Abstract
In this minireview, we will highlight work in the last 30 years that has clearly demonstrated that the O-GlcNAc modification is nutrient-responsive and plays multiple roles in metabolic regulation of signaling and gene expression. Further, we will examine recent studies that have investigated the impact of O-GlcNAc in a variety of glucose- and insulin-responsive tissues and the roles attributed to O-GlcNAc in the induction of insulin resistance and glucose toxicity, the hallmarks of type II diabetes mellitus. We will also summarize potential causal roles for the O-GlcNAc modification in complications associated with diabetes.
Keywords: Diabetes, Insulin Resistance, O-GlcNAcylation, O-linked N-Acetylglucosamine (O-GlcNAc), Type 2 Diabetes, OGA, OGT, Hexosamine Biosynthetic Pathway
Introduction
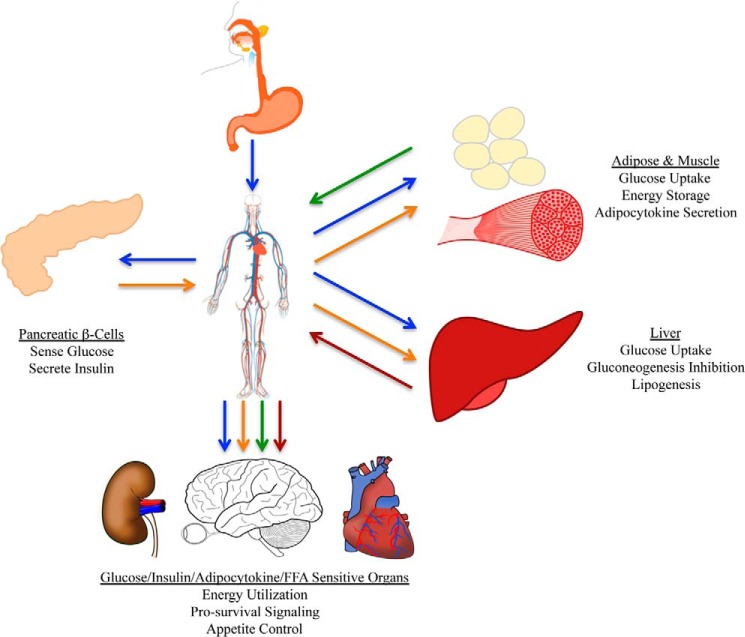
Type II diabetes, also referred to as non-insulin-dependent diabetes mellitus or adult-onset diabetes, is a metabolic disorder in which hyperglycemia and insulin resistance are the hallmarks (1, 2). This disease, which afflicts over 25 million United States citizens, is thought to result from a combination of predisposing genetic traits coupled with chronic low energy expenditure and overnutrition (1–3). Thus, type II diabetes displays traits of both an inherited as well as an acquired disease. Insulin resistance coupled with chronic glucose toxicity are thought to be responsible for the many complications associated with the disease (1, 2). Thus, understanding the mechanisms by which tissues sense and respond to changes in glucose levels and develop resistance to insulin-stimulated signaling is key to developing therapeutics for this multisystem disease of energy metabolism (Fig. 1).
FIGURE 1.
Whole organism energy dynamics and the diabetic phenotype. Glucose (blue arrows) is absorbed into the bloodstream from the duodenum. Glucose is sensed by the β-cells of the pancreas that respond by secreting insulin into the bloodstream (orange arrows). Insulin triggers adipose and muscle cells to take glucose out of the bloodstream and promotes energy storage. Insulin also modulates adipocytokine secretion (green arrows). In the liver, insulin promotes glucose uptake and inhibition of gluconeogenesis and promotes lipogenesis and FFA secretion (red arrows). Glucose, insulin, adipocytokines, and FFA all act on responsive organs, including the brain, eye, heart, kidney, and circulatory system, to promote/inhibit a variety of processes and responses. In the diabetic phenotype, all of the tissues and organs of the organism become affected due to a disconnect between sugar metabolism and insulin-dependent signal transduction.
The Hexosamine Biosynthetic Pathway (HBP) as a Glucose Sensor and Modulator of Insulin Signaling
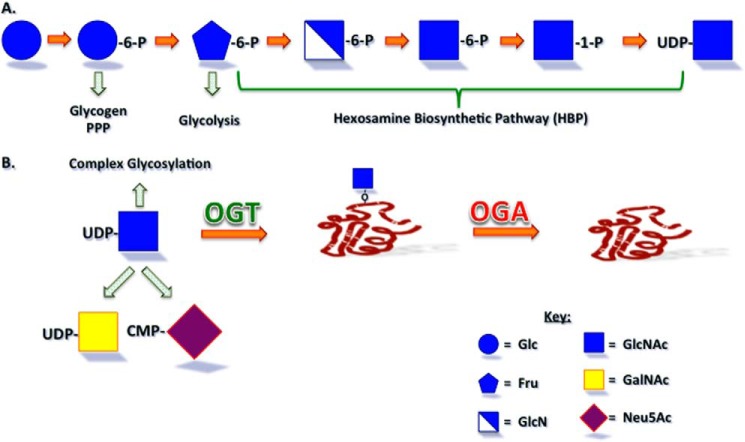
Once glucose enters a cell, it is rapidly converted to glucose 6-phosphate, which can be further utilized in multiple metabolic pathways (Fig. 2A). This intermediate can be utilized for glycogen synthesis, the pentose phosphate pathway, or converted to fructose 6-phosphate. The vast majority of fructose 6-phosphate is converted to fructose 1,6-bisphosphate and thus committed to glycolysis. However, a small percentage (2–5%) is converted to glucosamine 6-phosphate by the rate-limiting enzyme l-glutamine d-fructose 6-phosphate amidotransferase (GFAT)2 to commit it to the HBP that generates UDP-GlcNAc (4, 5). Groundbreaking work by multiple laboratories demonstrated that the HBP was a glucose sensor and that decreasing/increasing flux through this path could hamper or induce, respectively, the insulin-resistant state (4, 6–11). For example, in adipocytes, treatment with glucosamine that feeds directly into the HBP, via the generation of glucosamine 6-phosphate via phosphorylation, can abrogate the need for high glucose in the induction of insulin resistance (6). Further, overexpression of GFAT in peripheral insulin-responsive tissues leads to an insulin-resistant phenotype in mice (12). Pathways that utilize UDP-GlcNAc, the end product of the HBP, have been investigated as the mechanism by which the HBP acts as a nutrient sensor and modulator of signaling.
FIGURE 2.
The hexosamine biosynthetic pathway and UDP-GlcNAc utilization. A, glucose entering a cell can be utilized in glycogen synthesis, the pentose phosphate pathway (PPP), and glycolysis. Approximately 2–5% of entering glucose is shunted into the hexosamine biosynthetic pathway that generates the sugar nucleotide UDP-GlcNAc, the levels of which are proportional to that of the glucose entering the cells and are elevated in diabetic animals and patients. B, UDP-GlcNAc can be utilized for the generation of free, protein-linked, or lipid-linked complex carbohydrates or for the generation of other sugar nucleotides, UDP-GlcNAc and CMP-Neu5Ac, in humans. UDP-GlcNAc also serves as the sugar nucleotide donor for the OGT-catalyzed addition of O-GlcNAc to serine and threonine residues of nuclear and cytoplasmic proteins, which can be removed by the enzymatic action of OGA. Neu5Ac, N-acetylneuraminic acid.
The Regulatory O-GlcNAc Post-translational Modification
Although UDP-GlcNAc can be used for the generation of other sugar nucleotides and the synthesis of complex glycans that are free or attached to proteins and lipids, this sugar nucleotide also serves as the donor for the O-GlcNAc modification that was originally discovered Torres and Hart (13) in the mid-1980s (Fig. 2B). Unlike classical complex glycosylation, the O-GlcNAc modification of protein serine and threonine residues occurs in the nucleus and cytoplasm, and its addition and removal are catalyzed by nucleocytoplasmic enzymes, O-GlcNAc transferase (OGT) and neutral β-N-acetylglucosaminidase (OGA), respectively (14–18). Several excellent recent reviews have focused on this modification, the thousands of O-GlcNAc-modified proteins known, and the cycling enzymes (14, 19–25). Here, we will review the tissue-specific roles for the cycling enzymes and the O-GlcNAc modification in energy homeostasis and the type II diabetes mellitus phenotype.
Functions for the O-GlcNAc Modification in the Pancreatic β-Cell
The pancreas is a glucose-responsive endocrine tissue that secretes hormones in response to glucose levels (Fig. 1). Dysregulation of the β-cells of the pancreas, which are responsible for the secretion of insulin in response to hyperglycemia, is a major hallmark of the diabetic phenotype (1, 2). The O-GlcNAc modification of several proteins, primarily transcription factors, has been implicated in positively regulating the secretion of insulin from the β-cells of the pancreas. In particular, O-GlcNAc modification of NeuroD1, which enhances transcription of the insulin gene and promotes nuclear localization and O-GlcNAc modification of the transcription factor PDX-1, enhances DNA binding (26, 27). However, long-term elevation of O-GlcNAc levels via pharmacological approaches has been associated with β-cell apoptosis, although the mechanism is unclear but may involve the Akt pathway (28, 29). Further studies aimed at uncovering the roles of acute and chronic hyperglycemia that can be assigned to increases in O-GlcNAc levels in the glucose-responsive pancreas are still required.
Functions for the O-GlcNAc Modification in Skeletal Muscle and Adipose Tissue (Insulin-responsive Glucose Uptake and Metabolism)
Skeletal muscle and adipose tissue are responsible for clearing the majority of glucose from the bloodstream in response to insulin (1, 2) (Fig. 1). Insulin promotes energy storage in these tissues as well as the secretion of adipocytokines from adipose tissue (2, 19) (Fig. 1). Pioneering studies using metabolic, pharmacological, and genetic approaches clearly demonstrated an important role for the HBP in peripheral insulin resistance (4, 6–11). These studies were followed up by pharmacological and genetic studies that suggested that elevation in the O-GlcNAc modification leads to adipose and skeletal muscle insulin resistance (30, 31). This work included demonstration that a transgenic mouse overexpressing OGT in skeletal muscle displayed insulin resistance as well as hyperleptinemia (30). Cell culture studies discovered similar insulin-resistant phenotypes upon elevation in O-GlcNAc levels via pharmacological approaches (31–33). Further work uncovered that multiple proteins involved in the insulin signaling pathway were functionally O-GlcNAc-modified (34–38) and that the O-GlcNAc cycling enzymes genetically interacted with components of the insulin-like signaling pathway in the model organism Caenorhabditis elegans (39–44). In addition to defects in insulin-dependent glucose uptake and energy storage, the HBP and more specifically the O-GlcNAc modification are also functionally implicated in the secretion of adipocytokines that modulate a number of energy homeostatic functions in the organism, including feeding behavior (30, 33, 45–47) (Fig. 1). For example, Rossetti's group (47) clearly demonstrated that leptin transcription, expression, and secretion were regulated by the HBP, and later, McClain et al. (30) demonstrated using genetic approaches that this regulation was directly due to the O-GlcNAc modification. Work by our own group using pharmacological manipulation followed by quantitative mass spectrometry has further determined that multiple mammalian adipocytokines are regulated by levels of the O-GlcNAc modification in adipocytes (45, 46). Thus, multiple groups using a variety of approaches and systems have determined strong links between the O-GlcNAc modification and peripheral insulin resistance. Work by Buse and colleagues (48), who demonstrated that overexpression of OGA did not ameliorate hyperglycemia/chronic insulin-induced insulin resistance in cultured adipocytes, and work by Vocadlo and colleagues (49, 50), who demonstrated that a specific inhibitor of OGA did not induce peripheral insulin resistance, clearly show that the field still has much to uncover to fully understand the role of the O-GlcNAc modification in modulating peripheral tissue insulin responsiveness. In particular, the field needs to acknowledge and address that metabolic treatments (for example, the addition of glucose, glucosamine, GlcNAc, and/or glutamine), pharmacological treatments (inhibition of OGT, OGA, and GFAT via inhibitors with various mechanisms and specificities, for example), and genetic manipulation (O-GlcNAc/HBP enzyme overexpression and knockdown/knock-out) are not necessarily comparable. For example, pharmacological inhibition of OGA potentially elevates O-GlcNAc levels by breaking the dynamic on/off cycle, whereas overexpression of OGT likely elevates O-GlcNAc levels by shifting the equilibrium toward modification.
Functions for the O-GlcNAc Modification in the Liver (Insulin-suppressed Gluconeogenesis)
The liver plays a central role in glucose homeostasis and is insulin-responsive (Fig. 1). In addition to insulin-responsive glucose uptake, insulin also inhibits gluconeogenesis and promotes FFA secretion in the liver (1). Perhaps some of the most compelling studies for the role of O-GlcNAc in the liver and the diabetic phenotype were conducted by the Montiminy and Evans groups (51, 52), who showed that overexpression of OGA in the liver rescued circulating glucose levels in a diabetic mouse model and that overexpression of OGT in the liver induced insulin resistance and dyslipidemia in a normal mouse, respectively. Elegant work by Hart and colleagues (53, 54) has demonstrated that expression of key gluconeogenic enzymes is regulated at the level of transcription by the O-GlcNAc modification, and this work has been recently followed up on and extended by Yang's group (55). O-GlcNAc modification of transcription factors is also centrally involved in the regulated expression of lipogenic enzymes (56, 57). Thus, in the liver, metabolic regulation of gene expression via the O-GlcNAc modification appears to be a central theme, and it has been established in multiple systems that the O-GlcNAc modification is a regulator of transcription (58, 59).
Functions for the O-GlcNAc Modification in Organs/Tissues Associated with Diabetic Complications
Multiple tissues and organs are involved in the diabetic phenotype, including the kidney, eye, brain, heart, and vascular system (1, 2) (Fig. 1). Retinopathy and nephropathy that lead to blindness and kidney failure are major complications of diabetes mellitus (60, 61). Insulin plays a pro-survival role in large part by activation of the anti-apoptotic PI3K-Akt pathway (62). Flux through the HBP and elevation in O-GlcNAc modification of proteins have been demonstrated to attenuate this pathway in multiple systems and thus may play a significant role in the induction of apoptosis in these tissues (31, 34, 37, 52, 63). Alternatively or perhaps additively, the O-GlcNAc modification is known to modulate NO production in endothelial cells, promoting macro- and microvascular complications that may lead to organ failure (64, 65). In alignment with the role of O-GlcNAc in vascular regulation, several groups have demonstrated a role for O-GlcNAc in the heart, and recent studies have covered these findings (23, 25, 66–68). Diabetic cardiomyopathy is responsible for a large number of the deaths associated with diabetes mellitus (69). Recent work has demonstrated that the O-GlcNAc modification of proteins in cardiac tissue is a mechanism for hyperglycemia/insulin-resistant-induced mitochondrial dysfunction, contractile defects, and atherosclerosis as well as perturbations in calcium loading and oxidative stress responses (23, 25, 66–68). Further, OGA protein levels and O-GlcNAc-modified proteins are increased in erythrocytes of pre-diabetic and diabetic patients, paving the way for O-GlcNAc levels being used as a biomarker for early detection and efficacious treatment of diabetes (70, 71). Future studies are needed to uncover the roles for O-GlcNAc in mediating glucose toxicity and the normal physiological roles for this modification in modulating energy utilization and responsiveness to extracellular cues in organs associated with the diabetic phenotype.
Conclusions and Future Directions
As illustrated in Fig. 1, the organism as a whole must be considered when investigating energy homeostasis and its dysregulation in diabetes. This is especially true for the O-GlcNAc modification that has been increasingly implicated as a metabolic sensor that regulates gene expression, i.e. an epigenetic mark, of secreted factors. In this review, we have highlighted several such examples, including O-GlcNAc levels regulating insulin secretion in the pancreas, which goes on to regulate multiple processes in other organs, and the O-GlcNAc modification regulating insulin-dependent leptin secretion at the level of transcription in adipose tissue, which then circulates to the brain to regulate eating behavior (Fig. 1). Thus, a major challenge in the O-GlcNAc and diabetes field is elucidating not only direct effects of O-GlcNAc modification on particular proteins in specific cell types, but equally importantly, elucidating indirect effects of changes in O-GlcNAc levels in one tissue having metabolic consequences in another tissue that impact the overall energy dynamics and health of the entire organism.
Acknowledgments
We are indebted to members of the Wells laboratory, past and present, for scientific discussions.
This work was supported, in whole or in part, by National Institutes of Health Grants P41GM103490 and P01GM107012 (to L. W.) through the NIGMS. This is the sixth article in the Minireview Series on the Thirtieth Anniversary of Research on O-GlcNAcylation of Nuclear and Cytoplasmic Proteins: Nutrient Regulation of Cellular Metabolism and Physiology by O-GlcNAcylation.
- GFAT
- l-glutamine:d-fructose-6-phosphate amidotransferase
- HBP
- hexosamine biosynthetic pathway
- O-GlcNAc
- O-linked β-N-acetylglucosamine
- OGT
- O-GlcNAc transferase
- OGA
- neutral β-N-acetylglucosaminidase
- PPP
- pentose phosphate pathway.
REFERENCES
- 1. Abdul-Ghani M. A. (2013) Type 2 diabetes and the evolving paradigm in glucose regulation. Am. J. Manag. Care 19, S43–S50 [PubMed] [Google Scholar]
- 2. Brownlee M. (2001) Biochemistry and molecular cell biology of diabetic complications. Nature 414, 813–820 [DOI] [PubMed] [Google Scholar]
- 3. Centers for Disease Control and Prevention (2014) National Diabetes Statistics Report, Centers for Disease Control and Prevention, Atlanta, GA [Google Scholar]
- 4. Traxinger R. R., Marshall S. (1991) Coordinated regulation of glutamine:fructose-6-phosphate amidotransferase activity by insulin, glucose, and glutamine: role of hexosamine biosynthesis in enzyme regulation. J. Biol. Chem. 266, 10148–10154 [PubMed] [Google Scholar]
- 5. Kornfeld R. (1967) Studies on l-glutamine d-fructose 6-phosphate amidotransferase: I. Feedback inhibition by uridine diphosphate-N-acetylglucosamine. J. Biol. Chem. 242, 3135–3141 [PubMed] [Google Scholar]
- 6. Marshall S., Bacote V., Traxinger R. R. (1991) Discovery of a metabolic pathway mediating glucose-induced desensitization of the glucose transport system: Role of hexosamine biosynthesis in the induction of insulin resistance. J. Biol. Chem. 266, 4706–4712 [PubMed] [Google Scholar]
- 7. Marshall S., Garvey W. T., Traxinger R. R. (1991) New insights into the metabolic regulation of insulin action and insulin resistance: role of glucose and amino acids. FASEB J. 5, 3031–3036 [DOI] [PubMed] [Google Scholar]
- 8. McClain D. A. (2002) Hexosamines as mediators of nutrient sensing and regulation in diabetes. J. Diabetes Complications 16, 72–80 [DOI] [PubMed] [Google Scholar]
- 9. McClain D. A., Crook E. D. (1996) Hexosamines and insulin resistance. Diabetes 45, 1003–1009 [DOI] [PubMed] [Google Scholar]
- 10. McClain D. A., Hazel M., Parker G., Cooksey R. C. (2005) Adipocytes with increased hexosamine flux exhibit insulin resistance, increased glucose uptake, and increased synthesis and storage of lipid. Am. J. Physiol. Endocrinol. Metab. 288, E973–E979 [DOI] [PubMed] [Google Scholar]
- 11. Wells L., Vosseller K., Hart G. W. (2003) A role for N-acetylglucosamine as a nutrient sensor and mediator of insulin resistance. Cell. Mol. Life Sci. 60, 222–228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hebert L. F., Jr., Daniels M. C., Zhou J., Crook E. D., Turner R. L., Simmons S. T., Neidigh J. L., Zhu J. S., Baron A. D., McClain D. A. (1996) Overexpression of glutamine:fructose-6-phosphate amidotransferase in transgenic mice leads to insulin resistance. J. Clin. Invest. 98, 930–936 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Torres C. R., Hart G. W. (1984) Topography and polypeptide distribution of terminal N-acetylglucosamine residues on the surfaces of intact lymphocytes. Evidence for O-linked GlcNAc. J. Biol. Chem. 259, 3308–3317 [PubMed] [Google Scholar]
- 14. Hart G. W., Haltiwanger R. S., Holt G. D., Kelly W. G. (1989) Nucleoplasmic and cytoplasmic glycoproteins. Ciba Found. Symp. 145, 102–112, discussion 112–108 [DOI] [PubMed] [Google Scholar]
- 15. Kreppel L. K., Blomberg M. A., Hart G. W. (1997) Dynamic glycosylation of nuclear and cytosolic proteins: cloning and characterization of a unique O-GlcNAc transferase with multiple tetratricopeptide repeats. J. Biol. Chem. 272, 9308–9315 [DOI] [PubMed] [Google Scholar]
- 16. Lubas W. A., Frank D. W., Krause M., Hanover J. A. (1997) O-Linked GlcNAc transferase is a conserved nucleocytoplasmic protein containing tetratricopeptide repeats. J. Biol. Chem. 272, 9316–9324 [DOI] [PubMed] [Google Scholar]
- 17. Gao Y., Wells L., Comer F. I., Parker G. J., Hart G. W. (2001) Dynamic O-glycosylation of nuclear and cytosolic proteins: cloning and characterization of a neutral, cytosolic β-N-acetylglucosaminidase from human brain. J. Biol. Chem. 276, 9838–9845 [DOI] [PubMed] [Google Scholar]
- 18. Wells L., Gao Y., Mahoney J. A., Vosseller K., Chen C., Rosen A., Hart G. W. (2002) Dynamic O-glycosylation of nuclear and cytosolic proteins: further characterization of the nucleocytoplasmic β-N-acetylglucosaminidase, O-GlcNAcase. J. Biol. Chem. 277, 1755–1761 [DOI] [PubMed] [Google Scholar]
- 19. Teo C. F., Wollaston-Hayden E. E., Wells L. (2010) Hexosamine flux, the O-GlcNAc modification, and the development of insulin resistance in adipocytes. Mol. Cell. Endocrinol. 318, 44–53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Hart G. W., Slawson C., Ramirez-Correa G., Lagerlof O. (2011) Cross talk between O-GlcNAcylation and phosphorylation: roles in signaling, transcription, and chronic disease. Annu. Rev. Biochem. 80, 825–858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Hanover J. A., Krause M. W., Love D. C. (2012) Bittersweet memories: linking metabolism to epigenetics through O-GlcNAcylation. Nat. Rev. Mol. Cell Biol. 13, 312–321 [DOI] [PubMed] [Google Scholar]
- 22. Vocadlo D. J. (2012) O-GlcNAc processing enzymes: catalytic mechanisms, substrate specificity, and enzyme regulation. Curr. Opin. Chem. Biol. 16, 488–497 [DOI] [PubMed] [Google Scholar]
- 23. Zachara N. E. (2012) The roles of O-linked β-N-acetylglucosamine in cardiovascular physiology and disease. Am. J. Physiol. Heart Circ. Physiol. 302, H1905–H1918 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Ma J., Hart G. W. (2014) O-GlcNAc profiling: from proteins to proteomes. Clin. Proteomics 11, 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Vaidyanathan K., Durning S., Wells L. (2014) Functional O-GlcNAc modifications: implications in molecular regulation and pathophysiology. Crit. Rev. Biochem. Mol. Biol. 49, 140–163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Andrali S. S., Qian Q., Ozcan S. (2007) Glucose mediates the translocation of NeuroD1 by O-linked glycosylation. J. Biol. Chem. 282, 15589–15596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Gao Y., Miyazaki J., Hart G. W. (2003) The transcription factor PDX-1 is post-translationally modified by O-linked N-acetylglucosamine and this modification is correlated with its DNA binding activity and insulin secretion in min6 β-cells. Arch. Biochem. Biophys. 415, 155–163 [DOI] [PubMed] [Google Scholar]
- 28. Kang E. S., Han D., Park J., Kwak T. K., Oh M. A., Lee S. A., Choi S., Park Z. Y., Kim Y., Lee J. W. (2008) O-GlcNAc modulation at Akt1 Ser473 correlates with apoptosis of murine pancreatic β-cells. Exp. Cell Res. 314, 2238–2248 [DOI] [PubMed] [Google Scholar]
- 29. Liu K., Paterson A. J., Chin E., Kudlow J. E. (2000) Glucose stimulates protein modification by O-linked GlcNAc in pancreatic β-cells: linkage of O-linked GlcNAc to β-cell death. Proc. Natl. Acad. Sci. U.S.A. 97, 2820–2825 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. McClain D. A., Lubas W. A., Cooksey R. C., Hazel M., Parker G. J., Love D. C., Hanover J. A. (2002) Altered glycan-dependent signaling induces insulin resistance and hyperleptinemia. Proc. Natl. Acad. Sci. U.S.A. 99, 10695–10699 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Vosseller K., Wells L., Lane M. D., Hart G. W. (2002) Elevated nucleocytoplasmic glycosylation by O-GlcNAc results in insulin resistance associated with defects in Akt activation in 3T3-L1 adipocytes. Proc. Natl. Acad. Sci. U.S.A. 99, 5313–5318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Arias E. B., Kim J., Cartee G. D. (2004) Prolonged incubation in PUGNAc results in increased protein O-linked glycosylation and insulin resistance in rat skeletal muscle. Diabetes 53, 921–930 [DOI] [PubMed] [Google Scholar]
- 33. Hazel M., Cooksey R. C., Jones D., Parker G., Neidigh J. L., Witherbee B., Gulve E. A., McClain D. A. (2004) Activation of the hexosamine signaling pathway in adipose tissue results in decreased serum adiponectin and skeletal muscle insulin resistance. Endocrinology 145, 2118–2128 [DOI] [PubMed] [Google Scholar]
- 34. Park S. Y., Ryu J., Lee W. (2005) O-GlcNAc modification on IRS-1 and Akt2 by PUGNAc inhibits their phosphorylation and induces insulin resistance in rat primary adipocytes. Exp. Mol. Med. 37, 220–229 [DOI] [PubMed] [Google Scholar]
- 35. Gandy J. C., Rountree A. E., Bijur G. N. (2006) Akt1 is dynamically modified with O-GlcNAc following treatments with PUGNAc and insulin-like growth factor-1. FEBS Lett. 580, 3051–3058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Soesanto Y. A., Luo B., Jones D., Taylor R., Gabrielsen J. S., Parker G., McClain D. A. (2008) Regulation of Akt signaling by O-GlcNAc in euglycemia. Am. J. Physiol. Endocrinol. Metab. 295, E974–E980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Whelan S. A., Dias W. B., Thiruneelakantapillai L., Lane M. D., Hart G. W. (2010) Regulation of insulin receptor substrate 1 (IRS-1)/AKT kinase-mediated insulin signaling by O-linked β-N-acetylglucosamine in 3T3-L1 adipocytes. J. Biol. Chem. 285, 5204–5211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Wang S., Huang X., Sun D., Xin X., Pan Q., Peng S., Liang Z., Luo C., Yang Y., Jiang H., Huang M., Chai W., Ding J., Geng M. (2012) Extensive crosstalk between O-GlcNAcylation and phosphorylation regulates Akt signaling. PLoS One 7, e37427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Hanover J. A., Forsythe M. E., Hennessey P. T., Brodigan T. M., Love D. C., Ashwell G., Krause M. (2005) A Caenorhabditis elegans model of insulin resistance: altered macronutrient storage and dauer formation in an OGT-1 knockout. Proc. Natl. Acad. Sci. U.S.A. 102, 11266–11271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Forsythe M. E., Love D. C., Lazarus B. D., Kim E. J., Prinz W. A., Ashwell G., Krause M. W., Hanover J. A. (2006) Caenorhabditis elegans ortholog of a diabetes susceptibility locus: oga-1 (O-GlcNAcase) knockout impacts O-GlcNAc cycling, metabolism, and dauer. Proc. Natl. Acad. Sci. U.S.A. 103, 11952–11957 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Lee J., Kim K. Y., Lee J., Paik Y. K. (2010) Regulation of Dauer formation by O-GlcNAcylation in Caenorhabditis elegans. J. Biol. Chem. 285, 2930–2939 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Love D. C., Ghosh S., Mondoux M. A., Fukushige T., Wang P., Wilson M. A., Iser W. B., Wolkow C. A., Krause M. W., Hanover J. A. (2010) Dynamic O-GlcNAc cycling at promoters of Caenorhabditis elegans genes regulating longevity, stress, and immunity. Proc. Natl. Acad. Sci. U.S.A. 107, 7413–7418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Rahman M. M., Stuchlick O., El-Karim E. G., Stuart R., Kipreos E. T., Wells L. (2010) Intracellular protein glycosylation modulates insulin mediated lifespan in C. elegans. Aging 2, 678–690 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Hanover J. A., Wang P. (2013) O-GlcNAc cycling shows neuroprotective potential in C. elegans models of neurodegenerative disease. Worm 2, e27043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Lim J. M., Sherling D., Teo C. F., Hausman D. B., Lin D., Wells L. (2008) Defining the regulated secreted proteome of rodent adipocytes upon the induction of insulin resistance. J. Proteome Res. 7, 1251–1263 [DOI] [PubMed] [Google Scholar]
- 46. Lim J. M., Wollaston-Hayden E. E., Teo C. F., Hausman D., Wells L. (2014) Quantitative secretome and glycome of primary human adipocytes during insulin resistance. Clin. Proteomics 11, 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Wang J., Liu R., Hawkins M., Barzilai N., Rossetti L. (1998) A nutrient-sensing pathway regulates leptin gene expression in muscle and fat. Nature 393, 684–688 [DOI] [PubMed] [Google Scholar]
- 48. Robinson K. A., Ball L. E., Buse M. G. (2007) Reduction of O-GlcNAc protein modification does not prevent insulin resistance in 3T3-L1 adipocytes. Am. J. Physiol. Endocrinol. Metab. 292, E884–E890 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Macauley M. S., He Y., Gloster T. M., Stubbs K. A., Davies G. J., Vocadlo D. J. (2010) Inhibition of O-GlcNAcase using a potent and cell-permeable inhibitor does not induce insulin resistance in 3T3-L1 adipocytes. Chem. Biol. 17, 937–948 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Macauley M. S., Shan X., Yuzwa S. A., Gloster T. M., Vocadlo D. J. (2010) Elevation of global O-GlcNAc in rodents using a selective O-GlcNAcase inhibitor does not cause insulin resistance or perturb glucohomeostasis. Chem. Biol. 17, 949–958 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Dentin R., Hedrick S., Xie J., Yates J., 3rd, Montminy M. (2008) Hepatic glucose sensing via the CREB coactivator CRTC2. Science 319, 1402–1405 [DOI] [PubMed] [Google Scholar]
- 52. Yang X., Ongusaha P. P., Miles P. D., Havstad J. C., Zhang F., So W. V., Kudlow J. E., Michell R. H., Olefsky J. M., Field S. J., Evans R. M. (2008) Phosphoinositide signalling links O-GlcNAc transferase to insulin resistance. Nature 451, 964–969 [DOI] [PubMed] [Google Scholar]
- 53. Housley M. P., Rodgers J. T., Udeshi N. D., Kelly T. J., Shabanowitz J., Hunt D. F., Puigserver P., Hart G. W. (2008) O-GlcNAc regulates FoxO activation in response to glucose. J. Biol. Chem. 283, 16283–16292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Housley M. P., Udeshi N. D., Rodgers J. T., Shabanowitz J., Puigserver P., Hunt D. F., Hart G. W. (2009) A PGC-1α-O-GlcNAc transferase complex regulates FoxO transcription factor activity in response to glucose. J. Biol. Chem. 284, 5148–5157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Ruan H. B., Han X., Li M. D., Singh J. P., Qian K., Azarhoush S., Zhao L., Bennett A. M., Samuel V. T., Wu J., Yates J. R., 3rd, Yang X. (2012) O-GlcNAc transferase/host cell factor C1 complex regulates gluconeogenesis by modulating PGC-1α stability. Cell Metab. 16, 226–237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Anthonisen E. H., Berven L., Holm S., Nygård M., Nebb H. I., Grønning-Wang L. M. (2010) Nuclear receptor liver X receptor is O-GlcNAc-modified in response to glucose. J. Biol. Chem. 285, 1607–1615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Guinez C., Filhoulaud G., Rayah-Benhamed F., Marmier S., Dubuquoy C., Dentin R., Moldes M., Burnol A. F., Yang X., Lefebvre T., Girard J., Postic C. (2011) O-GlcNAcylation increases ChREBP protein content and transcriptional activity in the liver. Diabetes 60, 1399–1413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Ozcan S., Andrali S. S., Cantrell J. E. (2010) Modulation of transcription factor function by O-GlcNAc modification. Biochim. Biophys. Acta 1799, 353–364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Lewis B. A. (2013) O-GlcNAcylation at promoters, nutrient sensors, and transcriptional regulation. Biochim. Biophys. Acta 1829, 1202–1206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Sun Y. M., Su Y., Li J., Wang L. F. (2013) Recent advances in understanding the biochemical and molecular mechanism of diabetic nephropathy. Biochem. Biophys. Res. Commun. 433, 359–361 [DOI] [PubMed] [Google Scholar]
- 61. Ola M. S., Nawaz M. I., Siddiquei M. M., Al-Amro S., Abu El-Asrar A. M. (2012) Recent advances in understanding the biochemical and molecular mechanism of diabetic retinopathy. J. Diabetes Complications 26, 56–64 [DOI] [PubMed] [Google Scholar]
- 62. Vasudevan K. M., Garraway L. A. (2010) AKT signaling in physiology and disease. Curr. Top. Microbiol. Immunol. 347, 105–133 [DOI] [PubMed] [Google Scholar]
- 63. Nakamura M., Barber A. J., Antonetti D. A., LaNoue K. F., Robinson K. A., Buse M. G., Gardner T. W. (2001) Excessive hexosamines block the neuroprotective effect of insulin and induce apoptosis in retinal neurons. J. Biol. Chem. 276, 43748–43755 [DOI] [PubMed] [Google Scholar]
- 64. Musicki B., Kramer M. F., Becker R. E., Burnett A. L. (2005) Inactivation of phosphorylated endothelial nitric oxide synthase (Ser-1177) by O-GlcNAc in diabetes-associated erectile dysfunction. Proc. Natl. Acad. Sci. U.S.A. 102, 11870–11875 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Beleznai T., Bagi Z. (2012) Activation of hexosamine pathway impairs nitric oxide (NO)-dependent arteriolar dilations by increased protein O-GlcNAcylation. Vascul. Pharmacol. 56, 115–121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Dassanayaka S., Jones S. P. (2014) O-GlcNAc and the cardiovascular system. Pharmacol. Ther. 142, 62–71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Durgan D. J., Pat B. M., Laczy B., Bradley J. A., Tsai J. Y., Grenett M. H., Ratcliffe W. F., Brewer R. A., Nagendran J., Villegas-Montoya C., Zou C., Zou L., Johnson R. L., Jr., Dyck J. R., Bray M. S., Gamble K. L., Chatham J. C., Young M. E. (2011) O-GlcNAcylation, novel post-translational modification linking myocardial metabolism and cardiomyocyte circadian clock. J. Biol. Chem. 286, 44606–44619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Jones S. P., Zachara N. E., Ngoh G. A., Hill B. G., Teshima Y., Bhatnagar A., Hart G. W., Marbán E. (2008) Cardioprotection by N-acetylglucosamine linkage to cellular proteins. Circulation 117, 1172–1182 [DOI] [PubMed] [Google Scholar]
- 69. Bugger H., Abel E. D. (2014) Molecular mechanisms of diabetic cardiomyopathy. Diabetologia 57, 660–671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Park K., Saudek C. D., Hart G. W. (2010) Increased expression of β-N-acetylglucosaminidase in erythrocytes from individuals with pre-diabetes and diabetes. Diabetes 59, 1845–1850 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Wang Z., Park K., Comer F., Hsieh-Wilson L. C., Saudek C. D., Hart G. W. (2009) Site-specific GlcNAcylation of human erythrocyte proteins: potential biomarker(s) for diabetes. Diabetes 58, 309–317 [DOI] [PMC free article] [PubMed] [Google Scholar]