Abstract
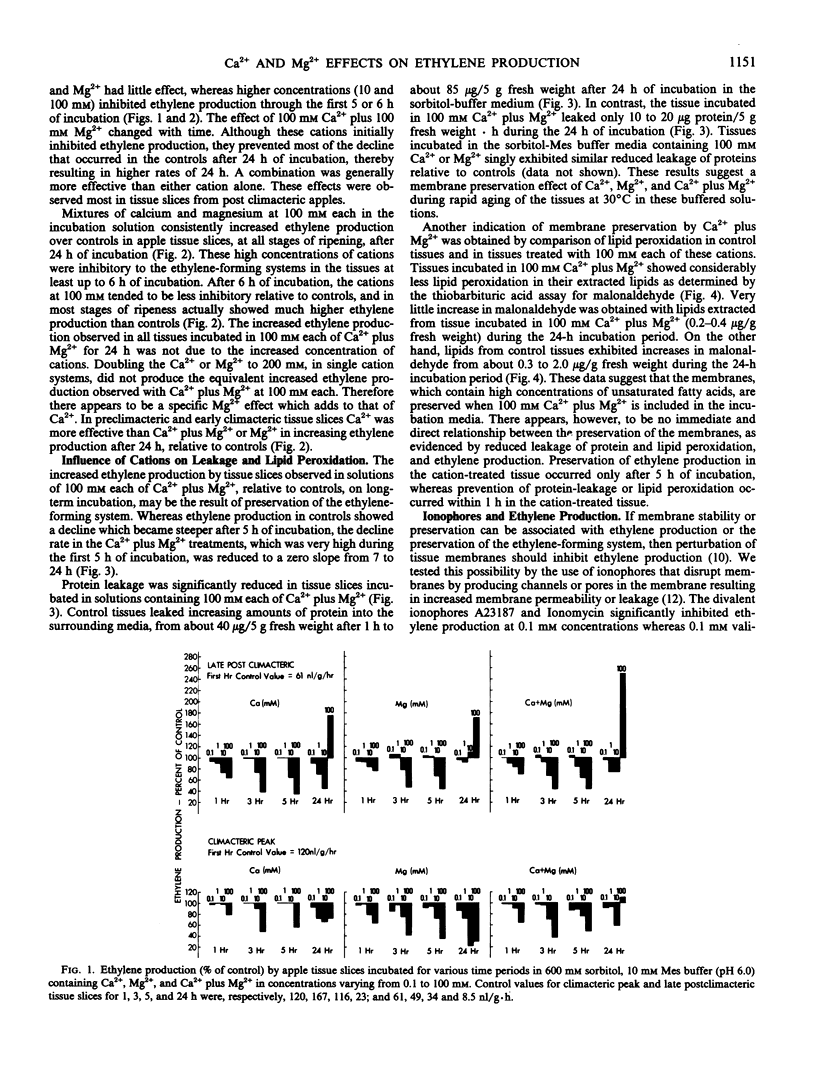
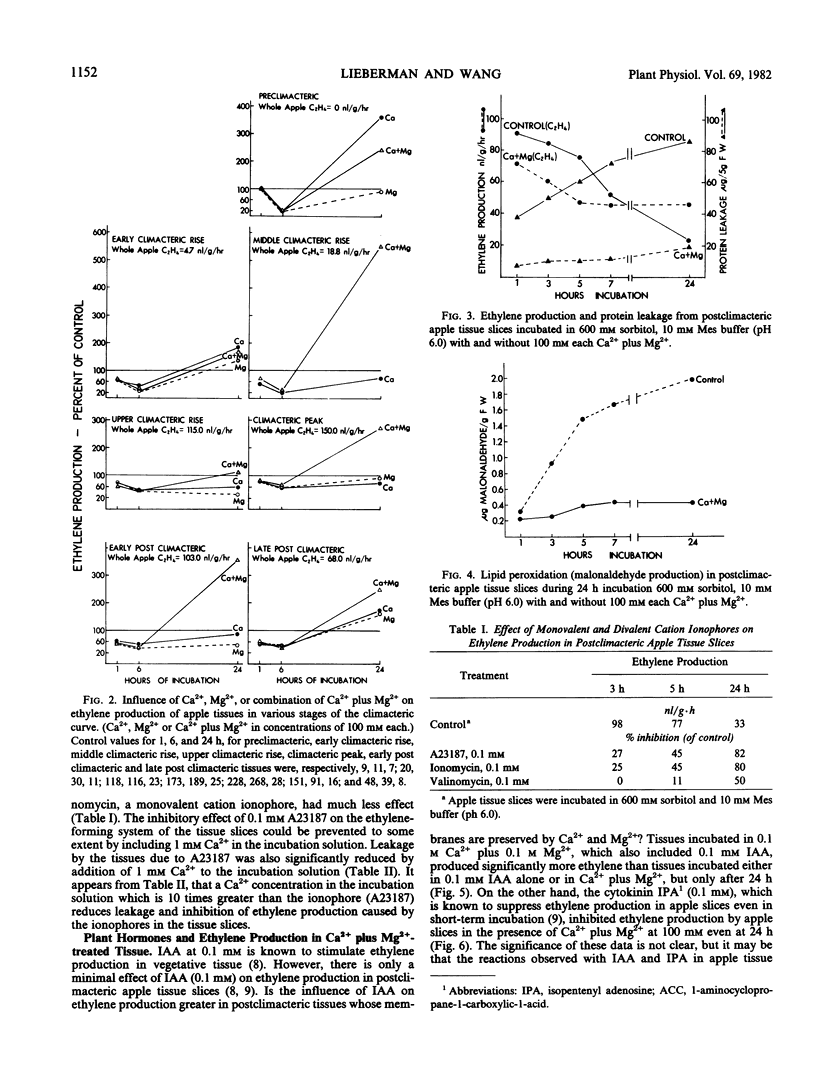
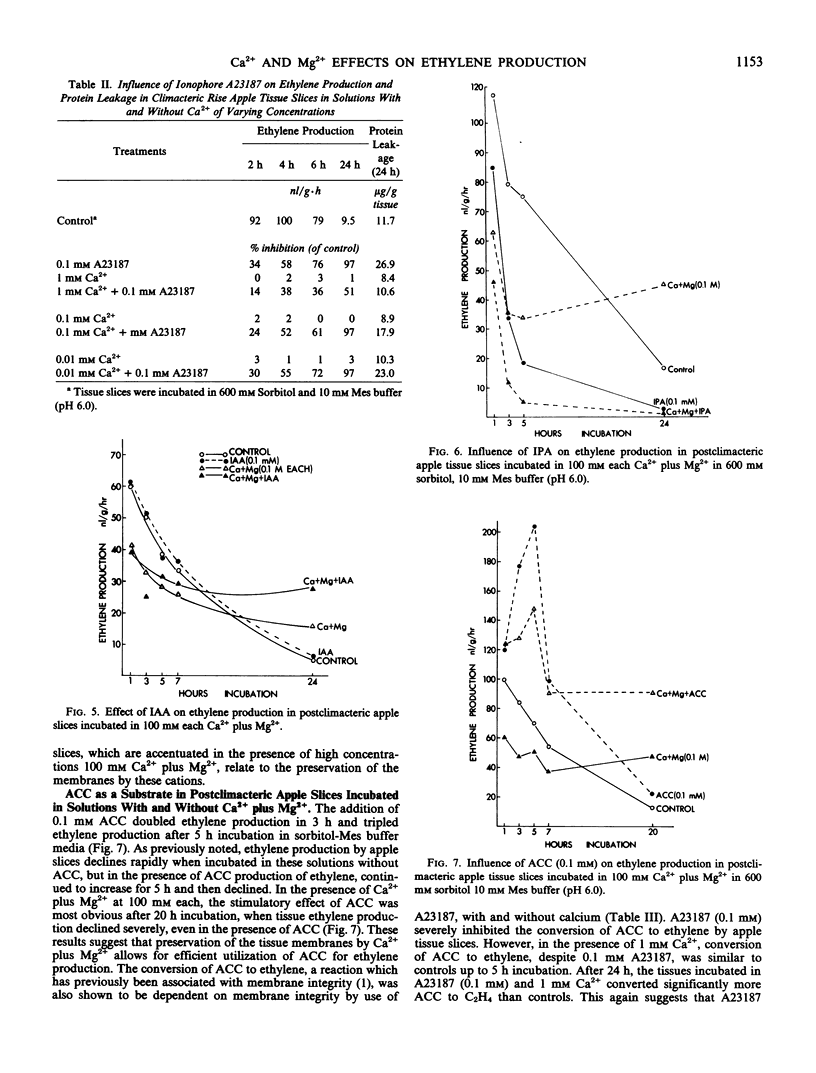
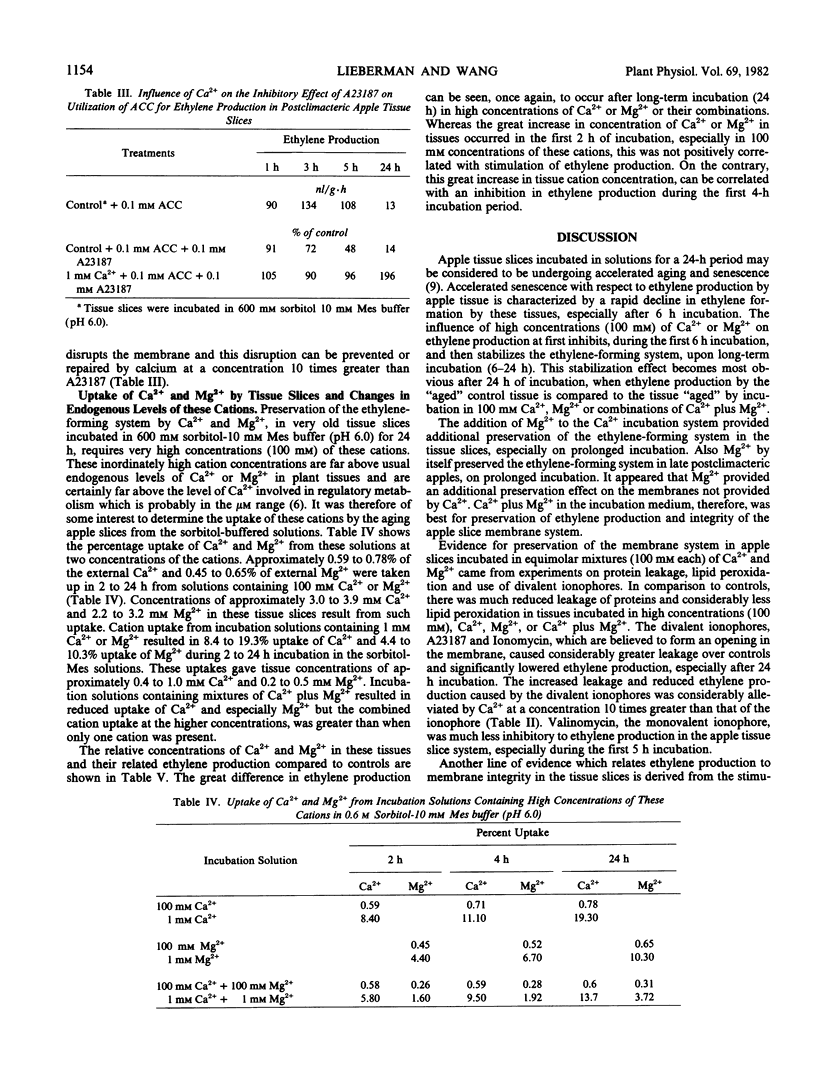
The decline in ethylene production in apple (Pyrus malus L. cv. Golden Delicious) tissue slices during 24 hours incubation in 600 millimolar sorbitol and 10 millimolar 2-(N-morpholino)ethanesulfonic acid buffer (pH 6.0) is recognized as a senescent phenomenon. The inclusion of very high concentrations (100 millimolar) of Ca2+, Mg2+, or Ca2+ plus Mg2+ severely inhibited ethylene production during the first 6 hours of incubation. However, after 6 hours and up to 24 hours the ethylene-forming system was stablized. These high concentrations of Ca2+, Mg2+, or Ca2+ plus Mg2+ virtually eliminated lipid peroxidation and protein leakage from these slices. Also conversion of 1-aminocyclopropane-1-carboxylic-1-acid to ethylene and the influence of indoleacetic acid on ethylene production was stabilized after 24 hours of incubation by these high concentrations of Ca2+, Mg2+, and Ca2+ plus Mg2+. Addition of divalent ionophores severely inhibited ethylene production, but this inhibition was prevented by Ca2+ in concentrations greater than the ionophore. These data suggest that the loss of ethylene production by aging tissue slices results from degradation of membranes. They support previous work that indicates that the ethylene-forming system, perhaps the segment of the pathway from 1-aminocyclo-propane-1-carboxylic-1-acid to ethylene, resides in the plasma membrane.
Full text
PDF
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Apelbaum A., Burgoon A. C., Anderson J. D., Solomos T., Lieberman M. Some Characteristics of the System Converting 1-Aminocyclopropane-1-carboxylic Acid to Ethylene. Plant Physiol. 1981 Jan;67(1):80–84. doi: 10.1104/pp.67.1.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beeler T. J., Jona I., Martonosi A. The effect of ionomycin on calcium fluxes in sarcoplasmic reticulum vesicles and liposomes. J Biol Chem. 1979 Jul 25;254(14):6229–6231. [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Lieberman M., Baker J. E., Sloger M. Influence of Plant Hormones on Ethylene Production in Apple, Tomato, and Avocado Slices during Maturation and Senescence. Plant Physiol. 1977 Aug;60(2):214–217. doi: 10.1104/pp.60.2.214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lieberman M., Kunishi A. T. Ethylene-forming Systems in Etiolated Pea Seedling and Apple Tissue. Plant Physiol. 1975 Jun;55(6):1074–1078. doi: 10.1104/pp.55.6.1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poovaiah B. W., Leopold A. C. Deferral of leaf senescence with calcium. Plant Physiol. 1973 Sep;52(3):236–239. doi: 10.1104/pp.52.3.236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poovaiah B. W., Leopold A. C. Inhibition of abscission by calcium. Plant Physiol. 1973 May;51(5):848–851. doi: 10.1104/pp.51.5.848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pressman B. C. Biological applications of ionophores. Annu Rev Biochem. 1976;45:501–530. doi: 10.1146/annurev.bi.45.070176.002441. [DOI] [PubMed] [Google Scholar]


