Abstract
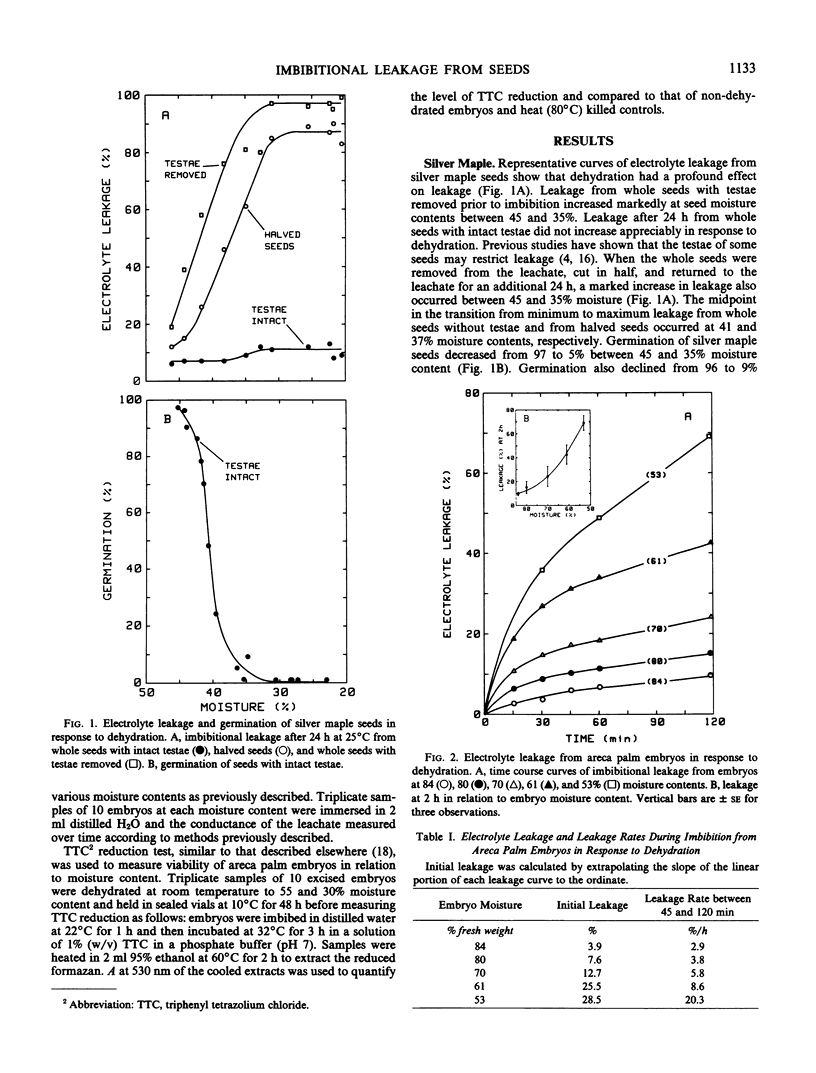
Changes in electrolyte leakage and viability in response to dehydration stress were examined in two species of seeds that do not survive desiccation. Leakage from silver maple (Acer saccharinum L.) seeds increased markedly as seed moisture contents decreased from 45 to 35% (fresh weight basis) and germination decreased from 97 to 5%, coincidentally. Time course curves of imbibitional leakage from areca palm (Chrysalido-carpus lutescens [Bory] Wendl.) embryos showed an increase in both initial leakage and steady-state leakage rates in response to dehydration from an original moisture content of 84 to as low as 53%. Absorbance at 530 nanometers of extracts from triphenyl tetrazolium chloride stained embryos of areca palm was used as a measure of viability. Absorbance decreased significantly in response to dehydration as embryo moisture content decreased from 80 to 30%. Collectively, the data suggest that membranes in the desiccation-sensitive seed tissues studied are damaged by dehydration below a critical moisture content, 40% in silver maple seed and 55% in areca palm embryos, and that the membrane damage contributes to loss of viability.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bramlage W. J., Leopold A. C., Parrish D. J. Chilling Stress to Soybeans during Imhibition. Plant Physiol. 1978 Apr;61(4):525–529. doi: 10.1104/pp.61.4.525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duke S. H., Kakefuda G. Role of the testa in preventing cellular rupture during imbibition of legume seeds. Plant Physiol. 1981 Mar;67(3):449–456. doi: 10.1104/pp.67.3.449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graziani Y., Livne A. Dehydration, water fluxes, and permeability of tobacco leaf tissue. Plant Physiol. 1971 Nov;48(5):575–579. doi: 10.1104/pp.48.5.575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larson L. A. The effect soaking pea seeds with or without seedcoats has on seedling growth. Plant Physiol. 1968 Feb;43(2):255–259. doi: 10.1104/pp.43.2.255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leopold A. C., Musgrave M. E., Williams K. M. Solute leakage resulting from leaf desiccation. Plant Physiol. 1981 Dec;68(6):1222–1225. doi: 10.1104/pp.68.6.1222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leopold A. C. Temperature effects on soybean imbibition and leakage. Plant Physiol. 1980 Jun;65(6):1096–1098. doi: 10.1104/pp.65.6.1096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKersie B. D., Stinson R. H. Effect of Dehydration on Leakage and Membrane Structure in Lotus corniculatus L. Seeds. Plant Physiol. 1980 Aug;66(2):316–320. doi: 10.1104/pp.66.2.316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parrish D. J., Leopold A. C. On the mechanism of aging in soybean seeds. Plant Physiol. 1978 Mar;61(3):365–368. doi: 10.1104/pp.61.3.365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parrish D. J., Leopold A. C. Transient changes during soybean imbibition. Plant Physiol. 1977 Jun;59(6):1111–1115. doi: 10.1104/pp.59.6.1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steponkus P. L., Lanphear F. O. Refinement of the triphenyl tetrazolium chloride method of determining cold injury. Plant Physiol. 1967 Oct;42(10):1423–1426. doi: 10.1104/pp.42.10.1423. [DOI] [PMC free article] [PubMed] [Google Scholar]



