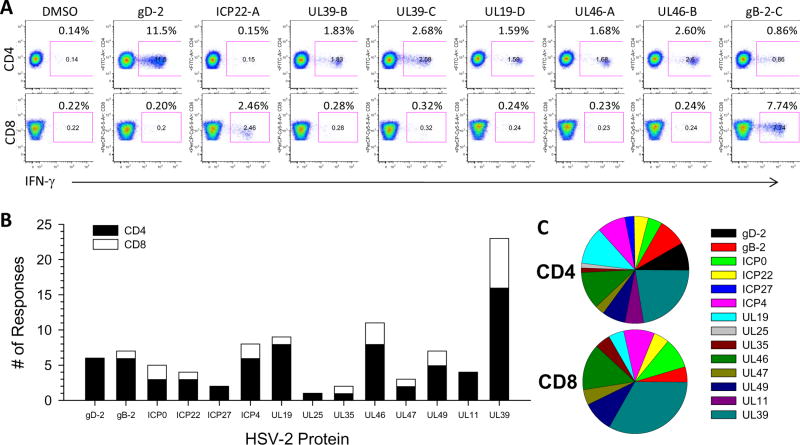
To determine whether cervical T cells responding to the HSV-2 peptide pools were CD4+ or CD8+, cervical T cell lines were incubated with the HSV-2 peptide pools that tested positive by IFN-γ ELISPOT and phenotypes of the responding cells were measured by ICS and flow cytometry. As displayed in representative histograms in Figure 7A, 7 of the HSV-2 peptide pools that were positive in the 7/19/10 cytobrush from HSV-2 Participant 17 were recognized by CD4+ T cells (gD-2, UL39-B, UL39-C, UL19-D, UL46-A, UL46-B, gB-2-C) while 2 pools were recognized by CD8+ T cells (ICP22-A and gB-2-C). In total, 28 cervical T cell lines from 17 participants were analyzed for the phenotype of the responding T cells: 4 participants had 3 cervical T cell lines, 3 participants had 2 cervical T cell lines and 10 participants had 1 cervical T cell analyzed. There were a total of 133 positive responses to individual HSV-2 peptide pools that could be measured by ICS and flow cytometry from all 28 cervical T cell lines; 105 of these were CD4+ and 28 were CD8+. On an individual basis, CD4 T cells derived from a cervical T cell line were reactive to an average of 3 different HSV-2 peptide pools (range 0-13) compared to CD8 T cells which were reactive to an average of one HSV-2 peptide pool (range 0-4) (P=0.00044). In Figure 7B, all CD4 and CD8 responses to individual HSV-2 proteins from all 17 participants were grouped together to assess the CD4/CD8 phenotype of T cells responding to the HSV-2 proteins; when a CD4 or CD8 response to an individual HSV-2 protein was detected in more than one cytobrush sample from the same individual, we only included this response once. On a population level, CD4 responses were directed at a broader range of HSV-2 proteins compared to CD8 responses (Figure 7B). Figure 7C displays pie graphs depicting the percentage of the total number of CD4 (top) or CD8 (bottom) responses directed at individual HSV-2 proteins. UL39 was the most common HSV-2 protein recognized by both CD4 and CD8 T cells derived from the cervix (Figure 7B and C). CD8 responses were also commonly directed at ICP0, ICP4, UL46 and UL49 (Figure 7B and C) while CD4 responses were commonly directed at gB-2, gD-2, UL19, UL46 and ICP4 (Figure 7B and C). Taken together, these data suggest a greater antigenic diversity in the cervically-derived CD4 T cell response compared to the cervically-derived CD8 T cell response to HSV-2 on both an individual and population basis. Thus, not only do CD4+ T cells comprise a greater percentage of the HSV-2 specific CD3+/IFN-γ+ population within the cervical T cell lines (Table 3; Figure 6B) but they are also directed at a broader range of HSV-2 proteins as compared to the CD8+ T cells derived from the cervix (Figure 7B and 7C).
Figure 7. Antigenic diversity of HSV-2 specific CD4 and CD8 T cell responses in cervical T cell lines.
(A) The expanded cervical T cell line from HSV-2+ Participant 17 from the cytobrush sample obtained on 7/19/10 (Figure 3A, top graph) was stimulated with DMSO only (negative control) or one of the HSV-2 peptide pools that were positive in IFN-γ ELISPOT assays and the phenotype of reactive cells was determined using ICS/flow cytometry. Top row of graphs are CD4 versus IFN-γ and the bottom row of graphs are CD8 versus IFN-γ; the percentage of CD4+/IFN-γ+ or CD8+/IFN-γ+ cells is displayed. (B) Positive CD4 and CD8 responses to individual HSV-2 proteins measured in cervical T cell lines from all HSV-2+ participants determined by ICS/flow cytometry were collated. Black bars, CD4+/IFN-γ+ responses, open bar, CD8+/IFN-γ+ responses. (C) The percentage of CD4+/IFN-γ+ responses (top pie graph) and CD8+/IFN-γ+ responses (bottom pie graph) directed at individual HSV-2 proteins present in cervical T cell lines from HSV-2+ participants is displayed.
Discussion
This study markedly extends the knowledge of HSV-2 specific T cells populating the human female genital tract. HSV-specific T cells were frequently detected in the cervix and approximately 3% of all T cells expanded from the cervical cytobrush samples were directed at HSV-2. The HSV-2 specific T cell response was predominantly comprised of CD4 T cells, which were directed at a broader range of HSV-2 proteins compared to CD8 T cells. HSV-2 specific T cells appeared to be enriched in the cervix compared to the blood compartment and T cell responses directed at the same HSV-2 protein and peptide pool were often detected in serial cytobrush samples as well as in PBMC.
Total CD4+ T cells and HSV-2 specific CD4+ CD4+ T cells were consistently detected in the cervical T cell lines and at high frequencies compared to CD8+ T cells, which were present at significantly lower frequencies and sometimes undetectable. This observation is consistent with a comprehensive analysis of memory T cell subsets within various peripheral tissues which demonstrated that memory CD4+ T cells represent the majority subset in mucosal tissue including the lung and intestinal tract, although the female genital tract was not analyzed (21). CD4+ T cells were shown to be present in high numbers in the endocervix in a distinct band underneath the epithelium (22) and a study of 30 healthy Caucasian women showed that CD3+ T cells made up approximately 1% of the total cells isolated from the endocervix and of those, 61% were CD4+ (23). A recent study by McKinnon et al. showed a 2:1 CD4:CD8 ratio in T cells isolated from healthy women in Seattle, Chicago and Nairobi (24). Our study demonstrates that approximately 75% of the total CD3+ population within the cervical T cell lines were CD4+ (Figure 6C). These data suggest that the total T cell population obtained upon cytobrushing is skewed toward CD4+ T cells. This dominance in CD4+ T cells is more pronounced in the HSV-2 specific CD3+ population where a median of 91% of HSV-2 specific CD3+ T cells were CD4+ (Figure 6B). The presence of sexually transmitted infections (STI) has been shown to influence the T cell composition in the cervix: higher numbers of CD4+ T cells were measured in the endocervix in subjects with non-ulcerative STI including chlamydia, gonorrhea and trichomoniasis (25). However, because the non-specific stimulation of T cells isolated from the cervix may preferentially expand T cells with higher proliferative capacity, the most accurate assessment of the T cell composition of both total T cells and HSV-2 specific T cells would be performed ex vivo. It is likely that a significant fraction of CD8+ T cells are resident memory T cells that have limited proliferative capacity and thus unlikely to expand in our protocol requiring cells to undergo multiple rounds of cell division. The impact of expansion methods on the maturational state of cervical T cells in HIV+ women suggested that expansion using anti-CD3, similar to the current study, led to the accumulation of effector memory T cells (CD45R0+CCR7-CD27-) after 7 days (26); however these studies did not measure virus specific responses but instead total CD4 and CD8 T cells. Our limited data analyzing cytobrush-derived T cells ex vivo for HSV-2 specific CD4+ and CD8+ T cells suggest that CD8+ T cells were at lower frequencies than CD4+ T cells or undetectable, similar to the phenotype of cervical T cell lines generated upon in vitro expansion (unpublished data). Interestingly, higher numbers of CD8+ T cells were present in ectocervical biopsy specimens compared to endocervical cytobrush specimens obtained from healthy women (24) suggesting that CD8+ T cells may reside at tissue locations not sampled during cytobrushing and perhaps providing another possibility as to why low frequencies of HSV-2 specific CD8+ T cells were measured. In any event, while the presence of high frequencies of HSV-2 specific CD4+ T cells in the cervix may suggest an important role in the local control of genital HSV-2 infection, it may also have significant implications for HIV acquisition since HSV-2 increases the risk of HIV acquisition, possibly due in part to increased CD4+ T cell activation in the cervix and an increased expression of HIV susceptibility markers, CCR5 and α4β7 (27-29).
HSV-2 disease is characterized by frequent clinical and subclinical shedding. The frequent detection and high frequency of HSV-specific T cells in the cervix suggests ongoing exposure to antigen although cervical shedding of HSV-2 tends to occur at lower rates than from other areas of the lower genital tract (30). The current study detected HSV-2 DNA in only 3 of the cytobrush samples (5% of samples); this is similar to what was observed in a cross-sectional study of 509 HSV-2 seropositive women where 7% of all CVL samples were positive for HSV-2 DNA (31). The antimicrobial activity of CVL, which increases at the time of clinical HSV-2 outbreaks, has been proposed as a mechanism to prevent the spread of HSV-2 from external genital sites to the upper genital tract (32). The high frequency of HSV-2 specific cervical T cells detailed in the current study may contribute to the control of HSV-2 spread in the female genital tract; anecdotally, HSV-2 DNA was not detected in any CVL with a correspondingly high level of HSV-2 specific LP responses in the cytobrush samples. A more intense study of mucosal sampling, including multiple external and internal genital sites, and local T cells is warranted to assess the relationship between local mucosal HSV-specific T cell immunity and viral shedding in order to determine the mechanism of viral control at the site of infection and reactivation.
Short-term polyclonal expansion of the T cells obtained from cytobrushing provided sufficient cells to analyze the antigenic repertoire of cervical T cell lines. In general, T cell recovery was too low to perform ex vivo functional and other phenotypic T cell studies. We have recently obtained cervical biopsies which may provide a larger source of cells that can be tested ex vivo to determine the memory/effector phenotype, cytokine profile and lytic function of the cervical resident T cells; such studies are best done ex vivo to prevent changes in biologically relevant mechanisms that may be altered upon short-term and long-term cell culture (33, 34). These ex vivo studies will aid in the determination of the mechanisms utilized by local T cells to limit or prevent HSV reactivation and spread in HSV-2 infected participants or protection from infection in HSV resistant populations. Recently, our group demonstrated that CD8αα+ T cells are the dominant resident population of dermal-epidermal junction CD8+ T cells that persist at the site of previous reactivation in skin near the genital region (17). Importantly, these cells (1) lacked the expression of CCR7 and S1PR1, suggesting that they may be tissue resident T cells, and (2) possessed gene signatures of T cell activation and antiviral activity suggesting a role in immune surveillance and in the containment of HSV-2 reactivation in human peripheral tissue (17). It will be important to determine if these CD8αα+ cells also persist in the human female genital tract as a means to control local HSV-2 reactivation; presumably, these cells lack significant proliferative potential and may not be expanded using the techniques employed in this study but instead may only be detected if assessed in situ or ex vivo.
UL39 and ICP4 were the most common HSV-2 proteins recognized by cervical T cell lines, including both CD4+ and CD8+ T cells, from HSV-2+ participants. This is similar to what we have previously reported in blood-derived HSV-2 specific CD8+ T cells from HSV-2+ participants (35, 36) and immune seronegative (IS) participants (37). Studying the antigenic repertoire of local T cell responses will be important for the rational design of prophylactic and therapeutic vaccines against HSV-2. Induction of local T cell immunity will be an essential feature of any HSV-2 vaccine; frequent cervical cytobrushing was well-tolerated by our study participants and may represent a viable strategy for assessing whether candidate HSV-2 vaccines elicit local adaptive immune responses. While the design of therapeutic vaccines may be informed by the study of HSV-infected participants with the lowest rates of mucosal HSV reactivation (“virus controllers”), strategies for the prevention of HSV infection require the elucidation of protective mechanisms employed by participants who resist infection, especially those who remain seronegative even after frequent exposure to HSV from infected sexual partners (37, 38). We are currently exploring whether local T cell responses are present in these HSVneg participants in order to assess whether these responses may be linked to protection from HSV infection at the genital mucosa.
The current study has utilized HSV-2 peptide pools representing 16 HSV-2 proteins commonly recognized by systemic and skin-derived T cells from HSV-2+ participants. HSV-2 encodes 80 open reading frames (ORF) and thus, the current study screens T cells directed at only 20% of the HSV-2 proteome. Dr. David Koelle has developed an elegant method to efficiently generate a genome-wide map of the responsiveness to HSV-1-specific T cells (39) and more recently, to HSV-2-specific T cells (unpublished data). This technology will provide a global analysis of both the CD4 and CD8 T cell response to HSV-2. It is likely to reveal novel T cell epitopes recognized by cervically-derived T cells and allow a precise comparison of the antigenic reactivity between T cells derived from the mucosa and blood.
In summary, this study assessed the frequency and antigenic profile of mucosal T cell responses to HSV-2 in the human female genital tract using non-specific in vitro polyclonally expanded cervical cytobrush-derived T cells from HSV-2 infected women. Local immunity at sites of HSV-2 exposure is likely to play an important role in preventing or controlling HSV reactivation or HSV infection and may inform the design of future HSV-2 vaccine strategies. Efficacious HSV-2 vaccines will require the elicitation of local T cell immunity and sampling the cervix for the detection of vaccine-induced T cells would be a feasible strategy and one of high priority to employ in future vaccine trials. While the study of polyclonally expanded T cells has provided valuable insight into the phenotype and antigenic profile of local T cell immunity to HSV-2, the study of local T cells ex vivo should provide mechanistic data that may lead to correlates of immune protection and novel strategies to suppress HSV-2 reactivation or prevent HSV-2 infection.
Methods
Study Population
Healthy HSV-1 and HSV-2 seronegative (HSVneg), HSV-1-seronegative/HSV-2 seropositive (HSV-1-/2+) and HSV-1-seropositive/HSV-2-seropositive (HSV-1+/2+) female participants were enrolled into IRB-approved protocols at the University of Washington Virology Research Clinic (VRC), Seattle, WA. All participants provided written informed consent. HSV Western blot to detect antibodies to HSV-1 and HSV-2 was performed as previously described (40, 41).
PBMC, DC, LCL and viruses
Peripheral blood mononuclear cells (PBMC) were isolated by Ficoll-Hypaque and cryopreserved within 8 hours of venipuncture as previously described (42). Dendritic cells (DC) were generated from PBMC using GM-CSF and IL-4 as previously described (35). Lymphoblastoid cell lines (LCL) were generated as previously described (43). HSV-1 strain E115 and HSV-2 strain 333 were used at an MOI of 10 or were UV-inactivated to use as viral antigen where indicated.
Cervical mucosal sample collection, processing and T cell expansion
Cervical cells were collected during speculum examination using a Cytobrush Plus® Cell Collector (Medscand, CA) inserted into the cervical os, rotated through 360° once, and immediately placed in 5 ml of cold transport medium RPMI-CVX (RPMI 1640 medium supplemented with 5mM l-glutamine, 50 U/ml penicillin, 50 μg/ml streptomycin, and 10% human serum, 10μl/ml amphotericin B, and 0.5 μl/ml ciprofloxacin). Each participant provided 2 cervical cytobrush samples spaced approximately one month apart; 5 HSV-2+ participants provided an additional cytobrush sample approximately 6-10 months post study entry. Cervical samples with visible red blood cell contamination were discarded. Under sterile conditions, cervical cells were isolated within 2 h of collection by gently vortexing the tube containing the cytobrush followed by rotating the cytobrush against the sides of the tube to dislodge cells. The transport medium was flushed through the cytobrush bristles and the cell suspension was passed through a 40 μM filter into a clean 50-ml centrifuge tube. Cells were pelleted at 250 × g for 10 min, resuspended in RPMI-CVX and counted. Cervical cells were cultured in 24-well plates (up to 2×106/well) along with 5×106 autologous irradiated PBMC, OKT3 (1μl in 25ml) and CD28 (0.5 μl/ml) (BD Biosciences, San Jose, CA) in a final volume of 2ml/well. IL-2 (50 U/ml) was added every 2-3 days in fresh RPMI-CVX.
Cervicovaginal lavage (CVL) fluid was obtained from each participant in order to measure HSV DNA by washing the cervical os and posterior vaginal wall with 10 ml of phosphate buffered saline (PBS, pH 7.0) as previously described (44). Samples were transported on ice and centrifuged at 1000 × g for 20 min at 4°C, aliquoted and stored at -80°C.
HSV-specific LP responses
Cervical T cell lines were washed twice and 1×105 cells were incubated in triplicate with 1×105 irradiated autologous PBMC as antigen presenting cells (APC) in 96-well round-bottomed plates. Cervical T cell lines were stimulated with 1:500 dilution of UV-inactivated (UV) HSV-1, UV-HSV-2, mock antigen (Ag) or purified PHA (0.4 μg/ml) (Murex, distributed by Remel, Lenexa, KS) as previously described (9). After 3 days, 1 μCi [3H]thymidine (New England Nuclear) was added to each well for 18 hrs and its incorporation into DNA was measured. Δcpm were calculated by subtracting cpm from mock Ag wells from cpm from UV-HSV wells. An LP response was considered positive if the Δcpm were >5,000 cpm.
Synthetic peptides and peptide pools
We selected 16 HSV-2 open reading frames (ORFs) for peptide synthesis as previously described (37). ORFs were mostly virion proteins (capsid, tegument, glycoprotein) or immediate-early (IE) proteins. Amino acid sequences were derived from the HSV-2 strain HG52 genome (GenBank accession no. NC-001798). Peptides were 15 amino acids long and overlapping by 11 amino acids and synthesized in crude form by either CBI/Mimotopes (San Diego, CA) (gD-2, ICP0, ICP4, ICP22, ICP27, UL39) or New England Peptide LLC (Gardner, MA) (UL19, UL25, UL35, UL46, UL47, UL49, UL11, UL27, UL29, US5) and lyophilized. Each peptide was dissolved in 10 mg/ml in sterile endotoxin-free DMSO (Sigma) and stored at 4°C. The mass of each peptide was approximately 4-5 mg. Control peptide pools included the CEF pool (CBI/Mimotopes) comprising immunodominant CD8+ T cell epitopes within CMV, EBV and influenza (37, 42, 45) and the CMV pp65 peptide mix containing 138 15 mers overlapping by 11 amino acids (Becton Dickinson, San Jose, CA).
Peptide pools (library and array) were prepared for each ORF by grouping peptides linearly across an ORF and were used to screen PBMC, cervical T cell lines or T cell clones. A total of 2,633 peptides were synthesized and grouped into pools composed of peptides ranging from 21-100 peptides/pool (median 85 peptides/pool) as previously described (37). Array pools, used to deconvolute library pools that were positive in the T cell screen, were prepared by arranging the peptides within a single library pool in a row-and-column format and pooling the peptides in each column or row.
IFN-γ ELISPOT
The production of IFN-γ by HSV-specific T cells obtained from PBMC or cervical T cell lines was analyzed by ELISPOT as previously described (37) with modifications. PBMC were thawed in R10 [RPMI 1640 supplemented with 10% FBS, 2 mM l-glutamine, 25 mM HEPES (N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid) buffer, 50 μg/ml streptomycin, 50 U/ml penicillin containing 50 U/ml benzonase (Novagen, Madison, WI)], and then washed and rested overnight in R10 at 37°C, 5% CO2 before assay. Cervical T cell lines were washed twice and counted. Using the IFN-γ ELISPOT kit (Mabtech, Cincinnati, OH) according to the manufacturer's instructions, PBMC were plated at 2×105 cells/well (no additional APC added) and cervical T cell lines were plated at 5×104 cells/well along with 5×104 irradiated autologous PBMC as APC. Peptides were added to the wells at a final concentration of 1 μg/ml for overnight stimulation. Wells containing R10 alone or R10 and DMSO served as negative controls, and those containing 1 μg/ml PHA served as positive controls. Negative controls were tested in 4 replicate wells, whereas HSV-2, CMV and CEF peptide pools and PHA were tested in duplicate wells. Spots were counted and analyzed using the automated Bioreader® (Biosys GmbH, Germany). Responses were considered positive if (1) the spot forming cells (SFC)/well were 4 times greater than the mean SFC in the 4 DMSO wells and (2) the SFC/well was ≥ 11 SFC/well (37, 46). Results are expressed as SFC per 106 cells.
ICS and flow cytometry
To determine the phenotype of HSV-2 peptide specific cervically-derived T cells, ICS and flow cytometry was performed using a 6-color ICS panel in a 96-well plate format modified from (47) as previously described (37). Briefly, in order to assess T cell responses to whole HSV-2, cervical T cell lines (0.5-1×106/well) were co-incubated with irradiated autologous LCL that were mock infected (negative control) or infected overnight with HSV-2 at a cell ratio of 10:1; in order to assess HSV-2 peptide-specific T cell responses, irradiated autologous LCL were co-incubated with HSV-2 peptide pools (1 μg/ml) or individual HSV-2 peptides (1 μg/ml). Controls included DMSO (negative control), SEB (positive control), CEF or CMV peptide pools. During the 6-hr incubation at 37°C, Brefeldin A (10 μg/ml, Sigma, St. Louis, MO) and the co-stimulatory antibodies CD28 and CD49d (each at 1 μg/ml, BD Biosciences) were included. Antibodies CD4-FITC, CD8 PerCP-Cy5.5, IFN-γ APC, and IL-2 PE were purchased from BD Biosciences, CD3 ECD was purchased from Beckman-Coulter (Marseille, France) and the LIVE/DEAD Fixable Violet Dead Cell Stain was purchased from Invitrogen/Molecular Probes (Eugene, OR). Samples were collected from 96-well plates using High Throughput Sample (HTS, BD) device for analysis by the LSRII and all FACS analyses were performed using FlowJo® software (Treestar, Inc., OR). To be considered positive, IFN-γ or IL-2 production needed to exceed background by a factor of at least two (48).
Measurement of HSV DNA in cervical secretions
CVL fluid collected at both clinic visits from HSVneg and HSV-2+ women was evaluated for HSV DNA by quantitative, real-time, fluorescence-based PCR as described (49).
Statistical analysis
The Wilcoxon Matched-Pair Signed Ranks test was employed to determine the differences between compartments. Statistical significance was defined as P<0.05 with 2-tailed α.
Acknowledgments
This study was supported by the National Institute of Allergy and Infectious Diseases, National Institutes of Health, grants R21 AI-083418 and R01 AI-091701 to C. Posavad, P01 AI-030731 to A. Wald and L. Corey. We thank Dr. Amalia Magaret for valuable advice regarding statistical analyses, Dr. Betsy Herold for sharing her expertise related to the collection of CVL, Michael Remington for sample collection, Kaile Ross and Emma Robinson for participant scheduling, Stacy Selke for clinical data management, Cindy Hirano and Elizabeth Morrigan for assistance with Institutional Review Board protocols, Kerry Laing for supplying some HSV-2 peptide pools, and Anna Rashevsky for performing the HSV PCR.
AW has received research funding from Gilead, Agenus, Genocea and Vical and has been a consultant for Aicuris.
Footnotes
Conflict of Interest Statement: CMP and LC are coinventors on several patents involving potential HSV vaccine candidates. LC is on the scientific advisory board for and holds stock (<1% of company) in Immune Design Corp.
References
- 1.Freeman EE, Weiss HA, Glynn JR, Cross PL, Whitworth JA, Hayes RJ. Herpes simplex virus 2 infection increases HIV acquisition in men and women: systematic review and meta-analysis of longitudinal studies. AIDS. 2006;20(1):73–83. doi: 10.1097/01.aids.0000198081.09337.a7. [DOI] [PubMed] [Google Scholar]
- 2.Xu F, Sternberg MR, Kottiri BJ, McQuillan GM, Lee FK, Nahmias AJ, et al. Trends in herpes simplex virus type 1 and type 2 seroprevalence in the United States. JAMA. 2006;296(8):964–73. doi: 10.1001/jama.296.8.964. [DOI] [PubMed] [Google Scholar]
- 3.Gupta R, Warren T, Wald A. Genital herpes. Lancet. 2007;370(9605):2127–37. doi: 10.1016/S0140-6736(07)61908-4. [DOI] [PubMed] [Google Scholar]
- 4.Corey L, Langenberg AG, Ashley R, Sekulovich RE, Izu AE, Douglas JM, Jr, et al. Recombinant glycoprotein vaccine for the prevention of genital HSV-2 infection: two randomized controlled trials. Chiron HSV Vaccine Study Group. JAMA. 1999;282(4):331–40. doi: 10.1001/jama.282.4.331. [DOI] [PubMed] [Google Scholar]
- 5.Stanberry LR, Spruance SL, Cunningham AL, Bernstein DI, Mindel A, Sacks S, et al. Glycoprotein-D-adjuvant vaccine to prevent genital herpes. N Engl J Med. 2002;347(21):1652–61. doi: 10.1056/NEJMoa011915. [DOI] [PubMed] [Google Scholar]
- 6.Johnston C, Koelle DM, Wald A. HSV-2: in pursuit of a vaccine. J Clin Invest. 2011;121(12):4600–9. doi: 10.1172/JCI57148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Johnston C, Saracino M, Kuntz S, Magaret A, Selke S, Huang ML, et al. Standard-dose and high-dose daily antiviral therapy for short episodes of genital HSV-2 reactivation: three randomised, open-label, cross-over trials. Lancet. 2012;379(9816):641–7. doi: 10.1016/S0140-6736(11)61750-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bagdades EK, Pillay D, Squire SB, O'Neil C, Johnson MA, Griffiths PD. Relationship between herpes simplex virus ulceration and CD4+ cell counts in patients with HIV infection. AIDS. 1992;6(11):1317–20. doi: 10.1097/00002030-199211000-00012. [DOI] [PubMed] [Google Scholar]
- 9.Posavad CM, Koelle DM, Shaughnessy MF, Corey L. Severe genital herpes infections in HIV-infected individuals with impaired herpes simplex virus-specific CD8+ cytotoxic T lymphocyte responses. Proc Natl Acad Sci U S A. 1997;94(19):10289–94. doi: 10.1073/pnas.94.19.10289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Posavad CM, Wald A, Kuntz S, Huang ML, Selke S, Krantz E, et al. Frequent reactivation of herpes simplex virus among HIV-1-infected patients treated with highly active antiretroviral therapy. J Infect Dis. 2004;190(4):693–6. doi: 10.1086/422755. [DOI] [PubMed] [Google Scholar]
- 11.Koelle DM, Posavad CM, Barnum GR, Johnson ML, Frank JM, Corey L. Clearance of HSV-2 from recurrent genital lesions correlates with infiltration of HSV-specific cytotoxic T lymphocytes. J Clin Invest. 1998;101(7):1500–8. doi: 10.1172/JCI1758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Koelle DM, Abbo H, Peck A, Ziegweid K, Corey L. Direct recovery of herpes simplex virus (HSV)-specific T lymphocyte clones from recurrent genital HSV-2 lesions. J Infect Dis. 1994;169(5):956–61. doi: 10.1093/infdis/169.5.956. [DOI] [PubMed] [Google Scholar]
- 13.Posavad CM, Huang ML, Barcy S, Koelle DM, Corey L. Long term persistence of herpes simplex virus-specific CD8+ CTL in persons with frequently recurring genital herpes. J Immunol. 2000;165(2):1146–52. doi: 10.4049/jimmunol.165.2.1146. [DOI] [PubMed] [Google Scholar]
- 14.Koelle DM, Chen HB, Gavin MA, Wald A, Kwok WW, Corey L. CD8 CTL from genital herpes simplex lesions: recognition of viral tegument and immediate early proteins and lysis of infected cutaneous cells. J Immunol. 2001;166(6):4049–58. doi: 10.4049/jimmunol.166.6.4049. [DOI] [PubMed] [Google Scholar]
- 15.Zhu J, Koelle DM, Cao J, Vazquez J, Huang ML, Hladik F, et al. Virus-specific CD8+ T cells accumulate near sensory nerve endings in genital skin during subclinical HSV-2 reactivation. J Exp Med. 2007;204(3):595–603. doi: 10.1084/jem.20061792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Peng T, Zhu J, Phasouk K, Koelle DM, Wald A, Corey L. An effector phenotype of CD8+ T cells at the junction epithelium during clinical quiescence of herpes simplex virus 2 infection. J Virol. 2012;86(19):10587–96. doi: 10.1128/JVI.01237-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhu J, Peng T, Johnston C, Phasouk K, Kask AS, Klock A, et al. Immune surveillance by CD8alphaalpha+ skin-resident T cells in human herpes virus infection. Nature. 2013;497(7450):494–7. doi: 10.1038/nature12110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schiffer JT, Abu-Raddad L, Mark KE, Zhu J, Selke S, Koelle DM, et al. Mucosal host immune response predicts the severity and duration of herpes simplex virus-2 genital tract shedding episodes. Proc Natl Acad Sci U S A. 2010;107(44):18973–8. doi: 10.1073/pnas.1006614107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ouwendijk WJ, Laing KJ, Verjans GM, Koelle DM. T-cell immunity to human alphaherpesviruses. Curr Opin Virol. 2013 doi: 10.1016/j.coviro.2013.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Koelle DM, Schomogyi M, Corey L. Antigen-specific T cells localize to the uterine cervix in women with genital herpes simplex virus type 2 infection. J Infect Dis. 2000;182(3):662–70. doi: 10.1086/315749. [DOI] [PubMed] [Google Scholar]
- 21.Sathaliyawala T, Kubota M, Yudanin N, Turner D, Camp P, Thome JJ, et al. Distribution and compartmentalization of human circulating and tissue-resident memory T cell subsets. Immunity. 2013;38(1):187–97. doi: 10.1016/j.immuni.2012.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Johansson EL, Rudin A, Wassen L, Holmgren J. Distribution of lymphocytes and adhesion molecules in human cervix and vagina. Immunology. 1999;96(2):272–7. doi: 10.1046/j.1365-2567.1999.00675.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kyongo JK, Jespers V, Goovaerts O, Michiels J, Menten J, Fichorova RN, et al. Searching for lower female genital tract soluble and cellular biomarkers: defining levels and predictors in a cohort of healthy Caucasian women. PLOS One. 2012;7(8):e43951. doi: 10.1371/journal.pone.0043951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.McKinnon LR, Hughes SM, De Rosa SC, Martinson JA, Plants J, Brady KE, et al. Optimizing viable leukocyte sampling from the female genital tract for clinical trials: an international multi-site study. PLOS One. 2014;9(1):e85675. doi: 10.1371/journal.pone.0085675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Levine WC, Pope V, Bhoomkar A, Tambe P, Lewis JS, Zaidi AA, et al. Increase in endocervical CD4 lymphocytes among women with nonulcerative sexually transmitted diseases. J Infect Dis. 1998;177(1):167–74. doi: 10.1086/513820. [DOI] [PubMed] [Google Scholar]
- 26.Bere A, Denny L, Hanekom W, Burgers WA, Passmore JA. Comparison of polyclonal expansion methods to improve the recovery of cervical cytobrush-derived T cells from the female genital tract of HIV-infected women. J Immunol Methods. 354(1-2):68–79. doi: 10.1016/j.jim.2010.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Martinelli E, Tharinger H, Frank I, Arthos J, Piatak M, Jr, Lifson JD, et al. HSV-2 infection of dendritic cells amplifies a highly susceptible HIV-1 cell target. PLoS Pathog. 2011;7(6):e1002109. doi: 10.1371/journal.ppat.1002109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.McKinnon LR, Kaul R. Quality and quantity: mucosal CD4+ T cells and HIV susceptibility. Curr Opin HIV AIDS. 2012;7(2):195–202. doi: 10.1097/COH.0b013e3283504941. [DOI] [PubMed] [Google Scholar]
- 29.Rebbapragada A, Wachihi C, Pettengell C, Sunderji S, Huibner S, Jaoko W, et al. Negative mucosal synergy between Herpes simplex type 2 and HIV in the female genital tract. AIDS. 2007;21(5):589–98. doi: 10.1097/QAD.0b013e328012b896. [DOI] [PubMed] [Google Scholar]
- 30.Tanton C, Weiss HA, LeGoff J, Changalucha J, Clayton TC, Ross DA, et al. Patterns of herpes simplex virus shedding over 1 month and the impact of acyclovir and HIV in HSV-2-seropositive women in Tanzania. Sex Transm Infect. 2011;87(5):406–11. doi: 10.1136/sti.2010.048496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Aumakhan B, Hardick A, Quinn TC, Laeyendecker O, Gange SJ, Beyrer C, et al. Genital herpes evaluation by quantitative TaqMan PCR: correlating single detection and quantity of HSV-2 DNA in cervicovaginal lavage fluids with cross-sectional and longitudinal clinical data. Virol J. 2010;7:328. doi: 10.1186/1743-422X-7-328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Keller MJ, Madan RP, Shust G, Carpenter CA, Torres NM, Cho S, et al. Changes in the soluble mucosal immune environment during genital herpes outbreaks. J Acquir Immune Defic Syndr. 2012;61(2):194–202. doi: 10.1097/QAI.0b013e31826867ae. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bere A, Denny L, Burgers WA, Passmore JA. Polyclonal expansion of cervical cytobrush-derived T cells to investigate HIV-specific responses in the female genital tract. Immunology. 2010;130(1):23–33. doi: 10.1111/j.1365-2567.2009.03172.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bere A, Denny L, Hanekom W, Burgers WA, Passmore JA. Comparison of polyclonal expansion methods to improve the recovery of cervical cytobrush-derived T cells from the female genital tract of HIV-infected women. J Immunol Methods. 2010;354(1-2):68–79. doi: 10.1016/j.jim.2010.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hosken N, McGowan P, Meier A, Koelle DM, Sleath P, Wagener F, et al. Diversity of the CD8+ T-cell response to herpes simplex virus type 2 proteins among persons with genital herpes. J Virol. 2006;80(11):5509–15. doi: 10.1128/JVI.02659-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Laing KJ, Magaret AS, Mueller DE, Zhao L, Johnston C, De Rosa SC, et al. Diversity in CD8(+) T cell function and epitope breadth among persons with genital herpes. J Clin Immunol. 2010;30(5):703–22. doi: 10.1007/s10875-010-9441-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Posavad CM, Remington M, Mueller DE, Zhao L, Magaret AS, Wald A, et al. Detailed characterization of T cell responses to herpes simplex virus-2 in immune seronegative persons. J Immunol. 2010;184(6):3250–9. doi: 10.4049/jimmunol.0900722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Posavad CM, Wald A, Hosken N, Huang ML, Koelle DM, Ashley RL, et al. T cell immunity to herpes simplex viruses in seronegative subjects: silent infection or acquired immunity? J Immunol. 2003;170(8):4380–8. doi: 10.4049/jimmunol.170.8.4380. [DOI] [PubMed] [Google Scholar]
- 39.Jing L, Haas J, Chong TM, Bruckner JJ, Dann GC, Dong L, et al. Cross-presentation and genome-wide screening reveal candidate T cells antigens for a herpes simplex virus type 1 vaccine. J Clin Invest. 2012;122(2):654–73. doi: 10.1172/JCI60556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Morrow RA, Friedrich D. Inaccuracy of certain commercial enzyme immunoassays in diagnosing genital infections with herpes simplex virus types 1 or 2. Am J Clin Pathol. 2003;120(6):839–44. doi: 10.1309/AKWH-F62Y-YJ05-4HA6. [DOI] [PubMed] [Google Scholar]
- 41.Morrow RA, Friedrich D, Meier A, Corey L. Use of “biokit HSV-2 Rapid Assay” to improve the positive predictive value of Focus HerpeSelect HSV-2 ELISA. BMC Infect Dis. 2005;5:84. doi: 10.1186/1471-2334-5-84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bull M, Lee D, Stucky J, Chiu YL, Rubin A, Horton H, et al. Defining blood processing parameters for optimal detection of cryopreserved antigen-specific responses for HIV vaccine trials. J Immunol Methods. 2007;322(1-2):57–69. doi: 10.1016/j.jim.2007.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Posavad CM, Rosenthal KL. Herpes simplex virus-infected fibroblasts are resistant to and inhibit cytotoxic T-lymphoyte activity. J Virol. 1992;66(11):6264–72. doi: 10.1128/jvi.66.11.6264-6272.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.John M, Keller MJ, Fam EH, Cheshenko N, Hogarty K, Kasowitz A, et al. Cervicovaginal secretions contribute to innate resistance to herpes simplex virus infection. J Infect Dis. 2005;192(10):1731–40. doi: 10.1086/497168. [DOI] [PubMed] [Google Scholar]
- 45.Currier JR, Kuta EG, Turk E, Earhart LB, Loomis-Price L, Janetzki S, et al. A panel of MHC class I restricted viral peptides for use as a quality control for vaccine trial ELISPOT assays. J Immunol Methods. 2002;260(1-2):157–72. doi: 10.1016/s0022-1759(01)00535-x. [DOI] [PubMed] [Google Scholar]
- 46.Moodie Z, Huang Y, Gu L, Hural J, Self SG. Statistical positivity criteria for the analysis of ELISpot assay data in HIV-1 vaccine trials. J Immunol Methods. 2006;315(1-2):121–32. doi: 10.1016/j.jim.2006.07.015. [DOI] [PubMed] [Google Scholar]
- 47.Horton H, Thomas EP, Stucky JA, Frank I, Moodie Z, Huang Y, et al. Optimization and validation of an 8-color intracellular cytokine staining (ICS) assay to quantify antigen-specific T cells induced by vaccination. J Immunol Methods. 2007;323(1):39–54. doi: 10.1016/j.jim.2007.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kaul R, Thottingal P, Kimani J, Kiama P, Waigwa CW, Bwayo JJ, et al. Quantitative ex vivo analysis of functional virus-specific CD8 T lymphocytes in the blood and genital tract of HIV-infected women. AIDS. 2003;17(8):1139–44. doi: 10.1097/00002030-200305230-00004. [DOI] [PubMed] [Google Scholar]
- 49.Jerome KR, Huang ML, Wald A, Selke S, Corey L. Quantitative stability of DNA after extended storage of clinical specimens as determined by real-time PCR. J Clin Microbiol. 2002;40(7):2609–11. doi: 10.1128/JCM.40.7.2609-2611.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]