Abstract
Purpose
To better understand oral human papillomavirus (HPV) infection and cancer risk among long-term sexual partners of patients with HPV-positive oropharyngeal cancer (HPV-OPC).
Patients and Methods
An oral rinse sample, risk factor survey, cancer history, and oral examination (partners only) were collected from patients with HPV-OPC and their partners. Oral rinse samples were evaluated for 36 types of HPV DNA using PGMY 09/11 primers and line-blot hybridization and HPV16 copy number using quantitative polymerase chain reaction. Oral HPV prevalence was compared with infection among those age 45 to 65 years using National Health and Nutrition Examination Survey (NHANES) 2009-2010.
Results
A total of 164 patients with HPV-OPC and 93 of their partners were enrolled. Patients were primarily men (90%), were never-smokers (51%), and had performed oral sex (97%), with a median age of 56 years; they had a high prevalence of oncogenic oral HPV DNA (61%) and oral HPV16 DNA (54%) at enrollment. Female partners had comparable oncogenic oral HPV prevalence compared with members of the general population of the same age (1.2% v 1.3%). Among the six male partners, no oncogenic oral HPV infections were detected. No precancers or cancers were identified during partner oral cancer screening examinations. However, a history of cervical disease was reported by nine partners (10.3%) and two female patients (11.8%), and three patients (2.0%) reported a previous partner who developed invasive cervical cancer.
Conclusion
Oral HPV16 DNA is commonly detected among patients with HPV-OPC at diagnosis, but not among their partners. Partners of patients with HPV-OPC do not seem to have elevated oral HPV infection compared with the general population.
INTRODUCTION
The incidence of human papillomavirus (HPV) –positive oropharyngeal squamous cell cancer (HPV-OPC) has increased dramatically over the past three decades.1–4 Despite the newly appreciated importance of HPV16 as the primary cause of oropharyngeal squamous cell cancer (OPC), we have a limited understanding of how oncogenic oral HPV infections are transmitted or progress into cancer. Case-control studies have indicated oral HPV infection is the principal risk factor for HPV-OPC.5–7
Sexual behavior is consistently associated with oral HPV infection. Indeed, in many cross-sectional studies, having performed oral sex on a higher number of recent or lifetime partners is associated with increased odds of prevalent oral HPV.8–11 However, because sexual behaviors are generally colinear, it is difficult to distinguish which sexual behaviors are responsible for HPV transmission from the genital tract to the mouth. Deep kissing (ie, French kissing)9,12 rimming (ie, oral-anal contact),11 autoinoculation,13,14 and peripartum exposure15 have also been associated with prevalent oral HPV infection, albeit inconsistently.16
Many patients and their partners have anxiety about HPV transmission and partners' cancer risk. Therefore, we evaluated oral HPV prevalence among patients with HPV-OPC and their partners to better understand these risks.
PATIENTS AND METHODS
Study Design and Participants
This was a multicenter prospective study of 164 patients with HPV-OPC and 93 of their spouses or long-term partners (hereafter called partners). Only patients with incident OPC with tumor testing indicating HPV positivity were included in this analysis (as described in Laboratory Methods). Participants were enrolled between October 2009 and May 2013 in head and neck cancer clinics at four study sites: Johns Hopkins Hospital (JHH; Baltimore, MD), Mount Sinai Medical System (MSMS; New York, NY), Dana-Farber Cancer Institute (DFCI; Boston, MA), and Oregon Health and Science University (OHSU; Portland, OR). Nested within this case series was a partner study of the partners of these patients with HPV-OPC. Patients with incident, previously untreated OPC were eligible for enrollment, regardless of whether they currently had a partner. Partners were self-identified by patients as an individual with whom they had been sexually active for ≥ 2 years.
At two study sites (MSMS, OHSU), a convenience sample of healthy volunteers was enrolled from individuals attending free oral cancer screening events in May 2011 and April 2012. These healthy volunteers had one study visit only, where they provided an oral rinse and blood sample and answered the same study survey questions as the patients and partners.
Sample Collection
At each study visit, oral exfoliated cells were collected using a 30-second oral rinse and gargle method with 10 mL of Scope (P&G, Cincinnati, OH) mouthwash. A detailed computer-assisted self-interview (CASI) risk factor survey was also collected at each visit, including questions on demographics, tobacco and alcohol use, and detailed sexual behaviors. At study baseline, a blood sample and cancer history were also collected. When a history of previous HPV-positive cancer was reported by a patient or partner, we attempted to obtain medical record confirmation of the cancer. In addition, partners had a head and neck examination to screen for cancer by a head and neck surgeon or medical oncologist. Medical record abstraction of treatment and local tumor testing results were also available for patients. The study was approved by the institutional review board at each study site.
Laboratory Methods
HPV DNA detection in oral rinse samples.
Oral rinse samples were tested for 36 types of HPV DNA using PGMY 09/11 primers and line-blot hybridization as previously described.17 In brief, DNA was purified from oral exfoliated cells using a magnetic bead–based automated platform (QIAsymphony SP; Qiagen, Venlo, the Netherlands) and then analyzed for 36 different HPV DNA genotypes using PGMY09/11 polymerase chain reaction (PCR) primer pools and primers for β-globin, followed by reverse line-blot hybridization to the Roche linear array. The same method and laboratory were used to generate oral HPV infection data in the National Health and Nutrition Examination Survey (NHANES) 2009-2010.8 All oral rinse test results presented were β-globin positive.
Baseline oral rinse samples were also evaluated for HPV16 viral load using TaqMan quantitative (qPCR) in the ABI 7300 real-time PCR systems (Applied Biosystems, Foster City, CA) as previously described.18 Detection of HPV DNA in oral exfoliated cells cannot distinguish infectious viral particles from intracellular DNA (ie, whether it is active HPV infection able of being transmitted or HPV DNA sloughed off from tumor cell where DNA has been integrated and is not infectious).19
Methods for HPV antibody detection in blood samples.
Blood samples (1:100) were tested centrally for HPV16 L1, E6, and E7 immunoglobulin G (IgG) antibodies by programmable enzyme-linked immunosorbent assay in the Anderson laboratory as previously described,20 modified as single-plex assays in 96-well plates.21 Proteins were expressed using a human HeLa cell lysate in vitro transcription/translation system (Thermo Scientific, Waltham, MA) and blocked with 10% Escherchia coli DH5α lysate prepared by sonication. Luminescence was measured in relative light units (RLUs) as a ratio to GST-antigen control. Cutoff values for positive serology were defined as the mean plus three standard deviations of the RLU ratio observed among healthy volunteers (n = 81).
Methods for tumor HPV detection and classification.
The 164 patients with HPV-OPC were classified as HPV positive based on centralized testing (n = 55) for HPV16 by in situ hybridization (ISH),22 institutional oncogenic HPV ISH testing (n = 66), or institutional p16 immunohistochemistry (n = 43; 25 of whom underwent institutional PCR testing; of these 25, 88% [n = 22] were HPV16 positive), given that both ISH and p16 are currently being used in clinical settings to identify HPV-OPC. Sixteen additional patients and their partners were excluded from analysis based on negative centralized testing for HPV16 (n = 8) or negative local p16 (n = 8) results (data not shown).
Analytic Methods
Characteristics of patients with and without enrolled partners were compared using the χ2 test for categorical and test of medians for continuous variables. Oral HPV prevalence in patients and partners were compared with the weighted prevalence in a population-based sample from NHANES 2009-2010 data, restricted to individuals 45 to 65 years of age, for comparability with the study participants.
The oral rinse samples were considered HPV positive for any oral HPV if any of the 36 HPV types evaluated were detected on line blot. Prevalence of any oncogenic HPV was defined as detection of any of the following: HPV16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59, 68, or 73. HPV16 positivity was also evaluated using qPCR, where positivity was defined using the usual laboratory cutoff (copy number > three in 2 μL of oral rinse sample tested); results when copy number was > zero are also presented because of the research hypothesis regarding possible low-level transmission of oral HPV DNA.
RESULTS
Of the 164 enrolled patients with HPV-OPC, 93 (57%) had a partner enrolled. Patients were primarily men (90%), had performed oral sex (97%), and were never-smokers (51%), with a median age of 56 years (Table 1). Most patients had a tonsil (48%) or base of tongue (40%) subsite. The American Joint Committee on Cancer stage distribution for patients was as follows: primarily stage IV (79.9%) but included some stages III (14.5%), II (4.4%), and I (1.3%) diagnoses. Most cases of stage IV disease (115 of 127; 90.6%) were N2M0 (Table 1). Four patients (2.4%) had metastatic disease at diagnosis. All 164 patients included had either oncogenic HPV detected in their tumor by ISH (71%) or p16 (29%) detection, and most patients had testing documenting both HPV and p16 positivity in their tumors (67%). Partners were primarily women (94%), had performed oral sex (98%), and were never-smokers (57%), with a median age of 53 years (Table 1).
Table 1.
Demographic and Behavioral Characteristics of Patients With HPV-OPC and Their Partners
| Characteristic | Partners (n = 93) |
Patients |
P* | ||||
|---|---|---|---|---|---|---|---|
| With Partner Enrolled (n = 93) |
No Partner Enrolled (n = 71) |
||||||
| No. | % | No. | % | No. | % | ||
| Cancer stage | .613 | ||||||
| I | 2 | 2.2 | 0 | 0.0 | |||
| II | 3 | 3.3 | 4 | 5.8 | |||
| III | 14 | 15.6 | 9 | 13.0 | |||
| IVa (N2M0) | 63 | 70.0 | 52 | 75.4 | |||
| IVb (N3M0) | 6 | 6.7 | 2 | 2.9 | |||
| IVc (any N; metastasized) | 2 | 2.2 | 2 | 2.9 | |||
| Cancer subsite | .573 | ||||||
| Tonsil | 42 | 47.7 | 37 | 54.4 | |||
| BOT | 41 | 46.6 | 25 | 36.8 | |||
| Tonsil and BOT | 1 | 1.1 | 2 | 2.9 | |||
| Other OP or unknown primary | 4 | 4.6 | 4 | 5.9 | |||
| Study site | .001 | ||||||
| DFCI | 17 | 18.3 | 17 | 18.3 | 29 | 40.9 | |
| JHH | 38 | 40.9 | 38 | 40.9 | 11 | 15.5 | |
| OHSU | 23 | 24.7 | 23 | 24.7 | 15 | 21.1 | |
| MSMS | 15 | 16.1 | 15 | 16.1 | 16 | 22.5 | |
| Sex | .060 | ||||||
| Male | 6 | 6.5 | 87 | 93.6 | 60 | 84.5 | |
| Female | 87 | 93.6 | 6 | 6.5 | 11 | 15.5 | |
| Race | .877 | ||||||
| White | 87 | 93.6 | 87 | 93.6 | 64 | 91.4 | |
| Black | 3 | 3.2 | 3 | 3.2 | 3 | 4.3 | |
| Other | 3 | 3.2 | 3 | 3.2 | 3 | 4.3 | |
| Age, years | .983 | ||||||
| Median | 53 | 56 | 56 | ||||
| IQR | 46-59 | 51-61 | 50-64 | ||||
| Marital status | < .001 | ||||||
| Single | 0 | 0.0 | 0 | 0.0 | 12 | 16.9 | |
| Married or living as married | 93 | 100.0 | 93 | 100.0 | 37 | 52.1 | |
| Divorced, widowed, or separated | 0 | 0.0 | 0 | 0.0 | 22 | 31.0 | |
| Years with current sexual partner | < .001 | ||||||
| < 2 or no current partner | 0 | 0.0 | 0 | 0.0 | 20 | 32.8 | |
| 2-5 | 8 | 9.3 | 10 | 11.5 | 6 | 9.8 | |
| 6-10 | 13 | 15.1 | 13 | 14.9 | 7 | 11.5 | |
| 11-20 | 17 | 19.8 | 17 | 19.5 | 7 | 11.5 | |
| 21-30 | 26 | 30.2 | 27 | 31.0 | 17 | 27.9 | |
| ≥ 31 | 22 | 25.6 | 20 | 23.0 | 4 | 6.6 | |
| Education status | .373 | ||||||
| High school graduate or some high school | 29 | 31.2 | 37 | 39.8 | 30 | 42.3 | |
| College graduate | 36 | 38.7 | 31 | 33.3 | 27 | 38.0 | |
| Advanced degree | 22 | 23.7 | 18 | 19.4 | 8 | 11.3 | |
| Not reported | 6 | 6.5 | 7 | 7.5 | 6 | 8.5 | |
| Lifetime No. of oral sex partners | .185 | ||||||
| 0 | 2 | 2.4 | 2 | 2.4 | 2 | 3.2 | |
| 1 | 12 | 14.1 | 9 | 10.6 | 1 | 1.6 | |
| 2-5 | 47 | 55.3 | 29 | 34.1 | 17 | 27.4 | |
| 6-15 | 21 | 24.7 | 24 | 28.2 | 22 | 35.5 | |
| ≥ 16 | 3 | 3.5 | 21 | 24.7 | 20 | 32.3 | |
| Lifetime No. of vaginal sex partners | .141 | ||||||
| 1 | 11 | 13.1 | 7 | 8.1 | 0 | 0.0 | |
| 2-5 | 36 | 42.9 | 14 | 16.3 | 11 | 18.3 | |
| 6-15 | 28 | 33.3 | 30 | 34.9 | 20 | 33.3 | |
| ≥ 16 | 9 | 10.7 | 35 | 40.7 | 29 | 48.3 | |
| Lifetime No. of French kiss partners | .085 | ||||||
| 1-5 | 31 | 35.6 | 14 | 16.1 | 6 | 9.8 | |
| 6-15 | 32 | 36.8 | 29 | 33.3 | 13 | 21.3 | |
| ≥ 16 | 24 | 27.6 | 44 | 50.6 | 42 | 68.9 | |
| Age first performed oral sex, years | .189 | ||||||
| ≤ 16 | 13 | 15.1 | 15 | 17.2 | 12 | 18.8 | |
| 17-20 | 43 | 50.0 | 54 | 62.1 | 35 | 54.7 | |
| 21-25 | 17 | 19.8 | 15 | 17.2 | 9 | 14.1 | |
| ≥ 26 or never performed oral sex | 13 | 15.1 | 3 | 3.5 | 8 | 12.5 | |
| No. of oral sex partners in past year | .001 | ||||||
| 0 | 23 | 26.7 | 23 | 26.4 | 31 | 49.2 | |
| 1 | 60 | 69.8 | 59 | 67.8 | 22 | 34.9 | |
| 2 | 1 | 1.2 | 4 | 4.6 | 5 | 7.9 | |
| ≥ 3 | 2 | 2.3 | 1 | 1.2 | 5 | 7.9 | |
| Ever had sex with someone of same sex | .311 | ||||||
| Yes | 7 | 8.1 | 4 | 4.6 | 1 | 1.6 | |
| No | 79 | 91.9 | 83 | 95.4 | 62 | 98.4 | |
| Family history of HNC | .829 | ||||||
| Yes | 2 | 2.2 | 4 | 4.3 | 3 | 4.2 | |
| No | 53 | 57.0 | 54 | 58.1 | 48 | 67.6 | |
| Unknown | 38 | 40.9 | 35 | 37.6 | 20 | 28.2 | |
| Family income | .006 | ||||||
| < $50,000 | 11 | 11.9 | 10 | 10.7 | 17 | 24.0 | |
| $50,000-$99,000 | 21 | 22.6 | 21 | 22.6 | 23 | 32.4 | |
| ≥ $100,000 | 48 | 51.6 | 49 | 52.7 | 19 | 26.8 | |
| Refused to answer or unknown | 13 | 14.0 | 13 | 14.0 | 12 | 16.9 | |
| Mouthwash use† | .819 | ||||||
| Never | 22 | 28.4 | 27 | 31.0 | 23 | 36.5 | |
| Few times each week or month | 38 | 44.2 | 37 | 42.5 | 24 | 38.1 | |
| Once per day | 9 | 10.5 | 12 | 13.8 | 10 | 15.9 | |
| ≥ Twice per day | 6 | 7.0 | 11 | 12.6 | 6 | 9.5 | |
| Smoking status† | .423 | ||||||
| Never | 49 | 57.0 | 52 | 56.5 | 31 | 46.3 | |
| Former | 27 | 31.4 | 29 | 31.5 | 25 | 37.3 | |
| Current | 10 | 11.6 | 11 | 12.0 | 11 | 16.4 | |
| Smoking pack-years (among ever-smokers)† | .276 | ||||||
| > 0-19 | 23 | 57.6 | 17 | 43.6 | 19 | 57.6 | |
| ≥ 20 | 11 | 32.4 | 22 | 56.4 | 14 | 42.4 | |
| Alcohol use† | .632 | ||||||
| Never | 30 | 34.9 | 15 | 16.3 | 13 | 19.4 | |
| Former | 7 | 8.1 | 26 | 28.3 | 22 | 32.8 | |
| Current | 49 | 57.0 | 51 | 55.4 | 32 | 47.8 | |
| Marijuana status† | .998 | ||||||
| Never | 63 | 72.4 | 51 | 58.6 | 39 | 59.1 | |
| Former | 17 | 19.5 | 28 | 32.2 | 21 | 31.8 | |
| Current | 7 | 8.1 | 8 | 9.2 | 6 | 9.1 | |
Abbreviations: BOT, base of tongue; DFCI, Dana-Farber Cancer Institute; HNC, head and neck cancer; HPV-OPC, human papillomavirus–positive oropharyngeal cancer; IQR, interquartile range; JHH, Johns Hopkins Hospital; MSMS, Mount Sinai Medical System; OHSU, Oregon Health and Science University; OP, oropharyngeal.
Patients with versus without partners enrolled; P values calculated excluding those with unknown data for each variable.
Risk behavior (smoking, drinking, and marijuana) results presented exclude seven partners, two patients with partners, and five patients without partners who declined to provide this information.
Patients without enrolled partners (n = 71) were single (17%); divorced, separated, or widowed (31%); or married (52%) and had partners who were not available or not interested in participating. Patients with and without enrolled partners were similar in term of demographics, tobacco and alcohol use, and lifetime sexual behaviors (Table 1). However, those without enrolled partners were more likely to report multiple oral sexual partners in the past year (15.8% v 5.8%; P = .001).
Oral HPV Prevalence
Oral HPV DNA prevalence among patients and partners at enrollment is summarized in Table 2 and contrasted with prevalence in the general population of the same age and sex using NHANES data. Although all patients were HPV positive based on tumor HPV or p16 testing, only 61% of patients (100 of 164) had oncogenic HPV DNA detected in their exfoliated oral cells (ie, oral rinse samples) at diagnosis. Most (88 of 100) of the oncogenic oral HPV DNA detected among patients was HPV16. The median HPV16 copy number detected in these 88 oral rinses was 74 (interquartile range, eight to 470). Among 76 patients without HPV16 DNA detected in their oral rinses, only one (1.3%) had > three copies of HPV16 DNA detected by qPCR. When limited to the subset of patients who had tumor oncogenic HPV ISH testing performed, the proportion of those with oncogenic HPV (77 of 116; 66%) or HPV16 (66 of 116; 57%) detected in their oral rinse samples was similar to that detected among all patients with HPV-OPC (Appendix Table A1, online only). Oral HPV prevalence was similar in patients with and without partners enrolled (oncogenic HPV, 60% v 62%; P = .82; HPV16, 57% v 49%; P = .33). Oncogenic oral HPV prevalence was also similar among male (61%) and female patients (59%; P = .85) and among patients enrolled at each of the study sites (DFCI, 61%; JHH, 63%; OHSU, 60%; Sinai, 58%; P = .97).
Table 2.
Oral HPV DNA Prevalence in Patients With HPV-OPC and Their Partners Compared With General US Population of Similar Age (NHANES)
| NHANES Population (%)* | Partners |
Patients |
|||
|---|---|---|---|---|---|
| No. | % | No. | % | ||
| Baseline | (n = 93) | (n = 164) | |||
| Any oral HPV | 9.1 | 4† | 4.3 | 106 | 64.6 |
| Any oncogenic HPV | 4.7 | 1† | 1.1 | 100‡ | 61.0 |
| HPV16 | 1.4 | 0 | 0.0 | 88 | 53.7 |
| HPV16 qPCR ≥ 3§ | — | 0 | 0.0 | 71 | 45.5 |
| HPV16 qPCR > 0§ | — | 2 | 2.3 | 83 | 53.2 |
| Among women | (n = 87) | (n = 17) | |||
| Any oral HPV at baseline | 4.3 | 2 | 2.3 | 11 | 64.7 |
| Any oncogenic HPV | 1.3 | 1 | 1.2 | 10 | 58.8 |
| HPV16 at baseline | 0.5 | 0 | 0.0 | 9 | 52.9 |
| Among men | (n = 6) | (n = 147) | |||
| Any oral HPV at baseline | 14.0 | 2 | 33.3 | 95 | 64.3 |
| Any oncogenic HPV | 8.2 | 0 | 0.0 | 90 | 61.2 |
| HPV16 at baseline | 2.4 | 0 | 0.0 | 79 | 53.7 |
Abbreviations: HPV, human papillomavirus; HPV-OPC, human papillomavirus– positive oropharyngeal cancer; NHANES, National Health and Nutrition Examination Survey; qPCR, quantitative polymerase chain reaction.
Ages 45 to 65 years.
Oral HPV infection detected among partners included one infection with HPV51 (oncogenic), one infection with HPV83 (nononcogenic), two infections with HPV62 (nononcogenic), and two HPV16 infections detected by qPCR only.
Majority of oncogenic oral HPV infections detected in oral rinse samples of patients with HPV-OPC were HPV16 (88 of 100; 88%). Other oncogenic oral HPV infections detected included: 14 HPV52 infections (8.5%), four HPV33 infections (2.4%), three HPV73 infections (1.8%), two infections each for HPV59 and HPV51 (1.2%), and one infection each for HPV18, 39, 45, and 56 (0.6%).
All oral HPV results presented, including HPV16 results, were generated by line blot unless specifically indicated by qPCR.
None (0.0%) of the 93 partners had any oral HPV16 DNA detected by line blot. When qPCR assay was used, two female partners had low levels (< one copy) of oral HPV16 DNA detected, but both were below the normal laboratory threshold (ie, three copies) for positivity. Overall, oncogenic oral HPV prevalence among the 87 female partners was similar to that observed in the general population (1.2% v 1.3%; Table 2). There were only six male partners enrolled, two of whom had an oral HPV infection detected (HPV62 and 83), but neither was an oncogenic HPV type, and no HPV16 infections were detected among the male partners by either line-blot or qPCR testing (Table 2).
HPV Antibody Results
IgG antibodies to the HPV16 E6 and E7 antigens were measured at baseline in 119 patients with HPV-OPC and 44 partners in whom blood was collected and compared with 81 healthy volunteers (Fig 1). Healthy volunteers were individuals seen at OHSU or MSMS oral cancer screening events during the same time period as the study. Healthy volunteers were primarily women (61%), white non-Hispanic (89%), and never-smokers (56%), with a median age of 51 years and median number of lifetime oral sex partners self-reported as two to five.
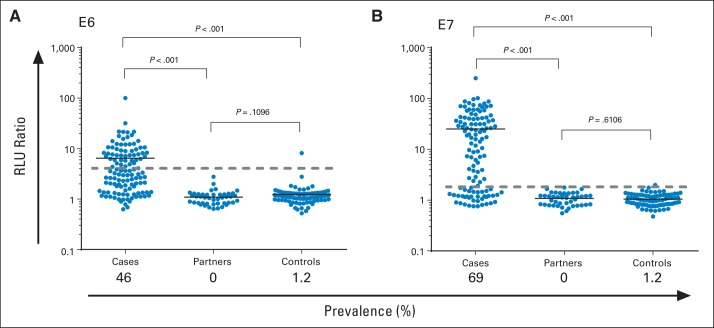
Fig 1.
Detection of HPV16 (A) E6 and (B) E7 antibodies in baseline serum from 136 patients with human papillomavirus (HPV) –positive oropharyngeal cancer, 48 partners, and 81 healthy volunteers. Relative light unit ratio of immunoglobulin G to specific HPV protein/control GST protein detected in sera is shown. Black line in each group represents median value in that group. Dashed gray line on each graph represents three standard deviations above mean in healthy volunteers (cutoff for positivity). Proportion of each group considered seropositive (ie, prevalence) listed on x-axis.
HPV16 E6 and E7 antibodies were significantly more common among patients than partners or healthy volunteers (each P < .001; Fig 1). Of 119 patients with blood samples, 55 (46%) were positive for E6-Abs (P < .001), and 82 (69%) were positive for E7-Abs (P < .001). Most patients had antibodies to either E6 and/or E7 detected (91 of 119; 76%), but only 34% of patients (40 of 119) had antibodies to both E6 and E7. Sensitivity (ie, proportion of patients with HPV-OPC with HPV16 E6 and/or E7 antibodies detected; 56 of 70; 80%) was similar when restricted to patients known to have HPV16 (as opposed to any oncogenic HPV) detected in the tumor. In contrast, no partners (zero of 44; 0%) and two healthy volunteers (two of 81; 2.5%) had HPV16 E6 and/or E7 antibodies detected (Fig 1).
Cancer History
We evaluated the cancer history of all participants and asked about cancer in their sexual partners. Most patients and partners reported no personal cancer history and no known history of HPV-positive cancer in their current or former partners. Oral abnormalities and precancers were searched for in partners using a visual surveillance examination by a head and neck surgeon or medical oncologist, and none were identified. One partner (1.1%) and one female patient (5.9%) had a documented history of invasive cervical cancer. In addition, eight partners (9.2%), and one female patient (5.9%) reported a history of cervical dysplasia (one confirmed by medical records and others by self-report only; Table 3). Other self-reported cancer history data included a diagnosis of prostate cancer by 4.1% (six of 147) of male patients and a history of breast cancer self-reported by 17.6% of female patients (three of 17) and 4.6% of female partners (four of 87; Table 3).
Table 3.
Cancer History in Patients With HPV-OPC and Their Partners by Sex*
| History | Partners (n = 93) |
Patients (n = 164) |
||||||
|---|---|---|---|---|---|---|---|---|
| Women (n = 87) |
Men (n = 6) |
Women (n = 17) |
Men (n = 147) |
|||||
| No. | % | No. | % | No. | % | No. | % | |
| Personal cancer history | ||||||||
| Cervical dysplasia† | 8 | 9.2 | 0 | 0.0 | 1 | 5.9 | 0 | 0.0 |
| Invasive cervical cancer† | 1 | 1.1 | 0 | 0.0 | 1 | 5.9 | 0 | 0.0 |
| Oropharyngeal cancer | 0 | 0.0 | 0 | 0.0 | — | — | — | — |
| Anal cancer | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 |
| Prostate or breast cancer | 4 | 4.6 | 0 | 0.0 | 3 | 17.6 | 6 | 4.1 |
| Cancer in current or former spouse or partner | ||||||||
| Invasive cervical cancer | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 3 | 2.0 |
| Oropharyngeal cancer | 1 | 1.1 | 0 | 0.0 | 0 | 0.0 | 2 | 1.4 |
| Anal cancer | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 1 | 0.7 |
Abbreviation: HPV-OPC, human papillomavirus–positive oropharyngeal cancer.
Including personal cancer history and history of malignancy in each participant's current and past spouses or partners (as known to participant).
Medical record confirmation was available for both cases of invasive cervical cancer one of nine cases of cervical dysplasia; history of dysplasia in other eight women based on self-report only.
Several patients with HPV-OPC reported a previous partner who had been diagnosed with an HPV-positive cancer, including invasive cervical cancer (n = 3; 2.0%), oropharyngeal cancer (n = 2; 1.4%), and anal cancer (n = 1; 0.7%), but these reports could not be confirmed, because these previous partners were not enrolled onto the study. In addition, one enrolled partner had a previous husband who died as a result of oropharyngeal cancer (before meeting her current partner, who also developed HPV-OPC).
DISCUSSION
As HPV has emerged as an increasingly important cause of oropharyngeal squamous cell cancer over the past two decades, some patients with HPV-OPC and their families have questions about how, when, and why they became infected with HPV and concerns about transmitting these infections.23 This study demonstrates that most partners of patients with HPV-OPC do not have any detectable oral HPV DNA, suggesting either that oral-oral transmission (ie, kissing) is rare and/or that most partners effectively clear any active infections to which they are exposed. Indeed, oncogenic oral HPV prevalence in partners in this study was similar to that in the general population of the same age. Furthermore, prevalence of both oral HPV16 DNA detection and HPV16 oncogene antibodies was rare among partners, suggesting their own risk of HPV-OPC remains low. However, given the moderate sensitivity of oral HPV16 DNA detection demonstrated in patients (61% had oncogenic oral HPV detected), the presence of undetected or quiescent infection in partners cannot be excluded.
Oral HPV concordance in couples has not been well explored. In one study of couples from a maternity unit, persistent oral HPV infection in one partner was associated with a 10-fold increased risk of persistent oncogenic oral HPV infection in the other partner.24 However, oral HPV concordance between partners was modest both in that study and in another study of women with cervical HPV infection and their partners,13 which is consistent with the low oral HPV concordance observed in our study.
Although most patients had no history of previous HPV-positive cancer, several had a previous or current partner with a history of cervical dysplasia or invasive cervical cancer. This is consistent with transmission of HPV to the oropharynx when performing oral sex on a partner with an oncogenic genital HPV infection. Therefore, cervical HPV and Pap testing for female partners of patients with HPV-OPC is appropriate at the time of diagnosis of HPV-OPC, followed by routine Pap screening consistent with general screening recommendations for all women. Recent research suggests that husbands of women with cervical cancer have approximately a two-fold increased risk of tonsillar cancer, supporting oral HPV transmission by performing oral sex.25 One report of a husband and wife with synchronous HPV-OPC caused by the same HPV infection has been reported,26 but this is uncommon.
Although all patients with HPV-OPC had either oncogenic HPV and/or p16 detected in their tumors, only 61% had oncogenic HPV DNA detected in their oral rinses, similar to other studies that have suggested moderate sensitivity and low specificity of oral HPV DNA detection for HPV-OPC.5 Because HPV is often integrated into the tumor DNA of these patients, much of the HPV DNA detected in oral rinses of patients may represent small DNA strands sloughed off from the tumor without the viral capsid and thus not infectious. Variability in reports of oral HPV prevalence among patients with HPV-OPC may also be partially explained by differences in sensitivity of available consensus primer sets and variable sampling techniques. To further explore partners' risk of HPV-related cancer, we measured HPV16 E6– and E7–specific antibodies. We observed no E6 or E7 antibodies in partners. In comparison, E6- and E7-Abs were detected at diagnosis in the majority of patients with OPC, consistent with our previous reports.5,20
This study had several limitations and strengths. HPV-OPC is more common in men, and there were small numbers of female patients and male partners, limiting conclusions on these subgroups. Furthermore, genital HPV infection was not tested. The convenience sample of healthy volunteers was not population based or matched to the demographics of patients or partners. The four clinical centers where patients and partners were enrolled may not be representative of patients seen elsewhere, although results were similar across these four distinct study sites. Strengths of the study include centralized testing of oral rinse and antibody HPV data as well as some centralized tumor testing. Furthermore, this study used a standardized risk factor survey across all study sites, collected risk factor information directly from participants using CASI, and restricted patients to those with incident confirmed HPV-positive disease.
This study demonstrates that oncogenic oral HPV DNA detection is common among patients with HPV-OPC but not among their partners. This is the first study to our knowledge to explore oral HPV prevalence and cancer history in partners of patients with HPV-OPC and suggests that cancer risk in these partners remains low. Partners may have been repeatedly exposed to oral HPV during the many years it presumably took for enrolled patients' oral HPV infections to progress to HPV-OPC. Therefore, the low oral HPV prevalence detected in partners is notable and suggests that most partners have cleared any oral HPV infections that they may have acquired. This is consistent with initial research that suggested most individuals are able to clear oral HPV infections within 1 or 2 years.27,28
Supplementary Material
Acknowledgment
We thank Alicia Wentz for her data analysis support.
Appendix
Table A1.
Prevalence of Oral HPV Infection in Subanalysis Restricted to 116 Patients With HPV-OPC With HPV16-Positive Tumor by ISH Testing
| Infection | All Patients |
HPV16 Positive by ISH |
||
|---|---|---|---|---|
| No. | % | No. | % | |
| Baseline | (n = 164) | (n = 116) | ||
| Any oral HPV | 106 | 64.6 | 82 | 70.7 |
| Any oncogenic HPV | 100 | 61.0 | 77 | 66.9 |
| HPV16 | 88 | 53.7 | 66 | 56.9 |
| HPV16 qPCR ≥ 3* | 71 | 45.5 | 64 | 57.1 |
| HPV16 qPCR > 0* | 83 | 53.2 | 56 | 50.0 |
| Among women | (n = 17) | (n = 12) | ||
| Any oral HPV at baseline | 11 | 64.7 | 9 | 75.0 |
| Any oncogenic HPV | 10 | 58.8 | 9 | 75.0 |
| HPV16 at baseline | 9 | 52.9 | 8 | 66.7 |
| Among men | (n = 147) | (n = 104) | ||
| Any oral HPV at baseline | 95 | 64.3 | 73 | 70.2 |
| Any oncogenic HPV | 90 | 61.2 | 68 | 65.4 |
| HPV16 at baseline | 79 | 53.7 | 58 | 55.8 |
Abbreviations: HPV, human papillomavirus; HPV-OPC, human papillomavirus–positive oropharyngeal cancer; ISH, in situ hybridization; qPCR, quantitative polymerase chain reaction.
All oral HPV results presented, including HPV16 results, were generated by line blot unless specifically indicated by qPCR.
Footnotes
Listen to the podcast by Dr D'Souza at www.jco.org/podcasts
Processed as a Rapid Communication manuscript.
Supported by the Johns Hopkins Richard Gelb Cancer Prevention Award (G.D.) and Research Grant No. U01CA117374 from the Early Detection Research Network (K.S.A.).
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
Clinical trial information: NCT01342978.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Although all authors completed the disclosure declaration, the following author(s) and/or an author's immediate family member(s) indicated a financial or other interest that is relevant to the subject matter under consideration in this article. Certain relationships marked with a “U” are those for which no compensation was received; those relationships marked with a “C” were compensated. For a detailed description of the disclosure categories, or for more information about ASCO's conflict of interest policy, please refer to the Author Disclosure Declaration and the Disclosures of Potential Conflicts of Interest section in Information for Contributors.
Employment or Leadership Position: Karen S. Anderson, Provista Diagnostics (U) Consultant or Advisory Role: Karen S. Anderson, Provista Diagnostics (C); Maura L. Gillison, GlaxoSmithKline (U), Bristol-Myers Squibb (U) Stock Ownership: Karen S. Anderson, Provista Diagnostics Honoraria: Maura L. Gillison, Merck Research Funding: None Expert Testimony: None Patents, Royalties, and Licenses: None Other Remuneration: None
AUTHOR CONTRIBUTIONS
Conception and design: Gypsyamber D'Souza, Karen S. Anderson, Maura L. Gillison, Marshall R. Posner
Financial support: Gypsyamber D'Souza, Karen S. Anderson, Marshall R. Posner
Administrative support: Gypsyamber D'Souza, Shirani Rajan, Jennifer Gerber
Provision of study materials or patients: Gypsyamber D'Souza, Neil D. Gross, Robert Haddad, Marshall R. Posner
Collection and assembly of data: All authors
Data analysis and interpretation: Gypsyamber D'Souza, Maura L. Gillison, Marshall R. Posner
Manuscript writing: All authors
Final approval of manuscript: All authors
REFERENCES
- 1.Chaturvedi AK, Engels EA, Anderson WF, et al. Incidence trends for human papillomavirus–related and –unrelated oral squamous cell carcinomas in the United States. J Clin Oncol. 2008;26:612–619. doi: 10.1200/JCO.2007.14.1713. [DOI] [PubMed] [Google Scholar]
- 2.Hammarstedt L, Dahlstrand H, Lindquist D, et al. The incidence of tonsillar cancer in Sweden is increasing. Acta Otolaryngol. 2007;127:988–992. doi: 10.1080/00016480601110170. [DOI] [PubMed] [Google Scholar]
- 3.Hammarstedt L, Lindquist D, Dahlstrand H, et al. Human papillomavirus as a risk factor for the increase in incidence of tonsillar cancer. Int J Cancer. 2006;119:2620–2623. doi: 10.1002/ijc.22177. [DOI] [PubMed] [Google Scholar]
- 4.Chaturvedi AK, Engels EA, Pfeiffer RM, et al. Human papillomavirus and rising oropharyngeal cancer incidence in the United States. J Clin Oncol. 2011;29:4294–4301. doi: 10.1200/JCO.2011.36.4596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.D'Souza G, Kreimer AR, Viscidi R, et al. Case-control study of human papillomavirus and oropharyngeal cancer. N Engl J Med. 2007;356:1944–1956. doi: 10.1056/NEJMoa065497. [DOI] [PubMed] [Google Scholar]
- 6.Smith EM, Ritchie JM, Summersgill KF, et al. Human papillomavirus in oral exfoliated cells and risk of head and neck cancer. J Natl Cancer Inst. 2004;96:449–455. doi: 10.1093/jnci/djh074. [DOI] [PubMed] [Google Scholar]
- 7.Zhu C, Ling Y, Dong C, et al. The relationship between oral squamous cell carcinoma and human papillomavirus: A meta-analysis of a Chinese population (1994-2011) PLoS One. 2012;7:e36294. doi: 10.1371/journal.pone.0036294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gillison ML, Broutian T, Pickard RK, et al. Prevalence of oral HPV infection in the United States, 2009-2010. JAMA. 2012;307:693–703. doi: 10.1001/jama.2012.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.D'Souza G, Agrawal Y, Halpern J, et al. Oral sexual behaviors associated with prevalent oral human papillomavirus infection. J Infect Dis. 2009;199:1263–1269. doi: 10.1086/597755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kreimer AR, Alberg AJ, Daniel R, et al. Oral human papillomavirus infection in adults is associated with sexual behavior and HIV serostatus. J Infect Dis. 2004;189:686–698. doi: 10.1086/381504. [DOI] [PubMed] [Google Scholar]
- 11.Beachler DC, Weber KM, Margolick JB, et al. Risk factors for oral HPV infection among a high prevalence population of HIV-positive and at-risk HIV-negative adults. Cancer Epidemiol Biomarkers Prev. 2012;21:122–133. doi: 10.1158/1055-9965.EPI-11-0734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pickard RK, Xiao W, Broutian TR, et al. The prevalence and incidence of oral human papillomavirus infection among young men and women, aged 18-30 years. Sex Transm Dis. 2012;39:559–566. doi: 10.1097/OLQ.0b013e31824f1c65. [DOI] [PubMed] [Google Scholar]
- 13.Widdice LE, Breland DJ, Jonte J, et al. Human papillomavirus concordance in heterosexual couples. J Adolesc Health. 2010;47:151–159. doi: 10.1016/j.jadohealth.2010.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Edelstein ZR, Schwartz SM, Hawes S, et al. Rates and determinants of oral human papillomavirus infection in young men. Sex Transm Dis. 2012;39:860–867. doi: 10.1097/OLQ.0b013e318269d098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hahn HS, Kee MK, Kim HJ, et al. Distribution of maternal and infant human papillomavirus: Risk factors associated with vertical transmission. Eur J Obstet Gynecol Reprod Biol. 2013;169:202–206. doi: 10.1016/j.ejogrb.2013.02.024. [DOI] [PubMed] [Google Scholar]
- 16.Kreimer AR, Villa A, Nyitray AG, et al. The epidemiology of oral HPV infection among a multinational sample of healthy men. Cancer Epidemiol Biomarkers Prev. 2011;20:172–182. doi: 10.1158/1055-9965.EPI-10-0682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Broutian TR, He X, Gillison ML. Automated high throughput DNA isolation for detection of human papillomavirus in oral rinse samples. J Clin Virol. 2011;50:270–275. doi: 10.1016/j.jcv.2010.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Koshiol J, Rotunno M, Gillison ML, et al. Assessment of human papillomavirus in lung tumortissue. J Natl Cancer Inst. 2011;103:501–507. doi: 10.1093/jnci/djr003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Agoston ES, Robinson SJ, Mehra KK, et al. Polymerase chain reaction detection of HPV in squamous carcinoma of the oropharynx. Am J Clin Pathol. 2010;134:36–41. doi: 10.1309/AJCP1AAWXE5JJCLZ. [DOI] [PubMed] [Google Scholar]
- 20.Anderson KS, Wong J, D'Souza G, et al. Serum antibodies to the HPV16 proteome as biomarkers for head and neck cancer. Br J Cancer. 2011;104:1896–1905. doi: 10.1038/bjc.2011.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ramachandran N, Anderson KS, Raphael JV, et al. Tracking humoral responses using self assembling protein microarrays. Proteomics Clin Appl. 2008;2:1518–1527. doi: 10.1002/prca.200800034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jordan RC, Lingen MW, Perez-Ordonez B, et al. Validation of methods for oropharyngeal cancer HPV status determination in US cooperative group trials. Am J Surg Pathol. 2012;36:945–954. doi: 10.1097/PAS.0b013e318253a2d1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fakhry C, D'Souza G. Discussing the diagnosis of HPV-OSCC: Common questions and answers. Oral Oncol. 2013;49:863–871. doi: 10.1016/j.oraloncology.2013.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rintala M, Grénman S, Puranen M, et al. Natural history of oral papillomavirus infections in spouses: A prospective Finnish HPV Family Study. J Clin Virol. 2006;35:89–94. doi: 10.1016/j.jcv.2005.05.012. [DOI] [PubMed] [Google Scholar]
- 25.Hemminki K, Dong C, Frisch M. Tonsillar and other upper aerodigestive tract cancers among cervical cancer patients and their husbands. Eur J Cancer Prev. 2000;9:433–437. doi: 10.1097/00008469-200012000-00010. [DOI] [PubMed] [Google Scholar]
- 26.Haddad R, Crum C, Chen Z, et al. HPV16 transmission between a couple with HPV-related head and neck cancer. Oral Oncol. 2008;44:812–815. doi: 10.1016/j.oraloncology.2007.09.004. [DOI] [PubMed] [Google Scholar]
- 27.Kreimer AR, Pierce Campbell CM, Lin HY, et al. Incidence and clearance of oral human papillomavirus infection in men: The HIM cohort study. Lancet. 2013;382:877–887. doi: 10.1016/S0140-6736(13)60809-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Beachler DC, D'Souza G. Oral human papillomavirus infection and head and neck cancers in HIV-infected individuals. Curr Opin Oncol. 2013;25:503–510. doi: 10.1097/CCO.0b013e32836242b4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.