Abstract
Background
Several QTLs have been identified for major economically relevant traits in livestock, such as growth and meat quality, revealing the complex genetic architecture of these traits. The use of network approaches considering the interactions of multiple molecules and traits provides useful insights into the molecular underpinnings of complex traits. Here, a network based methodology, named Association Weight Matrix, was applied to study gene interactions and pathways affecting pig conformation, growth and fatness traits.
Results
The co-association network analysis underpinned three transcription factors, PPARγ, ELF1, and PRDM16 involved in mesoderm tissue differentiation. Fifty-four genes in the network belonged to growth-related ontologies and 46 of them were common with a similar study for growth in cattle supporting our results. The functional analysis uncovered the lipid metabolism and the corticotrophin and gonadotrophin release hormone pathways among the most important pathways influencing these traits. Our results suggest that the genes and pathways here identified are important determining either the total body weight of the animal and the fat content. For instance, a switch in the mesoderm tissue differentiation may determinate the age-related preferred pathways being in the puberty stage those related with the miogenic and osteogenic lineages; on the contrary, in the maturity stage cells may be more prone to the adipocyte fate. Hence, our results demonstrate that an integrative genomic co-association analysis is a powerful approach for identifying new connections and interactions among genes.
Conclusions
This work provides insights about pathways and key regulators which may be important determining the animal growth, conformation and body proportions and fatness traits. Molecular information concerning genes and pathways here described may be crucial for the improvement of genetic breeding programs applied to pork meat production.
Introduction
About 43% of the meat consumed worldwide proceeds from pigs, thus representing the major source of meat for human food intake [1]. Moreover, pig serves as a model for metabolic diseases such as obesity in humans [2], [3]. For meat industry, carcass conformation and growth are economically important traits, determining the proportions of the different commercial cuts [4]. Understanding the interactions between genes defining body growth and conformation of pigs is therefore critical for an efficient pig production.
Over 553 quantitative trait loci (QTLs) for growth-related traits have been reported in pigs [http://www.animalgenome.org/cgi-bin/QTLdb/SS/index]. Moreover, a genome wide linkage analysis for growth and body composition carried out in an Iberian × Landrace cross (IBMAP) confirmed previous QTL regions and identified new ones in 10 of the 18 autosomes [5]. Despite the large number of QTLs identified by QTL scan and Genome-Wide Association Studies (GWAS) the genetic architecture of these complex traits is far from being understood [6]. The detection of SNPs having a clear effect on complex traits using GWAS is limiting, still being a challenging task. The main reason is because many genes have a little effect, moreover, the need for multiple tests correction methods may result in removing some interesting SNPs [7]. The power of single trait GWAS can be enhanced when considering simultaneously multiple phenotypes because complex traits generally have multiple correlated traits [7].
Hence, for complex traits, a systems biology approach that integrates the results into coherent network models offers many advantages over single trait approaches [8]. Recently, a framework for integrating the information of GWAS with network inference algorithms, named Association Weight Matrix (AWM), was developed to reveal and identify key regulatory elements, provide in silico information and generate gene networks with the aim to better understand the regulatory mechanisms of complex traits [9], [10]. However, few studies have been performed to date using system biology approaches and genotypic data in livestock species [9], [11]–[15].
Network biology approaches may substantially improve our knowledge about the diverse molecular pathways underlying complex traits. Using this methodology, the main objective of this work was to identify key regulators, gene interactions and pathways determining pig growth and conformation traits in order to improve our knowledge about the architecture of these complex traits.
Results and Discussion
Global growth network description and trait cluster analysis
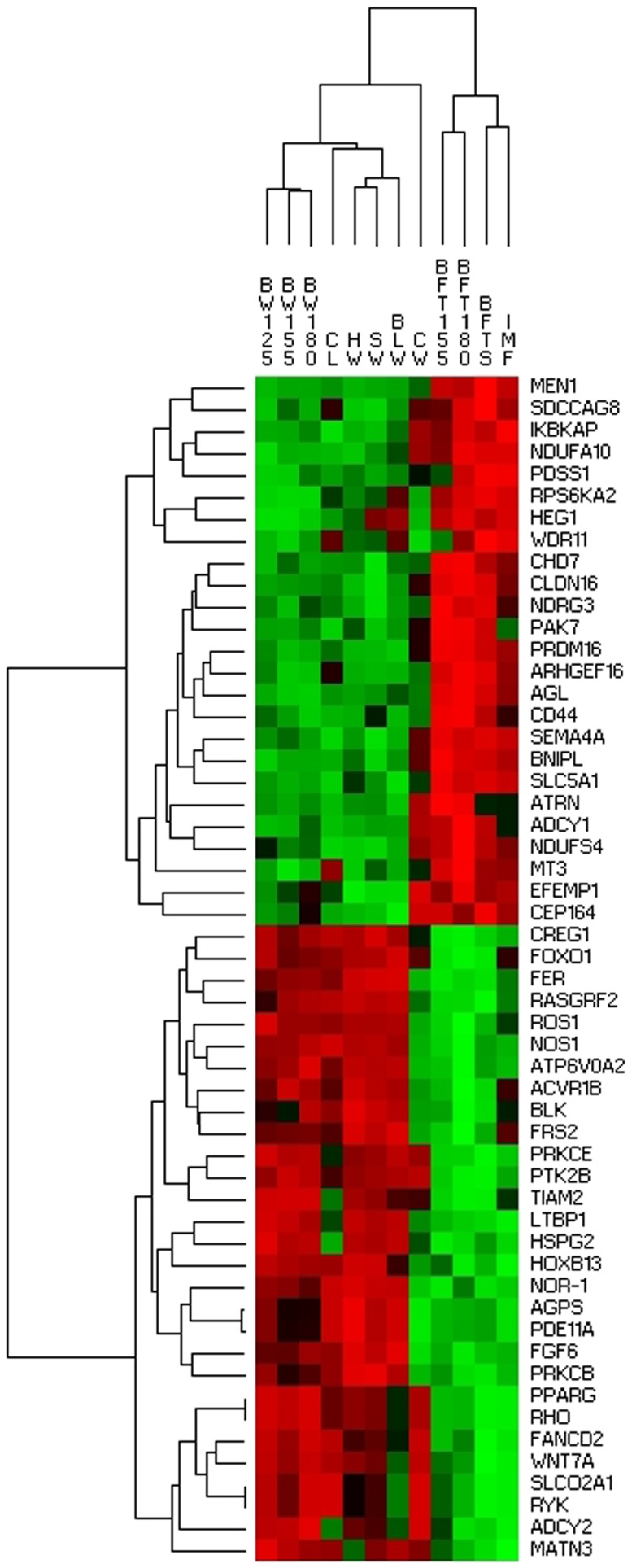
In the present study, we used a systems biology approach considering 12 growth-related phenotypes (Table 1). Given that primary cuts have economic impact in the Iberian pig production [16], ham weight was considered as the key trait for the AWM analysis. Among the genotypes of the 60K SNPs Porcine Beadchip, a total of 41,279 SNPs were retained for further analysis. Single-trait-single-SNP analysis by GWAS was performed for all traits (S1 Figure). The AWM approach captured a total of 1,747 annotated genes proximal to co-associated SNPs for conformation, growth and fatness traits. Therefore, an AWM with 1,747 nodes, representing genes, and a total of 316,166 edges, which account for the predicted interactions, was built (Fig. 1A). Interestingly, in the hierarchical cluster analysis two groups of phenotypic traits were formed showing a clear opposite directionality of the additive values. The first one containing the fatness traits (BFT155, BFT180, BFTS and IMF), whereas the second one encompassing the growth and conformation related traits (BW125, BW155, BW180, HW, SW, BLW, CW and CL) (S2 Figure). Additionally, a second cluster analysis considering only 54 genes (S1 Table) of the network which are known to be related with growth was performed, showing again, after clustering, two different groups for the additive values of growth and fatness traits (Fig. 2).
Table 1. Phenotypic traits registered in the BC1_LD (F1× Landrace) and in the BC (F2× Landrace) and F3 generations of the Iberian × Landrace cross.
| Trait | Abbreviation | Statistics | ||
| N | Mean | SD | ||
| Body weight at 125 days (kg) | BW125 | 270 | 58.11 | 8.71 |
| Body weight at 155 days (kg) | BW155 | 269 | 80.74 | 13.61 |
| Body weight at 180 days (kg) | BW180 | 269 | 100.10 | 14.91 |
| Carcass weight (kg) | CW | 271 | 74.46 | 11.07 |
| Carcass length (cm) | CL | 261 | 81.86 | 6.23 |
| Backfat thickness at 155 days (mm) | BFT155 | 269 | 13.34 | 3.08 |
| Backfat thickness at 180 days (mm) | BFT180 | 220 | 15.60 | 3.21 |
| Backfat thickness at slaughter (mm) | BFTS | 237 | 23.26 | 6.13 |
| Intramuscular fat percentage (%) | IMF | 247 | 1.52 | 0.78 |
| Weight of hams (kg) | HW | 271 | 21.62 | 3.39 |
| Weight of shoulders (kg) | SW | 271 | 10.04 | 1.72 |
| Weight of belly (kg) | BLW | 276 | 7.33 | 1.14 |
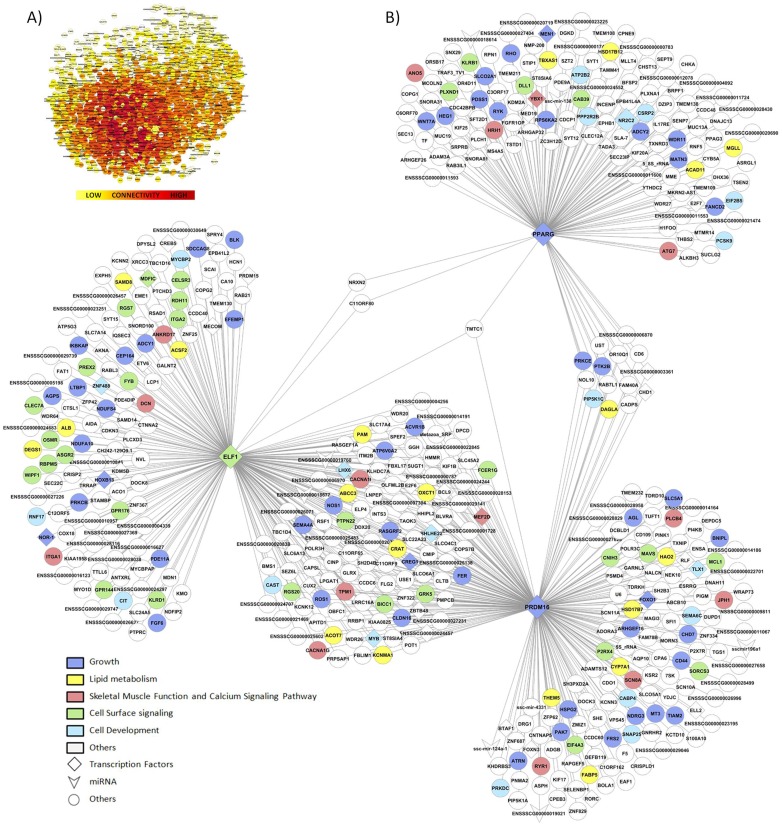
Figure 1. Co-association networks based on the AWM approach.
A) Full network formed by 1,747 nodes, representing genes and SNPs, and a total of 316,166 edges, accounting for the interactions among them. B) Network formed by 513 nodes and 639 edges representing genes and interactions among the top trio of transcription factors. Colours corresponded to different functions according to the legend.
Figure 2. Hierarchical cluster analysis considering only those genes in the network related with growth (S1 Table) among 12 phenotypic traits.
The green colour in the figure corresponds to negative SNP additive effect values and red to positive SNP additive effect values.
Next, in order to simplify and visualize the data with Cytoscape software, the number of interactions was reduced by selecting only the strongest co-associations, major than 0.86 (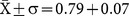 ). The resulting network had 53,200 predicted interactions and 1,703 genes.
). The resulting network had 53,200 predicted interactions and 1,703 genes.
Key transcription factors regulating growth traits
Within the 1,703 associated-genes, a total of 142 putative regulators (S2 Table) were identified. After exploring all the possible interconnected trios among regulators, the top trio which spanned most of the network topology with highest connectivity (a total of 26,160 connections) and minimum redundancy was formed by the Peroxisome Proliferator-Activated Receptor Gamma (PPARγ; PPARγDeg = 147), the E74-Like Factor 1 (Ets Domain Transcription Factor) (ELF1; ELF1Deg = 237), and the PR Domain Containing 16 (PRDM16; PRDM16Deg = 256) genes. In the resulting network, there were a total of 639 co-associations with the top trio of TF connecting 513 genes (Fig. 1B). Interestingly, ELF1 localized in a QTL on SSC11 identified for growth and body composition traits in the IBMAP cross [5], whereas no QTL was identified on SSC6 and SSC13 regions where the two other TF, PRDM16 and PPARG, were located. This result supports that the network methodology allowed the detection of potential variations affecting the analyzed traits that would have not been detected by using single-trait based approaches (Table 2).
Table 2. Additive effect and p-value of the SNPs representing the top trio of transcription factors.
| Gene | PRDM16 | ELF1 | PPARG | |||
| Representative SNP | MARC0030882 | MARC0000451 | ISU10000701 | |||
| Trait | Additive Effect | P-value | Additive Effect | P-value | Additive Effect | P-value |
| BW125 | −1.564 | 9.70E–02 | 1.767 | 2.89E–02 | 3.261 | 4.74E–05 |
| BW155 | −2.933 | 1.90E–02 | 2.217 | 4.09E–02 | 3.983 | 2.45E–04 |
| BW180 | −3.115 | 3.08E–02 | 1.762 | 1.59E–01 | 5.329 | 1.41E–05 |
| CW | −0.734 | 5.23E–01 | –0.008 | 9.95E–01 | 3.338 | 5.37E–04 |
| CL | −0.534 | 1.28E–01 | 0.589 | 6.15E–02 | 0.758 | 1.26E–02 |
| BFT155 | 0.630 | 4.78E–02 | −0.011 | 1.00E+00 | 0.148 | 5.65E–01 |
| BFT180 | 0.581 | 6.35E–02 | −0.297 | 2.99E–01 | 0.161 | 5.54E–01 |
| BFTS | 0.666 | 3.38E–01 | −0.526 | 3.46E–01 | −0.537 | 3.73E–01 |
| IMF | 0.010 | 8.81E–01 | −0.036 | 5.39E–01 | −0.054 | 4.17E–01 |
| HW | −0.580 | 1.16E–02 | 0.586 | 3.25E–03 | 0.546 | 6.01E–03 |
| SW | −0.282 | 1.27E–02 | 0.229 | 2.19E–02 | 0.249 | 8.74E–03 |
| BLW | −0.250 | 7.57E–03 | 0.174 | 3.24E–02 | 0.147 | 7.15E–02 |
In the network, PPARγ gene, which is a key regulator of adipocyte differentiation, glucose homeostasis and fatty acid metabolism, was highly connected presenting 147 co-associations with other genes. This gene plays a role in determining the energy balance and the fat deposition influencing growth and body size [17], [18]. Furthermore, PPARγ has been associated with obesity, diabetes and atherosclerosis [19], and it has been identified, using the same methodology, as a key transcription factor regulating cattle puberty-related traits [9]. Interestingly, in another study of our group, PPARγ was identified as over-expressed in pigs having more MUFA and SFA versus pigs with high PUFA content [20]. Other studies in pigs suggested that PPARγ is an excellent target for determining growth and fat deposition traits at a certain age in pigs [21], [22].
On the other hand, ELF1 gene is a major regulator of haematopoiesis and energy metabolism [23]. ELF1 has also been described to trigger the NF-κB pathway activation involved in cell growth and differentiation and in lipid metabolism [24], [25]. Noteworthy, other members of the ELF1 gene family (ETS transcription regulator family) are known to regulate adipocyte and osteoblast differentiation [26]. Remarkably, FOXP3 which interacts with ELF1 has been identified as a central transcription factor regulating IMF in cattle using the same methodology [13], [27].
Finally, PRDM16 gene is involved in the differentiation of the brown adipose tissue, specifically in the switch between myogenic and adipogenic lineages [28]. PRDM16 has been reported to control the myogenic cell fate into brown fat cells in mice, however, pigs lack in brown fat tissue [28], [29]. PRDM16 can also function by triggering nervous and haematopoietic systems and participates in the regulation of the oxidative stress [30].
The embryonic mesoderm is a multipotent tissue that differentiates into myocytes, osteocytes and adipocytes [26]. The three top TF identified in the network have in common that are key regulators of the mesoderm cell fate. For instance, the over-expression of PPARγ may activate adipogenesis, ELF1 may regulate adipocyte and osteoblast differentiation, meanwhile PRDM16 may trigger the switch between adipose tissue and myocytes [28].
Selecting both miRNAs and TF as putative regulators did not affect the results, being the same top trio of genes identified as key regulators. In fact, inferring transcriptional and miRNA-mediated regulatory networks is still a challenge, particularly in non-model species such as the pig where the miRNA annotation is poor when compared to human or cow [31].
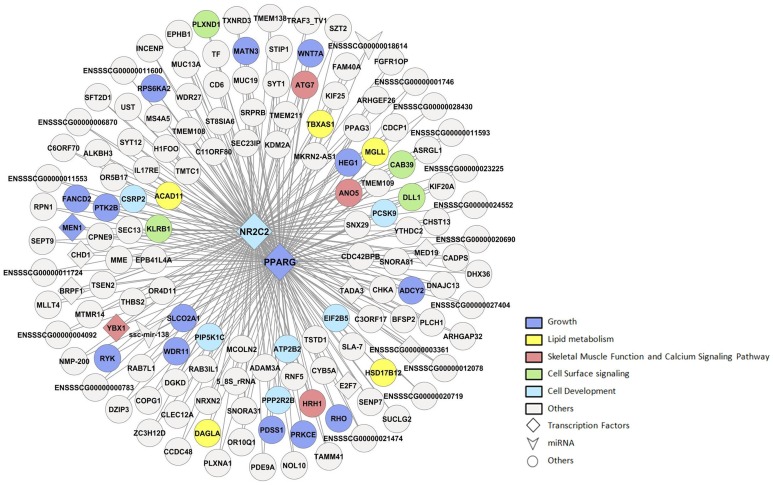
Additionally, a limitation of the AWM methodology is that only the nearest gene to the significant co-associated SNP is selected, discarding all other proximal genes. Linkage disequilibrium (LD) between molecular markers has to be taken into account for the AWM analysis. For instance, after exploring the network in more detail, a high co-association was observed between Nuclear Receptor Subfamily 2, Group C, Member 2 (NR2C2) and PPARγ sharing the same co-associated nodes (Fig. 3). The strong relationship between NR2C2 and PPARγ is supported by the literature, being NR2C2 a repressor of PPARγ activity [32]. Interestingly, a deficiency of NR2C2, which has been suggested to play a critical role in the regulation of energy and lipid homeostasis, in mice causes growth retardation [32], [33]. Remarkably, NR2C2 was also identified as co-associated in cattle growth network [12] and also in a network for fatness traits in cattle [13] and pig [11]. However, SNPs proximal to these genes (PPARγ and NR2C2) in our AWM network were separated by 1.27 Mb, being in complete LD (D′ = 1) (S3 Figure). Accordingly, LD can be a limitation to rule out which of these two genes play a key role regulating growth traits or if both genes are biologically relevant.
Figure 3. Network showing the shared-genes of NR2C2 and PPARγ.
Co-association network among the top TF
The parameters describing the network topology were calculated with CentiScaPe software, obtaining an average degree (Deg) of 62.44 and an average distance (AvDG) of 3.21; hence, showing a high degree of connection. A total of 54 genes out of the 513 nodes (Fig. 1B; blue colour in the network) belonged to growth-related gene ontologies (S1 Table). Noteworthy, a total of 20 genes were related to lipid metabolism (yellow colour in Fig. 1B). Among the 513 nodes setting up network connections, seven genes (COPS7B, EFEMP1, ETV6, FRS2, HSPG2, SH3PXD2A and TGS1) had been associated with human height, which is driven by growth and developmental processes [34], [35]. Interestingly, the indian hedgehog (IHH) gene product, identified in a human height GWAS study, binds to the patched domain containing 3 (PTCHD3) receptor, here identified as co-associated with ELF1 [36]. In addition, 46 of the 513 genes were common with a study for cattle growth trait using the same methodology (GRK5, NDRG3, RYK, FRS2, SCN8A, H1FOO, NALCN, EPB41L4A, LRRC16A, CNTNAP5, LTBP1, KHDRBS3, EPHB1, PRKCB, ATRN, TMEM108, PTK2B, RAPGEF5, RBPMS, SORCS3, SNX29, KCNN3, PLCH1, PLCB4, PDE11A, RGS7, NR2C2, WDR64, KCNMA1, DCN, SPEF2, CA10, MYB, RNF17, FYB, ETV6, CREB5, ZNF488, KSR2, SYT1, TBC1D16, SUCLG2, MLLT4, PLCXD3, VPS45, TUFT1) [16]. Central to this network, transmembrane and tetratricopeptide repeat containing 1 (TMTC1) gene appeared to be a common interaction factor for the 3 principal TFs. Not TMTC1 but transmembrane and tetratricopeptide repeat containing 2 (TMTC2) was also identified in the cattle growth network by Widmann et al. [12].
Aside from known interactions reported by the literature, our growth network allowed the identification of new interactions between genes that have not been previously described and may help in the understanding of such complex trait. In these sense, one of the top TF identified in the growth network, ELF1 gene, has not been reported to date to be involved in growth processes. This gene was identified to be co-associated with B Lymphoid Tyrosine Kinase (BLK) gene in the AWM analysis. It has been reported that ELF1 is a transcriptional activator of BLK and SRC kinases such as v-yes-1 Yamaguchi sarcoma viral related oncogene homolog (LYN) [37]. BLK is involved in the stimulation of insulin secretion in response to glucose [38]. In addition, the SRC family of protein tyrosine kinases (SFKs) interacts with growth factors [39] and cytokine receptors [40] and they are key mediators of PI3K and AKT signalling important for cell proliferation [41]. The LYN gene belonging to SFKs is required for rapid phosphorylation of Fer (Fps/Fes Related) Tyrosine Kinase (FER) [42]. In the AWM analysis we found FER also co-associated with ELF1.
Another interesting interaction identified in the co-association growth network was the ELF1 with HOXB13. Supporting this interaction, it has been described that Myeloid ecotropic viral integration site 1 (MEIS1) is a HOX cofactor which is regulated by ELF1 [43]. HOXB13 may play a role in growth repression and spinal cord formation [44], [45]. Furthermore, HOXB13 increases the androgen and favors the lipid accumulation in cells [46].
The transcription factor Forkhead box O1 (FOXO1) gene, also identified in the network co-associated with PRDM16, is activated in response to glucocorticoids and is blocked via the IGF/Akt pathway. FOXO1 is a target of insulin signalling and glucose metabolism, as well as it plays a role in myogenic growth and differentiation. Moreover, it has been observed that mice overexpressing FOXO1 in skeletal muscle had a reduced skeletal muscle mass when compared with wild-type mice [47]. When FOXO1 and PPARGC1A act together they promote gluconeogenesis. FOXO1 is known to repress PPARγ [48]. Another interesting gene was the miRNA ssc-miR-196a1, which was identified as co-associated with PRDM16, and has been recently reported to be associated with growth and development of skeletal muscle [49]. Other identified co-associated genes with PRDM16 were SH3PXD2A, ADAMTS12 and PTPN22. Interestingly, SH3PXD2A (SH3 And PX Domains 2A) is reported to bind the matrix metalloproteinases (ADAMs) and phosphoinositides [50]. Moreover, in human is associated with ADAM12 (the membrane-anchored protein corresponding to the secreted protein ADAMTS12) which is involved in skeletal muscle regeneration and mediates the neurotoxic effect of beta-amyloid peptide [51]. Finally, PTPN22 has been associated with diabetes in humans [52].
Biological pathways and functional analysis
Functional analysis using IPA program allowed us to identify the biological functions overrepresented considering the 513 co-associated genes related to the top trio of TF. Among the networks identified with IPA there were: “cell signalling, nucleic acid metabolism, cell-to-cell signalling and interaction”, “organism development, DNA replication, recombination and repair, and lipid metabolism” and “hereditary disorder, neurological disease and developmental disorder”, all of them having a score = 38, “cell-to-cell signalling and interaction, nervous system development and function, cellular assembly and organization” (score = 33) and “cellular development, nervous system development and function, behaviour” (score = 29) (S3 Table). Remarkably, the top molecular and cellular functions identified were: “post-translational modification” (p-value = 9.01×10−5), “cell-to-cell signalling and interaction” (p-value = 1.26×10−4), “molecular transport” (p-value = 1.26×10−4), “cellular development” (p-value = 2.89×10−4) and “cell morphology” (p-value = 3.10×10−4). Among the top physiological system development functions we observed the “organismal survival” (p-value = 1.18×10−4), “nervous system development and function” (p-value = 1.26×10−4), “tissue development” (p-value = 2.34×10−4) and “behaviour” (p-value = 2.88×10−4). These results are in agreement with those obtained by Widmann et al. [12] using the same methodology to study growth traits in cattle, where they identified similar biological processes (cell communication, signal transduction, cellular process, cell surface receptor signalling pathway and cell adhesion) suggesting that different genetic variants may be affecting the same pathways even in different species.
Among the most overrepresented pathways identified we observed D-myo-inositol (1,4,5)-triphosphate (Ins(1,4,5)P3) biosynthesis (p-value = 2.45×10−4), G-protein coupled receptor (GPCR) signalling (p-value = 5.88×10−3), corticotropin releasing (CRH) hormone signalling (p-value = 7.58×10−3), gonadotropin-releasing hormone (GnRH) signalling (p-value = 1.55×10−2), caveolar-mediated endocytosis signalling (p-value = 1.62×10−2), nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB) activation by viruses (p-value = 1.62×10−2), phospholipase C (PLC) signalling (p-value = 1.94×10−2) and neuronal nitric oxide synthase (nNOS) signalling in skeletal muscle cells (p-value = 3.46×10−2) pathways (S4 Table). Noteworthy, in the cattle growth network study, the GnRH signalling and the nitric oxide (NO) pathway were also identified [16]. Some of these pathways (S4 Table) are discussed in more detailed below.
GPCR, PLC and Ins(1,4,5)P3 signalling pathways
In mammals during growth and development there is a high requirement of lipids to increase in cell size and number. Lipids are the primary substrates which bind to certain GPCRs leading to an induced activity of PLC, which catalyse the hydrolysis of phosphatidylinositol 4,5-bisphosphate (PIP2) to inositol 1,4,5-trisphosphate (IP3) and 1,2-diacylglycerol (DAG) both having important second messenger functions [53]. DAG can be also used as a component of biological membranes or as a precursor to triacylglycerol (TAG) for energy storage [54]. The IP3 molecule binds to the Ins(1,4,5)P3 receptors (InsP3R) and trigger Ca2+ channel opening activating the ryanodine receptor-operated channel (RYR) [55], [56]. The IP3 signalling mechanism is crucial for normal cell physiology [57]. Moreover, GPCRs jointly with phosphatidylinositol kinases (PIPK) may be involved in feed signal transduction pathways [58]. Two PIPK genes (PIP5K1A and PIP5K1C) jointly with PLCB4 and RYR1 were identified as co-associated to PRDM16 gene. Thus, PRDM16 is a key transcriptional factor determining adiposity or miogenesis, and may also be necessary for the normal skeletal muscle development. Furthermore, the GPCRs, GPR144 and GPR176 were also identified in the growth network.
GnRH and CRH signalling pathway
Two hormone-related pathways, GnRH and CRH signalling pathways, were identified as overrepresented in our data. CRH is a peptide hormone secreted by the hypothalamus which controls adrenal secretion of cortisol and has been suggested to play a role in cell growth and survival. Interestingly, sheep with high cortisol response were prone to obesity [59]. Moreover, it has been observed that children treated with glucocorticoids showed growth retardation [60]. GnRH, which binds to GNRHR (GPCR member 7), is synthesized and released from neurons within the hypothalamus and regulates the production of gonadotropins, such as luteinizing hormone (LH) and follicle-stimulating hormone (FSH) in the pituitary gland, which, in turn triggers sexual maturation and promotes the secretion of endogenous sex hormones such as testosterone and estrogen from the gonads. Interestingly, growth-related traits in pig depend largely on gender [61]. In fact, GnRH agonists are used to treat central precocious puberty (CPP) characterized for developing an early puberty, larger growth of the skeleton and adult height [62]. Besides, the GnRF analog-diphtheria toxoid conjugate is used for castration and it is known to increase body weight at slaughter and improve average daily gain and feed conversion ratio [63]. The release of Ca2+ and DAG enhances the activation of protein kinases (PKC) to increase gonadotropin hormone secretion. Interestingly, in the network we can observe PRKCB co-associated to ELF-1 and PRKCE co-associated with PPARγ and PRDM16. These results underline an important function of the three key TF in our network having an important role in the puberty and bone growth development.
NF-κB and NO signalling pathway
NF-κB is a pleiotropic transcription factor being involved in many biological processes such as inflammation, immunity, differentiation, cell growth and apoptosis. This signalling pathway has also been identified in a study of human growth using gene expression data [64]. It is reported that ELF1 interacts with NF-κB via DNA binding domain [37]. Furthermore, the NF-κB activity may activate nNOS to generate NO [65]. NO may trigger GH secretion and affects other several pituitary peptides such as gonadotropins [66]. It is reported that a chronic exposure of NO may stimulate angiogenesis and adipocyte development [67]. Interestingly, we observed NOS1 co-associated with ELF1 in the growth network (Fig. 1B). Both, ELF1 and NOS1 genes may play a role in hematopoiesis and vascular development [68]. On the other hand, NO has the ability to enhance and regenerate diseased muscle, through determining the fibro-adipogenic progenitors fate and inhibiting adipogenesis [69]. In this direction, the NO production is thought to inhibit PPARγ expression [69]. Surprisingly, NOS1 was also identified as co-associated with PRDM16, but not with PPARγ.
General discussion: from network to phenotype inference
Iberian pigs are known to have higher IMF than Landrace pigs at the same growth stage [70]. Furthermore, the skeletal muscle grows faster in Landrace than in Iberian pigs, being less prone to obesity [70]. In this study, we analyzed backcrossed animals from an Iberian × Landrace cross, which differed in fat and growth traits. Growth refers to an increase in tissue mass and it can be plotted as a sigmoid curve depending on age and cumulative weight [71]. At the pre-mature phase, muscle mass, organ and bone formation are increased, meanwhile in the mature phase the animal is more prone to fattening and intramuscular deposition [71]. The top trio of TF identified in the network are key regulators for mesoderm cell differentiation in osteocytes, miocytes or adipocytes. Our results showed among the top molecular and cellular functions the cell development and interaction pathways which may be important in order to trigger tissue formation. Noteworthy, the hierarchical cluster analysis evidenced a clear division of the additive effects of the SNPs for the 12 growth phenotypic traits, between animal weight-related and fat-related traits (S2 Figure and Fig. 2). We hypothesize that the hormone releasing pathways here identified (GnRH and CRH) may be key for the regulation of conformation, growth and fatness traits in our animal material. An increased carcass weight with a reduced backfat thickness at a fixed age have been selection targets in commercial pig breeds, resulting in less mature animals as fat deposition rate is expected to increase in the puberty phase [72]. Given that Iberian breed is more prone to IMF and backfat deposition at the same growth stage lead to the hypothesis that Landrace animals may arrive later to the mature growth phase when compared to Iberian animals. What remains unclear is whether the signals for maturity switching are related to the GnRH or CRH hormone release pathways. Finally, the genes and pathways here identified had a high concordance with those reported by other authors studying growth metabolism in animals or height related traits in human. Our hypothesis are supported by the high concordance between our genes and pathways identified in the network and those reported by Fortes et al. [9] for puberty traits in cattle.
Conclusions
The processes regulating conformation, growth and fatness traits in pigs are complex and most of the mechanisms remain unknown despite being of great interest for the pig industry. The power of single trait GWAS can be enhanced when considering simultaneously multiple phenotypes taking advantage of system biology approaches. In the present study, the AWM gene co-association network analysis revealed key transcription factors, gene-gene interactions and pathways underpinning the regulation of pig conformation, growth and fatness. Network approaches represent a major step in understanding the genetics of complex diseases and traits. Further efforts should be made in order to study in more detail the new gene-gene interactions here identified, as well as, to study in more detail the key transcription factors and pathways involvement in the growth and conformation traits determination.
Material and Methods
Animal material and phenotypic classification
The animal material used belongs to several generations of the IBMAP population obtained from the cross of 3 Iberian boars (Guadyerbas) with 31 Landrace sows [73], [74]. For this study we used phenotypic records from 292 animals belonging to three different IBMAP generations: 159 BC1_LD animals (25% Iberian ×75% Landrace) from backcrossing five F1 males with 26 Landrace sows, 79 BC animals obtained by crossing 4 F2 boars and 22 Landrace sows and 54 F3 obtained by mating F2 animals. Animals were fed ad libitum and sacrificed at 180±2.8 days (average ± standard deviation) in a commercial slaughterhouse following national and institutional guidelines for the Good Experimental Practices and approved by the Ethical Committee of the Institution (IRTA- Institut de Recerca i Tecnologia Agroalimentàries).
Phenotypic records used in the analyses (Table 1) correspond to body weight (BW) measured at 125, 155 and 180 days (BW125, BW155, and BW180, respectively), backfat thickness (BFT) at the level of the fourth rib at 4 cm of the midline measured by ultrasounds at 155 and 180 days (BFT155 and BFT180) and measured with a rule at slaughter (BFTS), carcass length (CL) and carcass weight (CW), ham weight (HW), shoulder weight (SW), belly weight (BLW), and the intramuscular fat content (IMF) in the longissimus dorsi muscle.
Genetic markers and quality control
A total of 364 pigs, including their F0, F1 and F2 founder generations (72 animals), were genotyped with the Porcine SNP60K BeadChip [75] following the Infinium HD Assay Ultra protocol (Illumina Inc.; San Diego, CA, USA) and the genotypes were visualized with the GenomeStudio software (Illumina Inc.; San Diego, CA, USA). The quality control of the 62,163 SNPs was performed by using Plink [76] software removing markers with a minor allele frequency (MAF) <5% and animals with missing genotypes>5%. The SNP mapping and annotation was performed by using the pig assembly 10.2 [ftp://ftp.ncbi.hlm.nih.gov/genomes/Sus_scrofa/GEF/]. We also excluded markers which did not map in the Sscrofa10.2 version assembly. Pedstats program [77] was used to check Mendelian inheritance errors.
Genome-wide association analysis
Genome-wide association analysis (GWAS) for the twelve phenotypic growth traits were performed using a mixed model accounting for additive effects with Qxpak 5.0 software [78]:
in which yijlkm was the i-th individual record, sex (two levels) and batch (nine levels) were fixed effects, ß was a covariate coefficient with c being the covariate used in each case (described below), λl was a −1, 0, +1 indicator variable depending on the l-th individual genotype for the k-th SNP, ak represented the additive effect associated with the k-th SNP, ul represented the infinitesimal genetic effect with random distribution N(0, 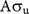 ) where A was a numerator of the the pedigree-based relationship matrix and eijlkm the residual.
) where A was a numerator of the the pedigree-based relationship matrix and eijlkm the residual.
Different covariates (c) were used for the analysis. Carcass weight was used as a covariate for CL, IMF, BFTS, HW, SW, and BLW. For BFT155 and BFT180 the covariates used were the body weight at their respective days. Meanwhile, for the body and the carcass weights the covariate used was the animal age.
Association weight matrix
The association weight matrix (AWM) was built from the GWAS results. First, the SNP additive effects were normalized with a z-score method using a R script and a matrix was constructed with these values, being SNPs in rows and traits in columns. Another matrix with the same format was generated for the p-values obtained in the GWAS. For the analysis, the ham weight was selected as the key phenotype. Subsequently, the AWM script [9] available from authors was used in R (http://www.r-project.org/). Those SNPs associated (nominal p-value <0.05) with the ham weight or with 3 or more traits were selected for further analysis. We included in the analysis the SNPs with a distance of minor than 2.5 kb (SNPs close) and major than 1,000 kb (SNPs far) from a gene. We also included SNPs located at less than 10 kb of miRNA. Finally, to facilitate the analysis, for SNPs clustering at less than 1 Mb of distance from each other, the SNP associated with the major number of characters was selected. The hierarchical clustering option of PermutMatrix software [79] was used to visualize the results of both traits and genes. The trio of putative regulators spanning most of the network topology with a minimum redundancy [10] was selected. In this study we took into account all the transcription factors (TF) from the list reported by Vaquerizas et al. [80]; additionally, those 22 genes belonging to the GO: 0050789 which accounts for the DNA binding TF activity were added. All miRNA annotated on Sscrofa10.2 assembly were also included in the analysis as potential regulators. PCIT algorithm [81] was used to construct a file containing the reported gene-gene interactions among the 3 TFs. The CentiScaPe plug-in [82] of Cytoscape software [83] was used to visualize the PCIT results and either to calculate the node centrality values (Deg) and network parameters.
Gene ontologies, pathways and network analysis
The Ingenuity Pathways Analysis software (IPA; Ingenuity Systems, Redwood city, CA, USA; www.ingenuity.com) was used to identify the most relevant biological functions and pathways in which the genes associated with the phenotypic traits were involved. IPA, which uses its own databases, allowed the identification of overrepresented pathways using the BH multiple testing correction [84] of p-value (FDR <0.05) and generating biological networks. The Mouse Genome Database (MGD; http://www.informatics.jax.org) was used in order to identify how mutant alleles driven in mice for the identified growth-related genes present in the network affected the phenotype.
Supporting Information
GWAS plot of the 12 traits: body weight measured at 125, 155 and 180 days (BW125, BW155, and BW180, respectively), backfat thickness measured at 155 and 180 days (BFT155 and BFT180) and measured at slaughter (BFTS), carcass length and weight (CL and CW), weight of the hams, shoulders and belly (HW, SW and BLW) and intramuscular fat (IMF) content. The horizontal green line represents the statistical significance (false discovery rate; set at q-value ≤0.05) calculated with the q-value library [85] implemented in R program (http://www.r-project.org/).
(DOCX)
Hierarchical cluster analysis among 12 phenotypic traits: body weight measured at 125, 155 and 180 days (BW125, BW155, and BW180, respectively), backfat thickness measured at 155 and 180 days (BFT155 and BFT180) and measured at slaughter (BFTS), carcass length and weight (CL and CW), weight of the hams, shoulders and belly (HW, SW and BLW) and the intramuscular fat (IMF) content.
(TIF)
Linkage disequilibrium among the PPARG and NR2C2 SNPs. Pattern of linkage disequilibrium analysis around ±2Mb of the SNPs in PPARG and NR2C2 . Figure colored from blue to red according to LD strength between consecutive markers. The green diamond-shape corresponds to the SNP in PPARG gene and the blue diamond-shape the SNP in NR2C2 gene.
(DOCX)
List of 54 growth-related genes in the network.
(XLSX)
List of 142 regulators (transcription factors and miRNAs) identified within the list of associated-genes.
(XLSX)
Top networks of molecular functions identified with IPA for the 513 genes.
(XLS)
Top pathways identified with IPA for the 513 genes.
(XLS)
Acknowledgments
The authors gratefully acknowledge J.L. Noguera (Institut de Recerca i Tecnologia Agroalimentàries; IRTA) for the animal material contribution.
Data Availability
The authors confirm that all data underlying the findings are fully available without restriction. All relevant data are within the paper and its Supporting Information files.
Funding Statement
This work has been funded by MICINN project AGL2011-29821-C02 (Ministerio de Economía y Competitividad), and by the Innovation Consolider-Ingenio 2010 Program (CSD2007-00036, Centre de Recerca en Agrigenòmica). APO was funded by a Personal Investigador en Formación (PIF) PhD grant from the Universitat Autònoma de Barcelona (458-01-1/2011). JC was funded by a Formación de Personal Investigador (FPI) PhD grant from Spanish Ministerio de Educación (BES-2009-018223), YRC by a Formación del Profesorado Universitario (FPU) PhD grant from the Spanish Ministerio (AP2008-01450), and MR by a Formació i Contractació de Personal Investigador Novell (FI-DGR) PhD grant from Generalitat de Catalunya (ECO/1639/2013). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.FAO (2008) Fats and fatty acids in human nutrition. Report of expert consultation. [PubMed]
- 2. Dodson M, Hausman G, Guan L, Du M, Rasmussen T, et al. (2010) Lipid metabolism, adipocyte depot physiology and utilization of meat animals as experimental models for metabolic research. Int J Biol Sci 6:691–699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Walters E, Wolf E, Whyte J, Mao J, Renner S, et al. (2012) Completion of the swine genome will simplify the production of swine as a large animal biomedical model. BMC Med Genomics 5:55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Gispert M, Font i Furnols M, Gil M, Velarde A, Diestre A, et al. (2007) Relationships between carcass quality parameters and genetic types. Meat Sci 77:397–404 10.1016/j.meatsci.2007.04.006 [DOI] [PubMed] [Google Scholar]
- 5. Fernandez A, Perez-Montarelo D, Barragan C, Ramayo-Caldas Y, Ibanez-Escriche N, et al. (2012) Genome-wide linkage analysis of QTL for growth and body composition employing the PorcineSNP60 BeadChip. BMC Genet 13:41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. McCarthy MI, Abecasis GR, Cardon LR, Goldstein DB, Little J, et al. (2008) Genome-wide association studies for complex traits: consensus, uncertainty and challenges. Nat Rev Genet 9:356–369 10.1038/nrg2344 [DOI] [PubMed] [Google Scholar]
- 7.Rao DC (2008) An Overview of the Genetic Dissection of Complex Traits. In: D. C Rao and C. Charles Gueditor. Advances in Genetics. Academic PressVol. Volume 60 . pp.3–34. Available: http://www.sciencedirect.com/science/article/pii/S0065266007004014. [DOI] [PubMed] [Google Scholar]
- 8. Wang K, Li M, Hakonarson H (2010) Analysing biological pathways in genome-wide association studies. Nat Rev Genet 11:843–854 10.1038/nrg2884 [DOI] [PubMed] [Google Scholar]
- 9. Fortes MRS, Reverter A, Zhang Y, Collis E, Nagaraj SH, et al. (2010) Association weight matrix for the genetic dissection of puberty in beef cattle. Proc Natl Acad Sci 107:13642–13647 10.1073/pnas.1002044107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Reverter A, Fortes MS (2013) Association Weight Matrix: A Network-Based Approach Towards Functional Genome-Wide Association Studies. In: Gondro C, van der Werf J, Hayes Beditors. Genome-Wide Association Studies and Genomic Prediction. Methods in Molecular Biology. Humana PressVol. 1019 . pp.437–447. Available: 10.1007/978-1-62703-447-0_20. [DOI] [PubMed] [Google Scholar]
- 11. Ramayo-Caldas Y, Ballester M, Fortes M, Esteve-Codina A, Castello A, et al. (2014) From SNP co-association to RNA co-expression: Novel insights into gene networks for intramuscular fatty acid composition in porcine. BMC Genomics 15:232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Widmann P, Reverter A, Fortes MR, Weikard R, Suhre K, et al. (2013) A systems biology approach using metabolomic data reveals genes and pathways interacting to modulate divergent growth in cattle. BMC Genomics 14:798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ramayo-Caldas Y, Fortes MRS, Hudson NJ, Porto-Neto LR, Bolormaa S, et al. (2014) A marker-derived gene network reveals the regulatory role of PPARGC1A, HNF4G and FOXP3in intramuscular fat deposition of beef cattle. J Anim Sci. Available: http://www.journalofanimalscience.org/content/early/2014/04/28/jas.2013-7484.abstract. [DOI] [PubMed]
- 14. Fortes MRS, Reverter A, Nagaraj SH, Zhang Y, Jonsson NN, et al. (2011) A single nucleotide polymorphism-derived regulatory gene network underlying puberty in 2 tropical breeds of beef cattle. J Anim Sci 89:1669–1683 10.2527/jas.2010-3681 [DOI] [PubMed] [Google Scholar]
- 15. Fortes MRS, Snelling WM, Reverter A, Nagaraj SH, Lehnert SA, et al. (2012) Gene network analyses of first service conception in Brangus heifers: Use of genome and trait associations, hypothalamic-transcriptome information, and transcription factors. J Anim Sci 90:2894–2906 10.2527/jas.2011-4601 [DOI] [PubMed] [Google Scholar]
- 16.Fernández A, García-Casco J, De Pedro E, Silió L, Rodríguez MC (2007) Genetic antagonism between intramuscular fat content and primal cuts in Iberian pigs? In: Casabianca F., Monin G., Audiot A., editors. 5. International Symposium on the Mediterranean Pig. Options Méditerranéennes: Série A. Séminaires Méditerranéens. Zaragoza: CIHEAMVol. 76 . pp.43–46. Available: http://om.ciheam.org/om/pdf/a76/00800557.pdf. [Google Scholar]
- 17. Cecil JE, Fischer B, Doney ASF, Hetherington M, Watt P, et al. (2005) The Pro12Ala and C–681G variants of the PPARG locus are associated with opposing growth phenotypes in young schoolchildren. Diabetologia 48:1496–1502 10.1007/s00125-005-1817-0 [DOI] [PubMed] [Google Scholar]
- 18. Rieusset J, Seydoux J, Anghel SI, Escher P, Michalik L, et al. (2004) Altered Growth in Male Peroxisome Proliferator-Activated Receptor γ (PPARγ) Heterozygous Mice: Involvement of PPARγ in a Negative Feedback Regulation of Growth Hormone Action. Mol Endocrinol 18:2363–2377 10.1210/me.2003-0325 [DOI] [PubMed] [Google Scholar]
- 19. Ahmadian M, Suh JM, Hah N, Liddle C, Atkins AR, et al. (2013) PPARγ signaling and metabolism: the good, the bad and the future. Nat Med 99:557–566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Puig-Oliveras A, Ramayo-Caldas Y, Corominas J, Estellé J, Pérez-Montarelo D, et al. (2014) Differences in Muscle Transcriptome among Pigs Phenotypically Extreme for Fatty Acid Composition. PLoS ONE 9:e99720 10.1371/journal.pone.0099720 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Chen Z, Zhao X, Jiang X, Guo X, Lv Z, et al. (2011) Association of PPARγ2 polymorphisms with carcass and meat quality traits in a Pietrain x Jinhua F2 population. Genet Mol Biol 34:56–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. O'Gorman CW, Stanko RL, Keisler DH, Garcia MR (2010) Effects of acute fasting and age on leptin and peroxisome proliferator-activated receptor gamma production relative to fat depot in immature and mature pigs. J Anim Physiol Anim Nutr 94:e266–e276 10.1111/j.1439-0396.2009.00968.x [DOI] [PubMed] [Google Scholar]
- 23. Calero-Nieto FJ, Wood AD, Wilson NK, Kinston S, Landry J-R, et al. (2010) Transcriptional regulation of Elf-1: locus-wide analysis reveals four distinct promoters, a tissue-specific enhancer, control by PU.1 and the importance of Elf-1 downregulation for erythroid maturation. Nucleic Acids Res 38:6363–6374 10.1093/nar/gkq490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Chang P-Y, Miyamoto S (2006) Nuclear Factor-κB Dimer Exchange Promotes a p21waf1/cip1 Superinduction Response in Human T Leukemic Cells. Mol Cancer Res 4:101–112 10.1158/1541-7786.MCR-05-0259 [DOI] [PubMed] [Google Scholar]
- 25. Jin E, Liu J, Suehiro J, Yuan L, Okada Y, et al. (2009) Differential roles for ETS, CREB, and EGR binding sites in mediating VEGF receptor 1 expression in vivo. Blood 114:5557–5566 10.1182/blood-2009-05-220434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Baek K, Baek J-H (2013) The transcription factors myeloid elf-1-like factor (MEF) and distal-less homeobox 5 (Dlx5) inversely regulate the differentiation of osteoblasts and adipocytes in bone marrow. Adipocyte 2:50–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Rudra D, deRoos P, Chaudhry A, Niec RE, Arvey A, et al. (2012) Transcription factor Foxp3 and its protein partners form a complex regulatory network. Nat Immunol 13:1010–1019 10.1038/ni.2402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Seale P, Bjork B, Yang W, Kajimura S, Chin S, et al. (2008) PRDM16 controls a brown fat/skeletal muscle switch. Nature 454:961–967 10.1038/nature07182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Berg F, Gustafson U, Andersson L (2006) The Uncoupling Protein 1 Gene (UCP1) Is Disrupted in the Pig Lineage: A Genetic Explanation for Poor Thermoregulation in Piglets. PLoS Genet 2:e129 10.1371/journal.pgen.0020129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Chuikov S, Levi BP, Smith ML, Morrison SJ (2010) Prdm16 promotes stem cell maintenance in multiple tissues, partly by regulating oxidative stress. Nat Cell Biol 12:999–1006 10.1038/ncb2101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Kim J, Cho I, Hong J, Choi Y, Kim H, et al. (2008) Identification and characterization of new microRNAs from pig. Mamm Genome 19:570–580 10.1007/s00335-008-9111-3 [DOI] [PubMed] [Google Scholar]
- 32. Kang HS, Okamoto K, Kim Y-S, Takeda Y, Bortner CD, et al. (2011) Nuclear Orphan Receptor TAK1/TR4-Deficient Mice Are Protected Against Obesity-Linked Inflammation, Hepatic Steatosis, and Insulin Resistance. Diabetes 60:177–188 10.2337/db10-0628 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Collins LL, Lee Y-F, Heinlein CA, Liu N-C, Chen Y-T, et al. (2004) Growth retardation and abnormal maternal behavior in mice lacking testicular orphan nuclear receptor 4. Proc Natl Acad Sci U S A 101:15058–15063 10.1073/pnas.0405700101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Gudbjartsson DF, Walters GB, Thorleifsson G, Stefansson H, Halldorsson BV, et al. (2008) Many sequence variants affecting diversity of adult human height. Nat Genet 40:609–615 10.1038/ng.122 [DOI] [PubMed] [Google Scholar]
- 35. Soranzo N, Rivadeneira F, Chinappen-Horsley U, Malkina I, Richards JB, et al. (2009) Meta-Analysis of Genome-Wide Scans for Human Adult Stature Identifies Novel Loci and Associations with Measures of Skeletal Frame Size. PLoS Genet 5:e1000445 10.1371/journal.pgen.1000445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Weedon MN, Lango H, Lindgren CM, Wallace C, Evans DM, et al. (2008) Genome-wide association analysis identifies 20 loci that influence adult height. Nat Genet 40:575–583 10.1038/ng.121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Oettgen P, Akbarali Y, Boltax J, Best J, Kunsch C, et al. (1996) Characterization of NERF, a novel transcription factor related to the Ets factor ELF-1. Mol Cell Biol 16:5091–5106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Borowiec M, Liew CW, Thompson R, Boonyasrisawat W, Hu J, et al. (2009) Mutations at the BLK locus linked to maturity onset diabetes of the young and β-cell dysfunction. Proc Natl Acad Sci 106:14460–14465 10.1073/pnas.0906474106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Sutton P, Borgia J, Bonomi P, Plate J (2013) Lyn, a Src family kinase, regulates activation of epidermal growth factor receptors in lung adenocarcinoma cells. Mol Cancer 12:76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Abram CL, Courtneidge SA (2000) Src Family Tyrosine Kinases and Growth Factor Signaling. Exp Cell Res 254:1–13 10.1006/excr.1999.4732 [DOI] [PubMed] [Google Scholar]
- 41. Parsons JT, Parsons SJ (1997) Src family protein tyrosine kinases: cooperating with growth factor and adhesion signaling pathways. Curr Opin Cell Biol 9:187–192 10.1016/S0955-0674(97)80062-2 [DOI] [PubMed] [Google Scholar]
- 42. Udell CM, Samayawardhena LA, Kawakami Y, Kawakami T, Craig AWB (2006) Fer and Fps/Fes Participate in a Lyn-dependent Pathway from FcεRI to Platelet-Endothelial Cell Adhesion Molecule 1 to Limit Mast Cell Activation. J Biol Chem 281:20949–20957 10.1074/jbc.M604252200 [DOI] [PubMed] [Google Scholar]
- 43. Xiang P, Lo C, Argiropoulos B, Lai CB, Rouhi A, et al. (2010) Identification of E74-like factor 1 (ELF1) as a transcriptional regulator of the Hox cofactor MEIS1. Exp Hematol 38:798–808.e2 10.1016/j.exphem.2010.06.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Economides KD, Zeltser L, Capecchi MR (2003) Hoxb13 mutations cause overgrowth of caudal spinal cordand tail vertebrae. Dev Biol 256:317–330 10.1016/S0012-1606(02)00137-9 [DOI] [PubMed] [Google Scholar]
- 45. Jung C, Kim R-S, Zhang H-J, Lee S-J, Jeng M-H (2004) HOXB13 Induces Growth Suppression of Prostate Cancer Cells as a Repressor of Hormone-Activated Androgen Receptor Signaling. Cancer Res 64:9185–9192 10.1158/0008-5472.CAN-04-1330 [DOI] [PubMed] [Google Scholar]
- 46. Norris JD, Chang C-Y, Wittmann BM, Kunder RS, Cui H, et al. (2009) The Homeodomain Protein HOXB13 Regulates the Cellular Response to Androgens. Mol Cell 36:405–416 10.1016/j.molcel.2009.10.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Kamei Y, Miura S, Suzuki M, Kai Y, Mizukami J, et al. (2004) Skeletal Muscle FOXO1 (FKHR) Transgenic Mice Have Less Skeletal Muscle Mass, Down-regulated Type I (Slow Twitch/Red Muscle) Fiber Genes, and Impaired Glycemic Control. J Biol Chem 279:41114–41123 10.1074/jbc.M400674200 [DOI] [PubMed] [Google Scholar]
- 48. Armoni M, Harel C, Karni S, Chen H, Bar-Yoseph F, et al. (2006) FOXO1 Represses Peroxisome Proliferator-activated Receptor-γ1 and -γ2 Gene Promoters in Primary Adipocytes: A Novel Paradigm to Increase the Insulin Sensitivity. J Biol Chem 281:19881–19891 10.1074/jbc.M600320200 [DOI] [PubMed] [Google Scholar]
- 49. Huang TH, Zhu MJ, Li XY, Zhao SH (2008) Discovery of porcine microRNAs and profiling from skeletal muscle tissues during development. PLoS One 3:e3225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Leyme A, Bourd-Boittin K, Bonnier D, Falconer A, Arlot-Bonnemains Y, et al. (2012) Identification of ILK as a new partner of the ADAM12 desintegrin and metalloprotease in cell adhesion and survival. Mol Biol Cell 23:: 17 3461–3472. doi: 10.1091/mbc.E11-11-0918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Laumet G, Petitprez V, Sillaire A, Ayral A-M, Hansmannel F, et al. (2010) A study of the association between the ADAM12 and SH3PXD2A (SH3MD1) genes and Alzheimer's disease. Neurosci Lett 468:1–2 10.1016/j.neulet.2009.10.040 [DOI] [PubMed] [Google Scholar]
- 52. Bottini N, Vang T, Cucca F, Mustelin T (2006) Role of PTPN22 in type 1 diabetes and other autoimmune diseases. Allelic Var Signal Elem Autoimmun 18:207–213 10.1016/j.smim.2006.03.008 [DOI] [PubMed] [Google Scholar]
- 53. Wymann MP, Schneiter R (2008) Lipid signalling in disease. Nat Rev Mol Cell Biol 9:162–176 10.1038/nrm2335 [DOI] [PubMed] [Google Scholar]
- 54. Carrasco S, Mérida I (2007) Diacylglycerol, when simplicity becomes complex. Trends Biochem Sci 32:27–36 10.1016/j.tibs.2006.11.004 [DOI] [PubMed] [Google Scholar]
- 55. Berridge MJ, Lipp P, Bootman MD (2000) The versatility and universality of calcium signalling. Nat Rev Mol Cell Biol 1:11–21 10.1038/35036035 [DOI] [PubMed] [Google Scholar]
- 56. Hume JR, McAllister CE, Wilson SM (2009) Caffeine inhibits InsP3 responses and capacitative calcium entry in canine pulmonary arterial smooth muscle cells. Vascul Pharmacol 50:89–97 10.1016/j.vph.2008.11.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Decrock E, De Bock M, Wang N, Gadicherla AK, Bol M, et al. (2013) IP3, a small molecule with a powerful message. 12th Eur Symp Calcium 1833:1772–1786 10.1016/j.bbamcr.2012.12.016 [DOI] [PubMed] [Google Scholar]
- 58. Bakthavatsalam D, Meijer HJG, Noegel AA, Govers F (2006) Novel phosphatidylinositol phosphate kinases with a G-protein coupled receptor signature are shared by Dictyostelium and Phytophthora. Trends Microbiol 14:378–382 10.1016/j.tim.2006.07.006 [DOI] [PubMed] [Google Scholar]
- 59. Lee TK, Lee C, Bischof R, Lambert GW, Clarke IJ, et al. (2014) Stress-induced behavioral and metabolic adaptations lead to an obesity-prone phenotype in ewes with elevated cortisol responses. Psychoneuroendocrinology 47:166–177 10.1016/j.psyneuen.2014.05.015 [DOI] [PubMed] [Google Scholar]
- 60. Allen DB (1996) Growth suppression by glucocorticoid therapy. Endocrinol Metab Clin North Am 25:699–717 10.1016/S0889-8529(05)70348-0 [DOI] [PubMed] [Google Scholar]
- 61.Serrano MP, Cámara L, Morales JI, Berrocoso JD, López Bote CJ, et al. (2012) Effect of gender, housing density and the interaction on growth performance and carcass and meat quality of pigs slaughtered at 110 kg body weight. Span J Agric Res Vol 11 No 1 2013. Available: http://revistas.inia.es/index.php/sjar/article/view/2869. [Google Scholar]
- 62. Mul D, Hughes IA (2008) The use of GnRH agonists in precocious puberty. Eur J Endocrinol 159:S3–S8 10.1530/EJE-08-0814 [DOI] [PubMed] [Google Scholar]
- 63. Kantas D, Papatsiros V, Tassis P, Tzika E, Pearce MC, et al. (2014) Effects of early vaccination with a gonadotropin releasing factor analog-diphtheria toxoid conjugate on boar taint and growth performance of male pigs. J Anim Sci 92:2251–2258 10.2527/jas.2013-6924 [DOI] [PubMed] [Google Scholar]
- 64. Stevens A, Hanson D, Whatmore A, Destenaves B, Chatelain P, et al. (2013) Human growth is associated with distinct patterns of gene expression in evolutionarily conserved networks. BMC Genomics 14:547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Morgan MJ, Liu Z (2011) Crosstalk of reactive oxygen species and NF-κB signaling. Cell Res 21:103–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Rubinek T, Rubinfeld H, Hadani M, Barkai G, Shimon I (2005) Nitric oxide stimulates growth hormone secretion from human fetal pituitaries and cultured pituitary adenomas. Endocrine 28:209–216 10.1385/ENDO:28:2:209 [DOI] [PubMed] [Google Scholar]
- 67. Dai Z, Wu Z, Yang Y, Wang J, Satterfield MC, et al. (2013) Nitric oxide and energy metabolism in mammals. BioFactors 39:383–391 10.1002/biof.1099 [DOI] [PubMed] [Google Scholar]
- 68. Dube A, Thai S, Gaspar J, Rudders S, Libermann TA, et al. (2001) ELF-1 Is a Transcriptional Regulator of the Tie2 Gene During Vascular Development. Circ Res 88:237–244 10.1161/01.RES.88.2.237 [DOI] [PubMed] [Google Scholar]
- 69. Cordani N, Pisa V, Pozzi L, Sciorati C, Clementi E (2014) Nitric Oxide Controls Fat Deposition in Dystrophic Skeletal Muscle by Regulating Fibro-Adipogenic Precursor Differentiation. STEM CELLS 32:874–885 10.1002/stem.1587 [DOI] [PubMed] [Google Scholar]
- 70. Serra X, Gil F, Pérez-Enciso M, Oliver M, Vázquez J, et al. (1998) A comparison of carcass, meat quality and histochemical characteristics of Iberian (Guadyerbas line) and Landrace pigs. Livest Prod Sci 56:215–223 10.1016/S0301-6226(98)00151-1 [DOI] [Google Scholar]
- 71. Owens FN, Dubeski P, Hanson CF (1993) Factors that alter the growth and development of ruminants. J Anim Sci 71:3138–3150. [DOI] [PubMed] [Google Scholar]
- 72. Gjerlaug-Enger E, Kongsro J, Ødegård J, Aass L, Vangen O (2012) Genetic parameters between slaughter pig efficiency and growth rate of different body tissues estimated by computed tomography in live boars of Landrace and Duroc. animal 6:9–18 10.1017/S1751731111001455 [DOI] [PubMed] [Google Scholar]
- 73. Pérez-Enciso M, Clop A, Noguera JL, Ovilo C, Coll A, et al. (2000) A QTL on pig chromosome 4 affects fatty acid metabolism: evidence from an Iberian by Landrace intercross. J Anim Sci 78:2525–2531. [DOI] [PubMed] [Google Scholar]
- 74. Ramayo-Caldas Y, Castello A, Pena R, Alves E, Mercade A, et al. (2010) Copy number variation in the porcine genome inferred from a 60 k SNP BeadChip. BMC Genomics 11:593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Ramos AM, Crooijmans RPMA, Affara NA, Amaral AJ, Archibald AL, et al. (2009) Design of a High Density SNP Genotyping Assay in the Pig Using SNPs Identified and Characterized by Next Generation Sequencing Technology. PLoS ONE 4:e6524 10.1371/journal.pone.0006524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Purcell S, Neale B, Todd-Brown K, Thomas L, Ferreira MAR, et al. (2007) PLINK: A Tool Set for Whole-Genome Association and Population-Based Linkage Analyses. Am J Hum Genet 81:559–575 10.1086/519795 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Wigginton JE, Abecasis GR (2005) PEDSTATS: descriptive statistics, graphics and quality assessment for gene mapping data. Bioinformatics 21:3445–3447 10.1093/bioinformatics/bti529 [DOI] [PubMed] [Google Scholar]
- 78. Perez-Enciso M, Misztal I (2011) Qxpak.5: Old mixed model solutions for new genomics problems. BMC Bioinformatics 12:202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Caraux G, Pinloche S (2005) PermutMatrix: a graphical environment to arrange gene expression profiles in optimal linear order. Bioinformatics 21:1280–1281 10.1093/bioinformatics/bti141 [DOI] [PubMed] [Google Scholar]
- 80. Vaquerizas JM, Kummerfeld SK, Teichmann SA, Luscombe NM (2009) A census of human transcription factors: function, expression and evolution. Nat Rev Genet 10:252–263 10.1038/nrg2538 [DOI] [PubMed] [Google Scholar]
- 81. Reverter A, Chan EKF (2008) Combining partial correlation and an information theory approach to the reversed engineering of gene co-expression networks. Bioinformatics 24:2491–2497 10.1093/bioinformatics/btn482 [DOI] [PubMed] [Google Scholar]
- 82. Scardoni G, Petterlini M, Laudanna C (2009) Analyzing biological network parameters with CentiScaPe. Bioinformatics 25:2857–2859 10.1093/bioinformatics/btp517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Shannon P, Markiel A, Ozier O, Baliga NS, Wang JT, et al. (2003) Cytoscape: A Software Environment for Integrated Models of Biomolecular Interaction Networks. Genome Res 13:2498–2504 10.1101/gr.1239303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Benjamini Y, Hochberg Y (1995) Controlling the false discovery rate: a pratical and powerful approach to multiple testing. J R Stat Soc Ser B Methodol 57:289–300. [Google Scholar]
- 85. Storey JD, Tibshirani R (2003) Statistical significance for genome-wide studies. Proc Natl Acad Sci 100:9440–9445 10.1073/pnas.1530509100 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
GWAS plot of the 12 traits: body weight measured at 125, 155 and 180 days (BW125, BW155, and BW180, respectively), backfat thickness measured at 155 and 180 days (BFT155 and BFT180) and measured at slaughter (BFTS), carcass length and weight (CL and CW), weight of the hams, shoulders and belly (HW, SW and BLW) and intramuscular fat (IMF) content. The horizontal green line represents the statistical significance (false discovery rate; set at q-value ≤0.05) calculated with the q-value library [85] implemented in R program (http://www.r-project.org/).
(DOCX)
Hierarchical cluster analysis among 12 phenotypic traits: body weight measured at 125, 155 and 180 days (BW125, BW155, and BW180, respectively), backfat thickness measured at 155 and 180 days (BFT155 and BFT180) and measured at slaughter (BFTS), carcass length and weight (CL and CW), weight of the hams, shoulders and belly (HW, SW and BLW) and the intramuscular fat (IMF) content.
(TIF)
Linkage disequilibrium among the PPARG and NR2C2 SNPs. Pattern of linkage disequilibrium analysis around ±2Mb of the SNPs in PPARG and NR2C2 . Figure colored from blue to red according to LD strength between consecutive markers. The green diamond-shape corresponds to the SNP in PPARG gene and the blue diamond-shape the SNP in NR2C2 gene.
(DOCX)
List of 54 growth-related genes in the network.
(XLSX)
List of 142 regulators (transcription factors and miRNAs) identified within the list of associated-genes.
(XLSX)
Top networks of molecular functions identified with IPA for the 513 genes.
(XLS)
Top pathways identified with IPA for the 513 genes.
(XLS)
Data Availability Statement
The authors confirm that all data underlying the findings are fully available without restriction. All relevant data are within the paper and its Supporting Information files.