Abstract
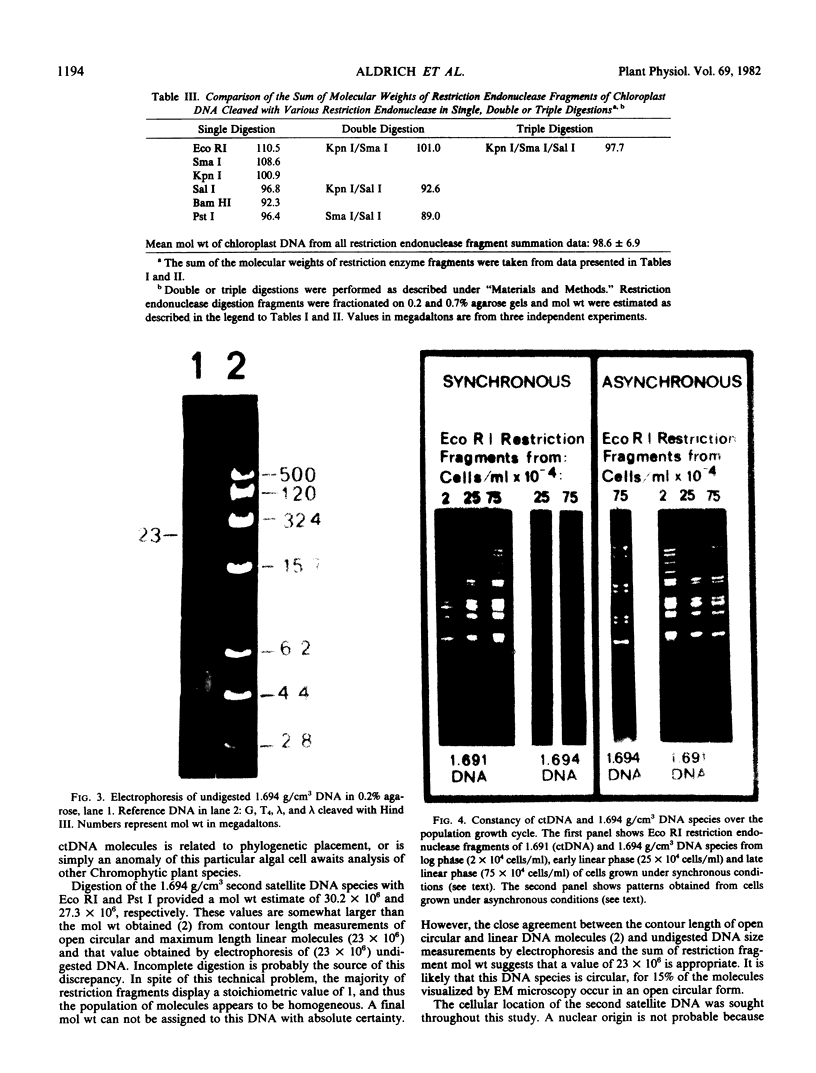
Two extranuclear DNA species have been isolated from the marine alga Olisthodiscus luteus. Rapid lysis of cells followed by the immediate addition of CsCl to the lysate was critical to the preservation of these satellite DNA species. Restriction endonuclease analysis demonstrates a molecular weight of 99 × 106 for chloroplast DNA and 23 × 106 for a second satellite species. The origin of the second satellite is not known. However, this smaller satellite DNA which originates from a nonnuclear, DNAse insensitive cellular component, displays no sequence homology with ctDNA by hybridization experiments. Constancy of restriction endonuclease fragment patterns of chloroplast and second satellite species during all phases of the growth cycle, whether cultures were maintained synchronously or asynchronously, was demonstrated.
Full text
PDF


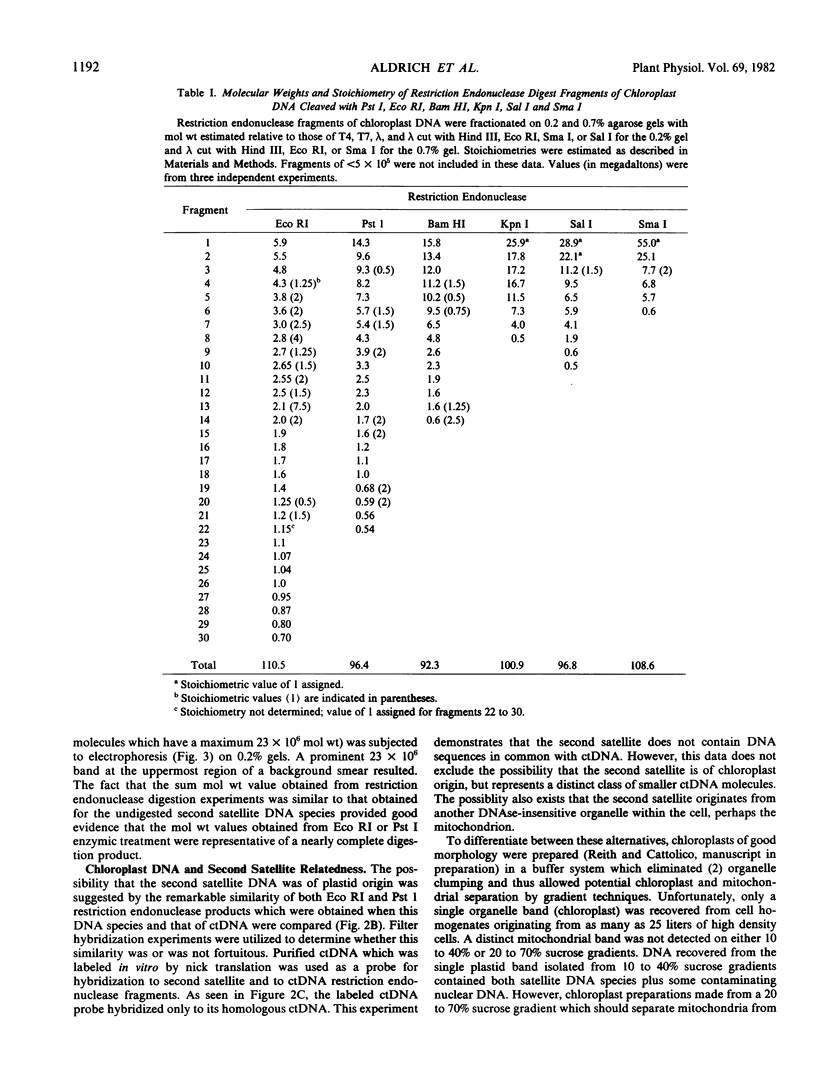
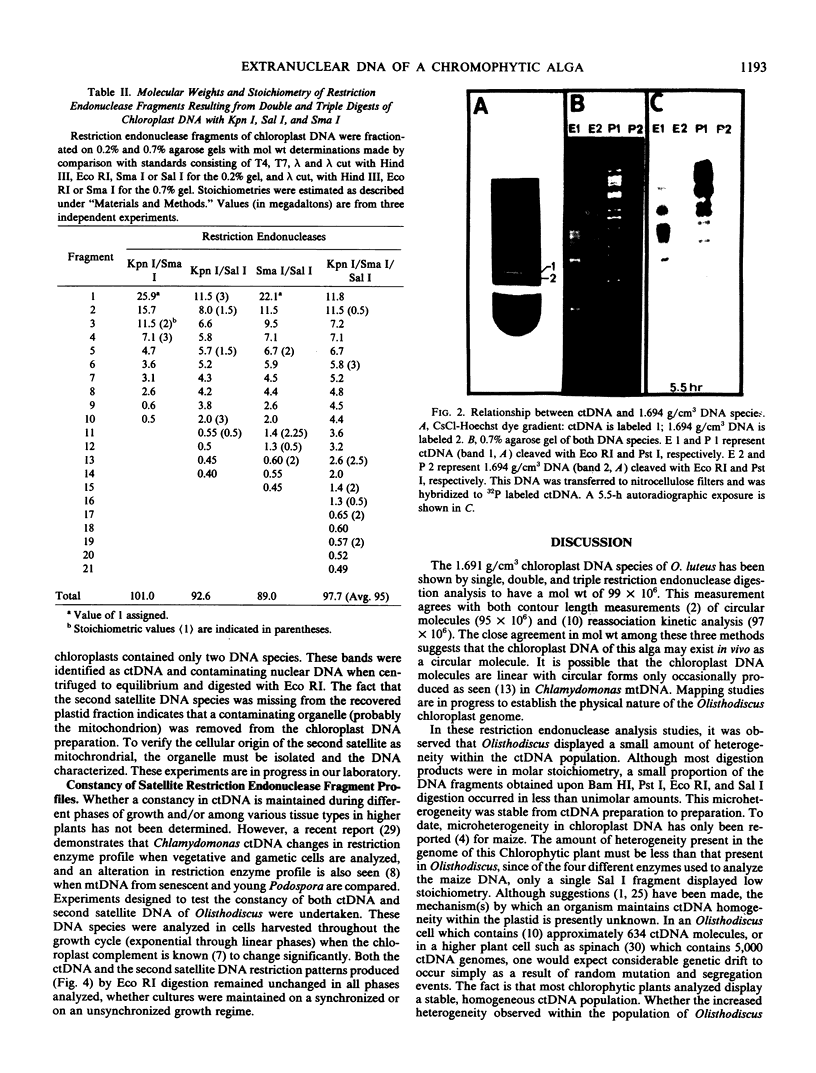

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams G. M. Chloroplast gene transmission in Chlamydomonas reinhardtii: a random choice model. Plasmid. 1978 Sep;1(4):522–535. doi: 10.1016/0147-619x(78)90009-4. [DOI] [PubMed] [Google Scholar]
- Aldrich J., Cattolico R. A. Isolation and Characterization of Chloroplast DNA from the Marine Chromophyte, Olisthodiscus luteus: Electron Microscopic Visualization of Isomeric Molecular Forms. Plant Physiol. 1981 Sep;68(3):641–647. doi: 10.1104/pp.68.3.641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bedbrook J. R., Bogorad L. Endonuclease recognition sites mapped on Zea mays chloroplast DNA. Proc Natl Acad Sci U S A. 1976 Dec;73(12):4309–4313. doi: 10.1073/pnas.73.12.4309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cattolico R. A. Variation in Plastid Number: Effect on Chloroplast and Nuclear Deoxyribonucleic Acid Complement in the Unicellular Alga Olisthodiscus luteus. Plant Physiol. 1978 Oct;62(4):558–562. doi: 10.1104/pp.62.4.558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cummings D. J., Belcour L., Grandchamp C. Mitochondrial DNA from Podospora anserina. II. Properties of mutant DNA and multimeric circular DNA from senescent cultures. Mol Gen Genet. 1979 Mar 27;171(3):239–250. doi: 10.1007/BF00267578. [DOI] [PubMed] [Google Scholar]
- Denhardt D. T. A membrane-filter technique for the detection of complementary DNA. Biochem Biophys Res Commun. 1966 Jun 13;23(5):641–646. doi: 10.1016/0006-291x(66)90447-5. [DOI] [PubMed] [Google Scholar]
- Fangman W. L. Separation of very large DNA molecules by gel electrophoresis. Nucleic Acids Res. 1978 Mar;5(3):653–665. doi: 10.1093/nar/5.3.653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillham N. W., Boynton J. E. Evolution of organelle genomes and protein-synthesizing systems. Ann N Y Acad Sci. 1981;361:20–43. doi: 10.1111/j.1749-6632.1981.tb46509.x. [DOI] [PubMed] [Google Scholar]
- Grant D., Chiang K. S. Physical mapping and characterization of Chlamydomonas mitochondrial DNA molecules: their unique ends, sequence homogeneity, and conservation. Plasmid. 1980 Jul;4(1):82–96. doi: 10.1016/0147-619x(80)90085-2. [DOI] [PubMed] [Google Scholar]
- Green B. R. Covalently closed minicircular DNA associated with Acetabularia chloroplasts. Biochim Biophys Acta. 1976 Oct 4;447(2):156–166. doi: 10.1016/0005-2787(76)90339-7. [DOI] [PubMed] [Google Scholar]
- Herrmann R. G., Bohnert H. J., Kowallik K. V., Schmitt J. M. Size, conformation and purity of chloroplast DNA of some higher plants. Biochim Biophys Acta. 1975 Jan 20;378(2):305–317. doi: 10.1016/0005-2787(75)90118-5. [DOI] [PubMed] [Google Scholar]
- Herrmann R. G., Kowallik K. V. Multiple amounts of DNA related to the size of chloroplasts. II. Comparison of electron-microscopic and autoradiographic data. Protoplasma. 1970;69(3):365–372. doi: 10.1007/BF01320301. [DOI] [PubMed] [Google Scholar]
- Kolodner R., Tewari K. K. Physicochemical characterization of mitochondrial DNA from pea leaves. Proc Natl Acad Sci U S A. 1972 Jul;69(7):1830–1834. doi: 10.1073/pnas.69.7.1830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolodner R., Tewari K. K. The molecular size and conformation of the chloroplast DNA from higher plants. Biochim Biophys Acta. 1975 Sep 1;402(3):372–390. doi: 10.1016/0005-2787(75)90273-7. [DOI] [PubMed] [Google Scholar]
- Maniatis T., Jeffrey A., Kleid D. G. Nucleotide sequence of the rightward operator of phage lambda. Proc Natl Acad Sci U S A. 1975 Mar;72(3):1184–1188. doi: 10.1073/pnas.72.3.1184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazza A., Casale A., Sassone-Corsi P., Bonotto S. A minicircular component of Acetabularia acetabulum chloroplast DNA replicating by the rolling circle. Biochem Biophys Res Commun. 1980 Apr 14;93(3):668–674. doi: 10.1016/0006-291x(80)91130-4. [DOI] [PubMed] [Google Scholar]
- McIntosh L., Cattolico R. A. Preservation of algal and higher plant ribosomal RNA integrity during extraction and electrophoretic quantitation. Anal Biochem. 1978 Dec;91(2):600–612. doi: 10.1016/0003-2697(78)90546-8. [DOI] [PubMed] [Google Scholar]
- Nass M. M., Ben-Shaul Y. A novel closed circular duplex DNA in bleached mutant and green strains of Euglena gracilis. Biochim Biophys Acta. 1972 Jun 22;272(1):130–136. doi: 10.1016/0005-2787(72)90041-x. [DOI] [PubMed] [Google Scholar]
- Oliver S. G. On the mutability of the yeast mitochondrial genome. J Theor Biol. 1977 Jul 21;67(2):195–201. doi: 10.1016/0022-5193(77)90193-x. [DOI] [PubMed] [Google Scholar]
- Pellegrini M. Three-dimensional reconstruction of organelles in Euglena gracilis Z. I. Qualitative and quantitative changes of chloroplasts and mitochondrial reticulum in synchronous photoautotrophic culture. J Cell Sci. 1980 Jun;43:137–166. doi: 10.1242/jcs.43.1.137. [DOI] [PubMed] [Google Scholar]
- Ryan R., Grant D., Chiang K. S., Swift H. Isolation and characterization of mitochondrial DNA from Chlamydomonas reinhardtii. Proc Natl Acad Sci U S A. 1978 Jul;75(7):3268–3272. doi: 10.1073/pnas.75.7.3268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sager R., Grabowy C., Sano H. The mat-1 gene in Chlamydomonas regulates DNA methylation during gametogenesis. Cell. 1981 Apr;24(1):41–47. doi: 10.1016/0092-8674(81)90499-2. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Talen J. L., Sanders J. P., Flavell R. A. Genetic complexity of mitochondrial DNA from Euglena gracilis. Biochim Biophys Acta. 1974 Dec 6;374(2):129–135. doi: 10.1016/0005-2787(74)90356-6. [DOI] [PubMed] [Google Scholar]
- Thomashow M. F., Nutter R., Montoya A. L., Gordon M. P., Nester E. W. Integration and organization of Ti plasmid sequences in crown gall tumors. Cell. 1980 Mar;19(3):729–739. doi: 10.1016/s0092-8674(80)80049-3. [DOI] [PubMed] [Google Scholar]
- Wong F. Y., Wildman S. G. Simple procedure for isolation of satellite DNA's from tobacco leaves in high yield and demonstration of minicircles. Biochim Biophys Acta. 1972 Jan 18;259(1):5–12. doi: 10.1016/0005-2787(72)90468-6. [DOI] [PubMed] [Google Scholar]