Abstract
Objective:
A family history of alcoholism is a significant risk factor for the development of alcohol use disorders (AUDs). Because common structural abnormalities are present in reward and affective brain regions in alcoholics and those with familial alcoholism, the current study examined the relationship between familial loading of AUDs and volumes of the amygdala and nucleus accumbens (NAcc) in largely alcohol-naive adolescents, ages 12–16 years (N = 140).
Method:
The amygdala and NAcc were delineated on each participant’s T1-weighted anatomical scan, using FMRIB Software Library’s FMRIB Integrated Registration & Segmentation Tool, and visually inspected for accuracy and volume outliers. In the 140 participants with accurate segmentation (75 male/65 female), subcortical volumes were represented as a ratio to intracranial volume (ICV). A family history density (FHD) score was calculated for each adolescent based on the presence of AUDs in first- and second-degree relatives (range: 0.03–1.50; higher scores represent a greater prevalence of familial AUDs). Multiple regressions, with age and sex controlled for, examined the association between FHD and left and right amygdala and NAcc volume/ICV.
Results:
There was a significant positive relationship between FHD and left NAcc volume/ICV (ΔR2 = .04, p = .02). Post hoc regressions indicated that this effect was only significant in females (ΔR2 = .11, p = .006).
Conclusions:
This finding suggests that the degree of familial alcoholism, genetic or otherwise, is associated with alterations in reward-related brain structure. Further work will be necessary to examine whether FHD is related to future alcohol-related problems and reward-related behaviors.
A family history of alcoholism is a major risk factor for developing an alcohol use disorder (AUD) (Cotton, 1979; Dawson et al., 1992; Merikangas et al., 1998). Individual risk for AUDs is further increased by having multiple first- and second-degree relatives with alcoholism (Dawson et al., 1992), and children with high familial loading of alcoholism are at greater risk for early-onset drinking (Hill & Yuan, 1999). This suggests that familial loading of alcoholism is an important determinant of overall risk susceptibility, which is also supported by studies that have found relationships between this measure and variation in alcoholism diagnosis and severity (Stoltenberg et al., 1998). Family history density (FHD) of AUDs likely reflects genetic loading risk, as scores for first- and second-degree relatives with alcoholism are assigned based on genetic relatedness to offspring with familial alcoholism (Stoltenberg et al., 1998). However, environmental contributions that influence risk may also be present, depending on the offspring’s exposure to the relative(s) with AUD(s), which may also moderate risk vulnerability (Jacob et al., 2003).
Several studies have explored volumetric brain characteristics in individuals at familial risk for alcohol dependence. Research using a region of interest (ROI) approach has found greater cerebellar volume (Hill et al., 2007, 2011) and consistently smaller amygdalar and hippocampal (Benegal et al., 2007; Hanson et al., 2010; Hill et al., 2001, 2013b) volumes in youth and young adults with familial alcoholism compared with controls. This suggests that morphometric characteristics of the brain may be particularly important markers of risk, but that structural variation related to risk may be present across brain areas. Some of these findings are specific to at-risk individuals who carry certain risk alleles (Hill et al., 2013b) or who have been diagnosed with an externalizing disorder (Hill et al., 2013a). For example, interactions with familial history risk status and externalizing disorders were reported in a study that found smaller caudate volume in youth and young adults from multiplex families with AUDs (Hill et al., 2013a). Importantly, studies that have examined brain volume in individuals with familial alcoholism have included samples of youth with little to no experience with alcohol (Benegal et al., 2007; Hanson et al., 2010) or individuals who were largely free of abuse or dependence diagnoses during childhood/adolescence (Hill et al., 2001, 2007, 2009, 2011, 2013a, 2013b), thereby controlling for confounding alcohol-related neurotoxic effects on the brain.
Interestingly, some of the findings in adolescents with familial alcoholism suggest lateralization effects. In a study of hippocampal volume, the authors found that male youth with a family history of alcoholism (i.e., those who were family history–positive [FHP]) had larger volumes of the left hippocampus than male youth without a family history of alcoholism (i.e., those who were family history–negative [FHN]) (Hanson et al., 2010). However, no differences were observed between FHP and FHN female adolescents. In another study, smaller right to left orbitofrontal cortex volume was found in high-risk youth from multiplex alcohol-dependent families compared with controls (Hill et al., 2009). The orbitofrontal cortex is an important structure associated with emotional regulation (Bechara et al., 2000) that has connections with the ventral striatum (Groenewegen et al., 1991). This may suggest that other structures associated with reward and affect pathways may have altered volume in at-risk youth.
In addition to volumetric brain studies of familial alcoholism, functional studies have also found differences between at-risk youth compared with their peers in some of the same areas in which structural differences have been observed. In fact, even in the absence of heavy alcohol use, adolescents with familial alcoholism have differences in brain activity in frontal, parietal, temporal, striatal, and cerebellar regions during functional magnetic resonance imaging (fMRI) tasks (Cservenka & Nagel, 2012; Cservenka et al., 2012, 2014b; Mackiewicz Seghete et al., 2013; Schweinsburg et al., 2004; Silveri et al., 2011). They also show differences in fronto-cerebellar, fronto-parietal, and nucleus accumbens (NAcc) synchrony in task-based connectivity studies (Herting et al., 2011; Spadoni et al., 2013; Weiland et al., 2013; Wetherill et al., 2012), as well as differences in ventral striatal (Cservenka et al., 2014a) and amygdalar (Cservenka et al., 2014b) resting state functional connectivity compared with their age-matched peers. Based on the multitude of studies observing functional differences within these brain regions, additional structural studies are warranted to better understand whether underlying pre-morbid alterations in brain volume may help explain the functional patterns observed.
Furthermore, studies have reported gray matter (GM) brain volumetric abnormalities in subcortical regions that encompass reward-related or limbic areas in adult alcoholics (Agartz et al., 1999; Durazzo et al., 2011; Fein & Fein, 2013; Le Berre et al., 2014; Makris et al., 2008; Ozsoy et al., 2013; Sullivan et al., 2005; Wrase et al., 2008), adolescent and young adult heavy drinkers (Howell et al., 2013; Squeglia et al., 2014), and alcohol-dependent youth (De Bellis et al., 2000, 2005; Fein et al., 2013; Nagel et al., 2005). It is possible that some of these subcortical abnormalities could have been present before the onset of heavy alcohol use and may have increased the risk for alcohol dependence. In support of this hypothesis, a few studies have found that some of the GM volume effects in alcoholics are related to a family history of alcoholism (Gilman et al., 2007; Sjoerds et al., 2013).
In studies of adolescents, increases in the sensitivity to reward may be related to volumetric alterations in subcortical brain structures across development. Specifically, although increases in reward sensitivity are seen from early to late adolescence, this sensitivity declines into young adulthood, accompanied by a decline in left NAcc volume, which displays an inverted U-shape, peaking in volume in late adolescence (ages 13–17) (Urošević et al., 2012). This suggests that increased risk taking and substance use during adolescence could be associated with volumetric changes that are taking place in regions that mediate reward-related behaviors. However, a more recent study found that smaller left NAcc volume in 15- to 18-year-old alcohol- and other substance-naive adolescents was predictive of future substance use initiation (Urošević et al., 2014). It is possible that other factors related to reward sensitivity, such as familial alcoholism (Yarosh et al., 2014), may influence subcortical brain volume and its association with future substance use across development. Thus, assessing the influence of risk status on brain structure during the adolescent period is warranted.
Reward and affect-related pathways implicated in addiction (Koob, 2013) may explain why subcortical regions, such as the amygdala and NAcc, are atypical in brain volumetric studies of alcoholics (Durazzo et al., 2011; Makris et al., 2008; Wrase et al., 2008), whereas some of these characteristics, such as smaller amygdalar volume, have also been observed in at-risk adolescents and young adults (Benegal et al., 2007; Hill et al., 2001, 2013b). The goal of the current study was to expand on the limited literature on brain volume in adolescents with familial alcoholism, in the absence of any experience with heavy alcohol use. We used an automated segmentation tool in a large sample of youth with a family history of alcoholism to examine brain volume of the NAcc and amygdala, both of which show smaller volumes in alcoholics compared with controls (Durazzo et al., 2011; Makris et al., 2008; Wrase et al., 2008). As the focus of this study was on bottom-up systems that may be associated with risk for AUDs, we chose to only segment select structures related to reward and emotional processing as opposed to brain regions involved in top-down functions associated with executive functioning and inhibitory control, such as the prefrontal cortex and caudate (Rubia et al., 2006). Based on findings in adult alcoholics, at-risk individuals, and individual differences in subcortical brain volume that were predictive of substance use initiation, we hypothesized that FHD of alcoholism would be negatively correlated with amygdalar and NAcc volumes.
Method
Participant recruitment and exclusionary criteria
Participants (ages 12–16 years) were recruited through the local community as part of an ongoing longitudinal study examining family history risk for developing an AUD (Cservenka & Nagel, 2012; Cservenka et al., 2012, 2014a, 2014b; Mackiewicz Seghete et al., 2013). To determine eligibility, each youth and one of their biological parents were screened separately using a comprehensive structured interview by telephone. The interview included the Diagnostic Interview Schedule for Children Predictive Scales (Lucas et al., 2001), the Family History Assessment Module (Rice et al., 1995), the Brief Lifetime version of the Customary Drinking and Drug Use Record (Brown et al., 1998), and the Structured Clinical Interview (Brown et al., 1994). Exclusionary criteria were left-handedness (Oldfield, 1971); MRI contraindications; significant head trauma (unconscious > 2 minutes); serious medical conditions; learning disabilities; diagnosis of a psychiatric disorder according to the Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition (American Psychiatric Association, 1994); psychotic illness in a biological parent (i.e., schizophrenia or bipolar I); lack of familial alcoholism in at least one second-degree relative of the youth participant; prenatal exposure to drugs or alcohol; and heavy alcohol or other substance use (>10 lifetime alcoholic drinks, >2 drinks on any occasion, >4 cigarettes/day, >5 occasions of marijuana use, or use of any other drugs). All parents and participants gave their written consent and assent, and the study was approved by the Oregon Health & Science University (OHSU) Institutional Review Board.
Participant demographics
Intellectual functioning was measured using the two-subtest form of the Wechsler Abbreviated Scale of Intelligence (Wechsler, 1999). Socioeconomic status (SES) was determined based on parental response to the Hollingshead Index of Social Position (Hollingshead, 1957). Youth reported their pubertal status using the Pubertal Development Scale (Petersen et al., 1988). Pubertal Development Scale scores were translated to Crockett Stages for each participant, where Stages 1–5 corresponded to pre-, early, mid-, late, and post-puberty (Carskadon & Acebo, 1993).
Family history density of alcoholism
FHD scores (Stoltenberg et al., 1998) were determined from the Family History Assessment Module (Rice et al., 1995) administered to at least one biological parent of each participating youth. Biological relatives with a history of AUD contributed to the score, with a biological parent adding 0.5, a grandparent adding 0.25, and aunts/uncles each adding 0.25 divided by the total number of aunts and uncles on that side of the family (Cservenka & Nagel, 2012). Based on the contribution of each of these relatives, a total FHD score was calculated for each participant. The FHD scores in our sample ranged from 0.03 to 1.50 (M = 0.42, SD = 0.29). Further, we present demographic and volume characteristics for the sample, divided by the risk for alcoholism. Youth were defined as FHP if they had at least one biological parent with an AUD or two or more second-degree relatives with an AUD on the same side of the family (Cservenka & Nagel, 2012). Youth were defined as family history–mild (FHM) if they had one second-degree relative with an AUD or two second-degree relatives with an AUD on different sides of the family. Based on these definitions, there were 78 FHP (MFHD = 0.59, SD = 0.28) and 62 FHM (MFHD = 0.21, SD = 0.11) youth in the current study.
Image acquisition
Participants were scanned at OHSU’s Advanced Imaging Research Center on a 3.0 Tesla MAGNETOM Trio, a Tim System, with a 12-channel head coil (Siemens Medical Solutions USA, Inc., Malvern, PA). Anatomical, high-resolution T1-weighted MPRAGE scans were collected in the sagittal plane (TR = 2,300 ms, TE = 3.58 ms, TI = 900 ms, flip angle = 10°, field of view = 240 × 256 mm, voxel size = 1 × 1 × 1.1 mm, 160 slices, acquisition time = 9:14). These T1-weighted scans were used for GM, white matter (WM), and cerebrospinal fluid (CSF) segmentation, as well as NAcc and amygdala delineation.
Image processing and analysis
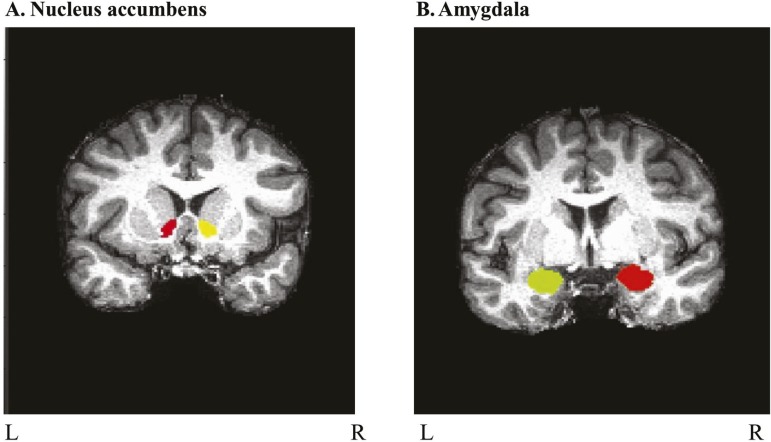
The NAcc and amygdala were defined for each participant using the FMRIB Integrated Registration and Segmentation Tool (FIRST) in FMRIB Software Library (FSL) (Patenaude et al., 2011). FIRST is an automated segmentation tool that uses the T1-weighted image to determine GM and WM boundaries and define ROIs. This tool has been used in previous publications examining subcortical volumes in alcoholics (Fein & Fein, 2013; Sameti et al., 2011). Each subcortical ROI was visually inspected for accuracy. Examples of inaccurate segmentations included grossly misplaced ROIs, incomplete ROIs, or ROIs that included other regions (i.e., amygdalar ROIs including portions of the hippocampus). When the amygdala or NAcc were not properly identified by FIRST, an affine matrix was included to improve anatomical registration and correct segmentation errors (N = 35; ∼25% of sample), which were subsequently confirmed for accuracy with visual inspection. FSL’s FMRIB Automated Segmentation Tool was used to segment GM, WM, and CSF for each subject (Zhang et al., 2001). Volumes in cubic millimeters extracted from each of these regions were added together to represent intracranial volume (ICV) for each youth. Left and right amygdala and NAcc volumes (mm3) were represented as a ratio to ICV for each participant to account for variation in ICV. Data from 190 youth (final sample N = 140) were processed using FIRST. Forty-five participants were excluded because of inaccurate ROI segmentation as were 5 outliers (an ROI/ICV value > 2.5 SD from mean), resulting in a final sample size of 140 participants (75 males/65 females). A representative delineation of bilateral NAcc and amygdala is presented for one of the participants in Figure 1.
Figure 1.
Representative delineation of nucleus accumbens and amygdala. Subcortical nuclei were delineated using FMRIB Software Library’s FMRIB Integrated Registration and Segmentation Tool on each youth’s anatomical scan and visually inspected for accuracy. Coronal views are shown of (A) nucleus accumbens and (B) amygdala on a representative participant’s T1-weighted anatomical scan.
Statistical analyses
For demographic variables and brain volumes, males and females were compared using independent samples t tests for normally distributed data and Mann–Whitney U tests for nonnormally distributed data (such as pubertal development). A chi-square test was used to compare the percentage of White participants by sex and risk status. Regression analyses were conducted to examine the relationship between FHD and brain volumes (ROI/ICV). All statistical analyses were conducted in IBM SPSS Statistics for Windows Version 20 (IBM Corp., Armonk, NY).
Results
Demographic characteristics and brain volumes
Participant demographics and brain volumes are presented in Table 1, divided by sex. Females in the sample were significantly older (t138 = 2.08, p = .04) and pubertally more mature compared with males (U138 = 964.50, Z = -6.65, p < .001). Males had significantly larger ICVs (t138 = -7.35, p < .001), with GM (t138 = -8.62, p < .001), WM (t138 = -6.78, p < .001), and CSF (t138 = -3.31, p = .001) all being significantly larger in males than in females. Females had significantly larger right NAcc volume/ICV compared with males (t138 = 2.42, p = .02). No other demographic or brain volume differences were present between the sexes. Overall, neither age, puberty, IQ, nor SES were significantly related to either left or right NAcc volume/ICV or amygdala volume/ICV (ps > .10). Table 2 presents demographic and brain volume characteristics, divided by family history risk status. FHP youth were significantly older (t138 = -2.26, p = .03) and pubertally more mature (U138 = 1,892.0, Z = -2.38, p = .02) than FHM youth. FHP adolescents also had significantly lower SES than FHM adolescents (t138 = -2.79, p = .01).
Table 1.
Demographic characteristics and brain volumes, divided by sex
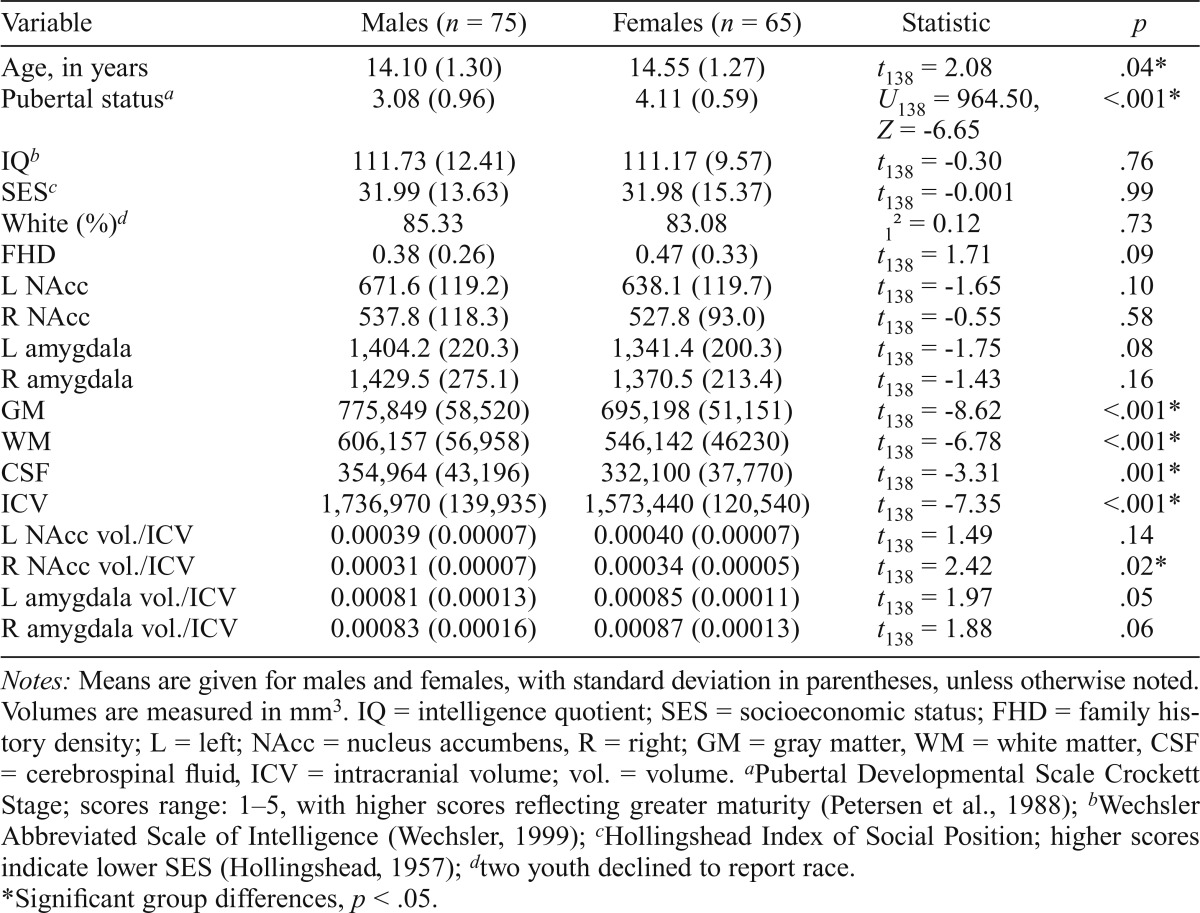
| Variable | Males (n = 75) | Females (n = 65) | Statistic | p |
| Age, in years | 14.10 (1.30) | 14.55 (1.27) | t138 = 2.08 | .04* |
| Pubertal statusa | 3.08 (0.96) | 4.11 (0.59) | U138 = 964.50, Z = -6.65 | <.001* |
| IQb | 111.73 (12.41) | 111.17 (9.57) | t138 = -0.30 | .76 |
| SESc | 31.99 (13.63) | 31.98 (15.37) | t138 = -0.001 | .99 |
| White (%)d | 85.33 | 83.08 | 12 = 0.12 | .73 |
| FHD | 0.38 (0.26) | 0.47 (0.33) | t138 = 1.71 | .09 |
| L NAcc | 671.6 (119.2) | 638.1 (119.7) | t138 = -1.65 | .10 |
| R NAcc | 537.8 (118.3) | 527.8 (93.0) | t138 = -0.55 | .58 |
| L amygdala | 1,404.2 (220.3) | 1,341.4 (200.3) | t138 = -1.75 | .08 |
| R amygdala | 1,429.5 (275.1) | 1,370.5 (213.4) | t138 = -1.43 | .16 |
| GM | 775,849 (58,520) | 695,198 (51,151) | t138 = -8.62 | <.001* |
| WM | 606,157 (56,958) | 546,142 (46230) | t138 = -6.78 | <.001* |
| CSF | 354,964 (43,196) | 332,100 (37,770) | t138 = -3.31 | .001* |
| ICV | 1,736,970 (139,935) | 1,573,440 (120,540) | t138 = -7.35 | <.001* |
| L NAcc vol./ICV | 0.00039 (0.00007) | 0.00040 (0.00007) | t138 = 1.49 | .14 |
| R NAcc vol./ICV | 0.00031 (0.00007) | 0.00034 (0.00005) | t138 = 2.42 | .02* |
| L amygdala vol./ICV | 0.00081 (0.00013) | 0.00085 (0.00011) | t138 = 1.97 | .05 |
| R amygdala vol./ICV | 0.00083 (0.00016) | 0.00087 (0.00013) | t138 = 1.88 | .06 |
Notes: Means are given for males and females, with standard deviation in parentheses, unless otherwise noted. Volumes are measured in mm3. IQ = intelligence quotient; SES = socioeconomic status; FHD = family history density; L = left; NAcc = nucleus accumbens, R = right; GM = gray matter, WM = white matter, CSF = cerebrospinal fluid, ICV = intracranial volume; vol. = volume.
Pubertal Developmental Scale Crockett Stage; scores range: 1–5, with higher scores reflecting greater maturity (Petersen et al., 1988);
Wechsler Abbreviated Scale of Intelligence (Wechsler, 1999);
Hollingshead Index of Social Position; higher scores indicate lower SES (Hollingshead, 1957);
two youth declined to report race.
Significant group differences, p < .05.
Table 2.
Demographic characteristics and brain volumes, divided by risk status
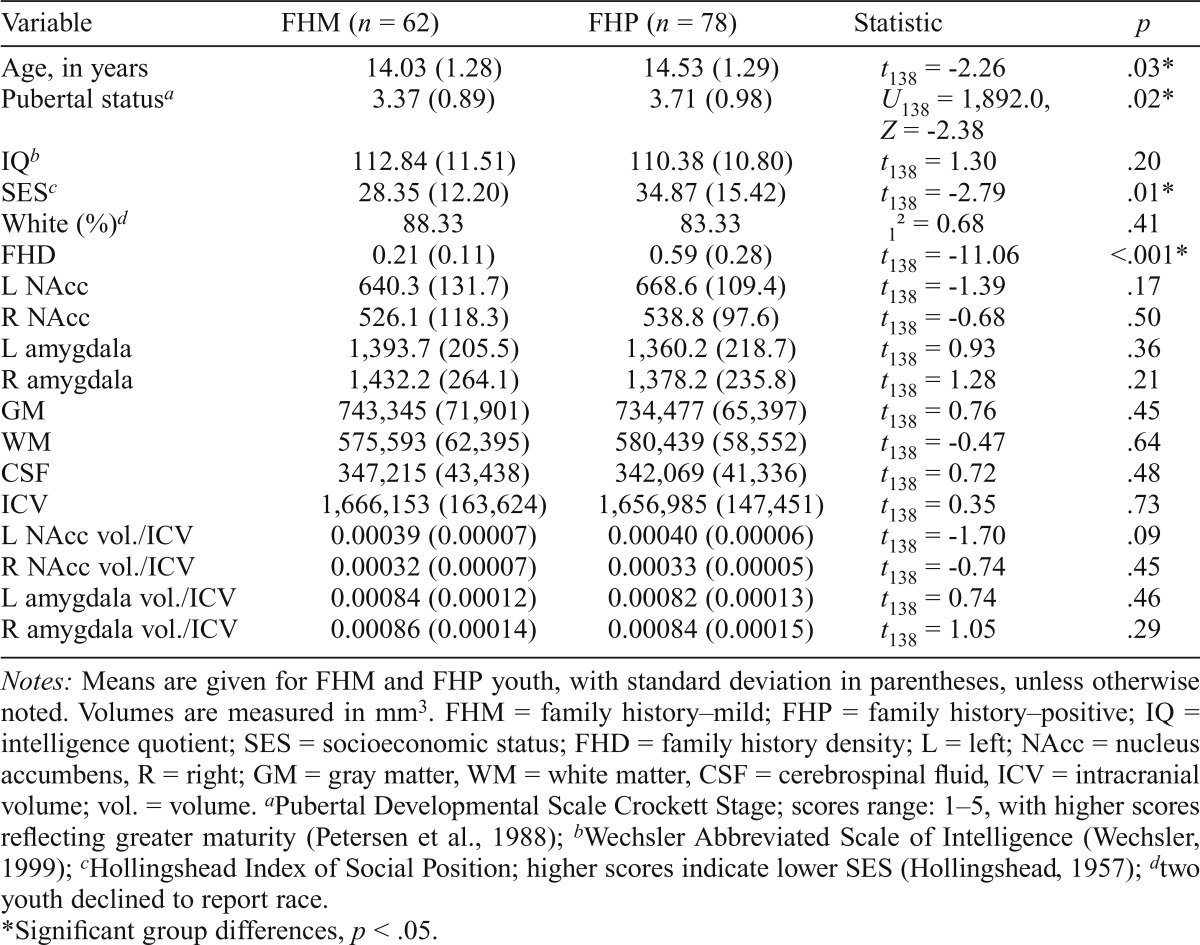
| Variable | FHM (n = 62) | FHP (n = 78) | Statistic | P |
| Age, in years | 14.03 (1.28) | 14.53 (1.29) | t138 = -2.26 | .03* |
| Pubertal statusa | 3.37 (0.89) | 3.71 (0.98) | U138 = 1,892.0, Z = -2.38 | .02* |
| IQb | 112.84 (11.51) | 110.38 (10.80) | t138 = 1.30 | .20 |
| SESc | 28.35 (12.20) | 34.87 (15.42) | t138 = -2.79 | .01* |
| White (%)d | 88.33 | 83.33 | 12 = 0.68 | .41 |
| FHD | 0.21 (0.11) | 0.59 (0.28) | t138 = -11.06 | <.001* |
| L NAcc | 640.3 (131.7) | 668.6 (109.4) | t138 = -1.39 | .17 |
| R NAcc | 526.1 (118.3) | 538.8 (97.6) | t138 = -0.68 | .50 |
| L amygdala | 1,393.7 (205.5) | 1,360.2 (218.7) | t138 = 0.93 | .36 |
| R amygdala | 1,432.2 (264.1) | 1,378.2 (235.8) | t138 = 1.28 | .21 |
| GM | 743,345 (71,901) | 734,477 (65,397) | t138 = 0.76 | .45 |
| WM | 575,593 (62,395) | 580,439 (58,552) | t138 = -0.47 | .64 |
| CSF | 347,215 (43,438) | 342,069 (41,336) | t138 = 0.72 | .48 |
| ICV | 1,666,153 (163,624) | 1,656,985 (147,451) | t138 = 0.35 | .73 |
| L NAcc vol./ICV | 0.00039 (0.00007) | 0.00040 (0.00006) | t138 = -1.70 | .09 |
| RNAccvol./ICV | 0.00032 (0.00007) | 0.00033 (0.00005) | t138 = -0.74 | .45 |
| L amygdala vol./ICV | 0.00084 (0.00012) | 0.00082 (0.00013) | t138 = 0.74 | .46 |
| R amygdala vol./ICV | 0.00086 (0.00014) | 0.00084 (0.00015) | t138 = 1.05 | .29 |
Notes: Means are given for FHM and FHP youth, with standard deviation in parentheses, unless otherwise noted. Volumes are measured in mm3. FHM = family history–mild; FHP = family history–positive; IQ = intelligence quotient; SES = socioeconomic status; FHD = family history density; L = left; NAcc = nucleus accumbens, R = right; GM = gray matter, WM = white matter, CSF = cerebrospinal fluid, ICV = intracranial volume; vol. = volume.
Pubertal Developmental Scale Crockett Stage; scores range: 1–5, with higher scores reflecting greater maturity (Petersen et al., 1988);
Wechsler Abbreviated Scale of Intelligence (Wechsler, 1999);
Hollingshead Index of Social Position; higher scores indicate lower SES (Hollingshead, 1957);
two youth declined to report race.
Significant group differences, p < .05.
Correlations between family history density and demographic characteristics
FHD was significantly related to IQ (r = -.24, p = .004) and SES (r = .32, p < .001), indicating that higher FHD was correlated with lower IQ and lower SES (higher Hollings-head score). IQ and SES were significantly correlated with each other (r = -.18, p = .03), indicating that higher IQ was related to higher SES (lower Hollingshead score). FHD was not significantly related to age (p = .58) or puberty (p = .07).
Family history density relationships with brain volumes
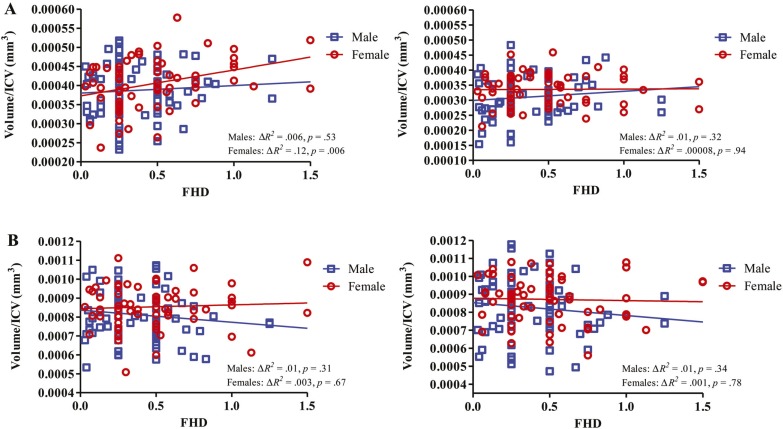
Hierarchical regressions, with age (mean centered) and sex (dummy coded) controlled for, examined the effect of FHD on brain volume. FHD was significantly related to left NAcc volume/ICV, F(3, 136) = 3.29, p = .02, ΔR2 = .04; FHD: β = .21, t = 2.47, p = .02. To examine whether this effect might be different in males and females, follow-up regression analyses were conducted looking at each sex separately. Post hoc regressions, with age controlled for, suggested that the relationship between FHD and left NAcc volume/ICV (Figure 2) was significantly positively associated in females (ΔR2 = .11, β = .34, t = 2.87, p = .006), whereas no significant relationship was present in males (ΔR2 = .005, β = .07, t = 0.63, p = .53). We examined whether the regression remained significant in females, with age controlled for, after excluding youth with FHD > 1.00. The association between FHD and left NAcc volume/ICV still held for females (ΔR2 = .10; FHD: β = .32, t = 2.64, p = .01) and remained nonsignificant in males (ΔR2 = .001; FHD: β = .04, t = 0.30, p = .76).
Figure 2.
Family history density (FHD) relationship with nucleus accumbens volume/intracranial volume (ICV) and amygdala volume/ICV. FHD was significantly related to left nucleus accumbens volume/ICV (A: left), with age and sex controlled for. Post hoc separate regressions in males and females, with age controlled for, indicate this relationship is significant in females but not males. Relationships between FHD and the other three regions of interest (A: right; B), with age controlled for, are illustrated separately for each sex.
No other significant relationships were observed with sub-cortical brain volumes—right NAcc volume/ICV: F(3, 136) = 2.29, p = .08, ΔR2 = .004; FHD: β = .06, t = 0.76, p = .45; left amygdala volume/ICV: F(3, 136) = 1.43, p = .24, ΔR2 = .001; FHD: β = -.04, t = -0.43, p = .67; right amygdala volume/ICV: F(3, 136) = 1.49, p = .22, ΔR2 = .006; FHD: β = -.08, t = -0.89, p = .38. Further, FHD did not contribute significantly in predicting GM, WM, CSF, or ICV above and beyond age and sex: GM: F(3, 136) = 24.58, p < .001, ΔR2 < .001; FHD: β = -.02, t = -0.27, p = .79; WM: F(3, 136) = 17.04, p < .001, ΔR2 = .003; FHD: β = .06, t = 0.76, p = .45; CSF: F(3, 136) = 11.10, p < .001, ΔR2 = .003; FHD: β = -.05, t = -0.68, p = .50; ICV: F(3, 136) = 19.37, p < .001, ΔR2 < .001; FHD: β = -.001, t = -0.02, p = .99.
Using the same FHD criteria as defined above, we calculated the FHD of major depressive disorder (MDD) for each youth to assess whether this, as opposed to the FHD of AUDs, may be related to the volume of the amygdala, a brain region that has shown a larger volume in individuals with a familial history of depression (Boccardi et al., 2010; Romanczuk-Seiferth et al., 2014). Information on the FHD of MDD was only available for 76 youth in this sample. However, the FHD of MDD did not significantly contribute beyond age and sex to left amygdala volume/ICV: F(3, 72) = 2.87, p = .04, ΔR2 = .02; FHD: β = -.14, t = -1.22, p = .23, whereas neither age and sex nor the addition of the FHD of MDD to the regression model was significantly related to right amygdala volume/ICV: F(3, 72) = 1.06, p = .37, ΔR2 = .02; FHD: β = .12, t = 1.05, p = .30.
Discussion
The purpose of this study was to examine whether brain volume is related to the degree of FHD of AUDs in a large sample (N = 140) of adolescents who have not begun initiating heavy alcohol or other substance use, thereby examining whether brain characteristics in these youth may be related to their degree of risk for developing alcoholism. The findings suggest that the density of alcoholism in the youth’s family is positively related to the volume of the left NAcc volume/ICV, and this relationship is significant only in girls with familial alcoholism.
Interestingly, this finding is the opposite of our hypothesis, as we had expected to find a negative relationship between FHD and subcortical brain volumes, given the findings related to volume reductions in these regions in alcoholics (Durazzo et al., 2011; Makris et al., 2008; Wrase et al., 2008) as well as youth and adults with a family history of alcoholism (Benegal et al., 2007; Hill et al., 2001, 2013b). Some of the previously published studies examined differences in brain volume between groups in a dichotomous fashion, such as comparing youth with and without familial alcoholism (Hanson et al., 2010), whereas others took a similar approach but included ultra-high-risk youth with multiplex familial AUDs in the family history group, thereby comparing high- and low-risk groups with very distinct levels of vulnerability toward AUDs (Hill et al., 2001, 2007, 2009, 2011, 2013a, 2013b). However, the current study examined the degree of familial risk for alcoholism and its relationship with brain volume in a large cohort of youth all considered at risk because of the presence of at least one second-degree relative with an AUD. These differences in sample characteristics and analytic strategies may explain in part the differences in findings. For example, although previous studies found smaller amygdalar volume in high-risk adolescents and young adults compared with controls, the lack of replication of this finding may be related to the choice of amygdalar delineation method. Former studies used manual tracing of the amygdala (Benegal et al., 2007; Hill et al., 2001, 2013b), whereas the current study implemented an automated segmentation technique. Correlations between manual tracing and automated segmentation tools are not always robust (Morey et al., 2009), which may suggest reduced reliability of an automated approach for some brain regions.
In support of the current results, a recent study in heavy drinking young adults showed larger ventral striatum volume in users compared with controls (Howell et al., 2013). The authors of this study suggested that the findings, opposite to what has been reported in alcoholics, could represent a phenotypic risk factor for more severe alcohol-related problems. It is possible that pre-morbid larger NAcc volumes may lead to problem behaviors, such as binge drinking in at-risk youth, and longitudinal studies will be able to better answer this question.
It is also important to consider the current findings within a developmental context. The NAcc is thought to be particularly important for the escalation of adolescent reward response and heightened risk taking (Ernst et al., 2005, 2006; Galvan, 2010; Galvan et al., 2006). It may be that larger NAcc volumes in at-risk youth increase their propensity for alcoholism because of alterations in reward-related response in individuals with a family history of alcoholism, which has been reported in some (Andrews et al., 2011; Yau et al., 2012), but not all, studies of familial risk (Bjork et al., 2008). In addition, although one study of NAcc volume across adolescence found that declines in left NAcc volume from late adolescence to early adulthood were accompanied by declines in reward sensitivity (Urošević et al., 2012), another study suggested that smaller left NAcc volume in late adolescence is predictive of future substance use initiation (Urošević et al., 2014). The current findings complement the former study in suggesting that the highest risk youth with larger left NAcc volumes could perhaps be more reward sensitive and thus more likely to initiate heavy alcohol use. It will be important to conduct longitudinal studies that examine the confluence of both the degree of family history risk and personality factors to understand their contributions to NAcc volume before and after the initiation of alcohol use.
Interestingly, the positive FHD association with left NAcc volume/ICV was driven by females in our sample of adolescents with familial alcoholism. These findings are similar to another study of family history of alcoholism and GM brain volume in youth without heavy alcohol use, in which a Family History × Sex interaction was found with hippocampal volume, such that at-risk males had larger left hippocampal volumes than males without familial alcoholism (Hanson et al., 2010). It is possible that multiple neurobiological risk factors for alcoholism differ by sex in those with familial AUDs since there are also differences by sex in WM volume relationships with neuropsychological functioning in youth with and without a family history of substance abuse/dependence (Silveri et al., 2008). Differences in the neurobiology between males and females with a family history of AUDs can also help explain why the development and progression of alcoholism has been shown to differ between the sexes (Keyes et al., 2010; Schuckit et al., 1998), which is likely related to sex differences in response to alcohol, variations in neurotransmitter systems between men and women, and different effects of alcohol-related neurotoxicity between the sexes (Ceylan-Isik et al., 2010).
Furthermore, the significant findings were lateralized for the NAcc, such that FHD was only significantly related to left NAcc volume/ICV. Lateralized differences in subcortical brain volume between at-risk youth or alcoholics and controls have previously been reported (Hanson et al., 2010; Hill et al., 2001; Makris et al., 2008); therefore, an association between FHD and brain volume in only one hemisphere is not unusual. Genetic susceptibility toward alcoholism could influence brain structural characteristics in a lateralized manner (Hanson et al., 2010; Hill et al., 2001, 2009), and sex differences in the development of subcortical structures have been previously documented (Giedd et al., 1997). Thus, it is plausible that sex and family history risk factors may interact to influence brain volume in one hemisphere but not the other. Genes previously associated with dopamine signaling and development have been shown to influence the volume of other basal ganglia structures, such as the caudate (Stein et al., 2011). Future research will need to examine whether genes involved in neuronal growth or dopamine signaling influence ventral striatal volume in a sex-specific fashion and whether this is moderated by family history status.
Although this study presents novel findings of a relationship between the degree of risk for alcoholism and brain volume, its limitations warrant discussion. The number of youth with families that had multiple relatives with AUDs was fairly low, as demonstrated by the low mean of the FHD scores. This may be attributable to difficulties in recruiting participants with families in which alcoholism is prevalent, as there may be challenges for those families to participate in a neuroimaging study. It is also possible that youth with higher FHD scores were ineligible because they exceeded alcohol or other substance use criteria at the time of the prescreen. Because detailed family history information is not available during this time, it is difficult to determine what the FHD scores of those youth may have been. Furthermore, although FIRST can be a fast and efficient method for delineation of subcortical structures in large samples, uncorrectable segmentation errors resulted in excluding participants from the analysis. Despite this limitation, the current sample of 140 youth is significantly larger than some of the other published neuroimaging studies of subcortical brain volume in familial alcoholism (Benegal et al., 2007; Hanson et al., 2010; Hill et al., 2001). As previously noted, the correlation between manual tracing and automated segmentation techniques can be low (Morey et al., 2009), but automated techniques may be advantageous for delineation of small nuclei, such as the ventral striatum, which does not have clear visually demarcated boundaries and was delineated using an automated method in another study of adolescents (Dennison et al., 2013). However, because of the low correlations of automated techniques with manual tracing for subcortical regions, it is possible that relationships between brain volume and risk status for other ROIs in the analysis could have been missed due to insufficient reliability of the automated technique.
The current study is the first to examine brain volume in a large sample of adolescents free of heavy alcohol consumption with detailed family history information to correlate the FHD of alcoholism with brain volume. The results suggest that reward-related structures implicated in alcohol dependence, such as the NAcc, may be associated with the degree of risk for alcoholism in females, a risk factor that likely reflects both genetic and environmental contributions to AUD vulnerability. Using longitudinal data, future work should examine if the neural markers that relate to the degree of risk are predictive of heavy alcohol use in later stages of adolescence or in adulthood. These findings would aid prevention scientists in targeting behaviors related to specific neural circuitry in adolescents who are at heightened vulnerability for problematic alcohol use because of the presence of multiple relatives with AUDs.
Footnotes
This research was supported by National Institute on Alcohol Abuse and Alcoholism Grants R01 AA017664 (to Bonnie J. Nagel) and U01 AA021691 (to Bonnie J. Nagel) as well as a pilot grant to Bonnie J. Nagel through funding awarded to the Portland Alcohol Research Center by the National Institute on Alcohol Abuse and Alcoholism (P60 AA010760 (John Crabbe).
References
- Agartz I, Momenan R, Rawlings RR, Kerich MJ, Hommer DW. Hippocampal volume in patients with alcohol dependence. Archives of General Psychiatry. 1999;56:356–363. doi: 10.1001/archpsyc.56.4.356. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 4th ed. Washington, DC: Author; 1994. [Google Scholar]
- Andrews MM, Meda SA, Thomas AD, Potenza MN, Krystal JH, Worhunsky P, Pearlson GD. Individuals family history positive for alcoholism show functional magnetic resonance imaging differences in reward sensitivity that are related to impulsivity factors. Biological Psychiatry. 2011;69:675–683. doi: 10.1016/j.biopsych.2010.09.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bechara A, Damasio H, Damasio AR. Emotion, decision making and the orbitofrontal cortex. Cerebral Cortex. 2000;10:295–307. doi: 10.1093/cercor/10.3.295. [DOI] [PubMed] [Google Scholar]
- Benegal V, Antony G, Venkatasubramanian G, Jayakumar PN. Gray matter volume abnormalities and externalizing symptoms in subjects at high risk for alcohol dependence. Addiction Biology. 2007;12:122–132. doi: 10.1111/j.1369-1600.2006.00043.x. [DOI] [PubMed] [Google Scholar]
- Bjork JM, Knutson B, Hommer DW. Incentive-elicited striatal activation in adolescent children of alcoholics. Addiction. 2008;103:1308–1319. doi: 10.1111/j.1360-0443.2008.02250.x. [DOI] [PubMed] [Google Scholar]
- Boccardi M, Almici M, Bresciani L, Caroli A, Bonetti M, Monchieri S, Frisoni GB. Clinical and medial temporal features in a family with mood disorders. Neuroscience Letters. 2010;468:93–97. doi: 10.1016/j.neulet.2009.10.067. [DOI] [PubMed] [Google Scholar]
- Brown SA, Myers MG, Lippke L, Tapert SF, Stewart DG, Vik PW. Psychometric evaluation of the Customary Drinking and Drug Use Record (CDDR): A measure of adolescent alcohol and drug involvement. Journal of Studies on Alcohol. 1998;59:427–438. doi: 10.15288/jsa.1998.59.427. [DOI] [PubMed] [Google Scholar]
- Brown SA, Myers MG, Mott MA, Vik PW. Correlates of success following treatment for adolescent substance abuse. Applied & Preventive Psychology. 1994;3:61–73. [Google Scholar]
- Carskadon MA, Acebo C. A self-administered rating scale for pubertal development. Journal of Adolescent Health. 1993;14:190–195. doi: 10.1016/1054-139x(93)90004-9. [DOI] [PubMed] [Google Scholar]
- Ceylan-Isik AF, McBride SM, Ren J. Sex difference in alcoholism: Who is at a greater risk for development of alcoholic complication? Life Sciences. 2010;87:133–138. doi: 10.1016/j.lfs.2010.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cotton NS. The familial incidence of alcoholism: A review. Journal of Studies on Alcohol. 1979;40:89–116. doi: 10.15288/jsa.1979.40.89. [DOI] [PubMed] [Google Scholar]
- Cservenka A, Casimo K, Fair DA, Nagel BJ. Resting state functional connectivity of the nucleus accumbens in youth with a family history of alcoholism. Psychiatry Research: Neuroimaging. 2014a;221:210–219. doi: 10.1016/j.pscychresns.2013.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cservenka A, Fair DA, Nagel BJ. Emotional processing and brain activity in youth at high risk for alcoholism. Alcoholism: Clinical and Experimental Research. 2014b;38:1912–1923. doi: 10.1111/acer.12435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cservenka A, Herting MM, Nagel BJ. Atypical frontal lobe activity during verbal working memory in youth with a family history of alcoholism. Drug and Alcohol Dependence. 2012;123:98–104. doi: 10.1016/j.drugalcdep.2011.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cservenka A, Nagel BJ. Risky decision-making: An FMRI study of youth at high risk for alcoholism. Alcoholism: Clinical and Experimental Research. 2012;36:604–615. doi: 10.1111/j.1530-0277.2011.01650.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson DA, Harford TC, Grant BF. Family history as a predictor of alcohol dependence. Alcoholism: Clinical and Experimental Research. 1992;16:572–575. doi: 10.1111/j.1530-0277.1992.tb01419.x. [DOI] [PubMed] [Google Scholar]
- De Bellis MD, Clark DB, Beers SR, Soloff PH, Boring AM, Hall J, Keshavan MS. Hippocampal volume in adolescent-onset alcohol use disorders. American Journal of Psychiatry. 2000;157:737–744. doi: 10.1176/appi.ajp.157.5.737. [DOI] [PubMed] [Google Scholar]
- De Bellis MD, Narasimhan A, Thatcher DL, Keshavan MS, Soloff P, Clark DB. Prefrontal cortex, thalamus, and cerebellar volumes in adolescents and young adults with adolescent-onset alcohol use disorders and comorbid mental disorders. Alcoholism: Clinical and Experimental Research. 2005;29:1590–1600. doi: 10.1097/01.alc.0000179368.87886.76. [DOI] [PubMed] [Google Scholar]
- Dennison M, Whittle S, Yücel M, Vijayakumar N, Kline A, Simmons J, Allen NB. Mapping subcortical brain maturation during adolescence: Evidence of hemisphere- and sex-specific longitudinal changes. Developmental Science. 2013;16:772–791. doi: 10.1111/desc.12057. [DOI] [PubMed] [Google Scholar]
- Durazzo TC, Tosun D, Buckley S, Gazdzinski S, Mon A, Fryer SL, Meyerhoff DJ. Cortical thickness, surface area, and volume of the brain reward system in alcohol dependence: Relationships to relapse and extended abstinence. Alcoholism: Clinical and Experimental Research. 2011;35:1187–1200. doi: 10.1111/j.1530-0277.2011.01452.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ernst M, Nelson EE, Jazbec S, McClure EB, Monk CS, Leiben-luft E, Pine DS. Amygdala and nucleus accumbens in responses to receipt and omission of gains in adults and adolescents. NeuroImage. 2005;25:1279–1291. doi: 10.1016/j.neuroimage.2004.12.038. [DOI] [PubMed] [Google Scholar]
- Ernst M, Pine DS, Hardin M. Triadic model of the neurobiology of motivated behavior in adolescence. Psychological Medicine. 2006;36:299–312. doi: 10.1017/S0033291705005891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fein G, Fein D. Subcortical volumes are reduced in short-term and long-term abstinent alcoholics but not those with a comorbid stimulant disorder. NeuroImage: Clinical. 2013;3:47–53. doi: 10.1016/j.nicl.2013.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fein G, Greenstein D, Cardenas VA, Cuzen NL, Fouche J-P, Ferrett H, Stein DJ. Cortical and subcortical volumes in adolescents with alcohol dependence but without substance or psychiatric comorbidities. Psychiatry Research: Neuroimaging. 2013;214:1–8. doi: 10.1016/j.pscychresns.2013.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galvan A. Adolescent development of the reward system. Frontiers in Human Neuroscience. 2010;4:6. doi: 10.3389/neuro.09.006.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galvan A, Hare TA, Parra CE, Penn J, Voss H, Glover G, Casey BJ. Earlier development of the accumbens relative to orbitofrontal cortex might underlie risk-taking behavior in adolescents. Journal of Neuroscience. 2006;26:6885–6892. doi: 10.1523/JNEUROSCI.1062-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giedd JN, Castellanos FX, Rajapakse JC, Vaituzis AC, Rapoport JL. Sexual dimorphism of the developing human brain. Progress in Neuro-Psychopharmacology & Biological Psychiatry. 1997;21:1185–1201. doi: 10.1016/s0278-5846(97)00158-9. [DOI] [PubMed] [Google Scholar]
- Gilman JM, Bjork JM, Hommer DW. Parental alcohol use and brain volumes in early- and late-onset alcoholics. Biological Psychiatry. 2007;62:607–615. doi: 10.1016/j.biopsych.2006.10.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groenewegen HJ, Berendse HW, Wolters JG, Lohman AHM. The anatomical relationship of the prefrontal cortex with the striatopallidal system, the thalamus and the amygdala: Evidence for a parallel organization. Progress in Brain Research. 1991;85:95–118. doi: 10.1016/s0079-6123(08)62677-1. [DOI] [PubMed] [Google Scholar]
- Hanson KL, Medina KL, Nagel BJ, Spadoni AD, Gorlick A, Tapert SF. Hippocampal volumes in adolescents with and without a family history of alcoholism. American Journal of Drug and Alcohol Abuse. 2010;36:161–167. doi: 10.3109/00952991003736397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herting MM, Fair D, Nagel BJ. Altered fronto-cerebellar connectivity in alcohol-naïve youth with a family history of alcoholism. NeuroImage. 2011;54:2582–2589. doi: 10.1016/j.neuroimage.2010.10.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill SY, De Bellis MD, Keshavan MS, Lowers L, Shen S, Hall J, Pitts T. Right amygdala volume in adolescent and young adult offspring from families at high risk for developing alcoholism. Biological Psychiatry. 2001;49:894–905. doi: 10.1016/s0006-3223(01)01088-5. [DOI] [PubMed] [Google Scholar]
- Hill SY, Lichenstein S, Wang S, Carter H, McDermott M. Caudate volume in offspring at ultra high risk for alcohol dependence: COMT Val158Met, DRD2, externalizing disorders, and working memory. Advances in Molecular Imaging. 2013a;3:43–54. doi: 10.4236/ami.2013.34007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill SY, Muddasani S, Prasad K, Nutche J, Steinhauer SR, Scanlon J, Keshavan M. Cerebellar volume in offspring from multiplex alcohol dependence families. Biological Psychiatry. 2007;61:41–47. doi: 10.1016/j.biopsych.2006.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill SY, Wang S, Carter H, McDermott MD, Zezza N, Stiffler S. Amygdala volume in offspring from multiplex for alcohol dependence families: The moderating influence of childhood environment and 5-HTTLPR. Journal of Alcoholism and Drug Dependence. 2013b;S1:1–9. doi: 10.4172/2329-6488.S1-001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill SY, Wang S, Carter H, Tessner K, Holmes B, McDermott M, Stiffler S. Cerebellum volume in high-risk offspring from multiplex alcohol dependence families: Association with allelic variation in GABRA2 and BDNF. Psychiatry Research: Neuroimaging. 2011;194:304–313. doi: 10.1016/j.pscychresns.2011.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill SY, Wang S, Kostelnik B, Carter H, Holmes B, McDermott M, Keshavan MS. Disruption of orbitofrontal cortex laterality in offspring from multiplex alcohol dependence families. Biological Psychiatry. 2009;65:129–136. doi: 10.1016/j.biopsych.2008.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill SY, Yuan H. Familial density of alcoholism and onset of adolescent drinking. Journal of Studies on Alcohol. 1999;60:7–17. doi: 10.15288/jsa.1999.60.7. [DOI] [PubMed] [Google Scholar]
- Hollingshead AB. Two factor index of social position. New Haven, CT: Author; 1957. [Google Scholar]
- Howell NA, Worbe Y, Lange I, Tait R, Irvine M, Banca P, Voon V. Increased ventral striatal volume in college-aged binge drinkers. PLoS ONE. 2013;8(9):e74164. doi: 10.1371/journal.pone.0074164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacob T, Waterman B, Heath A, True W, Bucholz KK, Haber R, Fu Q. Genetic and environmental effects on offspring alcoholism: New insights using an offspring-of-twins design. Archives of General Psychiatry. 2003;60:1265–1272. doi: 10.1001/archpsyc.60.12.1265. [DOI] [PubMed] [Google Scholar]
- Keyes KM, Martins SS, Blanco C, Hasin DS. Telescoping and gender differences in alcohol dependence: New evidence from two national surveys. American Journal of Psychiatry. 2010;167:969–976. doi: 10.1176/appi.ajp.2009.09081161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF. Addiction is a reward deficit and stress surfeit disorder. Frontiers in Psychiatry. 2013;4:72. doi: 10.3389/fpsyt.2013.00072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Berre A-P, Rauchs G, La Joie R, Mézenge F, Boudehent C, Vabret F, Beaunieux H. Impaired decision-making and brain shrinkage in alcoholism. European Psychiatry. 2014;29:125–133. doi: 10.1016/j.eurpsy.2012.10.002. [DOI] [PubMed] [Google Scholar]
- Lucas CP, Zhang H, Fisher PW, Shaffer D, Regier DA, Narrow WE, Rubio-Stipec M. The DISC Predictive Scales (DPS): Efficiently screening for diagnoses. Journal of the American Academy of Child and Adolescent Psychiatry. 2001;40:443–449. doi: 10.1097/00004583-200104000-00013. [DOI] [PubMed] [Google Scholar]
- Mackiewicz Seghete KL, Cservenka A, Herting MM, Nagel BJ. Atypical spatial working memory and task-general brain activity in adolescents with a family history of alcoholism. Alcoholism: Clinical and Experimental Research. 2013;37:390–398. doi: 10.1111/j.1530-0277.2012.01948.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makris N, Oscar-Berman M, Jaffin SK, Hodge SM, Kennedy DN, Caviness VS, Harris GJ. Decreased volume of the brain reward system in alcoholism. Biological Psychiatry. 2008;64:192–202. doi: 10.1016/j.biopsych.2008.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merikangas KR, Stolar M, Stevens DE, Goulet J, Preisig MA, Fenton B, Rounsaville BJ. Familial transmission of substance use disorders. Archives of General Psychiatry. 1998;55:973–979. doi: 10.1001/archpsyc.55.11.973. [DOI] [PubMed] [Google Scholar]
- Morey RA, Petty CM, Xu Y, Hayes JP, Wagner HR, II, Lewis DV, McCarthy G. A comparison of automated segmentation and manual tracing for quantifying hippocampal and amygdala volumes. NeuroImage. 2009;45:855–866. doi: 10.1016/j.neuroimage.2008.12.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagel BJ, Schweinsburg AD, Phan V, Tapert SF. Reduced hippocampal volume among adolescents with alcohol use disorders without psychiatric comorbidity. Psychiatry Research: Neuroimaging. 2005;139:181–190. doi: 10.1016/j.pscychresns.2005.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oldfield RC. The assessment and analysis of handedness: The Edinburgh inventory. Neuropsychologia. 1971;9:97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- Ozsoy S, Durak AC, Esel E. Hippocampal volumes and cognitive functions in adult alcoholic patients with adolescent-onset. Alcohol. 2013;47:9–14. doi: 10.1016/j.alcohol.2012.09.002. [DOI] [PubMed] [Google Scholar]
- Patenaude B, Smith SM, Kennedy DN, Jenkinson M. A Bayesian model of shape and appearance for subcortical brain segmentation. NeuroImage. 2011;56:907–922. doi: 10.1016/j.neuroimage.2011.02.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen AC, Crockett L, Richards M, Boxer A. A self-report measure of pubertal status: Reliability, validity, and initial norms. Journal of Youth and Adolescence. 1988;17:117–133. doi: 10.1007/BF01537962. [DOI] [PubMed] [Google Scholar]
- Rice JP, Reich T, Bucholz KK, Neuman RJ, Fishman R, Rochberg N, Begleiter H. Comparison of direct interview and family history diagnoses of alcohol dependence. Alcoholism: Clinical and Experimental Research. 1995;19:1018–1023. doi: 10.1111/j.1530-0277.1995.tb00983.x. [DOI] [PubMed] [Google Scholar]
- Romanczuk-Seiferth N, Pöhland L, Mohnke S, Garbusow M, Erk S, Haddad L, Heinz A. Larger amygdala volume in first-degree relatives of patients with major depression. NeuroImage: Clinical. 2014;5:62–68. doi: 10.1016/j.nicl.2014.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubia K, Smith AB, Woolley J, Nosarti C, Heyman I, Taylor E, Brammer M. Progressive increase of frontostriatal brain activation from childhood to adulthood during event-related tasks of cognitive control. Human Brain Mapping. 2006;27:973–993. doi: 10.1002/hbm.20237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sameti M, Smith S, Patenaude B, Fein G. Subcortical volumes in long-term abstinent alcoholics: Associations with psychiatric comorbidity. Alcoholism: Clinical and Experimental Research. 2011;35:1067–1080. doi: 10.1111/j.1530-0277.2011.01440.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuckit MA, Daeppen J-B, Tipp JE, Hesselbrock M, Bucholz KK. The clinical course of alcohol-related problems in alcohol dependent and nonalcohol dependent drinking women and men. Journal of Studies on Alcohol. 1998;59:581–590. doi: 10.15288/jsa.1998.59.581. [DOI] [PubMed] [Google Scholar]
- Schweinsburg AD, Paulus MP, Barlett VC, Killeen LA, Caldwell LC, Pulido C, Tapert SF. An fMRI study of response inhibition in youths with a family history of alcoholism. Annals of the New York Academy of Sciences. 2004;1021:391–394. doi: 10.1196/annals.1308.050. [DOI] [PubMed] [Google Scholar]
- Silveri MM, Rogowska J, McCaffrey A, Yurgelun-Todd DA. Adolescents at risk for alcohol abuse demonstrate altered frontal lobe activation during Stroop performance. Alcoholism: Clinical and Experimental Research. 2011;35:218–228. doi: 10.1111/j.1530-0277.2010.01337.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silveri MM, Tzilos GK, Yurgelun-Todd DA. Relationship between white matter volume and cognitive performance during adolescence: Effects of age, sex and risk for drug use. Addiction. 2008;103:1509–1520. doi: 10.1111/j.1360-0443.2008.02272.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sjoerds Z, Van Tol M-J, Van den Brink W, Van der Wee NJA, Van Buchem MA, Aleman A, Veltman DJ. Family history of alcohol dependence and gray matter abnormalities in non-alcoholic adults. The World Journal of Biological Psychiatry. 2013;14:565–573. doi: 10.3109/15622975.2011.640942. [DOI] [PubMed] [Google Scholar]
- Spadoni AD, Simmons AN, Yang TT, Tapert SF. Family history of alcohol use disorders and neuromaturation: A functional connectivity study with adolescents. American Journal of Drug and Alcohol Abuse. 2013;39:356–364. doi: 10.3109/00952990.2013.818680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Squeglia LM, Rinker DA, Bartsch H, Castro N, Chung Y, Dale AM, Tapert SF. Brain volume reductions in adolescent heavy drinkers. Developmental Cognitive Neuroscience. 2014;9:117–125. doi: 10.1016/j.dcn.2014.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein JL, Hibar DP, Madsen SK, Khamis M, McMahon KL, de Zubicaray GI, Thompson PM. Discovery and replication of dopamine-related gene effects on caudate volume in young and elderly populations (N = 1198) using genome-wide search. Molecular Psychiatry. 2011;16:927–937. doi: 10.1038/mp.2011.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoltenberg SF, Mudd SA, Blow FC, Hill EM. Evaluating measures of family history of alcoholism: Density versus dichotomy. Addiction. 1998;93:1511–1520. doi: 10.1046/j.1360-0443.1998.931015117.x. [DOI] [PubMed] [Google Scholar]
- Sullivan EV, Deshmukh A, De Rosa E, Rosenbloom MJ, Pfefferbaum A. Striatal and forebrain nuclei volumes: Contribution to motor function and working memory deficits in alcoholism. Biological Psychiatry. 2005;57:768–776. doi: 10.1016/j.biopsych.2004.12.012. [DOI] [PubMed] [Google Scholar]
- Urošević S, Collins P, Muetzel R, Lim K, Luciana M. Longitudinal changes in behavioral approach system sensitivity and brain structures involved in reward processing during adolescence. Developmental Psychology. 2012;48:1488–1500. doi: 10.1037/a0027502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urošević S, Collins P, Muetzel R, Schissel A, Lim KO, Luciana M. Effects of reward sensitivity and regional brain volumes on substance use initiation in adolescence. Social Cognitive and Affective Neuroscience. 2014 doi: 10.1093/scan/nsu022. Advance online publication. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wechsler D. Wechsler Abbreviated Scale of Intelligence. San Antonio, TX: Psychological Corporation; 1999. [Google Scholar]
- Weiland BJ, Welsh RC, Yau WY, Zucker RA, Zubieta JK, Heitzeg MM. Accumbens functional connectivity during reward mediates sensation-seeking and alcohol use in high-risk youth. Drug and Alcohol Dependence. 2013;128:130–139. doi: 10.1016/j.drugalcdep.2012.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wetherill RR, Bava S, Thompson WK, Boucquey V, Pulido C, Yang TT, Tapert SF. Frontoparietal connectivity in substance-naïve youth with and without a family history of alcoholism. Brain Research. 2012;1432:66–73. doi: 10.1016/j.brainres.2011.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wrase J, Makris N, Braus DF, Mann K, Smolka MN, Kennedy DN, Heinz A. Amygdala volume associated with alcohol abuse relapse and craving. American Journal of Psychiatry. 2008;165:1179–1184. doi: 10.1176/appi.ajp.2008.07121877. [DOI] [PubMed] [Google Scholar]
- Yarosh HL, Hyatt CJ, Meda SA, Jiantonio-Kelly R, Potenza MN, Assaf M, Pearlson GD. Relationships between reward sensitivity, risk-taking and family history of alcoholism during an interactive competitive fMRI task. PLoS ONE. 2014;9(2):e88188. doi: 10.1371/journal.pone.0088188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yau WY, Zubieta JK, Weiland BJ, Samudra PG, Zucker RA, Heitzeg MM. Nucleus accumbens response to incentive stimuli anticipation in children of alcoholics: Relationships with precursive behavioral risk and lifetime alcohol use. Journal of Neuroscience. 2012;32:2544–2551. doi: 10.1523/JNEUROSCI.1390-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Brady M, Smith S. Segmentation of brain MR images through a hidden Markov random field model and the expectation-maximization algorithm. IEEE Transactions on Medical Imaging. 2001;20:45–57. doi: 10.1109/42.906424. [DOI] [PubMed] [Google Scholar]