Abstract
Aqueous solutions of the bovine eye lens protein gamma II (or gamma B)-crystallin at neutral pH show a gradual increase in phase separation temperature, Tph, when allowed to stand for several weeks at room temperature without reducing agents. In a typical experiment, the Tph of the protein solution (218 mg/ml) increases from 2.5 +/- 1 degree C to 32.5 +/- 1 degree C after 21 days, and a new protein species, gamma IIH, is formed. The Tph of pure gamma IIH is at least 40 degrees C higher than that of pure gamma II. The average apparent hydrodynamic radius is 36 A for gamma IIH compared to 26 A for gamma II. The molecular mass of gamma IIH is approximately 41.5 kDa compared to 20 kDa for native gamma II. Therefore, gamma IIH is probably a dimer of gamma II crystallin. gamma IIH has a lower thiol content than gamma II and is not formed in the presence of dithiothreitol. We conclude that gamma IIH is a thiol oxidation product of gamma II-crystallin and is a dimer containing an intermolecular disulfide crosslink. Thus, some oxidative modifications of protein thiol groups lead to an increase in net attractive interactions between proteins. As a result, Tph increases and protein aggregates are formed. These two microscopic changes produce the increased light scattering associated with lens opacification.
Full text
PDF

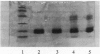
Images in this article
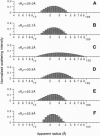
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Benedek G. B. The molecular basis of cataract formation. Ciba Found Symp. 1984;106:237–247. doi: 10.1002/9780470720875.ch14. [DOI] [PubMed] [Google Scholar]
- Bhat S. P., Spector A. Complete nucleotide sequence of a cDNA derived from calf lens gamma-crystallin mRNA: presence of Alu I-like DNA sequences. DNA. 1984 Aug;3(4):287–295. doi: 10.1089/dna.1.1984.3.287. [DOI] [PubMed] [Google Scholar]
- Broide M. L., Berland C. R., Pande J., Ogun O. O., Benedek G. B. Binary-liquid phase separation of lens protein solutions. Proc Natl Acad Sci U S A. 1991 Jul 1;88(13):5660–5664. doi: 10.1073/pnas.88.13.5660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark J. I., Benedek G. B. Phase diagram for cell cytoplasm from the calf lens. Biochem Biophys Res Commun. 1980 Jul 16;95(1):482–489. doi: 10.1016/0006-291x(80)90763-9. [DOI] [PubMed] [Google Scholar]
- Crompton M., Rixon K. C., Harding J. J. Aspirin prevents carbamylation of soluble lens proteins and prevents cyanate-induced phase separation opacities in vitro: a possible mechanism by which aspirin could prevent cataract. Exp Eye Res. 1985 Feb;40(2):297–311. doi: 10.1016/0014-4835(85)90014-4. [DOI] [PubMed] [Google Scholar]
- Garland D. Role of site-specific, metal-catalyzed oxidation in lens aging and cataract: a hypothesis. Exp Eye Res. 1990 Jun;50(6):677–682. doi: 10.1016/0014-4835(90)90113-9. [DOI] [PubMed] [Google Scholar]
- Garner M. H., Spector A. Selective oxidation of cysteine and methionine in normal and senile cataractous lenses. Proc Natl Acad Sci U S A. 1980 Mar;77(3):1274–1277. doi: 10.1073/pnas.77.3.1274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harding J. J., Beswick H. T., Ajiboye R., Huby R., Blakytny R., Rixon K. C. Non-enzymic post-translational modification of proteins in aging. A review. Mech Ageing Dev. 1989 Oct;50(1):7–16. doi: 10.1016/0047-6374(89)90054-7. [DOI] [PubMed] [Google Scholar]
- Harding J. J. Nonenzymatic covalent posttranslational modification of proteins in vivo. Adv Protein Chem. 1985;37:247–334. doi: 10.1016/s0065-3233(08)60066-2. [DOI] [PubMed] [Google Scholar]
- Hay R. E., Andley U. P., Petrash J. M. Expression of recombinant bovine gamma B-, gamma C- and gamma D-crystallins and correlation with native proteins. Exp Eye Res. 1994 May;58(5):573–584. doi: 10.1006/exer.1994.1052. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lou M. F., Dickerson J. E., Jr Protein-thiol mixed disulfides in human lens. Exp Eye Res. 1992 Dec;55(6):889–896. doi: 10.1016/0014-4835(92)90015-k. [DOI] [PubMed] [Google Scholar]
- McDermott M. J., Gawinowicz-Kolks M. A., Chiesa R., Spector A. The disulfide content of calf gamma-crystallin. Arch Biochem Biophys. 1988 May 1;262(2):609–619. doi: 10.1016/0003-9861(88)90413-4. [DOI] [PubMed] [Google Scholar]
- Najmudin S., Nalini V., Driessen H. P., Slingsby C., Blundell T. L., Moss D. S., Lindley P. F. Structure of the bovine eye lens protein gammaB(gammaII)-crystallin at 1.47 A. Acta Crystallogr D Biol Crystallogr. 1993 Mar 1;49(Pt 2):223–233. doi: 10.1107/S0907444992007601. [DOI] [PubMed] [Google Scholar]
- Pande J., McDermott M. J., Callender R. H., Spector A. Raman spectroscopic evidence for a disulfide bridge in calf gamma II crystallin. Arch Biochem Biophys. 1989 Feb 15;269(1):250–255. doi: 10.1016/0003-9861(89)90106-9. [DOI] [PubMed] [Google Scholar]
- Pande J., Ogun O., Nath C., Benedek G. Suppression of phase separation in bovine gamma IV crystallin solutions: effect of modification by charged versus uncharged polar groups. Exp Eye Res. 1993 Sep;57(3):257–264. doi: 10.1006/exer.1993.1123. [DOI] [PubMed] [Google Scholar]
- Siezen R. J., Benedek G. B. Controlled modulation of the phase separation and opacification temperature of purified bovine gamma IV-crystallin. Curr Eye Res. 1985 Oct;4(10):1077–1085. doi: 10.3109/02713688509003352. [DOI] [PubMed] [Google Scholar]
- Siezen R. J., Fisch M. R., Slingsby C., Benedek G. B. Opacification of gamma-crystallin solutions from calf lens in relation to cold cataract formation. Proc Natl Acad Sci U S A. 1985 Mar;82(6):1701–1705. doi: 10.1073/pnas.82.6.1701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siezen R. J., Kaplan E. D., Anello R. D. Superior resolution of gamma-crystallins from microdissected eye lens by cation-exchange high-performance liquid chromatography. Biochem Biophys Res Commun. 1985 Feb 28;127(1):153–160. doi: 10.1016/s0006-291x(85)80138-8. [DOI] [PubMed] [Google Scholar]
- Tanaka T., Rubin S., Sun S. T., Nishio I., Tung W. H., Chylack L. T., Jr Phase separation in rat lenses cultured in low glucose media. Invest Ophthalmol Vis Sci. 1983 Apr;24(4):522–525. [PubMed] [Google Scholar]
- Thomson J. A., Schurtenberger P., Thurston G. M., Benedek G. B. Binary liquid phase separation and critical phenomena in a protein/water solution. Proc Natl Acad Sci U S A. 1987 Oct;84(20):7079–7083. doi: 10.1073/pnas.84.20.7079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Truscott R. J., Augusteyn R. C. The state of sulphydryl groups in normal and cataractous human lenses. Exp Eye Res. 1977 Aug;25(2):139–148. doi: 10.1016/0014-4835(77)90126-9. [DOI] [PubMed] [Google Scholar]
- Truscott R. J., Martin F. The reaction of proteins with 3-hydroxyanthranilic acid as a possible model for senile nuclear cataract in man. Exp Eye Res. 1989 Dec;49(6):927–940. doi: 10.1016/s0014-4835(89)80017-x. [DOI] [PubMed] [Google Scholar]
- Weber K., Osborn M. The reliability of molecular weight determinations by dodecyl sulfate-polyacrylamide gel electrophoresis. J Biol Chem. 1969 Aug 25;244(16):4406–4412. [PubMed] [Google Scholar]
- Wistow G., Turnell B., Summers L., Slingsby C., Moss D., Miller L., Lindley P., Blundell T. X-ray analysis of the eye lens protein gamma-II crystallin at 1.9 A resolution. J Mol Biol. 1983 Oct 15;170(1):175–202. doi: 10.1016/s0022-2836(83)80232-0. [DOI] [PubMed] [Google Scholar]