Abstract
Rationale
While neurosteroids are well-described positive allosteric modulators of GABAA receptors, the binding sites that mediate these actions have not been definitively identified.
Objectives
To synthesize neurosteroid analogue photolabeling reagents that closely mimic the biological effects of endogenous neurosteroids and have photochemical properties that will facilitate their use as tools for identifying the binding sites for neurosteroids on GABAA receptors.
Results
Two neurosteroid analogues containing a trifluromethyl-phenyldiazirine group linked to the steroid C11 position were synthesized. These reagents, CW12 and CW14, are analogues of allopregnanolone (5α-reduced steroid) and pregnanolone (5β-reduced steroid), respectively. Both reagents were shown to have favorable photochemical properties with efficient insertion into the C–H bonds of cyclohexane. They also effectively replicated the actions of allopregnanolone and pregnanolone on GABAA receptor functions: they potentiated GABA-induced currents in Xenopus laevis oocytes transfected with α1β2γ2L subunits, modulated [35S]t-butylbicyclophosphorothionate binding in rat brain membranes and were effective anesthetics in Xenopus tadpoles. Studies using [3H]CW12 and [3H]CW14 showed that these reagents covalently label GABAA receptors in both rat brain membranes and in TSA cells expressing either α1 and β2 subunits or β3 subunits of the GABAA receptor. Photolabeling of rat brain GABAA receptors was shown to be both concentration-dependent and stereospecific.
Conclusions
CW12 and CW14 have the appropriate photochemical and pharmacological properties for use as photolabeling reagents to identify specific neurosteroid binding sites on GABAA receptors.
Keywords: Neurosteroids, photolabeling, GABAA receptors
Introduction
Certain endogenous pregnane steroids and their synthetic analogues (broadly termed neurosteroids) are potent, rapidly acting intravenous anesthetics in vertebrates (Atkinson et al. 1965; Gyermek and Soyka 1975; Selye 1941). The sedative/anesthetic actions of neurosteroids have been shown to be mediated by GABAA receptors (Harrison et al. 1987). At low concentrations, neurosteroids potentiate the effect of GABA on GABAA receptors whereas at higher concentrations they can also directly activate the ion channel. Evidence from multiple sources indicates that neurosteroid actions are mediated by two (or more) distinct classes of binding sites on the GABAA receptor: (1) Radioligand binding assays show that neurosteroids allosterically enhance [35S]TBPS binding to GABAA receptors at low concentrations (NS1 site) and inhibit it at higher concentrations (NS2 site) (Evers et al. 2010; Srinivasan et al. 1999). (2) Single channel electrophysiological studies demonstrate that prototypical neurosteroids such as allopregnanolone (3α5αP) produce several discrete kinetic effects on α1β2γ2 GABAA receptors that appear to occur at distinct, non-overlapping steroid concentrations and that specific neurosteroid analogues (e.g. B285, etiocholanolone) produce some, but not all of these single channel effects (Akk et al. 2010; Akk et al. 2007). These data indicate that different neurosteroid binding sites mediate mechanistically distinct components of the potentiation of GABA responses and that neurosteroid analogues can be selective agonists for specific neurosteroid binding sites (Akk et al. 2004; Li et al. 2007). (3) Site-directed mutagenesis studies have shown that mutations in the first (TM1; Q241) and fourth (TM4; Y410 and N407) transmembrane domains (TMDs) of the α1 subunit of the GABAA receptor prevent both neurosteroid potentiation and activation, whereas mutations in the TM1 (T236) region of the α1 subunit and the TM3 (Y284) region of the β2 subunit selectively prevent direct activation (Hosie et al. 2006). A model based on these data proposes a potentiating site between TM1 and TM4 of the α1 subunit, and an activating site in the cleft between TM1 of the α1 subunit and TM3 of the β2 subunit (Hosie et al. 2009b; Hosie et al. 2007). Electrophysiological experiments examining the effects of a variety of structurally modified neurosteroids on GABAA receptors with various mutations in the putative binding sites do not fully support this model, leaving it unclear whether these mutations represent portions of neurosteroid binding pockets or are sites involved in transduction of neurosteroid binding signal at a different site (Li et al. 2009).
Photoaffinity labeling with neurosteroid analogues provides a way to directly identify neurosteroid binding sites, rather than inferring their existence from changes in functional effects in mutagenesis experiments. We have previously reported on the use of the neurosteroid analogue photolabeling reagent 6-Azi-pregnanolone (6-AziP) to identify voltage-dependent ion channel-1 (VDAC) (Darbandi-Tonkabon et al. 2003; Darbandi-Tonkabon et al. 2004) and β-tubulin (Chen et al. 2012a) as neurosteroid binding proteins and to identify a neurosteroid binding site on the β3 subunit of homopentameric GABAA receptors (Chen et al. 2012b). The use of aliphatic diazirines, such as 6-AziP, as photolabeling reagents has some specific limitations: These reagents preferentially undergo intra- rather than intermolecular reactions limiting their efficiency as photolabeling reagents. Additionally, the mechanism through which aliphatic diazirines photolabel may be through generation of a diazo intermediate rather than through a carbene. Thus, aliphatic diazirines frequently only label nucleophilic amino acids. In contrast, reagents containing a trifluoromethyl-phenyldiazirine (TPD) group generate a carbene following ultraviolet irradiation; this enables them to insert into any C–H bond and to preferentially undergo intermolecular interactions. To address the limitation of 6-AziP, we have synthesized and tested two novel compounds, CW12 and CW14, as neurosteroid analogue photolabeling reagents. These reagents have a TPD group attached via an ether linkage at the C11 position on the steroid backbone. CW12 is a 5α-reduced steroid (analogue of allopregnanolone), whereas CW14 is 5β-reduced (analogue of pregnanolone (3α5βP)) to provide analogues of the two major endogenous neurosteroids, which have been shown to have similar but non-identical effects on GABAA receptors (Evers et al. 2010).
An effective photolabeling reagent must meet several criteria: First it must have photochemical properties that allow it to efficiently label any amino acid on a protein. Second, the reagent must closely reproduce the physiological effects of the compound it is designed to mimic. In this study we describe the photochemical properties of CW12 and CW14 and their neurosteroid-like effects on GABAA receptors and tadpoles. We also demonstrate that these regents photolabel GABAA receptors in both brain and in a heterologous expression system.
Methods
Synthesis of CW12 and CW14
The synthesis of CW12 (5α-reduced; compound 9a) and CW14 (5β-reduced; compound 9b) followed the reaction schemes shown in Supplemental Figures 1–2. Spectroscopic and elemental analysis data for CW12 and CW14 can also be found in the Supplemental Materials. The 3,20-dione precursors (compounds 10a and 10b) used for preparation of [3H]CW12 and [3H]CW14, were synthesized following the reaction scheme shown in Supplemental Figure 2. The radiosynthesis of [3H]CW12 and [3H]CW14 followed the reaction scheme shown in Supplemental Figure 3. The purity of CW12, CW14 and their tritiated analogues was determined using straight phase TLC. The non-radiolabeled compounds were visualized by charring and migrated as single bands. [3H]CW12 and [3H]CW14 were visualized by autoradiography as single bands that co-migrated with the non-radiolabeled standards (data not shown). These data indicate the purity of CW12 and CW14 and that the borohydride reductions produced the desired radiolabeled compounds and did not produce a diol (reduction of the 20-ketone).
[3H]CW12 was prepared from the 5α-reduced (3α)-3-hydroxy-11α-methyl-11β-(4-trifluoromethyldiazirinyl)benzyloxypregnan-3,20-dione as follows: 2 mg of this 3,20 dione was dissolved in ethanol with 25 mCi of [3H]-Na-borohydride (≈20 Ci/mmol) and reacted at room temperature for 3 hours in the dark. The reaction was terminated by acidification and the steroidal reaction products extracted into diethyl ether. The products (3α- and 3β-epimers) were separated by preparative straight-phase thin layer chromatography (TLC) (hexane:ethyl acetate:: 1:1) and visualized by autoradiography. CW12 was identified by co-migration with a non-radiolabeled standard which was visualized by charring the TLC plate following aerosol application of 5% sulfuric acid/95% ethanol. [3H]CW12 (the 3α-epimer) was scraped from the plate, eluted from the silica with chloroform:methanol::2:1 and stored in ethanol at −20°C. [3H]-CW14 was prepared in an analogous manner starting with the 5β-reduced (3α)-3-hydroxy-11α-methyl-11β-(4-trifluoromethyldizirinyl)benzyloxypregnan-3,20-dione. The 3α-epimer is the minor product in the preparation of [3H]CW12, whereas the 3α-epimer is the major product in the preparation of [3H]CW14 (Supplemental Figure 3).
To characterize the photoreactivity of the photolabeling reagents, [3H]CW12 and [3H]CW14 were dissolved in either ethanol or cyclohexane and irradiated for 5 min using a 450-watt Hanovia medium pressure mercury lamp filtered through a 1.5 cm thick saturated Cu2SO4 solution to absorb all light <315 nm (Darbandi-Tonkabon et al. 2003). The samples were then applied to reverse phase silica chromatography plates (Fisher Scientific, Pittsburgh, PA) and developed with a mobile phase of acetonitrile:H2O::95%:5%. Non-radioactive standards were visualized by charring and tritiated products by autoradiography.
Membrane preparation
Rat brain membranes were prepared from frozen rat brains (Pel Freez Biologicals) using previously described methods (Darbandi-Tonkabon et al. 2003). Briefly, rat brain cortex was homogenized in ice cold 0.32 M sucrose (10 ml/g of tissue) and centrifuged for 10 min at 1,500g. The supernatant was centrifuged for 30 min at 10,000g to obtain the P2 pellet. The P2 pellet was washed with 50 mM K-Phosphate/200 mM NaCl, pH 7.4. The pellet was re-suspended in 50 mM K-Phosphate/200 mM NaCl, pH 7.4 and recollected by centrifugation for 20 min at 10,000g. Aliquots of the membranes were stored at −80 °C.
Preparation of α1FLAGβ2 and β3FLAG GABAA receptors in TSA cell membranes
Transformed human embryonic kidney (TSA) cells were stably transfected with rat α1FLAG and β2 GABAA receptor subunits or with rat β3FLAG subunits using methods identical to those described for expression of GABA receptors in QT6 cells (Evers et al. 2010). Cells were grown in suspension culture in Dulbecco’s modified Eagle’s medium/F12 medium containing 50 mM HEPES and 10% fetal bovine serum. TSA cells were harvested and membranes were prepared by using the protocol previously described for preparation of QT6 cell membranes (Evers et al. 2010).
Photolabeling
Photolabeling of rat brain membranes and TSA cell membranes was performed as previously described (Darbandi-Tonkabon et al. 2003). Briefly, membranes (400 μg RBM or TSA cell) were incubated with [3H]CW12 or [3H]CW14 on ice for 30 min. The samples were then irradiated with UV light for 5 min using the Hanovia photoreactor. The membranes were collected by centrifugation and solubilized with SDS sample buffer (312.5 mM Tris-HCl, 5% SDS, 0.5 M dithiothreitol, 50% glycerol, and 0.1% bromphenol blue) or BN-PAGE loading buffer.
Blue native polyacrylamide gel electrophoresis (BN-PAGE) and SDS-polyacrylamide gel electrophoresis (SDS-PAGE)
BN-PAGE was carried out as previously described (Wittig et al. 2006). Samples were dissolved in 25 mM NaCl, 25 mM Imidazole/HCl, 0.5 mM EDTA, 7% digitonin, 10% glycerol, 1% Coomassie blue G-250 and 100 mM 6-aminohexanoic acid. After centrifugation at 12,000g for 15 min at 4°C, the solubilized proteins were loaded onto 5–13% gradient gels, which were cast using a gradient mixer at 4°C. The gel solutions contained 50 mM BisTris and 500 mM 6-Aminocaproic acid. The cathode buffer contained 50 mM Tricine, 15 mM BisTris, and 0.02% Coomassie blue G-250; and the anode buffer contained 50 mM BisTris, pH7.0. For SDS-PAGE, samples were solubilized in Laemmli Sample Buffer (62.5 mM Tris-HCl, pH 6.8, 2% SDS, 25% glycerol, 0.01% Bromophenol Blue).
Gel slicing
After electrophoresis, the gels were cut into vertical columns and sliced in 1 mm horizontal slices using a DE 113 manual gel slicer (Hoeffer Scientific Instruments, San Francisco, CA). Slices were digested with 4 ml of tissue solubilizer consisting of 3a20TM and TS-2 (ratio 9:1) for 48 hours, and the radioactivity in each slice was determined by scintillation spectrometry (Bureau and Olsen 1993).
Western blotting
Proteins from SDS-PAGE or BN-PAGE gels were transferred onto polyvinylidene fluoride membranes using a semi-dry transfer instrument. For BN-PAGE gels, the transfer solution contained 50 mM Tricine and 15 mM BisTris. For SDS-PAGE gels, the transfer solution contained 25 mM Tris, 192 mM glycine, pH 8.3, and 20% methanol. The membranes were blocked with 5% dried milk and incubated with antibodies to the α1 (Santa Cruz, 1:200), γ2 (Chemicon, 1:200) or β2/β3 subunits (bd17; Chemicon, 1:200) of the GABAA receptor, or to β-tubulin (Santa Cruz, 1:100), or voltage-dependent anion channel-1 (Abcam, 1:200) for 1 h followed by peroxidase-conjugated secondary antibody (Santa Cruz, 1:10,000). Immunoreactive bands were visualized using the ECL-plus western blotting detection system (Amersham Bioscience).
Autoradiography
SDS-PAGE gels were fixed for 30 minutes in isopropanol: water: acetic acid (25:65:10) at room temperature and then dried under vacuum. The dried gels were placed in cassettes and exposed to [3H]-sensitive ultra-film (Kodak Biomax light film) at −70 °C for periods ranging from five days to two weeks (Darbandi-Tonkabon et al. 2003; Darbandi-Tonkabon et al. 2004).
[35S]t-butylbicyclophosphorothionate ([35S]TBPS) binding
[35S]TBPS binding assays were performed using previously described methods (Covey et al. 2000; Hawkinson et al. 1994) with modification. Briefly, aliquots of membrane suspension (0.5 mg/ml final protein concentration in assay) were incubated with 1–2 nM [35S]TBPS (60-100Ci/mmol, Perkin Elmer Life Science, Boston, MA), 5 μl-aliquots of steroid in Me2SO solution (final steroid concentrations ranged from 1 nM to 10 μM) with or without 5μM GABA, in a total volume of 1 ml of assay buffer. The assay buffer was 50 mM K-phosphate buffer, pH 7.4, 200 mM NaCl. Nonspecific binding was defined as binding observed in the presence of 200 μM picrotoxin. Assay tubes were incubated for 2 hours at room temperature. A Brandel (Gaithersburg, MD) cell harvester was used to collect the membranes on Whatman glass fiber (GF/C) filter paper which was washed with 4 ml of ice-cold buffer three times, dried and dissolved in 4 ml ScintiVerse II (Fisher Scientific, Pittsburgh, PA). Radioactivity bound to the filters was measured by liquid scintillation spectrometry. Each data point was done in triplicate and all experiments were performed three times. The average specific binding values of each triplicate were used for curve fitting and IC50 and EC50 values are presented as the parameters of the curve fitting to the pooled data from the repeated experiments ± SEM. The data from binding experiments performed in the presence of GABA were fit to the Hill equation and data from binding experiments performed in the absence of GABA were fit to a 2-component binding equation using Sigma Plot version 8 (SPSS; Chicago IL) and Prism (GraphPad Software Inc, San Diego, CA) as previously described (Evers et al. 2010).
Xenopus oocyte electrophysiology
Stage V–VI oocytes were harvested from sexually mature female X. laevis (Xenopus One, Northland, MI) and defolliculated by shaking for 20 min at 37°C in collagenase (2 mg/ml). Capped mRNA encoding rat GABAA receptor α1, α1(Q241L) β2 and γ2L subunits were transcribed in vitro using the mMESSAGE mMachine Kit (Ambion, Austin, TX) from linearized pBluescript vectors containing receptor coding regions. The α1(Q241L) mutation was made using the QuikChange site-directed mutagenesis kit (Agilent Technologies, Santa Clara, CA) and mutated subunits were fully sequenced to confirm that only the desired mutation had been produced (Akk et al. 2008). Subunit transcripts were injected in equal parts (20–40 ng total RNA) 8–24 h following defolliculation. Oocytes were incubated up to 5 days at 18°C in ND96 medium containing (in mM) 96 NaCl, 1 KCl, 1 MgCl2, 2 CaCl2, and 5 HEPES at pH 7.4, supplemented with pyruvate (5 mM), penicillin (100 U/ml), streptomycin (100 μg/mL), and gentamycin (50 μg/ml). The cDNAs for the rat GABAA-receptor subunits were originally provided by A. Tobin, University of California, Los Angeles (α1); P. Malherbe, Hoffman-La Roche, Switzerland (β2); and C. Fraser, National Institute on Alcohol Abuse and Alcoholism (γ2L).
GABA currents were measured with a Warner OC725 two-electrode voltage-clamp amplifier 2–5 days following RNA injection in a bath of unsupplemented ND96 medium. Intracellular recording pipettes had a resistance of ~1 MΩ when filled with 3M KCl. Each oocyte was initially challenged with GABA alone and then with a single concentration of CW12 or CW14; this was done to provide a same-oocyte GABA comparator and to avoid the complication of slow neurosteroid washout. Control responses to another potentiator (a non-photolabeling steroid or pentobarbital) were obtained from the same oocyte. Compounds were applied alone (direct activation experiments) or co-applied with GABA (potentiation experiments) using a gravity-flow perfusion system. Holding potential was −70 mV, and peak current during 20 s drug applications was used for quantification. Data were acquired and analyzed with pCLAMP software (Molecular Devices, CA). Statistical differences were determined using a two-tailed Student’s t-test.
Tadpole anesthetic assay
Assays for neuroactive steroid-induced loss-of-righting reflex (LRR) and loss-of-swimming reflex (LSR) in Xenopus laevis tadpoles were performed and analyzed as previously described (Covey et al. 2000).
Animal experiments including tadpole anesthesia assays, Xenopus oocyte harvesting and use of rat brain tissue comply with ethical principles of laboratory animal care. The experimental protocols were approved by the Washington University Animal Studies committee and are in compliance with current United States law.
Results
Photochemistry of CW12 and CW14
To examine the ability of CW14 and CW12 to undergo bimolecular insertion reactions (essential for photolabeling), CW14, [3H]CW14 and its 3β-epimer (3β-[3H]CW14) were irradiated with UV light in either ethanol or cyclohexane and the photolysis products were analyzed on reverse-phase TLC. CW14, [3H]CW14 and 3β-[3H]CW14 all migrated as single bands in the absence of UV-irradiation, with CW14 (Rf = 0.38) running slightly slower (less polar) than its 3β-epimer (Rf = 0.4) (Figure 2). Following UV irradiation in ethanol, two major photolysis products were observed for CW14 (denoted by * and ** in Figure 2); both products were more polar than the parent compound. UV irradiation in cyclohexane also produced two major photolysis products, one slightly more polar (denoted by * in Figure 2) and one significantly less polar (denoted by # in Figure 2) than the parent compound. The non-polar product was uniquely observed in cyclohexane, indicating that CW14 can undergo carbene-mediated bimolecular insertion, forming an adduct with cyclohexane. Photolysis products of significantly higher polarity were also observed (top of the plate) following irradiation of [3H]CW14 in either ethanol or cyclohexane. These products were not further identified, but are likely to result from intramolecular reactions since they were observed following photolysis in either ethanol or cyclohexane. These products were not observed following photolysis of non-radiolabeled compounds. 3β-[3H]CW14 produced photolysis products in both ethanol and cyclohexane with migration patterns on reverse-phase TLC similar (but slightly more polar) to those observed with [3H]CW14 (Figure 2). [3H]CW12 showed a pattern of photolysis products in ethanol and cyclohexane similar to those observed with [3H]CW14, with clear evidence of formation of a cyclohexane adduct (not shown).
Figure 2. Photolysis products of CW14, [3 H]CW14 and [3H]3β-CW14.
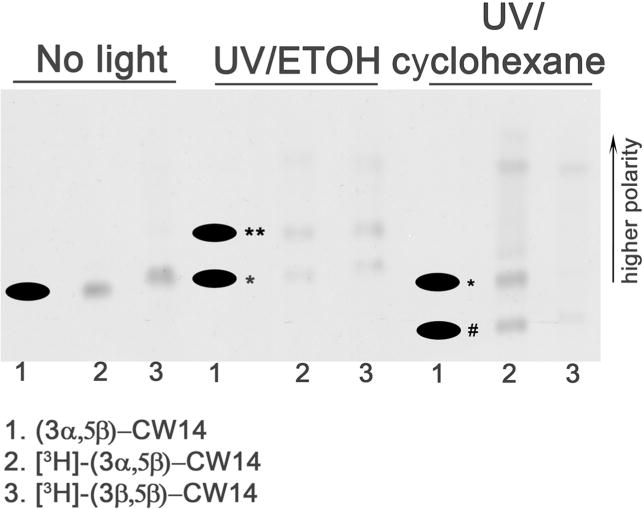
The figure shows a reverse-phase TLC (RP-TLC) plate with the origin at the bottom. More polar compounds migrate further up the plate. CW14 (1), [3H]-CW14 (2), and the 3β-epimer of [3H]CW14 (3) irradiated with ultraviolet light (UV) in either ethanol (ETOH, center) or cyclohexane (right) were analyzed by RP-TLC. Non UV-irradiated compounds 1, 2 and 3 were also analyzed by RP-TLC (left). An autoradiogram of the plate was produced to visualize radiolabeled compounds, followed by charring of the plate to visualize non-radiolabeled compounds. The image is an overlay of the autoradiogram with the charred plates (the black dots indicate the charred spots). The photolysis product slightly more polar than the parent compound (presumed intramolecular reaction) is denoted by *. The CW14-ethanol adduct is denoted by ** and the CW14-cyclohexane adduct is denoted by #.
The biological activity of CW12 and CW14
To determine whether CW12 and CW14 are neurosteroid-like modulators of GABAA receptors, we employed three criteria: (1) their ability to modulate GABA-elicited current in oocytes transfected with GABAA receptor subunits; (2) their ability to modulate [35S]TBPS binding to native GABAA receptors (rat brain membranes) with a pattern similar to that observed with endogenous neurosteroids and; (3) their ability to function as anesthetics in Xenopus tadpoles.
Electrophysiological studies
To study the effects of CW12 and CW14 on GABAA receptor currents, we first tested their modulation of GABA-elicited chloride currents in Xenopus laevis oocytes expressing α1β2γ2 subunits of GABAA receptors. Figure 3A shows representative traces of currents activated by 2 μM GABA in the absence and presence of neurosteroids. 2 μM GABA is well below the EC50 concentration of GABA, providing sufficient dynamic range to measure neurosteroid potentiation. GABA-elicited currents were enhanced by the endogenous neurosteroids, pregnanolone (3α5βP) and allopregnanolone (3α5αP). The average current produced by 0.5 µM 3α5αP + 2 µM GABA varied between 3.15 ± 0.36 times (n = 4; same oocyte comparator for CW14) and 2.16 ± 0.36 times greater than the current induced by 2 μM GABA alone (n = 7; same oocyte comparator for CW12). 0.5 μM CW14 (Fig. 3A1) and CW12 (Fig. 3A2) produced substantially greater potentiation of the GABA-elicited currents. The average current produced by 0.5 μM CW14 + 2 μM GABA (n=4) was 9.75 ± 1.94 times greater than the current induced by 2 μM GABA alone and the current elicited by 0.5 μM CW12 + 2 μM GABA (n = 7) was 7.12 ± 2.65 times greater. The potentiating effects of CW12 and CW14 on GABA-elicited currents were maximal at 0.5 µM (not shown), whereas 3α5αP and 3α5βP have EC50 values of 2.3 ± 0.03 μM (Wittmer et al. 1996) and 1.2 ± 0.40 μM (Covey et al. 2000) respectively. In contrast to 3α5αP and 3α5βP, the potentiating effects of CW14 and CW12 were not completely eliminated after washing with ND96 solution for 1 min. The higher hydrophobicity of CW14 and CW12 is likely the reason for the slow washout.
Figure 3. CW14 and CW12 modulate GABAA receptor function.
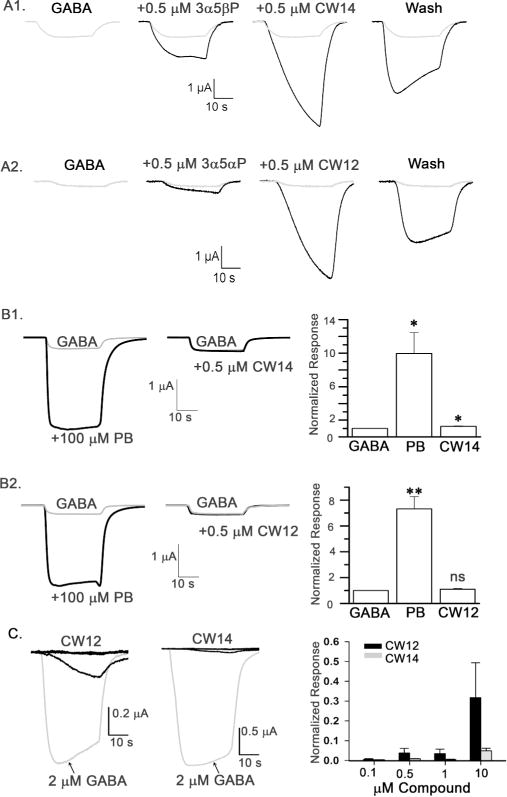
A. CW14 and CW12 potentiate GABA-elicited currents in Xenopus laevis oocytes expressing α1β2γ2 GABAA receptors. Representative traces of currents elicited by 2 μM GABA in the absence and presence of 0.5 μM pregnanolone (3α5βP) and CW14 (panel A1), or 0.5 μM allopregnanolone (3α5αP) and CW12 (panel A2). Membrane potential was −70 mV. B. CW14 and CW12 have minimal effect on GABA-induced currents in oocytes expressing α1(Q241L)β2γ2L GABAA receptors. Representative traces of currents elicited by 0.2 μM GABA in the absence and presence of 100 μM pentobarbital (PB, positive control) and 0.5 μM CW14 (panel B1) or 100 μM PB and 0.5 μM CW12 (panel B2). Summary graphs (right) show the effects (mean ± SEM) of PB and CW14 of (panel B1; n =5 oocytes) or PB and CW12 (panel B2; n =4 oocytes) relative to the current produced by 0.2 μM GABA alone. * = P< 0.05 compared to GABA alone; **P< 0.01 compared to GABA alone; ns = not significant compared to GABA alone. C. Direct activation of GABAA receptors by CW12 and CW14 in oocytes expressing α1β2γ2L GABAA receptors. Representative traces (left) of currents elicited by 0.1, 0.5, 1.0 and 10 μM CW12 and CW14 or 2 μM GABA (grey trace). Bar graph shows concentration-dependent activation of current by CW12 and CW14 (n=4 for each concentration) expressed as a fraction of the current elicited by 2 μM GABA.
There are numerous positive allosteric modulators of GABA-elicited currents that act at distinct sites on GABAA receptor. The α1(Q241L) mutation has been shown to selectively reduce or eliminate potentiation by numerous neuroactive steroids (Akk et al. 2008; Hosie et al. 2006), and can be thus employed as a specific indicator of the involvement of the classic neurosteroid site(s). To confirm that the steroid analogues CW14 and CW12 act via the classic neurosteroid potentiation site(s), we tested steroid-modulation in oocytes expressing α1(Q241L)β2γ2 receptors. As shown in Figure 3B1, coapplication of 0.5 μM CW14 with 0.2 μM GABA potentiated the peak response to 1.24 ± 0.07 (mean ± SEM; 5 cells) times that of GABA alone. While this potentiating effect was statistically significant (P<0.05; t-test vs. no effect), the magnitude of CW14 potentiation in the mutated receptors was markedly reduced in comparison to wild-type (compare Figure 3B1 vs 3A1). Application of 0.5 µM CW12 produced no significant potentiation (1.10 ± 0.07 times control, 4 cells) on the peak response elicited by 0.2 µM GABA in the α1(Q241L) containing receptors (Figure 3B2). Control experiments were conducted to demonstrate lack of effect of the α1(Q241L) mutation on potentiation by another allosteric potentiator, pentobarbital (PB), whose binding site (Chiara et al. 2013) does not overlap with the neurosteroid site. Coapplication of 100 μM PB with 0.2 μM GABA enhanced the maximal response to 8.78 ± 1.45 times control (pooled data from 5 cells used in experiments with CW14 and 4 cells used with CW12; Figure 3B1 and 3B2).
We also tested the ability of CW12 and CW14 to directly activate GABAA chloride currents in the absence of GABA (Figure 3C). 10 μM CW12 activated a current 0.32 ± 0.18 times the current elicited by 2 μM GABA (n=4); this is similar to the current directly elicited by 10 µM 3α5αP (0.37 ± 0.07; n=8). Markedly smaller current were directly elicited by 0.1 – 1.0 µM CW12 (n = 4 at each concentration). 10 μM CW14 directly activated a small GABAA chloride current (0.05 ± 0.01 times control; n = 4), similar to the current directly elicited by 10 µM 3α5βP (0.06 ± 0.03; n = 10).
Modulation of [35S]TBPS binding in rat brain membranes
Neurosteroids have a characteristic pattern by which they allosterically modulate binding of the convulsant [35S]TBPS to GABAA receptors (Evers et al. 2010; Srinivasan et al. 1999). To determine if CW12 and CW14 also produce this pattern, [35S]TBPS binding to rat brain membranes was measured in the presence of various concentrations of CW12 and CW14 in the presence and absence of 5 μM GABA (Figure 4A). In the presence of GABA, CW12 and CW14 produced single component inhibition of [35S]TBPS binding with IC50 values of 12.3 ± 3.9 nM and 15.9 ± 5.0 nM respectively. CW12 and CW14 appeared more potent than 3α5αP and 3α5βP which have IC50 values of 71 ± 18 nM and 74 ± 7 nM respectively (Covey et al. 2000). In the absence of GABA (Figures 4A1 and 4A2, control) CW12 and CW14 showed the characteristic neurosteroid pattern of allosteric modulation with enhancement of [35S]TBPS binding at low steroid concentrations and inhibition at higher concentrations (Evers et al. 2010). CW12 enhanced [35S]TBPS binding with an EC50= 57.3 ± 26.5 nM and inhibited binding with an IC50 = 204.9 ± 54.5 nM. CW14 had an EC50= 18.4 ± 31.4 nM and an IC50 = 1.3 ± 0.5 μM.
Figure 4. Biological actions of CW12 and CW14.
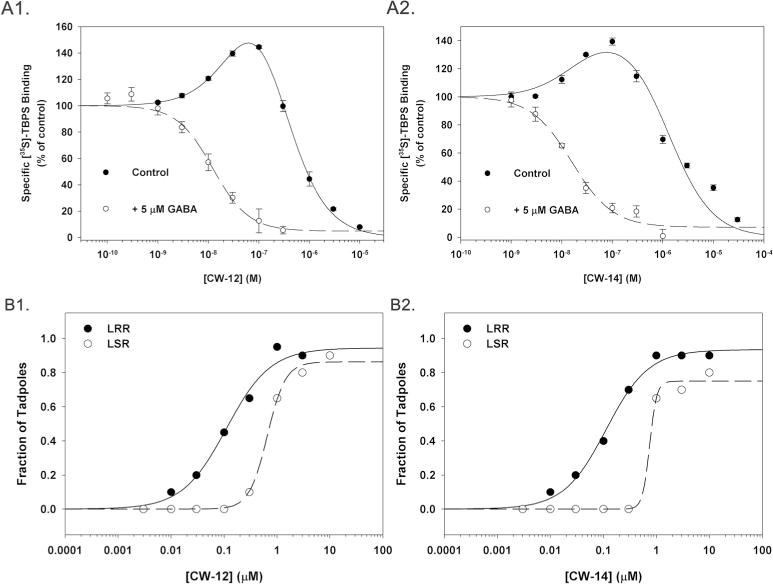
A. CW12 (panel A1) and CW14 (panel A2) modulation of [35S]TBPS binding in rat brain membranes. Each curve represents the mean ± SEM of three experiments with each point performed in triplicate. Closed circles (continuous lines) are from experiments performed in the absence of GABA and open circles with dashed lines from experiments performed in the presence of 5 μM GABA. B. Anesthetic effects of CW12 and CW14 in tadpoles. Loss of righting reflex (LRR) and loss of swimming reflex (LSR) in tadpoles induced by CW12 (B1) and CW14 (B2). The EC50 values for LRR by CW12 and CW14 were 110 ± 21 nM and 112 ± 14 nM respectively. The EC50 values for LSR by CW12 and CW14 were 655 ± 43 nM and 747 ± 364 nM, respectively.
Tadpole anesthesia
To test the anesthetic activity of CW12 and CW14, loss of righting reflex (LRR) and loss of swimming reflex (LSR) was measured in prelimbud stage Xenopus tadpoles (Fig. 4B). The EC50 values for LRR and LSR were 112 ± 14 nM and 747 ± 364 nM for CW14; and 110 ± 21 nM and 655 ± 43 nM for CW12 (n = 20 tadpoles at each concentration). These values are similar to those observed with 3α5αP (LRR = 390 ± 40 nM; LSR = 5.50 ± 0.48 μM) and 3α5βP (LRR = 63 ± 3 nM; LSR = 300 ± 0.05 nM).
CW12 and CW14 photolabeling of rat brain membranes
To determine if CW12 and CW14 are effective photolabeling reagents for neurosteroid binding proteins, rat brain membranes (400 μg) were photolabeled with [3H]CW12 or [3H]CW14, analyzed by SDS-PAGE and radiolabeled bands were identified by autoradiography. 1 μM [3H]CW12 photolabeled multiple proteins with the most prominent band at ≈52-kDa (Figure 5A). Photolabeling with [3H]CW14 was concentration-dependent with prominent bands at ≈35-kDa, ≈52-kDa, ≈66-kDa and ≈100-kDa (Figure 5B). While the bands at ≈35-kDa and ≈52-kDa are likely to be VDAC (Darbandi-Tonkabon et al. 2003) and a GABAA receptor subunit respectively, our previous work has shown that the ≈52-kDa band may represent other neurosteroid binding proteins, such as β-tubulin (Chen et al. 2012a).
Figure 5. SDS-PAGE autoradiograms of [3H]CW12 and [3H]CW14 photolabeled rat brain membranes.
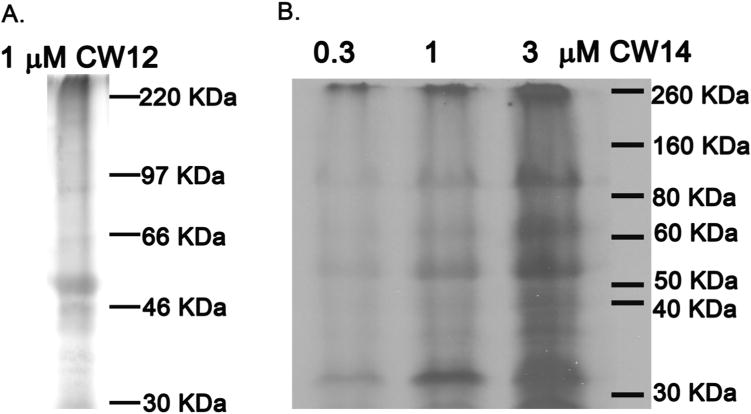
Rat brain membranes (400 μg) were photolabeled with either: (A) 1 μM [3H]CW12 or; (B) 0.3, 1 and 3 μM [3H]CW14. A band at ≈52-kDa is labeled by both [3H]CW12 and [3H]CW14. Photolabeling of the ≈52-kDa band by [3H]CW14 is concentration-dependent.
To clarify the identity of the photolabeled proteins, membranes were photolabeled with 10 μM [3H]CW14 and analyzed by blue-native electrophoresis, so that GABAA receptors would retain their pentameric structure (MW ≈250-kDa) and be identifiable by immunoblotting against multiple GABAA receptor subunits. As shown in Figure 6A, autoradiographic analysis of the blue native gel showed radiolabeled bands at ≈140, ≈240, ≈480 and ≈1200-kDa. Immunoblots to the α1, β2/3 and γ2 subunits identified the band at ≈240-kDa as the GABAA receptor, confirming that CW14 effectively photolabels this protein. The photolabeled band at ≈480-kDa was also stained by the GABAA receptor antibodies suggesting that it is a dimer of pentameric GABAA receptors. The photolabeled band at ≈140-kDa was identified as VDAC by immunoblot against VDAC-1. The photolabeled band at ≈1200-kDa was identified as β-tubulin by immunoblotting. The photolabeled bands between 480 and 1048-kDa were not identified, but may represent multimers of the GABAA receptor based on weak immunostaining with anti-GABAA subunit antibodies. One lane of the blue native gel was sliced and counted, indicating that the most prominently photolabeled protein was VDAC (≈140-kDa) followed by the GABAA receptor (≈240 and ≈480- kDa). Additionally, photolabeling of rat brain membranes with [3H]CW14 and its 3β-epimer ([3H]3β-CW14) were compared by BN-PAGE (Fig. 6B). The photolabeling of VDAC, GABAA receptors, and tubulin with [3H]CW14 demonstrated a concentration-dependent pattern (Fig. 6B left). However, minimal photolabeling was observed with 0.3 or 1 μM [3H]3β-CW14 (Fig. 6B right), indicating that photolabeling of both VDAC and the GABAA receptors was stereospecific.
Figure 6. Blue native-PAGE of [3H]CW 14 photolabeled rat brain membranes.
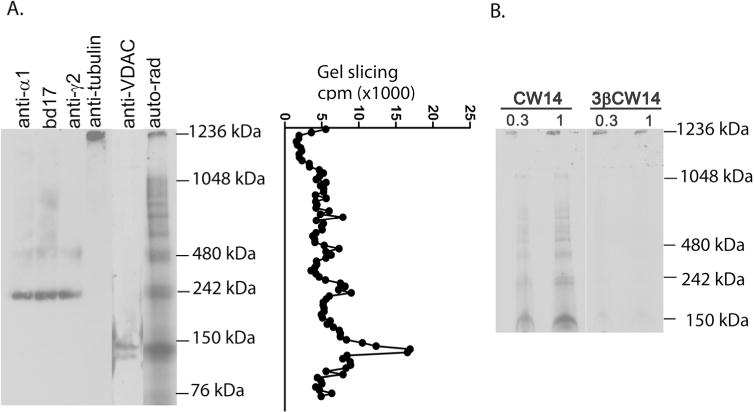
A. Rat brain membranes (400 μg) photolabeled with 3 μM [3H]CW14 were loaded onto a BN-PAGE followed by autoradiography, gel slicing or immunobloting with antibodies to the α1, β2/3 (bd17) or γ2 subunits of the GABAA receptor, to VDAC-1 or to β-tubulin. The photolabeled proteins correspond to GABAA receptors (≈240 and ≈480-kDa) as shown by anti-α1, bd17 and anti-γ2 antibodies; tubulin (≈1236 kDa); and VDAC (≈140 kDa). B. Autoradiogram of blue native-PAGE from rat brain membranes (400 μg) photolabeled with 0.3 and 1 μM [3H]CW14 or the 3β-epimer of [3H]CW14. [3H]CW14 photolabels GABAA receptors (≈240-kDa) in a concentration-dependent pattern. The photolabeling is stereospecific as evidenced by the minimal photolabeling of GABAA receptors and VDAC by [3H]3β-CW14.
CW14 photolabel recombinant GABAA receptors expressed in TSA cells
To further confirm that CW14 labels GABAA receptors, membranes (400 μg) from native TSA cells or from TSA cells expressing recombinant α1β2-heteromeric or β3-homomeric GABAA receptors were photolabeled with 10 μM [3H]CW14. The photolabeled proteins were separated by SDS-PAGE followed by gel slicing and scintillation counting. Two broad peaks of radioactivity, centered at ≈52-kDa and ≈100-kDa were observed in the α1β2 and β3-expressing membranes but not in the non-transfected TSA membranes (Figure 7). These peaks correspond to the molecular weights of monomers and dimers of GABAA receptor subunits. These results indicate that [3H]CW14 labels β3 and either β2 or α1 subunits of GABAA receptors.
Figure 7. CW14 photolabels GABAA receptors expressed in TSA cells.
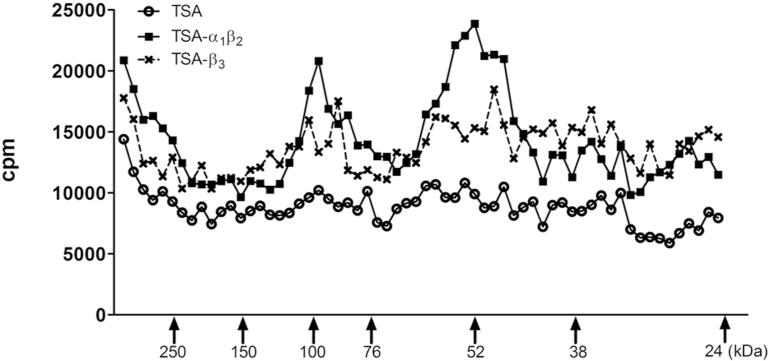
Membranes (400 μg protein) from wild-type TSA cells or TSA cells expressing α1β2 subunits or β3 subunits of the GABAA receptors were photolabeled with 10 μM [3H]CW14. Gel slicing demonstrated photolabeled protein peaks at ≈52-kDa and ≈100-kDa in the α1β2 and β3 containing membranes, corresponding to monomers and dimers of GABAA receptor subunits. No photolabeled peaks were observed in the wild-type membranes.
Discussion
This study was aimed at developing neurosteroid analogue photolabeling reagents for use in identifying pharmacologically relevant neurosteroid binding sites on GABAA receptors. This requires that the neurosteroid photoaffinity analogues bind in the same location on the GABAA receptor as do endogenous neurosteroids. Further, the photolabeling reagents must have photochemical properties enabling them to preferentially participate in intermolecular crosslinking rather than intramolecular reactions and to covalently insert at any proximate amino acid in an unbiased manner.
In a previous study, we used the neurosteroid analogue photolabeling reagent 6-AziP to identify a novel neurosteroid binding site (F301) in the second transmembrane domain of β-homomeric GABAA receptors (Chen et al. 2012b). This study did not identify the residues predicted to be involved in neurosteroid binding by site–directed mutagenesis (Hosie et al. 2009a; Hosie et al. 2006), indicating either that the residues identified by mutagenesis are relevant to transduction rather than binding of neurosteroids or that the predicted binding sites were simply not detected. One reason that 6-AziP may not label all neurosteroid binding sites is that the photolabeling moiety is an aliphatic diazirine. Diazirines are commonly used for protein photolabeling because they have an absorbance band centered at 350 nm, which is well away from the principal absorbance band of proteins (280 nm). Irradiation of a diazirine can, via elimination of molecular nitrogen, generate a carbene, which then participates in either an internal reaction (intramolecular) or a bimolecular insertion reaction. Carbenes can insert into any C–H bond and are thus not selective for specific amino acids. However, if an intramolecular reaction occurs, the carbene that is generated will be unavailable for bimolecular insertion. Irradiation can also convert a diazirine to a diazo intermediate that can then form a carbonium ion; the carbonium ion acts as an electrophile and can selectively label nucleophilic amino acid side chains (Brunner 1993). Aliphatic diazirines, such as 6-AziP, often undergo intramolecular reactions following carbene formation and may preferentially photolabel proteins through a diazo intermediate (Brunner 1993). This makes these reagents inefficient photolabeling reagents with a bias toward labeling nucleophilic amino acids (Pratt et al. 2000; Ziebell et al. 2004).
To overcome the photochemical limitations of 6-AziP, we synthesized neurosteroid analogues containing a 4-(3-trifluoromethyl-3H-diazirin-3-yl)-phenoxy (TPD) group. Following UV-irradiation, the TPD moiety preferentially generates a carbene (65%) rather than a diazoisomer (35%) and cannot undergo intramolecular rearrangement (Brunner et al. 1980). The TPD groups were placed at C-11 on the neurosteroid C-ring because previous studies had shown that neurosteroids with large hydrophobic substitutions at this position retain their ability to modulate GABAA receptors (Shu et al. 2007). Two photolabeling reagents with 11-TPD groups were synthesized: CW12, an analogue of the 5α-neurosteroid allopregnanolone and CW14, an analogue of the 5β-neurosteroid pregnanolone (Figure 1).
Figure 1.
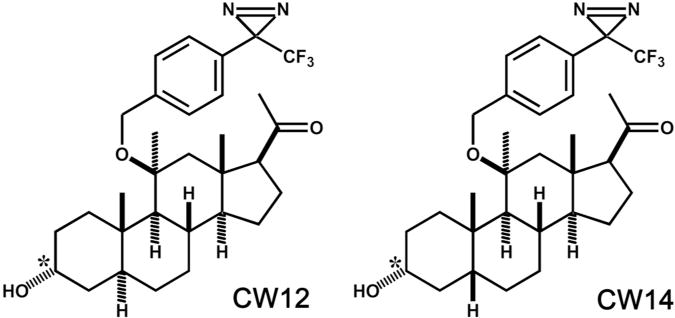
Structures of CW14 and CW12.
Photochemistry
To test whether these reagents generate a carbene that preferentially undergoes intermolecular reactions we examined the ability of CW12 and CW14 to form unique adducts with cyclohexane (a solvent in which the only bond available for attack is a C–H) following UV-irradiation. CW14 and its 3β-epimer both formed adducts with cyclohexane; these adducts migrated more slowly than the non-irradiated compounds on reverse-phase TLC indicating that they are less polar (denoted by # in Figure 2). Irradiation of CW14 in ethanol produced a product that was more polar than the non-irradiated compound (denoted by ** in Figure 2) indicating the probable formation of an ethanol-steroid adduct. Irradiation in both ethanol and cyclohexane produced a common product (denoted by * in figure 2), that migrated as a slightly more polar band than the non-irradiated CW14. The fact that this band was observed following photolysis in both solvents suggests it is produced by an intramolecular reaction. Since the TPD group cannot undergo a simple rearrangement following carbene formation, this suggests that the carbene may react with a proximate group on the neurosteroid, perhaps the 17-acetyl group. A similar pattern of photolabel incorporation was observed with CW12, with formation of a cyclohexane adduct as a major product and a common product formed in both ethanol and cyclohexane suggesting an intramolecular reaction product. Importantly, these data show that UV-irradiation of both CW14 and CW12 forms a carbene capable of intermolecular insertion in a C–H bond, indicating that these reagents should be efficient and unbiased photolabeling reagents.
Biological activity of CW12 and CW14
The GABAA receptor is affected by a diverse range of chemical compounds that almost certainly bind to a variety of distinct sites (Franks 2008). It is therefore imperative to demonstrate that the attachment of a photoreactive group does not substantially alter the interaction of the drug with its targets and that the photolabeling reagent closely mimics the effects of the parent compound. CW12 and CW14 were both shown to be efficacious anesthetics in pre-limb-bud stage tadpoles (a widely accepted assay for anesthetic action) with EC50 values very similar to those of the parent compounds (Figure 4B1 and 4B2), indicating that the photolabeling reagents retain the in vivo pharmacologic effect of neurosteroids. To assay the actions of CW12 and CW14 on GABAA receptors, we examined their effects on [35S]TBPS binding in rat brain membranes, because neurosteroids have a characteristic bimodal effect on [35S]TBPS binding. CW12 and CW14 both showed the characteristic pattern of modulation in the absence of GABA, with enhancement of binding at low steroid concentrations and complete inhibition of binding at higher concentrations (Figure 4A1 and 4A2). The low concentration enhancement of [35S]TBPS is hypothesized to result from neurosteroid binding to the site(s) responsible for potentiation of GABA action and the inhibition observed at higher concentrations is hypothesized to result from binding to the site(s) that mediate direct activation of the GABAA receptor (Evers et al. 2010). In the presence of GABA, CW12 and CW14 both showed single-component inhibition of [35S]TBPS binding, characteristic of neurosteroid effect (Figure 4A1 and 4A2). These results indicate that CW12 and CW14 replicate the effects of the parent neurosteroids and suggest that the photolabeling reagents bind to both the potentiating and direct activation sites.
Electrophysiological effects of CW12 and CW14 were studied in Xenopus oocytes expressing the α1, β2 and γ2L subunits of the GABAA receptor. Both CW12 and CW14 were potent and efficacious potentiators of GABA-elicited current. The slow washout of potentiating effects observed with CW12 and CW14 (Figure 3A) suggests that their high potency may arise, at least in part, from the hydrophobicity of CW12 and CW14 which results in a high membrane/aqueous partition coefficient, enhancing the apparent potency of the drug by presenting high concentrations to neurosteroid binding sites in the transmembrane domains (Akk et al. 2005; Chisari et al. 2010; Chisari et al. 2009). The ability of CW12 and CW14 to potentiate GABA-elicited current was markedly reduced by a mutation in the α1 subunit (Q241L) that has been shown to selectively prevent neurosteroid modulation of GABAA receptor currents (Figure 3B1 and 3B2), providing further evidence that these neurosteroid analogues act via the same mechanism as endogenous neurosteroids. CW12 directly activated GABAA currents (Figure 3C) quantitatively similar to its parent compound, allopregnanolone. CW14, like its parent compound pregnanolone, was a weak direct activator of GABAA currents (Figure 3C). The combined results of the [35S]TBPS binding studies and electrophysiological studies suggest that CW12 and CW14 interact with all of the pharmacologically relevant neurosteroid binding sites on the GABAA receptor.
Photolabeling GABAA receptors
While CW12 and CW14 appear to interact with neurosteroid binding sites on the GABAA receptor, they may still not covalently insert at these sites following UV irradiation. This could occur if the photolabeling moiety is not positioned sufficiently close to the walls of the binding pocket or if photolabeling is not adequately efficient. To determine if CW12 and CW14 can covalently label GABAA receptors, photolabeling studies were performed in native rat brain membranes and in membranes from TSA cells transfected with GABAA receptor subunits.
Both [3H]CW12 and [3H]CW14 labeled multiple proteins in rat brain membranes, with prominent labeling of a band at ≈52-kDA (Figure 5A). While this band is consistent with labeling of a GABAA receptor subunit, this identification is uncertain. To confirm that the GABAA receptor is photolabeled, we analyzed [3H]CW14 photolabeled rat brain membranes by blue-native gel electrophoresis. A photolabeled band at ≈242-kDa was observed on the blue native gel, consistent with intact GABAA receptor pentamers (Figure 6A). The identity of this photolabeled protein as a GABAA receptor was confirmed by immunoblots showing that the α1, β2/3 and γ2L subunits of the GABAA receptor all co-migrated with this photolabeled band. Photolabeling of the GABAA receptor was stereospecific as indicated by the finding that the 3β-epimer of [3H]CW14 did not label GABAA receptors (Figure 6B).
As a final confirmation that CW12 and CW14 are effective photolabeling reagents for the GABAA receptor, membranes from TSA cells expressing either α1β2 or β3-homomeric GABAA receptors were photolabeled with [3H]CW14 and analyzed by SDS-PAGE with gel slicing. Bands at ≈52- and ≈100-kDA were observed in the transfected, but not in wild-type TSA cell membranes (Figure 7). These data support [3H]CW14 photolabeling of GABAA receptor β-subunits, with the 100-kda band presumably reflecting a dimer of the β-subunit. It cannot be determined from these results if [3H]CW14 also labels α1 subunits.
In summary, CW12 and CW14 are novel photolabeling analogues of the endogenous neurosteroids allopregnanolone and pregnanolone, respectively. The photochemistry of these compounds is favorable; they can undergo intermolecular carbene insertion, allowing photolabeling of any proximate amino acid residue. CW12 and CW14 closely mimic the actions of their parent neurosteroids, both as anesthetics and as modulators of GABAA receptor function, fulfilling an essential criterion for a photolabeling reagent to identify neurosteroid binding sites. Finally, CW12 and CW14 photolabel β and possibly other subunits of the GABAA receptor. These reagents will be useful for identifying the specific amino acid residues with which neurosteroids interact on GABAA receptors, enabling the identification and modeling of their sites of action.
Supplementary Material
Acknowledgments
This work was supported by grant PO1-GM47969 from the National Institute of General Medical Sciences to A.S.E, D.F.C., J.H.S and C.F.Z and by grant MH099658 to S.M. from the National Institute of Mental Health. Washington University receives income and equity based on a license of related technology to Sage Therapeutics, Inc. D.F.C., C.F.Z. and A.S.E. have equity holdings in Sage Therapeutics, Inc. Sage Therapeutics, Inc. did not support this research or have any other role in the research or its conclusions.
References
- Akk G, Bracamontes JR, Covey DF, Evers A, Dao T, Steinbach JH. Neuroactive steroids have multiple actions to potentiate GABAA receptors. J Physiol. 2004;558:59–74. doi: 10.1113/jphysiol.2004.066571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akk G, Covey DF, Evers AS, Mennerick S, Zorumski CF, Steinbach JH. Kinetic and structural determinants for GABA-A receptor potentiation by neuroactive steroids. Current neuropharmacology. 2010;8:18–25. doi: 10.2174/157015910790909458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akk G, Covey DF, Evers AS, Steinbach JH, Zorumski CF, Mennerick S. Mechanisms of neurosteroid interactions with GABA(A) receptors. Pharmacol Ther. 2007;116:35–57. doi: 10.1016/j.pharmthera.2007.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akk G, Li P, Bracamontes J, Reichert DE, Covey DF, Steinbach JH. Mutations of the GABA-A receptor alpha1 subunit M1 domain reveal unexpected complexity for modulation by neuroactive steroids. Mol Pharmacol. 2008;74:614–27. doi: 10.1124/mol.108.048520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akk G, Shu HJ, Wang C, Steinbach JH, Zorumski CF, Covey DF, Mennerick S. Neurosteroid access to the GABAA receptor. J Neurosci. 2005;25:11605–13. doi: 10.1523/JNEUROSCI.4173-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atkinson RM, Davis B, Pratt MA, Sharpe HM, Tomich EG. Action of some steroids on the centtral nervous system of the mouse. II. Pharmacology. Journal of medicinal chemistry. 1965;8:426–32. doi: 10.1021/jm00328a004. [DOI] [PubMed] [Google Scholar]
- Brunner J. New photolabeling and crosslinking methods. Annu Rev Biochem. 1993;62:483–514. doi: 10.1146/annurev.bi.62.070193.002411. [DOI] [PubMed] [Google Scholar]
- Brunner J, Senn H, Richards FM. 3-Trifluoromethyl-3-phenyldiazirine. A new carbene generating group for photolabeling reagents. J Biol Chem. 1980;255:3313–8. [PubMed] [Google Scholar]
- Bureau MH, Olsen RW. GABAA receptor subtypes: ligand binding heterogeneity demonstrated by photoaffinity labeling and autoradiography. J Neurochem. 1993;61:1479–91. doi: 10.1111/j.1471-4159.1993.tb13643.x. [DOI] [PubMed] [Google Scholar]
- Chen ZW, Chen LH, Akentieva N, Lichti CF, Darbandi R, Hastings R, Covey DF, Reichert DE, Townsend RR, Evers AS. A neurosteroid analogue photolabeling reagent labels the colchicine-binding site on tubulin: a mass spectrometric analysis. Electrophoresis. 2012a;33:666–74. doi: 10.1002/elps.201100434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen ZW, Manion B, Townsend RR, Reichert DE, Covey DF, Steinbach JH, Sieghart W, Fuchs K, Evers AS. Neurosteroid analog photolabeling of a site in the third transmembrane domain of the β3 subunit of the GABAA receptor. Mol Pharmacol. 2012b;82:408–19. doi: 10.1124/mol.112.078410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiara DC, Jayakar SS, Zhou X, Zhang X, Savechenkov PY, Bruzik KS, Miller KW, Cohen JB. Specificity of intersubunit general anesthetic-binding sites in the transmembrane domain of the human α1β3γ2 gamma-aminobutyric acid type A (GABAA) receptor. J Biol Chem. 2013;288:19343–57. doi: 10.1074/jbc.M113.479725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chisari M, Eisenman LN, Covey DF, Mennerick S, Zorumski CF. The sticky issue of neurosteroids and GABA(A) receptors. Trends in neurosciences. 2010;33:299–306. doi: 10.1016/j.tins.2010.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chisari M, Eisenman LN, Krishnan K, Bandyopadhyaya AK, Wang C, Taylor A, Benz A, Covey DF, Zorumski CF, Mennerick S. The influence of neuroactive steroid lipophilicity on GABAA receptor modulation: evidence for a low-affinity interaction. J Neurophysiol. 2009;102:1254–64. doi: 10.1152/jn.00346.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Covey DF, Nathan D, Kalkbrenner M, Nilsson KR, Hu Y, Zorumski CF, Evers AS. Enantioselectivity of pregnanolone-induced gamma-aminobutyric acid(A) receptor modulation and anesthesia. J Pharmacol Exp Ther. 2000;293:1009–16. [PubMed] [Google Scholar]
- Darbandi-Tonkabon R, Hastings WR, Zeng CM, Akk G, Manion BD, Bracamontes JR, Steinbach JH, Mennerick SJ, Covey DF, Evers AS. Photoaffinity labeling with a neuroactive steroid analogue. 6-azi-pregnanolone labels voltage-dependent anion channel-1 in rat brain. J Biol Chem. 2003;278:13196–206. doi: 10.1074/jbc.M213168200. [DOI] [PubMed] [Google Scholar]
- Darbandi-Tonkabon R, Manion BD, Hastings WR, Craigen WJ, Akk G, Bracamontes JR, He Y, Sheiko TV, Steinbach JH, Mennerick SJ, Covey DF, Evers AS. Neuroactive steroid interactions with voltage-dependent anion channels: lack of relationship to GABA(A) receptor modulation and anesthesia. J Pharmacol Exp Ther. 2004;308:502–11. doi: 10.1124/jpet.103.058123. [DOI] [PubMed] [Google Scholar]
- Evers AS, Chen ZW, Manion BD, Han M, Jiang X, Darbandi-Tonkabon R, Kable T, Bracamontes J, Zorumski CF, Mennerick S, Steinbach JH, Covey DF. A synthetic 18-norsteroid distinguishes between two neuroactive steroid binding sites on GABAA receptors. J Pharmacol Exp Ther. 2010;333:404–13. doi: 10.1124/jpet.109.164079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franks NP. General anaesthesia: from molecular targets to neuronal pathways of sleep and arousal. Nature reviews Neuroscience. 2008;9:370–86. doi: 10.1038/nrn2372. [DOI] [PubMed] [Google Scholar]
- Gyermek L, Soyka LF. Steroid anesthetics. Anesthesiology. 1975;42:331–44. doi: 10.1097/00000542-197503000-00017. [DOI] [PubMed] [Google Scholar]
- Harrison NL, Majewska MD, Harrington JW, Barker JL. Structure-activity relationships for steroid interaction with the γ-aminobutyric acid A receptor complex. J Pharmacol Exp Ther. 1987;241:346–53. [PubMed] [Google Scholar]
- Hawkinson JE, Kimbrough CL, McCauley LD, Bolger MB, Lan NC, Gee KW. The neuroactive steroid 3 alpha-hydroxy-5 beta-pregnan-20-one is a two-component modulator of ligand binding to the GABAA receptor. Eur J Pharmacol. 1994;269:157–63. doi: 10.1016/0922-4106(94)90082-5. [DOI] [PubMed] [Google Scholar]
- Hosie AM, Clarke L, da Silva H, Smart TG. Conserved site for neurosteroid modulation of GABAA receptors. Neuropharmacology. 2009a;56:149–54. doi: 10.1016/j.neuropharm.2008.07.050. [DOI] [PubMed] [Google Scholar]
- Hosie AM, Clarke L, da Silva H, Smart TG. Conserved site for neurosteroid modulation of GABAA receptors. Neuropharmacology. 2009b;56:149–54. doi: 10.1016/j.neuropharm.2008.07.050. [DOI] [PubMed] [Google Scholar]
- Hosie AM, Wilkins ME, da Silva HM, Smart TG. Endogenous neurosteroids regulate GABAA receptors through two discrete transmembrane sites. Nature. 2006;444:486–9. doi: 10.1038/nature05324. [DOI] [PubMed] [Google Scholar]
- Hosie AM, Wilkins ME, Smart TG. Neurosteroid binding sites on GABA(A) receptors. Pharmacol Ther. 2007;116:7–19. doi: 10.1016/j.pharmthera.2007.03.011. [DOI] [PubMed] [Google Scholar]
- Li P, Bandyopadhyaya AK, Covey DF, Steinbach JH, Akk G. Hydrogen bonding between the 17β-substituent of a neurosteroid and the GABA(A) receptor is not obligatory for channel potentiation. Br J Pharmacol. 2009;158:1322–9. doi: 10.1111/j.1476-5381.2009.00390.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li P, Bracamontes J, Katona BW, Covey DF, Steinbach JH, Akk G. Natural and enantiomeric etiocholanolone interact with distinct sites on the rat α1β2γ2L GABAA receptor. Mol Pharmacol. 2007;71:1582–90. doi: 10.1124/mol.106.033407. [DOI] [PubMed] [Google Scholar]
- Pratt MB, Husain SS, Miller KW, Cohen JB. Identification of sites of incorporation in the nicotinic acetylcholine receptor of a photoactivatible general anesthetic. J Biol Chem. 2000;275:29441–51. doi: 10.1074/jbc.M004710200. [DOI] [PubMed] [Google Scholar]
- Selye H. Anesthetic effect of steroid hormones. Proc Soc Exp Biol Med. 1941;46:116–121. [Google Scholar]
- Shu HJ, Zeng CM, Wang C, Covey DF, Zorumski CF, Mennerick S. Cyclodextrins sequester neuroactive steroids and differentiate mechanisms that rate limit steroid actions. Br J Pharmacol. 2007;150:164–75. doi: 10.1038/sj.bjp.0706973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srinivasan S, Sapp DW, Tobin AJ, Olsen RW. Biphasic modulation of GABA(A) receptor binding by steroids suggests functional correlates. Neurochem Res. 1999;24:1363–72. doi: 10.1023/a:1022524421464. [DOI] [PubMed] [Google Scholar]
- Wittig I, Braun HP, Schagger H. Blue native PAGE. Nat Protoc. 2006;1:418–28. doi: 10.1038/nprot.2006.62. [DOI] [PubMed] [Google Scholar]
- Wittmer LL, Hu Y, Kalkbrenner M, Evers AS, Zorumski CF, Covey DF. Enantioselectivity of steroid-induced gamma-aminobutyric acidA receptor modulation and anesthesia. Mol Pharmacol. 1996;50:1581–6. [PubMed] [Google Scholar]
- Ziebell MR, Nirthanan S, Husain SS, Miller KW, Cohen JB. Identification of binding sites in the nicotinic acetylcholine receptor for [3H]azietomidate, a photoactivatable general anesthetic. J Biol Chem. 2004;279:17640–9. doi: 10.1074/jbc.M313886200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


