Abstract
Objective:
Medication-assisted treatment for substance use disorders (SUDs) is not widely used in treatment programs. The aims of the current study were to document the prevalence of adoption and implementation of extended-release injectable naltrexone, the newest U.S. Food and Drug Administration–approved medication for alcohol use disorder (AUD), in U.S. treatment programs and to examine associations between organizational and patient characteristics and adoption.
Method:
The study used interview data from a nationally representative sample of 307 U.S. SUD treatment programs to examine adoption and implementation of injectable naltrexone.
Results:
Thirteen percent of programs used injectable naltrexone for AUD, and 3% of programs used it for opioid use disorder. Every treatment program that offered injectable naltrexone to its patients used it in conjunction with psychosocial treatment, particularly cognitive behavioral therapy. Multivariate logistic regression results indicated that adoption was positively associated with the provision of wraparound services, the percentage of privately insured patients, and the presence of inpatient detoxification services. For-profit status and offering inpatient services were negatively associated with adoption. Within adopting programs, an average of 4.1% of AUD patients and 7.1% of patients with opioid use disorder were currently receiving the medication, despite clinical directors’ reports of positive patient outcomes, particularly for relapsers and for those who had been noncompliant with other medications. Cost was a significant issue for the majority of adopting organizations.
Conclusions:
The rate of adoption of injectable naltrexone in U.S. treatment programs remains limited. Researchers should continue to examine patient, organizational, and external characteristics associated with the adoption and implementation of injectable naltrexone over time.
Use of medications in the treatment of alcohol use disorders (AUDs) and other substance use disorders (SUDs) is rare, despite the availability of such medications since 1949 when the U.S. Food and Drug Administration (FDA) approved the use of disulfiram in treating AUD. Naltrexone (ReVia) was patented as a tablet medication in 1967 and approved as a treatment for AUD in 1994. Both animal and human studies through the 1980s and 1990s established the efficacy of naltrexone in treating AUD, and subsequent meta-analyses have confirmed the general consistency of this finding (Bouza et al., 2004; Roozen et al., 2006). Volpicelli et al. (1997) observed the problem of compliance in taking the tablets, with the suggestion that effective treatment with tablet naltrexone may be limited by the extent of patient motivation. The importance of compliance in using the tablets relative to treatment success has been found in multiple subsequent analyses (Gueorguieva et al., 2013; Kranzler et al., 2008; Swift et al, 2011).
Extended-release injectable naltrexone (Vivitrol) was developed to address the challenge of patient compliance by administering a 30-day dose in a single injection. The medication was approved by the FDA as a treatment for AUD in 2006 and as a treatment for opioid use disorder in 2010. The medication is intended for individuals with AUD who have stopped drinking and for opioid patients who have gone through detoxification treatment and are free of opioids or opioid-containing medications, including buprenorphine or methadone, for a minimum of 7–10 days.
Studies of the efficacy of injectable naltrexone in treating AUD patients indicate that it reduces drinking days, median drinks per day, and number of heavy drinking days and increases abstinence rates, percentage of days abstinent, length of continuous abstinence, time to first drink, and time to first heavy drinking day (Garbutt et al., 2005; Gastfriend et al., 2005; Kranzler et al, 2004; Lee et al, 2012; O’Malley et al, 2007). Recent meta-analyses of studies assessing injectable naltrexone’s effectiveness indicate that its use in AUD patients is linked to a reduction in heavy drinking days (Jonas et al, 2014), longer refill persistence, and longer medication persistence compared with acamprosate (Campral) and tablet naltrexone (Hartung et al, 2014). Similarly, the use of injectable naltrexone for opioid use disorder has been linked to abstinence rates, longer duration of opioid-free days, improved treatment retention, less craving, and lower relapse (Gastfriend, 2011; Syed & Keating, 2013).
Little is known about the rates and patterns of organizational adoption of injectable naltrexone since the 2010 FDA decision made it available for both AUD and opioid use disorder treatment. Studies before 2010 show relatively low rates of use in AUD treatment. Barriers to adoption included lack of knowledge about the medication, pharmacotherapy-resistant treatment ideology, financial constraints, and lack of access to prescribing staff (Ducharme et al., 2006; Fuller et al., 2005; Thomas et al., 2008).
To increase the availability of the full range of treatment options for both AUD and opioid use disorder, it is important to understand the types of organizations that are more likely to offer this innovative medication. This is the first study in a national sample of treatment programs, using onsite techniques of data collection, to examine the adoption of injectable naltrexone in SUD treatment programs following its FDA approval for both alcohol and opioid treatment. The first aim of the study was to document the prevalence of adoption of injectable naltrexone in U.S. treatment centers. The second aim was to examine associations between organizational and patient characteristics and adoption. In multivariate analyses, we consider whether these characteristics are associated with the odds of offering injectable naltrexone. We also provide information regarding implementation, delivery of other treatment services, and organizational leaders’ perceptions of the types of patients for whom injectable naltrexone has been found to be more successful.
Facilitators to the adoption of injectable naltrexone
There are multiple studies on treatment programs’ use of tablet naltrexone, the technological predecessor of injectable naltrexone. These studies have revealed that program features such as size, accreditation, and treatment philosophy are significant predictors of adoption (Heinrich & Cummings, 2014; Heinrich & Hill, 2008; Oser & Roman, 2008; Roman & Johnson, 2002). A study of early adoption of injectable naltrexone in a sample of SUD treatment centers primarily dependent on private funding revealed that organizational size, percentage of privately insured patients, and use of other AUD pharmacotherapies were significant predictors of adoption (Abraham & Roman, 2010). In the current study, based on more recent data, we build on existing research and hypothesize associations between adoption and several other organizational characteristics.
Wraparound services go beyond the direct treatment of SUD to address patients’ co-occurring medical, mental health, and other problems and are associated with better client retention and treatment outcomes (Chriqui et al., 2007; Ducharme et al., 2007). Rogers’ (2003) diffusion theory views innovations as practices that are considered new to an organization. In this model, the broad orientation to care reflected in wraparound services is an indicator of innovativeness. Despite well-known recommendations, wraparound services are not universally used in the SUD treatment field and are viewed by many as auxiliary to the goals of treatment. Research has found that using such services is associated with medication adoption (Heinrich & Hill, 2008); therefore, we expect that innovative organizations that provide wraparound services will also be likely to provide injectable naltrexone.
Patient payment methods have been consistently linked to medication adoption. A study examining the early adoption of injectable naltrexone for AUD found that the percentage of patients with private insurance was positively associated with adoption (Abraham & Roman, 2010). Patient payer source and revenues from private insurance have been linked to greater adoption of other medications for AUD as well (Abraham et al., 2011; Ducharme et al., 2006; Fuller et al., 2005; Thomas et al., 2003). Although public payer sources often cover the use of SUD medications (Lichtenberg and Philipson, 2002), injectable naltrexone is usually covered as a Medicaid medical benefit rather than a pharmacy benefit (American Society of Addiction Medicine [ASAM], 2013), unlike other medications, which are almost always covered under pharmacy benefits. Treatment services reimbursed through state medical benefits may be more difficult for centers to offer than those covered under pharmacy benefits because of associated billing complexity (McDonald, 2008).
It is also expected that treatment centers that attract a higher percentage of relapsers (i.e., those who have previously received formal treatment) may be more likely to adopt injectable naltrexone as part of their evidence-based treatment approach. A study on tablet naltrexone found that adoption was significantly associated with the percentage of relapsers represented in the caseload (Roman & Johnson, 2002). Although these data are more than a decade old, the extent to which other methods continue to be used before medications are attempted is a crucial question in understanding patterns of use of medications. Relapsers may be particularly challenging for treatment centers, and it may not seem rational to repeat the same therapy if it has already failed and an alternative is available (Krupitsky & Blokhina, 2010). Although the present study does not include data collection from patients, their attitudes are likely significant in accepting medications, and any frustration with the psychosocial treatments that they have observed to help others may facilitate their engagement with medication-assisted treatment (MAT).
In addition to considering facilitators of treatment programs’ use of injectable naltrexone, we also consider two characteristics that may be barriers to its use.
Barriers to the adoption of injectable naltrexone
The level of criminal justice referrals is expected to adversely affect centers’ adoption of injectable naltrexone. Because of the success of a variety of programs that are designed to divert certain offenders from prison into SUD treatment, an increasingly important source of SUD patients is the criminal justice system. This association with criminal justice and SUD treatment has implications for the type, scope, and duration of treatment that are yet to be fully explored. Perhaps as a function of this amalgamation of treatment and restrictive approaches, treatment centers with tighter linkages with the criminal justice system have been consistently shown to differ in treatment modalities, and research has found that centers with a greater reliance on criminal justice revenues have lower implementation of MAT (Knudsen & Roman, 2012). Further, SUD treatment programs within correctional institutions are often unwilling to adopt the use of medications to treat opioid use disorders (Kubiak et al., 2009; Rich et al., 2005; Smith-Rohrberg et al., 2004). In particular, the use of agonist SUD medications is limited by its being viewed in the criminal justice system as drug substitution (Friedmann et al., 2012; Marlowe, 2011; McMillan & Lapham, 2005; Walters et al., 2007). Other studies using samples of prisons, jails, or community corrections agencies indicate that MAT is rarely used (Friedmann et al., 2012; Matusow et al., 2013; Taxman et al., 2007). As a result, a treatment center’s connection with the criminal justice system has been shown to be associated with both less adoption and less sustained use of medications for AUD and opioid use disorder (Abraham et al., 2011; Ducharme et al., 2006; Knudsen & Roman, 2012).
Certain patterns of treatment ideology are also expected to adversely affect centers’ adoption of injectable naltrexone. Research has indicated that some centers respond to medication alternatives with an anti-pharmacotherapy stance that influences the services offered (Knudsen et al., 2005). A core set of principles and beliefs stem from the 12-step model, and a center’s emphasis on this model has been shown to act as a barrier to the adoption of pharmacotherapies for SUD treatment (Oser & Roman, 2008). For some, medications are incompatible with the 12-steps’ value system (Abraham et al., 2011), which emphasizes combinations of individual transformation and overall freedom from drugs as fundamental to successful recovery (Alcoholics Anonymous [AA], 1984). Several researchers found that 12-step groups have historically been seen as antithetical to the use of MAT (Mark et al., 2003; Swift et al., 1998), and members often experience pressure to stop taking medications (Rychtarik et al, 2000). Nevertheless, 12-step approaches do not necessarily preclude the use of any SUD medications, for other principles encourage those in recovery to follow physician recommendations, including being medically compliant (Alcoholics Anonymous, 1984).
Last, the use of injectable naltrexone could be related to levels of care and the extent to which medical involvement is indicated. Although some levels of care could facilitate its adoption, others could act as barriers to its use. On the one hand, given the more urgent medical needs of patients in inpatient detoxification services, treatment programs offering this level of care could have access to greater medical personnel, who are also needed to provide the medication. These greater resources are expected to be linked to adoption. On the other hand, the use of injectable naltrexone may be less likely in outpatient services, because it is more difficult to monitor patients in outpatient care.
Method
Data for this study were collected between June 2009 and January 2012 from a national sample of SUD treatment programs. Requirements for inclusion were that treatment programs were open to the general public and offered at least one level of care between ASAM Level I (structured outpatient treatment) and Level III (residential/inpatient treatment) services (Mee-Lee et al., 1996). Excluded from the study were counselors in private practice, transitional living facilities, court-ordered driver education classes or detoxification services, and methadone-only programs. To ensure programs were accessible to all community members, those in Veterans Health Administration facilities or in correctional facilities were also excluded from the sample. To ensure adequate tests of hypotheses regarding AUD treatment, programs were also required to have at least 25% of their clientele with a primary AUD. All research procedures were approved by the institutional review board of the University of Georgia.
Centers for the study were randomly selected using the Substance Abuse and Mental Health Services Administration treatment facility locator. Those that were screened as ineligible during a telephone screening were replaced by a random selection of alternate treatment centers. Data were collected using face-to-face interviews with the administrator and/or clinical director of each treatment program. Standardized questions related to the treatment center’s organization, management, and human resources were asked of the administrator, whereas questions related to levels of care, treatment philosophy, caseload characteristics, and use of pharmacological and behavioral therapies were asked of the clinical director. Interviews were combined whenever a treatment program’s administrator also served as the program’s clinical director. Before each in-person interview at the organization’s location, participants received a packet with a description of the study and two copies of the informed consent form. Field interviewers had at least a bachelor’s level of education and received extensive training in the administration of the instrument from the research team. Their training also ensured that they were familiar with both the contents of the standardized instrument and with terminology and emerging issues in SUD treatment. Most interviewers had multiple years of experience as a result of having worked on prior research projects. The final randomly selected sample resulted in 307 treatment programs, a response rate of 68% based on those that were eligible and contacted for participation in the study. All of the 48 contiguous states were represented in the data. Sixteen percent of the programs were located in the Northeast, 23.4% in the South, 28% in the Midwest, and 32.6% in the West.
Measures
Adoption of injectable naltrexone was a dichotomous variable indicating whether the use of the medication was an existing practice within the treatment program. An index ranging from 0 to 10 measured the sum of the following 10 wraparound services (National Institute on Drug Abuse, 2012): primary medical care, mental health, child care, family, housing/transportation, financial, legal, vocational, educational, and HIV/AIDS services. Three continuous measures of patient characteristics were included: percentage of patients referred by some unit of the criminal justice system, including probation, court-referred, and driving-under-the-influence patients; percentage of patients paying with private insurance; and percentage of patients who had previously been treated at any center (i.e., relapsers). A variable measuring the extent to which the program’s treatment was based on a 12-step model was included (0 = no extent, 5 = a very great extent). We also measured levels of care with a series of dichotomous variables for the following services: inpatient detoxification, inpatient treatment (30 days or less), residential treatment (more than 30 days), partial hospitalization, intensive outpatient treatment, and outpatient treatment.
Several organizational controls were included. Profit status was measured as a dichotomous variable and coded 1 for for-profit and 0 for nonprofit. Because programs based in general hospitals are in an environment more readily facilitating medication use (Roman & Johnson, 2002), we included a dichotomous indicator of whether the program was based in a hospital setting. Program size was measured by the number of full-time-equivalent employees. We also controlled for the percentage of private revenues. Funds from insurance reimbursements, including both commercial and Medicaid/Medicare coverage, client fees, and other income sources that were not considered “block” funding, such as government grants, were summed to measure the percentage of private funds. Medicaid and Medicare were not regarded as block funding because they are not guaranteed sources of revenue for treatment programs and thus are more similar to private sources. Last, we controlled for the percentage of patients with a primary AUD diagnosis.
Although the measures above, as well as the majority of the structured interview, consisted of closed-ended questions, we also included a series of closed- and open-ended questions regarding implementation that were asked of programs that prescribed injectable naltrexone. Responses were recorded on implementation rate, measured as the percentage of patients receiving the medication in the program, patient outcomes, delivery of other treatment services, and cost issues associated with the medication.
Analytic strategy
First, we examined descriptive statistics of the study variables. Second, we estimated the mean differences between prescribers of injectable naltrexone and nonprescribing programs using chi-square and t tests. Third, we used multivariate logistic regression to predict adoption of injectable naltrexone. Diagnostic tests revealed no evidence of multicollinearity. Last, open-ended responses to questions regarding implementation were examined for key themes.
We used expectation maximization to address covariates that had missing data. Every variable in our analysis had between 0% and 2% missing cases, with the exception of the variable for percentage of relapsers, which had 5% missing cases. To be conservative, we excluded two cases with missing data for the dependent variable (Allison, 2009). Little’s test indicated that cases were missing completely at random. Analyses were conducted with SPSS Version 21 statistical software (IBM Corp., Armonk, NY).
Results
Descriptive statistics
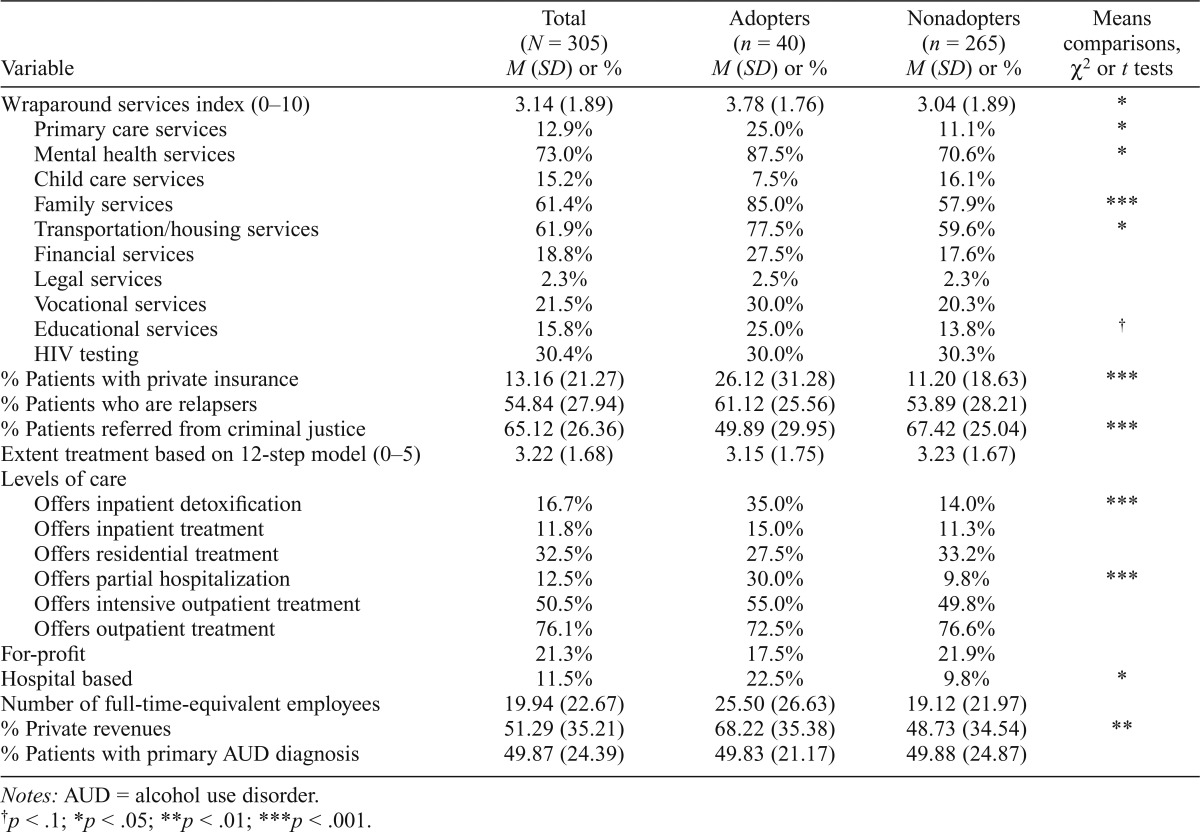
Table 1 presents descriptive statistics for the sample. Approximately 13% (n = 40) of the treatment centers in the total sample adopted injectable naltrexone. Roughly 43% of the total sample prescribed any medications, including medications for psychiatric conditions (not shown), suggesting that about a third (31%) of centers that used other medications also adopted injectable naltrexone. There were no centers that prescribed only injectable naltrexone and no other medications.
Table 1.
Descriptive statistics and comparisons between injectable naltrexone adopters and nonadopters
| Variable | Total (N = 305) M (SD) or % | Adopters (n = 40) M (SD) or % | Nonadopters (n = 265) M (SD) or % | Means comparisons, χ2 or t tests |
| Wraparound services index (0–10) | 3.14 (1.89) | 3.78 (1.76) | 3.04 (1.89) | * |
| Primary care services | 12.9% | 25.0% | 11.1% | * |
| Mental health services | 73.0% | 87.5% | 70.6% | * |
| Child care services | 15.2% | 7.5% | 16.1% | |
| Family services | 61.4% | 85.0% | 57.9% | *** |
| Transportation/housing services | 61.9% | 77.5% | 59.6% | * |
| Financial services | 18.8% | 27.5% | 17.6% | |
| Legal services | 2.3% | 2.5% | 2.3% | |
| Vocational services | 21.5% | 30.0% | 20.3% | |
| Educational services | 15.8% | 25.0% | 13.8% | † |
| HIV testing | 30.4% | 30.0% | 30.3% | |
| % Patients with private insurance | 13.16 (21.27) | 26.12 (31.28) | 11.20 (18.63) | *** |
| % Patients who are relapsers | 54.84 (27.94) | 61.12 (25.56) | 53.89 (28.21) | |
| % Patients referred from criminal justice | 65.12 (26.36) | 49.89 (29.95) | 67.42 (25.04) | *** |
| Extent treatment based on 12-step model (0–5) | 3.22 (1.68) | 3.15 (1.75) | 3.23 (1.67) | |
| Levels of care | ||||
| Offers inpatient detoxification | 16.7% | 35.0% | 14.0% | *** |
| Offers inpatient treatment | 11.8% | 15.0% | 11.3% | |
| Offers residential treatment | 32.5% | 27.5% | 33.2% | |
| Offers partial hospitalization | 12.5% | 30.0% | 9.8% | *** |
| Offers intensive outpatient treatment | 50.5% | 55.0% | 49.8% | |
| Offers outpatient treatment | 76.1% | 72.5% | 76.6% | |
| For-profit | 21.3% | 17.5% | 21.9% | |
| Hospital based | 11.5% | 22.5% | 9.8% | * |
| Number of full-time-equivalent employees | 19.94 (22.67) | 25.50 (26.63) | 19.12 (21.97) | |
| % Private revenues | 51.29 (35.21) | 68.22 (35.38) | 48.73 (34.54) | ** |
| % Patients with primary AUD diagnosis | 49.87 (24.39) | 49.83 (21.17) | 49.88 (24.87) |
Notes: AUD = alcohol use disorder.
p < .1;
p < .05;
p < .01;
p < .001.
Turning to our independent variables, on average, the use of wraparound services was greater in programs that adopted injectable naltrexone compared with nonadopting programs, t(303) = 2.32, p < .05. Specifically, adopters were more likely to offer primary care services, χ2(1) = 5.9, p < .05; mental health services, χ2(1) = 5.0, p < .05; family services, χ2(1) = 10.8, p < .001; and transportation/housing services, χ2(1) = 4.7, p < .05. Adopters also reported a substantially higher percentage of private revenues (68.2% vs. 48.7%), t(303) = 3.32, p < .01, and patients paying with private insurance (26.1% vs. 11.2%), t(303) = 4.25, p < .001, and a lower percentage of patients involved with the criminal justice system (49.9% vs. 67.4%), t(303) = 4.02, p < .001. Adopters were also more likely to offer inpatient detoxification, χ2(1) = 11.1, p < .001, and partial hospitalization, χ2(1) = 13.0, p < .001, compared with nonadopters. Last, a greater percentage of adopters were hospital based (22.5%) compared with nonadopters (9.8%), χ2(1) = 5.51, p < .05. The distributions for full-time-equivalent employees and percentage of patients with private insurance deviated from a normal distribution; therefore, a square-root transformation, which can be applied to zero values, was performed for the variables to better approximate a normal distribution (Howell, 2007; Tabachnick & Fidell, 2007). Subsequent analyses were performed using the transformed values.
Injectable naltrexone for opioid use disorder
Most adopting organizations were using injectable naltrexone for AUD only. Just 3% of the total sample, or 6.9% of centers that reported the use of any SUD medications, used injectable naltrexone specifically for opioid use disorder, although every program that offered the medication to their opiate clients also offered it to their AUD clients. It should be noted that 48.2% of the centers in our sample were interviewed before FDA approval of the medication for opioid use disorder in October 2010. The wait for approval apparently did not completely impede use, because almost half of all centers specifically using injectable naltrexone for opioid use patients had started doing so before FDA approval (44%). These centers can be thought of as the earliest adopters of injectable naltrexone for opioid use disorder. The number of these earliest adopters was too small to conduct further analyses examining their organizational and patient-level characteristics.
Use of other substance use disorder medications
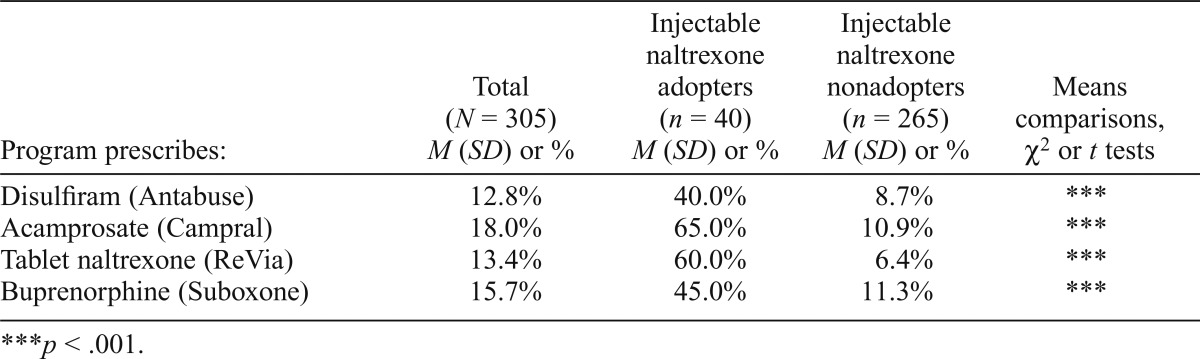
In Table 2, we consider whether nonadopters were more likely to rely on other FDA-approved medications for AUD and opioid use disorder in place of injectable naltrexone. We found that a significantly greater percentage of injectable naltrexone adopters also offered disulfiram, acamprosate, tablet naltrexone, and buprenorphine (Suboxone) compared with programs that did not use injectable naltrexone (ps < .001). Although use of each medication was less than 20% in the overall sample, among injectable naltrexone adopters, 40% also offered disulfiram, 65% used acamprosate, 60% also used tablet naltrexone, and 45% also offered buprenorphine.
Table 2.
Use of other medications for alcohol use disorder and opioid-use disorder among treatment programs
| Program prescribes: | Total (N = 305) M (SD) or % | Injectable naltrexone adopters (n = 40) M (SD) or % | Injectable naltrexone nonadopters (n = 265) M (SD) or % | Means comparisons, χ2 or t tests |
| Disulfiram (Antabuse) | 12.8% | 40.0% | 8.7% | *** |
| Acamprosate (Campral) | 18.0% | 65.0% | 10.9% | *** |
| Tablet naltrexone (ReVia) | 13.4% | 60.0% | 6.4% | *** |
| Buprenorphine (Suboxone) | 15.7% | 45.0% | 11.3% | *** |
p < .001.
Logistic regression results predicting adoption of injectable naltrexone
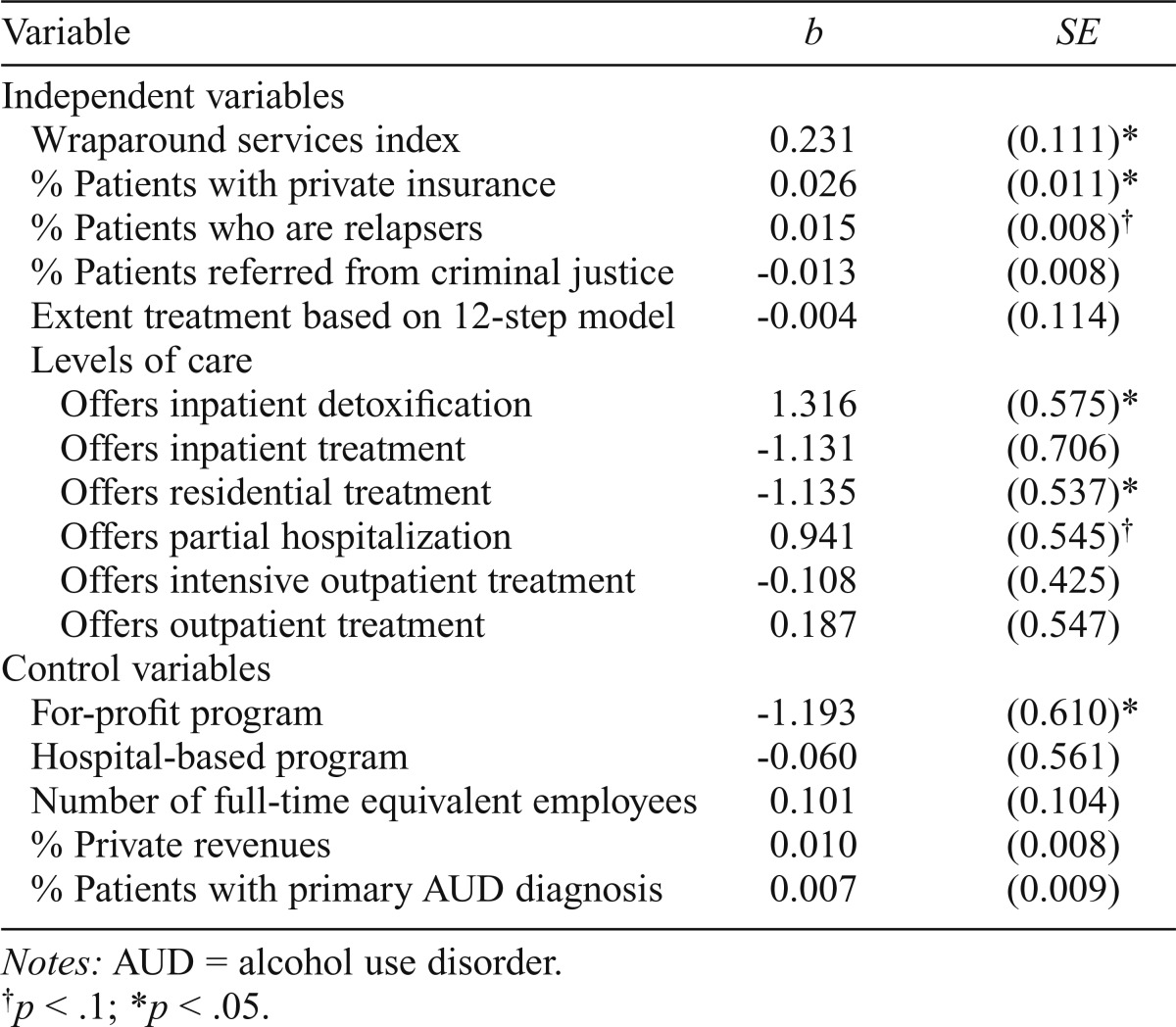
Table 3 reports the results of the multivariate logistic regression. We found that centers offering more wraparound services and centers with a greater percentage of privately insured patients were more likely to offer injectable naltrexone (b = 0.23 1, p < .05, and b = 0.026, p < .05, respectively). The percentage of relapsers was also positively associated with adoption, but this did not reach standard level significance (p < .1). Two levels of care were significant in the model (p < .05). Specifically, the odds of adoption were greater in programs that offered inpatient detoxification services and lower in centers with residential treatment services. Offering partial hospitalization also trended toward significance (p < .1), whereas for-profit status was negatively associated with offering the medication (b = -1.193, p < .05).
Table 3.
Multivariate logistic regression model of adoption of injectable naltrexone on organizational characteristics (N = 305)
| Variable | b | SE |
| Independent variables | ||
| Wraparound services index | 0.231 | (0.111)* |
| % Patients with private insurance | 0.026 | (0.011)* |
| % Patients who are relapsers | 0.015 | (0.008)† |
| % Patients referred from criminal justice | -0.013 | (0.008) |
| Extent treatment based on 12-step model | -0.004 | (0.114) |
| Levels of care | ||
| Offers inpatient detoxification | 1.316 | (0.575)* |
| Offers inpatient treatment | -1.131 | (0.706) |
| Offers residential treatment | -1.135 | (0.537)* |
| Offers partial hospitalization | 0.941 | (0.545)† |
| Offers intensive outpatient treatment | -0.108 | (0.425) |
| Offers outpatient treatment | 0.187 | (0.547) |
| Control variables | ||
| For-profit program | -1.193 | (0.610)* |
| Hospital-based program | -0.060 | (0.561) |
| Number of full-time equivalent employees | 0.101 | (0.104) |
| % Private revenues | 0.010 | (0.008) |
| % Patients with primary AUD diagnosis | 0.007 | (0.009) |
Notes: AUD = alcohol use disorder.
p < .1;
p < .05.
Implementation of injectable naltrexone
We examined implementation measures to supplement the finding regarding adoption. Within organizations that adopted injectable naltrexone, the average rate of implementation was just 4.1% (SD = 8.1) for AUD patients and 7.1% (SD = 8.4) for opioid patients. In fact, 85.7% of centers reported an implementation rate of less than 10%. In the average program, slightly more than half of AUD patients (M = 55.4, SD = 40.5) were considered candidates for injectable naltrexone, yet just 11.2% (SD = 22.2) of those who were considered candidates actually received the medication.
To further describe prescribing programs and the implementation of injectable naltrexone, respondents were asked several closed- and open-ended questions related to patient outcomes, delivery of other treatment services, and cost issues associated with the medication. Although the implementation rate was low, every center that prescribed injectable naltrexone reported that its patients either somewhat or very much improved in response to the medication. In an open-ended question, 52.4% of prescribers reported that the medication was more successful in a particular client population. One main theme emerged, which consisted of responses regarding relapse and noncompliance, in which clinical directors mentioned that they had seen success in patients who had been noncompliant with other medications as well as success in those who had prior relapses. Further, every center that prescribed injectable naltrexone used the medication in conjunction with psychosocial treatment, particularly cognitive behavioral therapy.
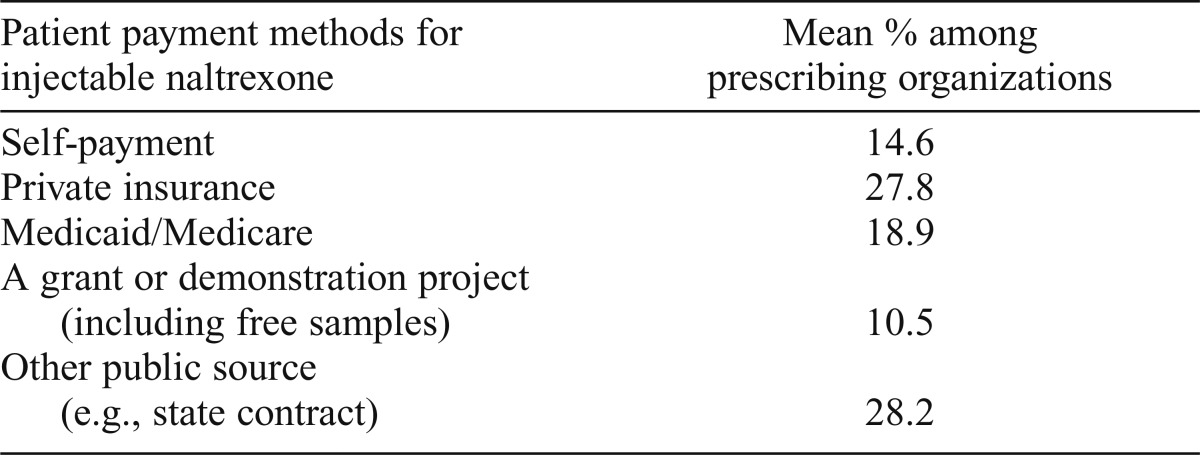
Despite the positive outcomes reported, cost was an issue that factored into the decision to prescribe injectable naltrexone among 90.5% of prescribing programs. In Table 4, we present information on how patients typically paid for injectable naltrexone. An average of 27.8% of a program’s patients paid for the medication with private insurance, and 14.6% paid out of pocket. Centers, on average, had 18.9% of their injectable naltrexone patients paying using either Medicaid or Medicare. Last, an average of 10.5% of adopting organizations’ patients received the medication through a grant or demonstration project (including receiving free samples), and 28.2% of patients used other public sources, such as state contracts.
Table 4.
Patient payment methods for injectable naltrexone
| Patient payment methods for injectable naltrexone | Mean % among prescribing organizations |
| Self-payment | 14.6 |
| Private insurance | 27.8 |
| Medicaid/Medicare | 18.9 |
| A grant or demonstration project (including free samples) | 10.5 |
| Other public source (e.g., state contract) | 28.2 |
Discussion
The data suggest that injectable naltrexone may still be in the early diffusion process, with just 13.1% of all treatment centers (and 30.8% of centers that use any medications) being adopters of the treatment for either AUD or opioid use disorder. An even smaller percentage of centers used it specifically for opioid use disorder, although this was expected given the much more recent approval of injectable naltrexone for opioid use disorder. Implementation was also low, with fewer than 10% of AUD or opioid patients, on average, receiving the medication in a treatment program. Additional information is needed to further understand the consideration of candidates for receiving the medication and the decision to prescribe it. Adopters were more likely than nonadopters to prescribe other medications as well, suggesting that nonadopters are not choosing to use another medication in place of injectable naltrexone. This pattern was also found in an early study of injectable naltrexone using a sample of privately funded centers (Abraham & Roman, 2010). The use of one innovation may facilitate the adoption of another one, similar to Rogers’ (2003) concept of technology clusters.
Multivariate logistic regression results indicated that adoption of injectable naltrexone was associated with four key organizational characteristics: wraparound services, percentage of patients with private insurance, levels of care, and for-profit status. Centers that offered more wraparound services, including care for medical and mental health issues, family services, and housing/transportation services, were more likely to be adopters of injectable naltrexone. This suggests a distinction between centers oriented toward multiple choices for treatment modalities in producing patient improvement, as well as treatment organizations that are more likely working with other systems rather than being independent. Diffusion efforts aimed at increasing the uptake of injectable naltrexone could be aimed at treatment programs with these characteristics.
The percentage of patients paying with private insurance was also a significant predictor of adoption, consistent with other studies on other medications. This finding is reasonable given the relatively costly nature of the drug. Centers serving patients who have more robust monetary resources in the form of private insurance may be more likely to incorporate it as a treatment option.
Our findings indicate that the cost of the medication seems to be an issue for many treatment centers. About 91% of adopters reported that the cost of injectable naltrexone factored into their decision to prescribe the medication to a particular patient. Research suggests that the monthly cost of injectable naltrexone is significantly higher than that of tablet naltrexone, buprenorphine, and methadone (Baser et al., 2011; Center for Substance Abuse Treatment, 2009). Nevertheless, a meta-analysis examining health care utilization and costs, among other outcomes, found that these were generally lower or as low for patients treated with injectable naltrexone relative to those treated with other AUD medications or with methadone (Hartung et al., 2014). This suggests that the higher monthly cost of the medication could potentially be offset by lower health care utilization (Hartung et al., 2014).
Given the intentions of the Affordable Care Act to increase access to behavioral health care and reduce hospital readmissions, the reduction in overall costs and health care utilization could lead to greater use of the medication. In some states, the medication may not be listed on the formulary, limiting its adoption within treatment programs. Even if the medication is covered, if it is listed under medical benefits, the program must first purchase the medication and may bill Medicaid only after it has been administered. The visits to receive a prescription or the injection itself could be handled separately as a medical benefit. Further, at least 20 states require prior authorization, and some states require documented noncompliance or prior failure with other medications in order to approve prior authorization for injectable naltrexone (American Society of Addiction Medicine, 2013). As a result, many treatment policies may be guided by state-specific policies as well as other insurance provider reimbursement options. Research examining these external characteristics is a needed next step toward greater understanding of the adoption of injectable naltrexone within treatment programs.
We also found that centers offering detoxification services were more likely to adopt injectable naltrexone. Centers with inpatient detoxification may be better able to medically manage and monitor patients who are being treated with medications. Interestingly, centers with residential services were less likely to offer injectable naltrexone than those without this level of care.
There were no significant results for 12-step treatment ideology. Given the widespread prevalence and apparent sustainability of the 12 steps in the presence of alternative approaches to treatment, these data suggest the possibility that a 12-step ideology does not necessarily exclude medication use. Every center in our sample that prescribed injectable naltrexone used the medication in conjunction with psychosocial treatment, undermining any suggestion that the use of MAT excludes behavioral treatments or counseling. Programs that heavily rely on 12-step treatment may support the use of injectable naltrexone to stop heavy drinking in the short run as long as it is in the context of a comprehensive treatment program that includes psychosocial interventions, which are associated with long-term sobriety in the 12-step model.
Although the percentage of relapsers was only significant at the .1 level in the multivariate logistic regression, respondents mentioned that they found injectable naltrexone to be particularly successful for relapsers and patients who had been noncompliant when using other medications. Treatment providers have little to guide them except their own experience in selecting the most appropriate candidates for treatment with injectable naltrexone. Given that the majority of centers that prescribed injectable naltrexone reported positive outcomes for their patients, more research is needed to examine why implementation remains limited among programs that have begun to offer the medication. Future research is also needed on the long-term outcomes of patients, including those with co-occurring alcohol and opioid problems, who are treated with injectable naltrexone.
Limitations
Several limitations of the current study should be noted. First, although the sample is representative of U.S. SUD treatment centers, the findings cannot be generalized to programs located in Veterans Health Administration facilities or based in correctional facilities. Second, although our response rate was satisfactory, there is a risk of nonresponse bias. Nevertheless, authors have suggested that higher response rates may have a limited impact on estimates (Davern et al., 2010; Mealing et al., 2010). Third, as is a common practice in federal surveys (e.g., National Survey of Substance Abuse Treatment Services [N-SSATS]) as well as other organizational-level research, our data are based on self-reports of administrative and clinical directors, which may be subject to social desirability and recall bias. No information was obtained directly from patients or from medical records to assess adoption and patient outcomes. Access to medical records could expand our analysis of adoption and implementation and ensure that rates are not overreported; however, the low reported rates of adoption and implementation suggest that this is unlikely to be a great concern. Last, these data are cross-sectional, restricting our ability to identify causal relationships. Examination of this sample over time will allow us to monitor the continued diffusion and implementation of injectable naltrexone.
Conclusions
We found that the rate of adoption of injectable naltrexone in a national sample of U.S. SUD treatment centers is limited (13%) despite federally approved use of the medication for both alcohol and opioid treatment. Given the medication’s promise, this may seem surprising. However, this rate is similar to the early adoption rates of other SUD medications (Ducharme et al., 2006; Fuller et al., 2005). Future research should consider the examination of specific implementation strategies aimed at engaging directors, counselors, and medical staff, as well as patients. Last, longitudinal research is needed to examine long-term implementation and sustainability of injectable naltrexone.
Footnotes
This research was supported by National Institute on Alcohol Abuse and Alcoholism Grant R01AA015974.
References
- Abraham AJ, Knudsen HK, Roman PM. A longitudinal examination of alcohol pharmacotherapy adoption in substance use disorder treatment programs: Patterns of sustainability and discontinuation. Journal of Studies on Alcohol and Drugs. 2011;72:669–677. doi: 10.15288/jsad.2011.72.669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abraham AJ, Roman PM. Early adoption of injectable naltrexone for alcohol-use disorders: Findings in the private-treatment sector. Journal of Studies on Alcohol and Drugs. 2010;71:460–466. doi: 10.15288/jsad.2010.71.460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anonymous Alcoholics. The AA member, medication, and other drugs. New York, NY: Author; 1984. [Google Scholar]
- Allison PD. “Missing data.”. In: Millsap RE, Maydeu-Olivares A, editors. The SAGE handbook of quantitative methods in psychology. London, England: Sage Publications Ltd; 2009. pp. 73–91. [Google Scholar]
- American Society of Addiction Medicine. Advancing access to addiction medications: Implications for opioid addiction treatment. 2013. Retrieved from http://www.asam.org/docs/advocacy/Implications-for-Opioid-Addiction-Treatment.
- Baser O, Chalk M, Fiellin DA, Gastfriend DR. Cost and utilization outcomes of opioid-dependence treatments. American Journal of Managed Care. 2011;17(Supplement 8):S235–S248. [PubMed] [Google Scholar]
- Bouza C, Angeles M, Muñoz A, Amate JM. Efficacy and safety of naltrexone and acamprosate in the treatment of alcohol dependence: A systematic review. Addiction. 2004;99:811–828. doi: 10.1111/j.1360-0443.2004.00763.x. [DOI] [PubMed] [Google Scholar]
- Center for Substance Abuse Treatment. Incorporating alcohol pharmacotherapies into medical practice. Treatment Improvement Protocol (TIP) Series, No. 49, (SMA) 09–4380. Rockville, MD: Substance Abuse and Mental Health Services Administration; 2009. [PubMed] [Google Scholar]
- Chriqui JF, Terry-McElrath Y, McBride DC, Eidson SS, Vander-Waal CJ. Does state certification or licensure influence outpatient substance abuse treatment program practices? Journal of Behavioral Health Services & Research. 2007;34:309–328. doi: 10.1007/s11414-007-9069-z. [DOI] [PubMed] [Google Scholar]
- Davern M, McAlpine D, Beebe TJ, Ziegenfuss J, Rockwood T, Call KT. Are lower response rates hazardous to your health survey? An analysis of three state telephone health surveys. Health Services Research. 2010;45:1324–1344. doi: 10.1111/j.1475-6773.2010.01128.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ducharme LJ, Knudsen HK, Roman PM. Trends in the adoption of medications for alcohol dependence. Journal of Clinical Psychopharmacology. 2006;26(Supplement 1):S13–S19. doi: 10.1097/01.jcp.0000246209.18777.14. [DOI] [PubMed] [Google Scholar]
- Ducharme LJ, Mello HL, Roman PM, Knudsen HK, Johnson JA. Service delivery in substance abuse treatment: Reexamining “comprehensive” care. Journal of Behavioral Health Services & Research. 2007;34:121–136. doi: 10.1007/s11414-007-9061-7. [DOI] [PubMed] [Google Scholar]
- Friedmann PD, Hoskinson R, Jr, Gordon M, Schwartz R, Kinlock T, Knight K, Frisman LK the MAT Working Group of CJ-DATS. Medication-assisted treatment in criminal justice agencies affiliated with the criminal justice-drug abuse treatment studies (CJ-DATS): Availability, barriers, and intentions. Substance Abuse. 2012;33:9–18. doi: 10.1080/08897077.2011.611460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuller BE, Rieckmann T, McCarty D, Smith KW, Levine H. Adoption of naltrexone to treat alcohol dependence. Journal of Substance Abuse Treatment. 2005;28:273–280. doi: 10.1016/j.jsat.2005.02.003. [DOI] [PubMed] [Google Scholar]
- Garbutt JC, Kranzler HR, O’Malley SS, Gastfriend DR, Pettinati HM, Silverman BL, Ehrich EW the Vivitrex Study Group. Efficacy and tolerability of long-acting injectable naltrexone for alcohol dependence: A randomized controlled trial. Journal of the American Medical Association. 2005;293:1617–1625. doi: 10.1001/jama.293.13.1617. [DOI] [PubMed] [Google Scholar]
- Gastfriend DR. Intramuscular extended-release naltrexone: Current evidence. Annals of the New York Academy of Sciences. 2011;1216:144–166. doi: 10.1111/j.1749-6632.2010.05900.x. [DOI] [PubMed] [Google Scholar]
- Gastfriend DR, Dong Q, Loewy J, Silverman B, Ehrich EW. Effect of long-acting injectable naltrexone on durability of drinking outcomes [Abstract] Alcoholism: Clinical and Experimental Research. 2005;29(Supplement S1):77A. [Google Scholar]
- Gueorguieva R, Wu R, Krystal JH, Donovan D, O’Malley SS. Temporal patterns of adherence to medications and behavioral treatment and their relationship to patient characteristics and treatment response. Addictive Behaviors. 2013;38:2119–2127. doi: 10.1016/j.addbeh.2013.01.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartung DM, McCarty D, Fu R, Wiest K, Chalk M, Gastfriend DR. Extended-release naltrexone for alcohol and opioid dependence: A meta-analysis of healthcare utilization studies. Journal of Substance Abuse Treatment. 2014;47:113–121. doi: 10.1016/j.jsat.2014.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinrich CJ, Cummings GR. Adoption and diffusion of evidence-based addiction medications in substance abuse treatment. Health Services Research. 2014;49:127–152. doi: 10.1111/1475-6773.12093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinrich CJ, Hill CJ. Role of state policies in the adoption of naltrexone for substance abuse treatment. Health Services Research. 2008;43:951–970. doi: 10.1111/j.1475-6773.2007.00812.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howell DC. Statistical methods for psychology. Belmont, CA: Thomson Wadsworth; 2007. [Google Scholar]
- Jonas DE, Amick HR, Feltner C, Bobashev G, Thomas K, Wines R, Garbutt JC. Pharmacotherapy for adults with alcohol use disorders in outpatient settings: A systematic review and meta-analysis. Journal of the American Medical Association. 2014;311:1889–1900. doi: 10.1001/jama.2014.3628. [DOI] [PubMed] [Google Scholar]
- Knudsen HK, Roman PM. Financial factors and the implementation of medications for treating opioid use disorders. Journal of Addiction Medicine. 2012;6:280–286. doi: 10.1097/ADM.0b013e318262a97a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knudsen HK, Roman PM, Ducharme LJ, Johnson JA. Organizational predictors of pharmacological innovation adoption: The case of disulfiram. Journal of Drug Issues. 2005;35:559–573. [Google Scholar]
- Kranzler HR, Stephenson JJ, Montejano L, Wang S, Gastfriend DR. Persistence with oral naltrexone for alcohol treatment: Implications for health-care utilization. Addiction. 2008;103:1801–1808. doi: 10.1111/j.1360-0443.2008.02345.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kranzler HR, Wesson DR, Billot L the Drug Abuse Sciences Naltrexone Depot Study Group. Naltrexone depot for treatment of alcohol dependence: A multicenter, randomized, placebo-controlled clinical trial. Alcoholism: Clinical and Experimental Research. 2004;28:1051–1059. doi: 10.1097/01.alc.0000130804.08397.29. [DOI] [PubMed] [Google Scholar]
- Krupitsky EM, Blokhina EA. Long-acting depot formulations of naltrexone for heroin dependence: A review. Current Opinion in Psychiatry. 2010;23:210–214. doi: 10.1097/YCO.0b013e3283386578. [DOI] [PubMed] [Google Scholar]
- Kubiak SP, Arfken CL, Gibson ES. Departments of corrections as purchasers of community-based treatment: A national study. Journal of Substance Abuse Treatment. 2009;36:420–427. doi: 10.1016/j.jsat.2008.08.009. [DOI] [PubMed] [Google Scholar]
- Lee JD, Grossman E, Huben L, Manseau M, McNeely J, Rotrosen J, Gourevitch MN. Extended-release naltrexone plus medical management alcohol treatment in primary care: Findings at 15 months. Journal of Substance Abuse Treatment. 2012;43:458–462. doi: 10.1016/j.jsat.2012.08.012. [DOI] [PubMed] [Google Scholar]
- Lichtenberg FR, Philipson TJ. The dual effects of intellectual property regulations: Within- and between-patent competition in the U.S. pharmaceuticals industry. Journal of Law & Economics. 2002;45:643–672. [Google Scholar]
- Mark TL, Kranzler HR, Poole VH, Hagen CA, McLeod C, Crosse S. Barriers to the use of medications to treat alcoholism. American Journal on Addictions. 2003;12:281–294. [PubMed] [Google Scholar]
- Marlowe DB. Evidence-based policies and practices for drug-involved offenders. The Prison Journal. 2011;91(Supplement):27S–47S. [Google Scholar]
- Matusow H, Dickman SL, Rich JD, Fong C, Dumont DM, Hardin C, Rosenblum A. Medication assisted treatment in US drug courts: Results from a nationwide survey of availability, barriers and attitudes. Journal of Substance Abuse Treatment. 2013;44:473–480. doi: 10.1016/j.jsat.2012.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald RC. Managing the intersection of medical and pharmacy benefits. Journal of Managed Care Pharmacy. 2008;14(Supplement):S7–S11. doi: 10.18553/jmcp.2008.14.S4-A.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMillan GP, Lapham SC. Staff perspectives on methadone maintenance therapy (MMT) in a large southwestern jail. Addiction Research and Theory. 2005;13:53–63. [Google Scholar]
- Mealing NM, Banks E, Jorm LR, Steel DG, Clements MS, Rogers KD. Investigation of relative risk estimates from studies of the same population with contrasting response rates and designs. BMC Medical Research Methodology. 2010;10:26. doi: 10.1186/1471-2288-10-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mee-Lee DL, Gartner L, Miller MM, Shulman GD, Wilford BB. Patient placement criteria for the treatment of substance related disorders. 2nd ed. Chevy Chase, MD: American Society of Addiction Medicine; 1996. [Google Scholar]
- National Institute on Drug Abuse. Principles of drug abuse treatment: A research-based guide (NIH Publication No. 02–4180) Rockville, MD: Author; 2012. [Google Scholar]
- O’Malley SS, Garbutt JC, Gastfriend DR, Dong Q, Kranzler HR. Efficacy of extended-release naltrexone in alcohol-dependent patients who are abstinent before treatment. Journal of Clinical Psychopharmacology. 2007;27:507–512. doi: 10.1097/jcp.0b013e31814ce50d. [DOI] [PubMed] [Google Scholar]
- Oser CB, Roman PM. A categorical typology of naltrexone-adopting private substance abuse treatment centers. Journal of Substance Abuse Treatment. 2008;34:433–442. doi: 10.1016/j.jsat.2007.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rich JD, Boutwell AE, Shield DC, Key RG, McKenzie M, Clarke JG, Friedmann PD. Attitudes and practices regarding the use of methadone in US state and federal prisons. Journal of Urban Health. 2005;82:411–419. doi: 10.1093/jurban/jti072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers EM. Diffusion of innovations. New York, NY: Free Press; 2003. [Google Scholar]
- Roman PM, Johnson JA. Adoption and implementation of new technologies in substance abuse treatment. Journal of Substance Abuse Treatment. 2002;22:211–218. doi: 10.1016/s0740-5472(02)00241-6. [DOI] [PubMed] [Google Scholar]
- Roozen HG, de Waart R, van der Windt DA, van den Brink W, de Jong CA, Kerkhof AJ. A systematic review of the effectiveness of naltrexone in the maintenance treatment of opioid and alcohol dependence. European Neuropsychopharmacology. 2006;16:311–323. doi: 10.1016/j.euroneuro.2005.11.001. [DOI] [PubMed] [Google Scholar]
- Rychtarik RG, Connors GJ, Dermen KH, Stasiewicz PR. Alcoholics Anonymous and the use of medications to prevent relapse: An anonymous survey of member attitudes. Journal of Studies on Alcohol. 2000;61:134–138. doi: 10.15288/jsa.2000.61.134. [DOI] [PubMed] [Google Scholar]
- Smith-Rohrberg D, Bruce RD, Altice FL. Research note—Review of corrections-based therapy for opiate dependent patients: Implications for buprenorphine treatment among correctional populations. Journal of Drug Issues. 2004;34:451–480. [Google Scholar]
- Swift RM, Duncan D, Nirenberg TD, Femino J. Alcoholic patients’ experience and attitudes on pharmacotherapy for alcoholism. Journal of Addictive Diseases. 1998;17:35–48. doi: 10.1300/J069v17n03_04. [DOI] [PubMed] [Google Scholar]
- Swift R, Oslin DW, Alexander M, Forman R. Adherence monitoring in naltrexone pharmacotherapy trials: A systematic review. Journal of Studies on Alcohol and Drugs. 2011;72:1012–1018. doi: 10.15288/jsad.2011.72.1012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Syed YY, Keating GM. Extended-release intramuscular naltrexone (VIVITROL®): A review of its use in the prevention of relapse to opioid dependence in detoxified patients. CNS Drugs. 2013;27:851–861. doi: 10.1007/s40263-013-0110-x. [DOI] [PubMed] [Google Scholar]
- Tabachnick BG, Fidell LS. Using multivariate statistics. Boston, MA: Allyn and Bacon; 2007. [Google Scholar]
- Taxman FS, Perdoni ML, Harrison LD. Drug treatment services for adult offenders: The state of the state. Journal of Substance Abuse Treatment. 2007;32:239–254. doi: 10.1016/j.jsat.2006.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas SE, Miller PM, Randall PK, Book SW. Improving acceptance of naltrexone in community addiction treatment centers: A pilot study. Journal of Substance Abuse Treatment. 2008;35:260–268. doi: 10.1016/j.jsat.2007.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas CP, Wallack SS, Lee S, McCarty D, Swift R. Research to practice: Adoption of naltrexone in alcoholism treatment. Journal of Substance Abuse Treatment. 2003;24:1–11. [PubMed] [Google Scholar]
- Volpicelli JR, Rhines KC, Rhines JS, Volpicelli LA, Alterman AI, O’Brien CP. Naltrexone and alcohol dependence. Role of subject compliance. Archives of General Psychiatry. 1997;54:737–742. doi: 10.1001/archpsyc.1997.01830200071010. [DOI] [PubMed] [Google Scholar]
- Walters ST, Clark MD, Gingerich R, Meltzer ML. Motivating offenders to change: A guide for probation and parole (NIC Accession number 022253) Washington, DC: National Institute of Corrections; 2007. [Google Scholar]


