Abstract
Background
Breakfast skipping (BS) is closely associated with overeating (in the evening), weight gain and obesity. It is unclear whether the addition of breakfast, with emphasis on dietary protein, leads to better appetite and energy intake regulation in adolescents.
Objective
The purpose of the study was to examine the impact of addition of a normal-protein (PN) breakfast vs protein-rich (PR) breakfast on appetite and food intake in ‘breakfast-skipping’ adolescents.
Subjects and Design
A total of 13 adolescents (age 14.3 ± 0.3 years; body mass index percentile 79 ± 4 percentile; skipped breakfast 5 ± 1× per week) randomly completed 3 testing days that included a PN (18 ± 1 g protein), PR (48 ± 2 g protein) or BS. Breakfast was 24% of estimated daily energy needs. Appetite, satiety and hormonal responses were collected over 5 h followed by an ad libitum lunch and 24-h food intake assessments.
Results
Perceived appetite was not different following PN vs BS; PR led to greater reductions vs BS (P<0.01) and PN (P< 0.001). Fullness was greater following both breakfast meals vs BS (P<0.01) but was not different between meals. Ghrelin was not different among treatments. Greater PYY concentrations were observed following both breakfast meals vs BS (P<0.01) but was not different between meals. Lunch energy intake was not different following PN vs BS; PR led to fewer kcal consumed vs BS (P<0.01) and PN (P<0.005). Daily food intake was not different among treatments.
Conclusions
Breakfast led to increased satiety through increased fullness and PYY concentrations in ‘breakfast skipping’ adolescents. A breakfast rich in dietary protein provides additional benefits through reductions in appetite and energy intake. These findings suggest that the addition of a protein-rich breakfast might be an effective strategy to improve appetite control in young people.
Keywords: breakfast skipping, appetite, ghrelin, PYY, increased dietary protein
Introduction
Over 17 million young people in the United States are currently overweight or obese.1 Due to the increased risks for a myriad of chronic diseases stemming from obesity, recent evidence suggests that this may be the first time in 200 years in which today's children may have a shorter life expectancy than their parents.2 Thus, more effort is needed to identify significant contributors and possible treatment and prevention strategies to combat this epidemic.
A primary contributor to obesity is the increase in unhealthy eating habits, with specific emphasis on breakfast skipping, which has been strongly associated with overeating, weight gain and obesity.3,4 Over the past 20 years, there has been a dramatic decline in breakfast consumption, which has closely paralleled the dramatic increase in obesity.5 Currently, up to 75% of older and overweight adolescents frequently skip breakfast.3,6 Numerous cross-sectional and observational studies in young people have examined the relationship between obesity and BS and found an inverse relationship between breakfast frequency and body mass index (i.e., the fewer the number of breakfast eating occasions, the greater the increases in body mass index).6–9 Adolescents who typically skip breakfast are more likely to consume more unhealthy foods/beverages including soft drinks, foods high in saturated fat and high calorie/high sugar snacks; they also tend to overeat throughout the day (especially during the evening hours).4,6,8,10 These common yet unhealthy practices are disconcerting at this sensitive life-stage given that dietary habits are developed and become solidified during adolescence and will most likely continue throughout adulthood, further impacting long-term body weight and overall health.9
It is currently unclear as to whether the daily consumption of breakfast might lead to better appetite control and energy intake regulation in adolescents. In addition, whether the macronutrient composition of the foods consumed at breakfast (for example, dietary protein) impacts these outcomes has not been explored.
In adults, diets containing a modest increase in dietary protein have led to greater reductions in total energy intake, body weight and fat mass while preserving lean body mass compared with eating a normal-protein diet.11,12 Protein-rich meals/diets also lead to acute and/or long-term alterations in perceived appetite,13–15 satiety13,14,16–19 and the appetite-regulating hormones ghrelin14 and PYY.20 The timing of protein consumption also appears to influence its satiating properties. In adults, dietary protein consumed at breakfast leads to greater initial and sustained feelings of fullness compared with when it is consumed at lunch or dinner.19 These data further support the concept of including a protein-rich breakfast for improved appetite control and food intake.
The purpose of this study was to examine the impact of consuming a normal-protein breakfast vs protein-rich breakfast on perceived appetite and satiety, hormonal responses and subsequent food intake in ‘breakfast-skipping’ adolescents.
Methods and procedures
Subjects (or materials) and methods
Adolescent boys and girls were recruited from the Kansas City, KS area through advertisements, flyers and e-mail list serves to participate in the research study. Eligibility was determined by the following inclusion criteria: (1) ages 13–17 years; (2) normal to overweight (body mass index-percentile: 50–94th percentile); (3) no metabolic diseases; (4) not currently or previously following a weight loss or other special diet in the past 6 months; and (5) frequently skips breakfast (i.e., no food/caloric beverage intake before 1100 hours) ≥5× per week. Fifty-five volunteers were interested in participating in the study; 17 met the screening criteria and began the study procedures; and 13 completed the study. Four subjects were unable to complete the study due to difficulty with the blood sampling procedures. The subject characteristics are presented in Table 1. All subjects and parents were informed of the study purpose, procedures and risks, and signed the consent/assent forms. The study procedures were approved by the KUMC-GCRC Advisory Committee and the Human Subjects Committee. The subjects received $120 for completing all study procedures.
Table 1. Subject characteristics of 13 ‘breakfast skipping’ adolescent boys and girls.
| Subject characteristics | Mean ± s.e.m. |
|---|---|
| Gender | |
| Males (n) | 7 |
| Females (n) | 6 |
| Age (years) | 14.3 ± 0.3 |
| Height (cm) | 164 ± 2 |
| Weight (kg) | 63.4 ± 3.5 |
| Body mass index | |
| Percentile for age and gender (%ile) | 79 ± 4 |
| Actual (kg m−2) | 23.5 ± 1.0 |
| Breakfast skipping (no. per week) | 5 ± 1 |
| First eating occasion of the day (h/min) | 11:40 ± 0:20 |
Experimental design
This study incorporated a randomized, crossover design consisting of 3, 5-h testing days. On separate days, the subjects randomly consumed a normal-protein (PN) breakfast, a protein-rich (PR) breakfast or skipped breakfast (BS). Pre- and postprandial perceived appetite, satiety and hormonal responses were measured throughout the 5-h period (Figure 1). Following the 5 h, the subjects were provided with an ad libitum lunch buffet and completed a food record documenting all food/beverages consumed over the remaining 24 h.
Figure 1.
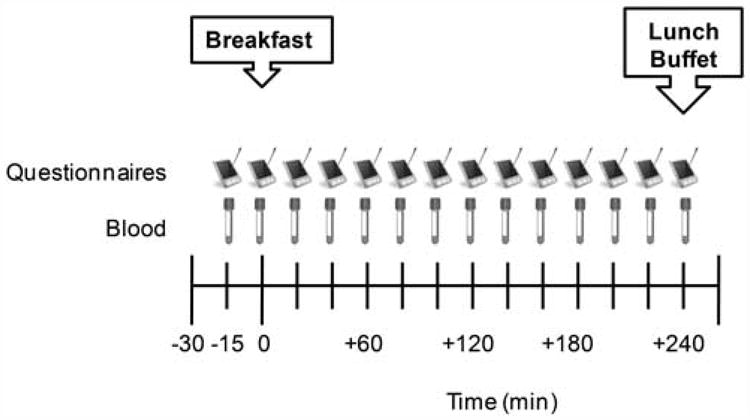
Testing day timeline.
Specific 5-h testing day procedures
For each testing day, the subjects reported to the KUMC-GCRC (between 0700 and 0900 hours) after an overnight fast (Figure 1). The subjects reclined in a chair and a catheter was inserted into an antecubital vein of the non-dominant arm. For the next 15 min, the subjects were familiarized with the testing day procedures. At time −15 min, a baseline (fasting) blood sample was drawn and a set of Palm-pilot-based questionnaires were completed. At time 0 min, a meal including water was provided if completing the PN or PR days and only water was provided if completing the BS day. The subjects were asked to consume the meal and/or water within 20 min. Blood sampling and questionnaires were performed over the remaining 4 h. At +240 min, the catheter was removed and an ad libitum lunch buffet was provided. The subjects were asked to consume this meal within 20 min and were instructed to eat as much or as little as desired until feeling ‘comfortably full.’ At the end of the testing day (+270 min), the subjects were permitted to leave the laboratory. There were 7 days (i.e., washout) in between each of the 3 testing days.
Baseline energy intake
Each subject completed food records during the 3 days immediately before the first testing day. This was used to document the subject's daily food intake and eating habits. Each subject was then required to consume the same foods and quantities recorded on the food records during the days before each subsequent testing day. This allowed us to standardize the energy intake consumed before each testing day without directly altering the foods and quantities typically consumed by each subject. The subjects were instructed to continue their normal breakfast skipping behavior throughout the study.
Test meals
The dietary characteristics of the breakfast meals are shown in Table 2. The meals contained 24% of estimated daily energy needs for normal to overweight adolescents.21 The PN meal contained 18.1 ± 0.9 g protein; macronutrient composition was 14% protein, 73% carbohydrates and 13% fat. The PR meal contained an additional 31 g of protein (total: 49.1 ± 2.5g protein); macronutrient composition was 38% protein, 49% carbohydrate and 13% fat. Both meals consisted of pancakes with butter and syrup, scrambled eggs with cheese and 266 ml of water. The meals were matched for total energy, dietary fat, fiber, sugar and energy density, with the only difference being the composition of the pancakes. For the PR meal, a portion of the flour in the PN pancakes was replaced with whey protein (See Table 2).
Table 2. Dietary characteristics of the breakfast meals.
| Dietary characteristicsa | Normal-protein breakfast | Protein-rich breakfast |
|---|---|---|
| Energy content (kcal) | 513 ± 26 | 512 ± 26 |
| PRO (g) | 18.1 ± 0.9 | 49.1 ± 2.5 |
| CHO (g) | 95.3 ± 4.9 | 62.8 ± 3.2 |
| Sugar (g) | 31.1 ± 1.6 | 30.7 ± 1.6 |
| Fiber (g) | 2.0 ± 0.1 | 2.1 ± 0.1 |
| Fat (g) | 7.5 ± 0.4 | 7.5 ± 0.4 |
| Energy density (kcalg−1) | 0.75 ± 0.01 | 0.74 ± 0.01 |
| Sample menu | Three Buttermilk Pancakes with syrup: | Three Whey Pancakes with syrup: |
| 2 Tbsp flour | 1 Tbsp flour | |
| Cup designer whey protein powder | ||
| Tsp baking powder | Tsp baking powder | |
| Tsp sugar | Tsp sugar | |
| Cup skim milk | 2 Tbsp skim milk | |
| 1 Tsp egg-whites only | Tsp egg whites-only | |
| 1 Tsp margarine | Tsp margarine | |
| Tsp vanilla extract | Tsp vanilla extract | |
| Tsp butter | Tsp butter | |
| 6 Tsp maple syrup | 6 Tsp maple syrup | |
| Scrambled eggs: | Scrambled eggs: | |
| Cup eggbeaters | Cup eggbeaters | |
| 1 Tsp cheese | 1 Tsp cheese |
Data are presented as mean ± s.e.m.
For the BS day, subjects were only provided with 266 ml of water. The BS day served as the subjects’ normal eating pattern and was used to identify the normal (baseline) perceived appetite, satiety, hormonal and food intake responses.
Questionnaires
Computerized questionnaires (AppetiteLog; US Department of Agriculture Laboratory/Western Human Nutrition Research Center, Davis, CA, USA) assessing perceived appetite (hunger, desire to eat, prospective food consumption), perceived satiety (fullness) and breakfast palatability (pleasantness) were downloaded onto a palm-pilot (Palm, Zire22, Palm, Sunnyvale, CA, USA) and completed throughout each testing period (Figure 1). The questionnaires contain validated visual analog scales incorporating a 100 mm horizontal line rating scale for each response.22 The questions are worded in the following manner: ‘how strong is your feeling of with anchors of ‘not at all’ to ‘extremely’.
Hormonal responses
Fourteen blood samples were drawn over the 5-h period (see Figure 1). The samples were collected in test tubes containing EDTA (ethylenediaminetetraacetic acid). Protease inhibitors were added to the sample to reduce protein degradation. Samples were centrifuged at −4 °C for 15 min; the plasma was separated and stored in microcentrifuge tubes at −80 °C for future analysis. Plasma active ghrelin and total PYY were measured with 2-plex Milliplex assay kits and Luminex technologies (Millipore/LINCO Research, St Charles, MO, USA).
Ad libitum lunch
The ad libitum lunch buffet occurred at the end of the testing day at + 240 min, which was between 1100 and 1300 hours depending on the testing day start time of each subject. We chose to provide the lunch buffet at this time due to the habitual dietary patterns of the subjects in this study, which typically included the consumption of their first meal between 1100 and 1230 hours (see Table 1).
Each subject was provided with the ad libitum lunch buffet in a quiet, self-contained room. The buffet contained a total of 3000 kcal and consisted of commonly eaten food items (i.e., crackers, fruits, vegetables, lunch-meats and string cheese). Subjects were required to consume 88.7 ml of water at lunch but were provided with additional water to drink ad libitum. Subjects were instructed to eat and drink as much or as little as desired until feeling ‘comfortably full’ within 20 min. Total food intake and water consumption were measured by weighing all items before and after the meal. Total energy and macronutrient composition were determined using the Nutrition Data System for Research (NDSR; 2006; Nutrition Coordinating Center; University of Minnesota School of Public Health).
24-h food intake
For the remaining 24 h after completing each of the testing days, the subjects recorded a dietary food record, recording all food and beverage items consumed until the following morning. Total energy and macronutrient composition were determined using NDSR.
Data and statistical analysis
To assess perceived appetite, satiety and hormonal responses following each of the testing days, 4-h postprandial incremental area under the curve was calculated from the fasting (baseline) time point and the 13 postprandial time points for each outcome. Area under the curve measurements were calculated using the trapezoidal rule.23 A composite area under the curve was calculated from the hunger, desire to eat and prospective food consumption responses to identify overall postprandial appetite, whereas the fullness area under the curve was used as the overall postprandial satiety response. To more extensively examine the time course of changes in these outcomes, specific time points immediately following the breakfast meal (+20min) and immediately before the ad libitum lunch (+240 min) were compared between treatments. The average palatability of each meal was also compared among breakfast treatments. A repeated measures analysis of variance was used to identify main effects of breakfast on all study outcomes. When main effects were detected, post hoc analyses were performed using Least Significant Difference procedures to identify differences among treatments. Pearson's correlations were computed to identify relationships between breakfast energy content, breakfast dietary protein, perceived appetite, satiety, hormonal responses and subsequent energy intake. Data are expressed as Mean ± s.e.m. P<0.05 was considered to be statistically significant. The sample size (n=13) provided >90% power to detect differences in the majority of the study outcomes among the breakfast treatments. In general, the effect size for the study outcomes ranged from modest to strong (0.267–0.471). Analyses were conducted using the Statistical Package for the Social Sciences (SPSS; version 16.0; SPSS, Chicago, IL, USA).
Results
Perceived appetite, satiety and palatability
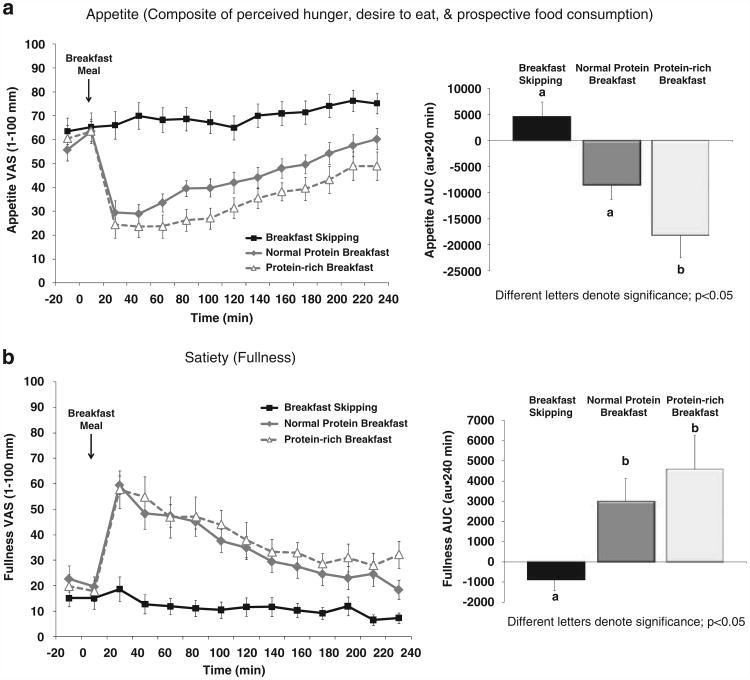
Perceived appetite and satiety throughout the 5 h testing days are shown in Figures 2a and b. BS led to sustained, elevated appetite throughout the testing period, whereas the addition of breakfast led to immediate declines in appetite followed by gradual increases throughout the remaining 4 h. BS also led to sustained, reduced fullness throughout the testing period, whereas the addition of breakfast led to immediate rises in fullness followed by gradual declines over 4 h.
Figure 2.
Perceived (a) appetite and (b) satiety throughout the 5-h testing days in 13 ‘breakfast skipping’ adolescent boys and girls.
An overall main effect of breakfast was observed for postprandial appetite (P<0.005) and satiety (P<0.01). Although the PN meal led to reduced postprandial appetite (−8473 ± 2995 mm·240 min), this was not different vs BS (4564 ± 3044 mm·240 min; P = 0.308, nonsignificant (NS)) (Figure 2a). The PR meal led to greater reductions in 4-h postprandial appetite (−13542 ± 3667 mm·240 min) vs BS (P<0.01) and PN (P<0.001). Both meals exhibited greater postprandial fullness (PN: 2996 ± 1223 mm·240 min; PR: 4597 ± 1783 mm·240 min) vs BS (−876 ± 572 mm·240 min; P<0.01) (Figure 2b). No difference in postprandial fullness was observed following PN vs PR (P = 0.343, NS).
With regard to the time course of changes in appetite, both breakfast meals led to similar reductions in appetite immediately following the respective meals (+ 20 min; Figure 2a). The PN and PR meals led to greater reductions in appetite (60.2 ± 4.4 and 48.9 ± 5.9 mm·240 min, respectively) immediately before lunch (+ 240 min) compared with BS (75.1 ± 4.4 mm·240 min; both meals, P<0.05). The PR meal led to the greatest reduction in appetite before lunch (PR vs PN, P<0.05). Similar, but opposite, findings were also observed with perceived fullness. Specifically, both breakfast meals led to similar increases in fullness immediately following the respective meals (+ 20 min; Figure 2b). The PN and PR meals led to greater increases in fullness (18.5 ± 4.0 and 32.1 ± 5.8 mm-240 min, respectively) immediately before lunch (+ 240 min) compared with BS (7.3 ± 2.2 mm·240 min; both meals, P<0.005). The PR meal led to the greatest increase in fullness before lunch (PR vs PN, P<0.005).
The PN and PR meals led to high palatability ratings (88 ± 2 and 87 ± 3 mm, respectively) indicating that the meals were rated as ‘very pleasant.’ No difference in palatability was found between meals.
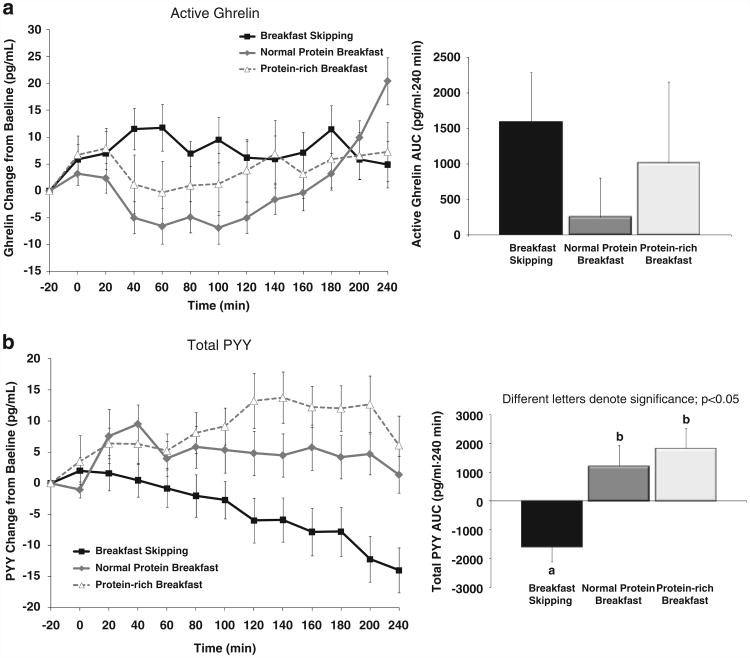
Hormonal responses
The appetite-stimulating hormone ghrelin and the appetite-suppressing (satiety) hormone PYY were assessed throughout the testing days (Figures 3a and b). BS led to gradual rises in active ghrelin along with gradual declines in PYY throughout the postprandial period. The addition of breakfast led to immediate declines in ghrelin followed by gradual increases throughout the remaining 4 h. The breakfast meals also led to gradual rises in PYY over 4 h.
Figure 3.
(a, b) Hormonal responses throughout the 5-h testing days in 13 ‘breakfast skipping’ adolescent boys and girls.
No main effect of breakfast was observed for postprandial active ghrelin (P = 0.590, NS) (Figure 3a). An overall main effect of breakfast was found for postprandial total PYY (P<0.001). Both meals showed greater postprandial total PYY concentrations (PN: 1202 ± 769 mm·240 min; PR: 1830 ± 718 mm·240 min) vs BS (−1587 ± 547 mm·240 min; P<0.01 and P<0.001, respectively) (Figure 3b). No difference in postprandial PYY was observed following PN vs PR (P = 0.446, NS).
With regard to the time course of changes in the hormonal responses, no differences in ghrelin were observed between any breakfast treatment at +20 or +240 min (Figure 3a). PYY concentrations were significantly higher immediately following the PR breakfast (+20 min) compared with BS (64.0 ± 3.6 vs 57.7 ± 4.1 pg ml−1, respectively, P<0.01). Immediately before lunch (+240 min), the PR meal led to greater PYY concentrations (69.7 ± 5.2pg ml−1) compared with BS (47.9 ± 4.6pg ml−1, P<0.001) and the PN meal (52.5 ± 4.6pg ml−1, P<0.001).
Subsequent food intake
Subsequent food intake measured from the ad libitum lunch and assessed through the 24-h food records is shown in Table 3. The BS and PN treatments led to ∼500 kcal consumed at lunch, whereas PR led to ∼370 kcal consumed. A main effect of breakfast was observed for the energy content consumed at the ad libitum lunch buffet (P<0.005). Although the energy content at lunch was not different between PN vs BS (P=0.780, NS), PR led to fewer kcal consumed vs BS (P<0.01) and PN (P<0.005). Total water intake at the ad libitum lunch was also measured. In general, the subjects consumed ∼200 ml (7 oz) of water at the ad libitum lunch buffet. No difference in water consumption was found among treatments.
Table 3. Subsequent food intake following each of the breakfast treatments.
| Energy intake | Breakfast skipping | Normal-protein breakfast | Protein-rich breakfast |
|---|---|---|---|
| Ad libitum luncha | |||
| Energy content (kcal) | 496 ± 68a | 503 ± 71a | 372± 54b |
| PRO (g) | 29 ± 4a | 24 ± 3b | 18 ± 2c |
| CHO (g) | 69 ± 10a | 78 ± 12b | 56 ± 10a |
| Fat (g) | 11 ± 1a | 11 ± 1a | 8 ± 1b |
| Water (ml) | 190 ± 40 | 216 ± 34 | 225 ± 39 |
| Total 24-h energy content (kcal)a,b | 2259 ± 280 | 2530 ± 212 | 2502 ± 284 |
Data are presented as mean ± s.e.m.; repeated measures analysis of variance; different letters denote significance; P<0.05.
Sum of breakfast, ad libitum lunch and post-testing food intake.
When all food intake was included over a 24-h period (i.e., including breakfast meal, ad libitum lunch buffet and the post-testing day food intake), no differences in daily energy intake were observed among treatments (main effect: P=0.580, NS) (Table 3).
Correlations
As shown in Table 4, energy content and dietary protein at breakfast were inversely associated with postprandial appetite and positively associated with postprandial fullness and PYY concentrations (all comparisons, P<0.05).
Table 4. Correlations between the dietary protein and energy content at the breakfast meals and specific appetite-related variables.
| Appetite-related variables | Dietary protein at breakfasta | Energy content at breakfasta |
|---|---|---|
| Appetite | −0.572b | −0.543c |
| Fullness | 0.435b | 0.424c |
| Active ghrelin | −0.050 | −0.128 |
| Total PYY | 0.482b | 0.530c |
| Subsequent energy intake | −0.114 | 0.040 |
Pearson's correlation coefficients (r) of the composite area under the curve/mean data during the 3 testing days.
Dietary protein at breakfast vs appetite-related variables; P<0.05.
Energy content at breakfast vs appetite-related variables; P<0.05.
Discussion
Although a strong relationship exists between breakfast skipping and obesity in adolescents, limited data exist concerning whether the addition of breakfast impacts appetite control and the regulation of energy intake in individuals who typically skip the morning meal. In this study, we found that the addition of breakfast, regardless of macronutrient composition, leads to increased satiety that was accompanied by increases in PYY concentrations. However, additional benefits through reductions in perceived appetite and subsequent-meal intake were observed after a breakfast meal rich in dietary protein. These findings are further strengthened by the correlation data indicating that increased energy content and increased dietary protein at breakfast are associated with reduced appetite, increased satiety and increased PYY concentrations. Although differences in subsequent energy intake were observed between the breakfast treatments, no differences in daily energy intake were noted. Take together, these findings support the addition of a protein-rich breakfast for better appetite control and food intake regulation over the short-term, but warrant additional research to identify whether the acute changes lead to long-term alterations in daily appetite control, food intake and energy regulation when breakfast is consumed on a daily basis.
The long-standing parental quote ‘eat your breakfast; it's the most important meal of the day’ is continuing to acquire scientific support. Breakfast is defined as the first eating occasion of the day, occurring within 2 h of waking (before 1000 hours). The breakfast meal generally consists of approximately 18–35% of total daily energy needs7,24 and has been associated with numerous health benefits.6 Some of these include reduced body weight, body mass index and less weight re-gain following weight loss.6,25 Several observational studies have identified whether energy intake has a role in this relationship. Specifically, consuming breakfast on a daily basis has been associated with better diet quality (that is, increased consumption of dietary fiber, fruits, vegetables and milk) and reduced consumption of soft drinks, foods high in saturated fat and high calorie/high sugar snacks.6 In addition, breakfast consumption has been associated with reduced overall food intake and reduced overeating in the evening.4
Only a few intervention-based studies have focused on the dietary habit of breakfast skipping.9 Of these studies, only Farshchi et al.26 examined the impact of breakfast skipping on appetite and subsequent food intake. In this particular study, 10 breakfast-consuming women were asked to follow 14 days of BS or consuming a 500 kcal carbohydrate-rich breakfast.26 Despite the additional kcal consumed at breakfast, daily food intake was lower following the 14 days of eating breakfast (1663 ± 141 kcal day−1) vs skipping breakfast (1754 ± 155 kcal day−1; P<0.05).26 No difference in appetite or satiety was observed between these patterns.26
In our current study, we found that eating breakfast led to similar daily food intake compared with skipping breakfast; however, appetite and satiety differed between breakfast patterns. The conflicting food intake and appetite outcomes between this study and our current study are most likely due to the different study subject populations and research questions. The previous study examined the impact of breakfast skipping in those who consumed breakfast on a daily basis, whereas our current study examined the impact of consuming breakfast in ‘breakfast skippers.’ Thus, future research examining whether the removal of the breakfast meal has a greater impact on appetite and food intake compared with when breakfast is re-introduced into a healthy diet plan would provided new insight into the topic of breakfast skipping. This concept is further supported by the meal patterning/meal frequency studies, which illustrate that the addition of meals (i.e., increased eating frequency) leads to little, if any, improvements in appetite control, whereas skipping meals significantly and negatively impacts appetite and the regulation of food intake.27
Another possible reason for the lack of differences in daily energy intake following breakfast skipping vs breakfast consumption in our current study may be due to the method of data collection. While the lunch energy intake was quantified through measurements of how much food was consumed at the ad libitum buffet, we used a single-day, food record-assisted dietary recall approach to estimate 24-h energy intake. Although this technique has been validated in children and adolescents,28,29 this method can lead to substantial under-reporting (ranging from 18 to 54%) which may have impacted our findings.30 Alternately, it is also plausible that energy intake is not the primary modulator regarding the association between breakfast consumption, body weight, and obesity. Further work is needed to more accurately and comprehensively assess habitual energy intake and eating patterns/behaviors in adolescents.
We were also interested in identifying whether the macronutrient composition of the foods (specifically dietary protein) consumed at breakfast impacts these outcomes. Previous studies from our laboratory and others have found reduced appetite, increased satiety and reduced subsequent food intake following protein-rich (28–65 g protein per meal) vs normal-protein (12–17 g protein per meal) meals.11,14,16,18,31–33 We also recently report that when additional protein (64 g protein) is provided at breakfast, lunch or dinner, satiety is greater following a protein-rich breakfast compared with protein-rich lunch and dinner meals.31 Our current study builds upon previous findings and indicates that a protein-rich breakfast leads to greater reductions in postprandial appetite and subsequent-meal food intake compared with a normal-protein breakfast.
Dietary protein is critical to the health and well-being of individuals of all ages but is particularly important for growing children and adolescents.21 The recommended dietary allowance for dietary protein is 0.85 g protein kg−1 day−1 for adolescents aged 14–18 years with protein making up between 10 and 30% of total energy intake.21 In this study, the protein-rich breakfast meal contained an additional 31 g of protein to that of the normal-protein meal. Theoretically, if the subjects consumed the recommended dietary allowance for dietary protein at both lunch and dinner, this would lead to a daily protein intake of 1.30 g protein kg−1 day−1 (17% of total energy intake), which is well-within the normal protein requirements for growing adolescents, 0.85–2.9 g protein kg−1 day−1.21 Thus, our current findings suggest that a modest, healthy increase in dietary protein leads to additional benefits surrounding appetite control and subsequent food intake when provided at the breakfast meal.
One possible mechanism contributing to the satiating properties of breakfast and dietary protein may involve the energy and macronutrient-responsive, gastrointestinal hormones that regulate ingestive behavior. Several studies report that food intake (with specific emphasis on increased dietary protein) leads to initial and sustained reductions in postprandial ghrelin and increases in postprandial PYY concentrations.14,20,34,35 In our current study, ghrelin exhibited breakfast meal-related declines compared with breakfast skipping; however, no significant differences were found. Our inability to detect differences in the hormonal responses between the breakfast treatments may have resulted from the somewhat large variability with the ghrelin responses, which may have been due to the varied sample population including normal weight to overweight adolescent boys and girls. In regard to the alterations in PYY concentrations, we found that the addition of breakfast led to an increase in postprandial PYY compared with breakfast skipping, a finding that is consistent with the current literature and the perceived satiety responses. Although there were no differences in PYY concentrations between the breakfast meals, we did observe a significant relationship between the quantity of dietary protein consumed at breakfast and postprandial satiety and PYY concentrations. These data illustrate that although postprandial PYY changes moderately contribute to the satiating effects of dietary protein, they do not appear to be highly sensitive, fine-tuned biomarkers. To more comprehensively examine the underlying mechanisms involved with breakfast and dietary protein, future research including the measurements of other energy and macronutrient-responsive hormones as well as thermogenesis (postprandial energy expenditure) is necessary.
Additional limitations
One of the fundamental questions surrounding the topic of ‘breakfast skipping’ includes the reasons as to why adolescents skip this meal. Several cross-sectional studies report that the main reason for this behavior is ‘lack of hunger in the morning.’ Secondary reasons include ‘not having enough time to eat breakfast’ and ‘using this as a way to lose weight.’36,37 Unfortunately, we did not access the reasons as to why the subjects in our study skipped breakfast. However, the elevated perceived appetite and reduced satiety responses during the 5-h breakfast skipping trial demonstrate that the subjects were experiencing a moderate-to-high level of hunger and very low satiety. Thus, other reasons not including a ‘lack of hunger’ appear to be involved in this group.
The subjects in this study were only provided with the normal-protein and protein-rich breakfast meals during the morning of each testing day. It is unclear as to whether an acclimation period would lead to differential appetitive and food intake responses; however, the lack of change in daily (24-h) food intake suggests that a longer intervention period is required to see changes in this outcome measure. Although we appreciate the need to extend these findings into practical outcomes involving long-term changes in appetite control, energy intake and body weight, we have chosen to complete as a first, but vital step, an acute study to determine whether the addition of a protein-rich breakfast does in fact result in alterations in appetite and hormonal responses. We are currently completing a long-term intervention that will potentially confirm the present findings, document changes in chronic food intake and identify the implications for energy regulation and body weight.
Finally, our current study included normal to overweight adolescents. Because individuals with varying obesity status experience different eating habits, attitudes and thoughts towards food,33 future studies targeting overweight and obese adolescents are critical to determine whether similar responses would be observed.
Conclusions
Although the incorporation of breakfast leads to beneficial changes in acute appetite control and food intake in ‘breakfast skipping’ adolescents, additional benefits are experienced with a protein-rich version.
Acknowledgments
We thank the study participants for their dedication and compliance; GCRC Administrative (Judy Otey, Jo Denton), Nursing (Jody Mahan, Denise Schaeffer), Bionutrition (Debra Sullivan, Jeannine Goetz) and other support (Kristy Anderson, Joel Pratt) staff for assisting with catheter insertions, blood collections and processing, and/or other screening/testing-day procedures and activities. We would also like to thank Candice Coffey, KUMC-School of Medicine medical student, who assisted with the testing-day procedures as well. This study was funded through the KUMC-GCRC Grant M01 RR023940 NCRR/NIH and the KUMC-School of Allied Health Research Award.
Footnotes
Conflict of interest: The authors declare no conflict of interest.
References
- 1.Ogden CL, Carroll MD, Curtin LR, McDowell MA, Tabak CJ, Flegal KM. Prevalence of overweight and obesity in the United States, 1999–2004. JAMA. 2006;295:1549–1555. doi: 10.1001/jama.295.13.1549. [DOI] [PubMed] [Google Scholar]
- 2.Zimmet P, Alberti KG, Kaufman F, Tajima N, Silink M, Arslanian S, et al. The metabolic syndrome in children and adolescents—an IDF consensus report. Pediatr Diabetes. 2007;8:299–306. doi: 10.1111/j.1399-5448.2007.00271.x. [DOI] [PubMed] [Google Scholar]
- 3.Affenito SG. Breakfast: a missed opportunity. J Am Dietetic Assoc. 2007;107:565–569. doi: 10.1016/j.jada.2007.01.011. [DOI] [PubMed] [Google Scholar]
- 4.de Castro JM. The time of day and the proportions of macronutrients eaten are related to total daily food intake. Br J Nutr. 2007;98:1077–1083. doi: 10.1017/S0007114507754296. [DOI] [PubMed] [Google Scholar]
- 5.Siega-Riz AM, Popkin BM, Carson T. Trends in breakfast consumption for children in the United States from 1965–1991. Am J Clin Nutr. 1998;67:748S–756S. doi: 10.1093/ajcn/67.4.748S. [DOI] [PubMed] [Google Scholar]
- 6.Rampersaud G. Breakfast habits, nutritional status, body weight, and academic performance in children and adolescents. J Am Dietetic Assoc. 2005;105:743–760. doi: 10.1016/j.jada.2005.02.007. [DOI] [PubMed] [Google Scholar]
- 7.Timlin M. Breakfast eating and weight change in a 5-year prospective analysis of adolescents: project EAT (eating among teens) Pediatrics. 2008;121:e638–e645. doi: 10.1542/peds.2007-1035. [DOI] [PubMed] [Google Scholar]
- 8.Fiore H, Travis S, Whalen A, Auinger P, Ryan S. Potentially protective factors associated with healthful body mass index in adolescents with obese and nonobese parents: a secondary data analysis of the third national health and nutrition examination survey, 1988–1994. J Am Dietetic Assoc. 2006;106:55–64. doi: 10.1016/j.jada.2005.09.046. quiz 76-9. [DOI] [PubMed] [Google Scholar]
- 9.Giovannini M, Verduci E, Scaglioni S, Salvatici E, Bonza M, Riva E, et al. Breakfast: a good habit, not a repetitive custom. J Int Med Res. 2008;36:613–624. doi: 10.1177/147323000803600401. [DOI] [PubMed] [Google Scholar]
- 10.Speiser PW, Rudolf MC, Anhalt H, Camacho-Hubner C, Chiarelli F, Eliakim A, et al. Childhood obesity. J Clin Endocrinol Metab. 2005;90:1871–1887. doi: 10.1210/jc.2004-1389. [DOI] [PubMed] [Google Scholar]
- 11.Halton TL, Hu FB. The effects of high protein diets on thermogenesis, satiety and weight loss: a critical review. J Am Col Nutr. 2004;23:373–385. doi: 10.1080/07315724.2004.10719381. [DOI] [PubMed] [Google Scholar]
- 12.Westerterp-Plantenga MS, Nieuwenhuizen A, Tome D, Soenen S, Westerterp KR. Dietary protein, weight loss, and weight maintenance. Annu Rev Nutr. 2009;29:21–41. doi: 10.1146/annurev-nutr-080508-141056. [DOI] [PubMed] [Google Scholar]
- 13.Lejeune MP, Westerterp KR, Adam TC, Luscombe-Marsh ND, Westerterp-Plantenga MS. Ghrelin and glucagon-like peptide 1 concentrations, 24-h satiety, and energy and substrate metabolism during a high-protein diet and measured in a respiration chamber. Am J Clin Nutr. 2006;83:89–94. doi: 10.1093/ajcn/83.1.89. [DOI] [PubMed] [Google Scholar]
- 14.Leidy HJ, Mattes RD, Campbell WW. Effects of acute and chronic protein intake on metabolism, appetite, and ghrelin during weight loss. Obesity (Silver Spring, Md) 2007;15:1215–1225. doi: 10.1038/oby.2007.143. [DOI] [PubMed] [Google Scholar]
- 15.Lejeune MP, Kovacs EM, Westerterp-Plantenga MS. Additional protein intake limits weight regain after weight loss in humans. Br J Nutr. 2005;93:281–289. doi: 10.1079/bjn20041305. [DOI] [PubMed] [Google Scholar]
- 16.Leidy HJ, Carnell NS, Mattes RD, Campbell WW. Higher protein intake preserves lean mass and satiety with weight loss in pre-obese and obese women. Obesity (Silver Spring, Md) 2007;15:421–429. doi: 10.1038/oby.2007.531. [DOI] [PubMed] [Google Scholar]
- 17.Veldhorst M, Smeets A, Soenen S, Hochstenbach-Waelen A, Hursel R, Diepvens K, et al. Protein-induced satiety: effects and mechanisms of different proteins. Physiol Behav. 2008;94:300–307. doi: 10.1016/j.physbeh.2008.01.003. [DOI] [PubMed] [Google Scholar]
- 18.Smeets A. Energy expenditure, satiety, and plasma ghrelin, glucagon-like peptide 1, and peptide tyrosine-tyrosine concentrations following a single high-protein lunch. J Nutr. 2008;138:698–702. doi: 10.1093/jn/138.4.698. [DOI] [PubMed] [Google Scholar]
- 19.Leidy HJ, Bossingham MJ, Mattes RD, Campbell WW. Increased dietary protein consumed at breakfast leads to an initial and sustained feeling of fullness during energy restriction compared to other meal times. Br J Nutr. 2009;101:798–803. doi: 10.1017/s0007114508051532. [DOI] [PubMed] [Google Scholar]
- 20.Batterham RL, Heffron H, Kapoor S, Chivers JE, Chandarana K, Herzog H, et al. Critical role for peptide YY in protein-mediated satiation and body-weight regulation. Cell Metabolism. 2006;4:223–233. doi: 10.1016/j.cmet.2006.08.001. [DOI] [PubMed] [Google Scholar]
- 21.Lupton JR, Brooks JA, Butte NF, Caballero B, Flatt JP, Fried SK, et al. Dietary Reference Intakes for Energy, Carbohydrate, Fiber, Fat, Fatty Acids, Cholesterol, Protein, and Amino Acids. Vol. 5. National Academy Press; Washington, DC, USA: 2002. pp. 589–768. [Google Scholar]
- 22.Flint A, Raben A, Blundell JE, Astrup A. Reproducibility, power and validity of visual analogue scales in assessment of appetite sensations in single test meal studies. Int J Obes Relat Metab Disord. 2000;24:38–48. doi: 10.1038/sj.ijo.0801083. [DOI] [PubMed] [Google Scholar]
- 23.Wolever TM, Bolognesi C. Prediction of glucose and insulin responses of normal subjects after consuming mixed meals varying in energy, protein, fat, carbohydrate and glycemic index. J Nutr. 1996;126:2807–2812. doi: 10.1093/jn/126.11.2807. [DOI] [PubMed] [Google Scholar]
- 24.Pereira MA, Jacobs DR, Jr, Van Horn L, Slattery ML, Kartashov AI, Ludwig DS. Dairy consumption, obesity, and the insulin resistance syndrome in young adults: the CARDIA study. JAMA. 2002;287:2081–2089. doi: 10.1001/jama.287.16.2081. [DOI] [PubMed] [Google Scholar]
- 25.Wyatt HR, Grunwald GK, Mosca CL, Klem ML, Wing RR, Hill JO. Long-term weight loss and breakfast in subjects in the national weight control registry. Obes Res. 2002;10:78–82. doi: 10.1038/oby.2002.13. [DOI] [PubMed] [Google Scholar]
- 26.Farshchi HR, Taylor MA, Macdonald IA. Deleterious effects of omitting breakfast on insulin sensitivity and fasting lipid profiles in healthy lean women. Am J Clin Nutr. 2005;81:388–396. doi: 10.1093/ajcn.81.2.388. [DOI] [PubMed] [Google Scholar]
- 27.Leidy HJ. Exper Biol. New Orleans Convention Center; New Orleans: Apr 19, 2009. Eating frequency and energy regulation in controlled feeding studies. [Google Scholar]
- 28.Weber JL, Lytle L, Gittelsohn J, Cunningham-Sabo L, Heller K, Anliker JA, et al. Validity of self-reported dietary intake at school meals by American Indian children: the pathways study. J Am Diet Assoc. 2004;104:746–752. doi: 10.1016/j.jada.2004.02.029. [DOI] [PubMed] [Google Scholar]
- 29.Lytle LA, Nichaman MZ, Obarzanek E, Glovsky E, Montgomery TN, Zive M, et al. Validation of 24-hour recalls assisted by food records in third-grade children. J Am Diet Assoc. 1993;93:1431–1436. doi: 10.1016/0002-8223(93)92247-u. [DOI] [PubMed] [Google Scholar]
- 30.Macdiarmid J, Blundell JE. Assessing dietary intake: who, what, and why of under-reporting. Nutr Res Rev. 1998;11:231–253. doi: 10.1079/NRR19980017. [DOI] [PubMed] [Google Scholar]
- 31.Leidy HJ, Bossingham MJ, Mattes RD, Campbell WW. Increased dietary protein consumed at breakfast leads to an initial and sustained feeling of fullness during energy restriction compared to other meal times. Br J Nutr. 2008;101:798–803. doi: 10.1017/s0007114508051532. [DOI] [PubMed] [Google Scholar]
- 32.Araya H. Short-term satiety in preschool children: a comparison between high protein meal and a high complex carbohydrate meal. Int J Food Sci Nutr. 2000;51:119–124. doi: 10.1080/096374800100813. [DOI] [PubMed] [Google Scholar]
- 33.Barkeling B, Rossner S, Bjorvell H. Effects of a high-protein meal (meat) and a high-carbohydrate meal (vegetarian) on satiety measured by automated computerized monitoring of subsequent food intake, motivation to eat and food preferences. Int J Obes. 1990;14:743–751. [PubMed] [Google Scholar]
- 34.Diepvens K, Haberer D, Westerterp-Plantenga M. Different proteins and biopeptides differently affect satiety and anorexi-genic/orexigenic hormones in healthy humans. Int J Obesity (2005) 2008;32:510–518. doi: 10.1038/sj.ijo.0803758. [DOI] [PubMed] [Google Scholar]
- 35.Foster-Schubert KE, Overduin J, Prudom CE, Liu J, Callahan HS, Gaylinn BD, et al. Acyl and total ghrelin are suppressed strongly by ingested proteins, weakly by lipids, and biphasically by carbohydrates. J Clin Endocrinol Metab. 2008;93:1971–1979. doi: 10.1210/jc.2007-2289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shaw ME. Adolescent breakfast skipping: an Australian study. Adolescence. 1998;33:851–861. [PubMed] [Google Scholar]
- 37.Reddan J, Wahlstrom K, Reicks M. Children's perceived benefits and barriers in relation to eating breakfast in schools with or without universal school breakfast. J Nutr Educ Behav. 2002;34:47–52. doi: 10.1016/s1499-4046(06)60226-1. [DOI] [PubMed] [Google Scholar]