Background: Micro RNA plays a role in the pathogenesis of glioma.
Results: Glioma-derived miR-92a induces IL-6+ IL-10+ NKT cells, which suppress CD8 cells.
Conclusion: Glioma-derived miR-92a compromises the antitumor immunity.
Significance: miR-92a may be a therapeutic target in the treatment of glioma.
Keywords: Cellular Immune Response, Glioblastoma, Lymphocyte, RNA, Tolerance
Abstract
Therapeutic outcomes of glioma are currently not encouraging. Tumor tolerance plays an important role in the pathogenesis of glioma. It is reported that micro RNAs (miR) are associated with tumor development. This study aims to investigate the role of miR-92a in the development of tolerant natural killer T (NKT) cells. In this study, U87 cells (a human glioma cell line) and primary glioma cells were prepared. The assessment of miR-92a was performed by real time RT-PCR. The expression of interleukin (IL)-10 and IL-6 in NKT cells was evaluated by flow cytometry. Results showed that abundant IL-6+ IL-10+ NKT cells were detected in glioma tissue. Cultures of glioma cells and NKT cells induced the expression of IL-6 and IL-10 in NKT cells. Glioma cells expressed miR-92a; the latter played a critical role in the induction of IL-6 and IL-10 expression in NKT cells. The expression of the antitumor molecules, including perforin, Fas ligand, and interferon-γ, was significantly attenuated compared with control NKT cells. The IL-6+ IL-10+ NKT cells showed less capability in the induction of apoptosis in glioma cells, but showed the immune suppressor functions on CD8+ T cell activities. We conclude that glioma-derived miR-92a induces IL-6+ IL-10+ NKT cells; this fraction of NKT cells can suppress cytotoxic CD8+ T cells.
Introduction
Glioma is a malignant tumor in the brain that stems from glial cells. The pathogenesis of glioma needs to be further investigated. Because of the anatomical feature, it is not easy to diagnose glioma in its early stages. Most glioma is diagnosed in its advanced stages. The therapeutic outcomes of glioma is currently not encouraging (1).
One of the factors of tumor pathogenesis is the tumor tolerance. Specific immune regulatory cells, such as regulatory T cells and regulatory B cells, are localized in tumor tissue (2). The mechanism by which immune regulatory cells develop in tumor is not yet fully understood.
Natural killer T (NKT)3 cells are a sub-fraction of T cells. A large number of published studies demonstrate that NKT cells have miscellaneous functions in immune regulation, one of which is that NKT cells are a tumor cell killer, based on the production of antitumor cytokines, including IL-4, IFN-γ, Fas ligand (FasL), IL-13, perforin, etc (3). Besides contributing to immune protection, NKT cells are also involved in immune tolerance in the body (4); the underlying mechanism of the tolerant NKT cell induction is unclear. Whether NKT cells contribute, tumor tolerance in glioma is unknown.
Micro RNAs (miR) are a kind of endogenous small RNA. The length of miR is about 20–24 nucleotides of RNA. Published data indicate that several miRs can regulate the same genes. A gene expression may be regulated by the combination of several miRNAs. Presumably, miRNA regulates human 1/3 gene (5). miRs are involved in the development and functions of regulatory immune cells (6), expression of IL-6 and IL-10, as well as the pathogenesis of cancer (7, 8). Based on the above information, we hypothesize that glioma cells can contribute to tumor tolerance. Indeed, the results of the present study showed that glioma cells produced miR-92a; the latter induced IL-6+ IL-10+ NKT cells. This fraction of NKT cells showed immune suppressor functions on CD8+ T cell activities.
MATERIALS AND METHODS
Reagents
The α-GalCer analog 7, anti-CD3 (F7.2.38), and anti-CD28 (CD28.1) antibodies were purchased from Santa Cruz Biotechnology (Shanghai, China). The miRCURY RNA Isolation Kit™ was purchased from Exiqon (Shanghai, China). The primers and antisense of miR-92a was synthesized by GenScript (Guangzhou, China). The fluorochrome-labeled antibodies for flow cytometry were purchased from BD Biosciences (Shanghai, China). The annexin V reagent kit was purchased from Sigma Aldrich. The immune cell isolation kits were purchased from Miltenyi Biotech (Guangzhou, China). The reagents for real time PCR was purchased from Invitrogen. The neutralizing antibodies of IL-6/IL-10, IL-2, IL-15 were purchased from R&D Systems (Guangzhou, China).
U87 Cell Culture
U87 cells (a human glioma cell line; ATCC) were cultured in DMEM supplemented with 10% fetal bovine serum, 100 units/ml penicillin, 0.1 mg/ml streptomycin, and 2 mm l-glutamine. The medium was changed every 2–3 days. The cell viability was greater than 99% before use for further experiments, as assessed by Trypan Blue exclusion assay.
Research Ethic Statement
The use of human tissue in the present study was approved by the Human Research Ethic Committee at Guilin Medical University. The experiments were carried out in accordance with these guidelines. An informed written consent was obtained from each subject.
Preparation of Primary Glioma Cells
Surgically removed glioma tissue was collected from the operation room, cut into small pieces (2 × 2 × 2 mm), and incubated for 2 h at 37 °C in the presence of 0.5 mg/ml collagenase IV. The tissue slurry was passed through a cell strainer (70 μm). After washing with culture medium three times, cells were incubated with magnetic bead-conjugated antibodies to separate immune cells, including CD3+ cells, CD14+ cells, and CD11c+ cells from glioma cells. As assessed by Trypan Blue exclusion assay, the viability of the cells was greater than 98%.
Flow Cytometry
For surface staining, the cells were blocked by 1% bovine serum albumin (BSA) for 30 min and incubated with fluorochrome-labeled antibodies for 1 h on ice. For intracellular staining, the cells were fixed with 2% paraformaldehyde containing 0.1% Triton X-100 for 2 h. After washing, the cells were blocked with 1% BSA for 30 min. The cells were incubated with the primary antibodies (labeled with fluorochromes anti-6B11 = 100 ng/ml; anti-CD3 = 500 ng/ml; anti-IL-10 = 200 ng/ml; anti-IL-6 = 200 ng/ml) for 1 h at room temperature. After washing, the cells were analyzed by flow cytometry. Results were analyzed with software FlowJo (TreeStar, Ashland OR).
Assessment of miR-92a
The total RNA was extracted from glioma cells with a miRCURY RNA Isolation kit. The cDNA was synthesized using a reverse transcription kit. Real-time PCR was performed on a MiniOpticon real-time PCR device (Bio-Rad) using the SYBR Green master mix using snRNA U6 as an internal control. Results were calculated by the 2−ΔΔCt method and normalized to a percentage of U6.
Immune Cell Isolation
NKT cells and CD8+ T cells were purified by the magnetic cell sorting (MACS) with commercial reagent kits following the manufacturer's instructions. The purity of the cells was greater than 98% as assessed by flow cytometry.
Generation of IL-6+ IL-10+ NKT Cells
NKT cells were isolated from the peripheral blood of healthy subjects and cultured in RPMI1640 medium in the presence of miR-92a (5 μg/ml), IL-2 (20 ng/ml), IL-15 (20 ng/ml). The medium was changed every 3 days. Cells were collected on day 9 to be used in further experiments. The frequency of IL-6+ IL-10+ NKT cells was greater than 90% as checked by flow cytometry.
Assessment of DNA Demethylation of the IL-6 and IL-10 Promoters
The total DNA was extracted from NKT cells using a DNA extraction reagent kit and treated with bisulfite to convert unmethylated cytosine residues to uracil. The methylation-specific PCR was performed on a real-time PCR device with the SYBR Green master mix and the following primers. IL-6 promoter, methylated primers: Forward, GATGTTTGAGGTTTATTTTGTTTTC; reverse, AAAACCTACCTCTACTACTAACGCC. Demethylated primers: forward, TGTTTGAGGTTTATTTTGTTTTTGA; reverse, AAAACCTACCTCTACTACTAACACC. IL-10 promoter primers, methylated: Forward, TTTGGAATATATTTTGTGATTTCGT; reverse, TCAACTATAAATTCTCATTCGCGTA. Demethylated: TTTGGAATATATTTTGTGATTTTGT; reverse, CCCTCAACTATAAATTCTCATTCACA. β-actin: Forward, catccgcaaagacctgtacg; reverse, cctgcttgctgatccacatc. Results were calculated with the 2−ΔΔCt method and normalized to a percentage of the internal control β-actin.
Enzyme-linked Immunosorbent Assay (ELISA)
The cytokine levels were determined by ELISA with commercial reagent kits following the manufacturer's instructions.
Apoptotic Assay
Cells were stained with an annexin V reagent kit and analyzed by flow cytometry.
Immune Suppression Assay
CD8+ CD25− T cells were isolated from the peripheral blood of healthy subjects, stained with carboxyl fluoroscein succinimidyl ester (CFSE), and cultured with the IL-6+ IL-10+ NKT cells at a ratio of 1:1 for 3 days in the presence of anti-CD3/CD28 antibodies. Cells were collected at the end of the culture and analyzed by flow cytometry (the CFSE-dilution assay).
Statistics
The data are presented as mean ± S.D. Differences between groups were determined by Student's t test or ANOVA if there were more than two groups. p < 0.05 was set as a significance criterion.
RESULTS
IL-10+ NKT Cells in Glioma Tissue
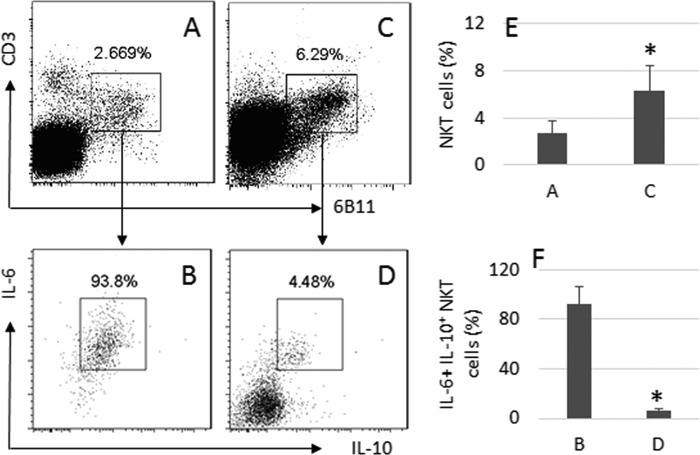
Surgically removed glioma tissue was collected from 12 patients with glioma. Single cells were prepared with the tissue and analyzed by flow cytometry. CD3+ 6B11+ NKT cells were gated from the single cells (Fig. 1, A and E), in which about 92.4% cells were IL-6+ IL-10+ (Fig. 1, B and F). We then analyzed the peripheral CD3+ T cells from the same patients (Fig. 1, C and E). The results showed that only 5.79% peripheral CD3+ 6B11+ NKT cells expressed IL-6 and IL-10 (Fig. 1, D and F). Results implicate that the environment of glioma tissue facilitates the differentiation of IL-6+ IL-10+ NKT cells.
FIGURE 1.
Frequency of IL-10+ NKT cells in glioma. Surgically removed glioma tissue was collected from 12 patients. Single cells were prepared from the tissue and analyzed by flow cytometry. A and C, gated dot plots indicate the frequency of NKT cells. B and D, dot plots indicate the frequency of IL-6+ IL-10+ NKT cells in the gated NKT cells of A and C. E and F, bars indicate the frequency of IL-10+ NKT cells of A and C (E), or B and D (F). The data are presented as mean ± S.D. *, p < 0.01, compared with A (E) or B (F). Samples from individual patients were processed separately. Data are representative of 12 independent experiments.
Glioma Cells Express miR-92a
Based on published data that miR-92a is associated with the pathogenesis of cancer (9), we hypothesize that glioma cells produce miR-92a to induce the IL-6+ IL-10+ NKT cells. To test the hypothesis, we analyzed the expression of miR-92a in glioma cells (including U87 cells and primary glioma cells). Results showed that the miR-92a was detectable in glioma cells, but at low levels. We then nonspecifically activated glioma cells by PMA. Results showed that the expression of miR-92a in NKT cells was markedly enhanced. Since glioma cells express several Toll-like receptors (TLR), we added SEB, or poly I:C, or LPS, to the glioma cell culture to activate the cells, which dramatically enhanced the expression of miR-92a in glioma cells (Fig. 2). Results suggest that glioma cells produce miR-92a that can be up-regulated by activation.
FIGURE 2.
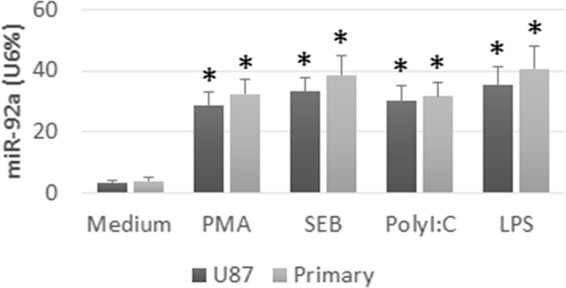
Levels of miR-92a in glioma cells. U87 cells and primary glioma cells (primary) were cultured in medium alone, or in the presence of PMA (50 ng/ml), or SEB (20 ng/ml), or polyI:C (30 ng/ml), or LPS (100 ng/ml), for 24 h, and analyzed by RT-qPCR. Bars indicate the levels of miR-92a in glioma cells. Data are presented as mean ± S.D. *, p < 0.01, compared with the medium group. Data are representative of three independent experiments.
Glioma Cells Induce IL-6+ IL-10+ NKT Cells
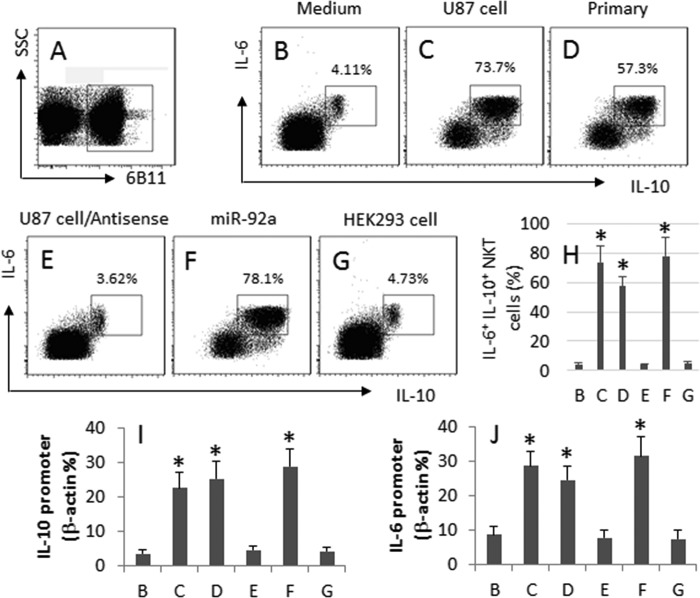
Based on the results of Fig. 1, we hypothesize that glioma cells induce the IL-6+ IL-10+ NKT cells. To test the hypothesis, we isolated 6B11+ NKT cells from PBMC of healthy volunteers; the NKT cells were cultured with U87 cells (a glioma cell line) for 6 days. The cells were analyzed by flow cytometry. The NKT cells (Fig. 3, A and H) were gated and further analyzed. Results showed that the culture of NKT cells with medium alone did not apparently induce IL-6+ IL-10+ NKT cells (Fig. 3, B and H). Culture with U87 cells (Fig. 3, C and H) or primary glioma cells (Fig. 3, D and H) markedly increased the frequency of the IL-6+ IL-10+ NKT cells, which was abolished by the presence of the antisense of miR-92a (Fig. 3, E and H). To strengthen the results, we added miR-92a to the culture, which markedly increased the frequency of IL-6+ IL-10+ NKT cells (Fig. 3, F and H). Used as a control, we cultured peripheral NKT cells with HEK293 cells, which did not induce any IL-6+ IL-10+ NKT cells (Fig. 3, G and H). Results indicate that glioma cells can induce IL-6+ IL-10+ NKT cells via miR-92a.
FIGURE 3.
Glioma cells induce IL-10 expression in NKT cells. The 6B11+ NKT cells were isolated from healthy subjects (the purity was >98%) and cultured in the presence of PMA (50 ng/ml); the additional treatment is denoted above the histograms. Cells were analyzed by flow cytometry. A, gated cells are NKT cells. B–F, histograms indicate the frequency of IL-6+ IL-10+ cells in the gated NKT cells in panel A. G, bars indicate the summarized data of B–F. I and J, bars indicate the demethylated DNA levels of the IL-10 promoter (I) and IL-6 promoter (J) that were determined by methylation-specific PCR. Data are presented as mean ± S.D. *, p < 0.01, compared with group B. Data are representative of three independent experiments.
To take a further insight into the mechanism of the induction of the IL-6+ IL-10+ NKT cells, we assessed the demethylation of the promoter of IL-6 and IL-10 in the NKT cells after the procedures described in Fig. 3, B--G. Results showed that the levels of the demethylated promoter DNA were correlated to the frequency of the IL-6+ IL-10+ NKT cells (Fig. 3, I and J).
Antitumor Capability Is Suppressed in IL-10+ NKT Cells
We then analyzed the antitumor cytokines in the IL-6+ IL-10+ NKT cells. IL-6+ IL-10+ NKT cells and control NKT cells were exposed individually to α-GalCer in culture for 48 h. As analyzed by flow cytometry, the levels of antitumor cytokines, including perforin, FasL, and IFN-γ, were significantly lower in IL-6+ IL-10+ NKT cells than control NKT cells (Fig. 4A). Results indicate that the expression of antitumor cytokines is suppressed in IL-6+ IL-10+ NKT cells and suggest that the antitumor capability of the cells may be attenuated or lost. To test this inference, we stained the above cells with annexin V reagent; the cells were analyzed by flow cytometry. Results showed that the activated NKT cells induced about 60% glioma cell apoptosis while only 5% glioma cells were apoptotic after culture with IL-6+ IL-10+ NKT cells, which was similar to the glioma cells cultured with medium alone (Fig. 4B). These results indicate that the IL-6+ IL-10+ NKT cells do not conserve the antitumor functions.
FIGURE 4.
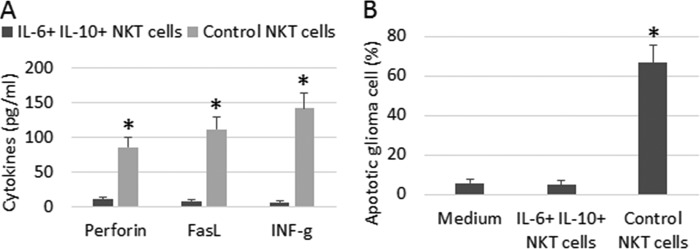
IL-6+ IL-10+ NKT cells show less antitumor capability. IL-6+ IL-10+ NKT cells and control NKT cells were prepared as described in the text. The two cell populations were cultured individually (106 cells/ml) in the presence of α-GalCer for 48 h. A, bars indicate the levels of antitumor cytokines (as denoted on the x axis) in the culture supernatant. B, U87 cells were cultured with either IL-6+ IL-10+ NKT cells or control NKT cells (at a ratio of 1:1) in the presence of α-GalCer for 48 h. Cells were analyzed by flow cytometry. Bars indicate the frequency of annexin V+ glioma cells. Data are presented as mean ± S.D. *, p < 0.01, compared with the group of IL-6+ IL-10+ NKT group (A), or medium group (B). Data are representative of three independent experiments.
Glioma-derived NKT Cells Have Immune Suppressor Functions
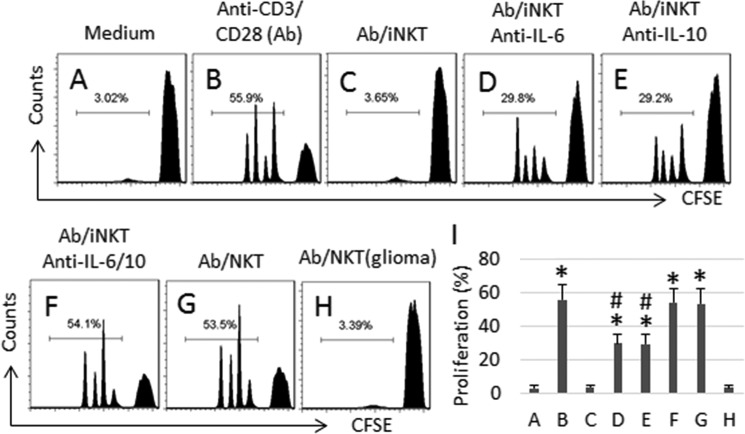
Based on the above results, we infer that the IL-6+ IL-10+ NKT cells have immune suppressor functions. To test the hypothesis, we cultured IL-6+ IL-10+ NKT cells with CD8+ CD25− T cells. Results showed that the presence of IL-6+ IL-10+ NKT cells significantly suppressed the anti-CD3/CD28 Ab-induced CD8+ T cell proliferation (Fig. 5, A–C, and I), which was attenuated by the addition of neutralizing antibodies of IL-6 (Fig. 5, D and I) or IL-10 (Fig. 5, E and I), and abolished by the addition of both anti-IL-6 and anti-IL-10 antibodies (Fig. 5, F and I). The control NKT cells (NKT cells isolated from healthy subjects and activated by α-GalCer) did not affect the anti-CD3/CD28-induced CD8+ T cell proliferation (Fig. 5, G and I). To strengthen the results, we isolated NKT cells from glioma tissue; NKT cells also suppressed the anti-CD3/CD28 Ab-induced CD8+ T cell proliferation. Results suggest that the IL-6+ IL-10+ NKT cells have immune suppressor functions.
FIGURE 5.
IL-6+ IL-10+ NKT cells suppress CD8+ T cell activities. CD8+ CD25− T cells were isolated from PBMC of healthy subjects, labeled with CFSE, and culture with the α-GalCer-activated control NKT cells (NKT) or IL-6+ IL-10+ NKT cells (iNKT) for 3 days at a ratio of 106 cells/106 cells (CD8 T cell/NKT cell)/sample. The additional treatment is denoted above each histogram. A–H, the histograms indicate the frequency of CD8+ T cell proliferation. I, bars indicate the summarized data of A–H. Dose of anti-IL-6 (1 μg/ml). Dose of anti-IL-10 (1 μg/ml). NKT (glioma): NKT cells were isolated from glioma tissue. Data are presented as mean ± S.D. *, p < 0.01, compared with group A. #, p < 0.01, compared with group F. Data are representative of three independent experiments.
DISCUSSION
It is proposed that tumor-specific tolerance contributes to tumor survival; the development of tumor tolerance is not fully understood yet. The present study has provided novel evidence to show that a novel fraction of NKT cells has immune suppressor functions on CD8+ T cell activities. The IL-6+ IL-10+ NKT cells show low levels of antitumor cytokines and do not induce glioma cell apoptosis. Glioma cells can induce the expression of IL-6+ IL-10 in NKT cells in which miR-92a plays a critical role.
The tumor immune tolerance has been recognized for a long time; it plays a critical role in the tumor escaping from the immune surveillance. The cellular components of the tumor immune tolerance mainly include regulatory T cells (10), regulatory B cells (11), and macrophages (12). The present study adds novel information to this point by showing that the intraglioma NKT cells also have the immune suppressive feature. Similar data have been reported by other investigators. Sag et al. indicate that, after activation, NKT cells express IL-10; the IL-10+ NKT cells have immune suppressor functions (13). Our data show that the glioma-derived NKT cells not only express IL-10, but more than 90% cells also express IL-6. The fact suggests that the glioma-derived NKT cells are different from those reported by Sag et al. (13).
NKT cells have miscellaneous functions; one of which is the antitumor function by releasing a number of antitumor cytokines, including those from Th1 cells, Th2 cells, and cytotoxic CD8+ T cells. Our studies indicate that the present data show that glioma-derived NKT cells also express IL-6; more than 90% glioma-derived NKT cells are IL-6+ IL-10+. Since IL-10 is an immune suppressive cytokine, we tested the immune suppressive function on CD8+ T cell proliferation. Although the expected suppressive effect on CD8+ T cell proliferation, blocking either IL-10 or IL-6 only partially attenuated the suppressive effect, which was almost completely suppressed in the presence of both anti-IL-6 and anti-IL-10 antibodies. Results were supported by the data of the IL-6 and IL-10 promoter demethylation. Others also have noted that IL-6 was involved in the pathogenesis of glioma (14).
Cumulative reports indicate that miRs are involved in the pathogenesis of cancer and glioma. Li et al. indicate that a core module comprising 14 miRNAs and five pathways that can predict the survival of glioma patients and represent potential targets for glioma therapy (15). Wang et al. report that overexpression of miR-143 decreased glioma cell migration, invasion, tube formation, and slowed tumor growth and angiogenesis and propose that overexpression of miR-143 may be a novel remedy in the treatment of glioma (16). Our study reveals another aspect that miR-92a is also involved in the pathogenesis of glioma by inducing IL-6+ IL-10+ NKT cells; the latter suppresses antitumor cells such as cytotoxic CD8+ T cells.
In summary, the present data show that there is a novel fraction of IL-6+ IL-10+ NKT cells in glioma tissue. The glioma-derived miR-92a is capable of inducing differentiation of the IL-6+ IL-10+ NKT cells. This fraction of NKT cells shows immune suppressive effects on CD8+ T cell proliferation.
This work was supported by the National Natural Science Foundation of China (No. 81360367), The Guangxi Provincial University Science and Technology Research Projects (2013ZD046), the Health Department of Guangxi Traditional Chinese Medical Science and Technology Special Project (QT2013025), and the development project of Guangxi Provincial Key Laboratory of Molecular Medicine of Liver Injury and Repair (*SYS2013009).
- NKT
- natural killer T
- miR
- micro RNA
- MACS
- magnetic cell sorting
- PMA
- phorbol myristate acetate.
REFERENCES
- 1. Janz C., Buhl R. (2014) Astroblastoma: Report of two cases with unexpected clinical behavior and review of the literature. Clin. Neurol. Neurosurg. 125, 114–124 [DOI] [PubMed] [Google Scholar]
- 2. Ye Z. P., He H. Y., Wang H., Li W. S., Luo L., Huang Z. C., Guo Y. (2014) Glioma-Derived ADAM10 Induces Regulatory B Cells to Suppress CD8+ T Cells. PLoS ONE 9, e105350. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 3. Berzins S. P., Ritchie D. S. (2014) Natural killer T cells: drivers or passengers in preventing human disease? Nat. Rev. Immunol. 14, 640–646 [DOI] [PubMed] [Google Scholar]
- 4. Hegde S., Fox L., Wang X., Gumperz J. E. (2010) Autoreactive natural killer T cells: promoting immune protection and immune tolerance through varied interactions with myeloid antigen-presenting cells. Immunology 130, 471–483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. O'Connell R. M., Rao D. S., Chaudhuri A. A., Baltimore D. (2010) Physiological and pathological roles for microRNAs in the immune system. Nat. Rev. Immunol. 10, 111–122 [DOI] [PubMed] [Google Scholar]
- 6. Skinner J. P. J., Keown A. A., Chong M. M. W. (2014) The miR-17–92a Cluster of MicroRNAs Is Required for the Fitness of Foxp3+ Regulatory T Cells. PLoS ONE 9, e88997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Hanin G., Shenhar-Tsarfaty S., Yayon N., Hoe Y. Y., Bennett E. R., Sklan E. H., Rao D., Rankinen T., Bouchard C., Geifman-Shochat S., Shifman S., Greenberg D. S., Soreq H. (2014) Competing targets of microRNA-608 affect anxiety and hypertension. Hum. Mol. Genet. 23, 4569–4580 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Xie N., Cui H., Banerjee S., Tan Z., Salomao R., Fu M., Abraham E., Thannickal V. J., Liu G. (2014) miR-27a Regulates Inflammatory Response of Macrophages by Targeting IL-10. J. Immunol. 193, 327–334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Li M., Guan X., Sun Y., Mi J., Shu X., Liu F., Li C. (2014) miR-92a family and their target genes in tumorigenesis and metastasis. Exp. Cell Res. 323, 1–6 [DOI] [PubMed] [Google Scholar]
- 10. Quezada S. A., Peggs K. S., Simpson T. R., Allison J. P. (2011) Shifting the equilibrium in cancer immunoediting: from tumor tolerance to eradication. Immunol. Rev. 241, 104–118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Biragyn A., Lee-Chang C., Bodogai M. (2014) Generation and identification of tumor-evoked regulatory B cells. Methods Mol. Biol. 1190, 89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Pan Y. F., Tan Y. X., Wang M., Zhang J., Zhang B., Yang C., Ding Z. W., Dong L. W., Wang H. Y. (2013) Signal regulatory protein α is associated with tumor-polarized macrophages phenotype switch and plays a pivotal role in tumor progression. Hepatology 58, 680–691 [DOI] [PubMed] [Google Scholar]
- 13. Sag D., Krause P., Hedrick C. C., Kronenberg M., Wingender G. (2014) IL-10GÇôproducing NKT10 cells are a distinct regulatory invariant NKT cell subset. J. Clin. Invest. 124, 3725–3740 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Yin Z., Lu G., Xiao Z., Liu T., He X., Wang Q., Lin C., Zhang S. (2014) Antitumor efficacy of argon-helium cryoablation-generated dendritic cell vaccine in glioma. Neuroreport. 25(4):199–204 [DOI] [PubMed] [Google Scholar]
- 15. Li R., Li X., Ning S., Ye J., Han L., Kang C., Li X. (2014) Identification of a Core miRNA-Pathway Regulatory Network in Glioma by Therapeutically Targeting miR-181d, miR-21, miR-23b, beta Catenin, CBP, and STAT3. PLoS ONE 9, e101903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Wang L., Shi Z. M., Jiang C. F., Liu X., Chen Q. D., Qian X., Li D. M., Ge X., Wang X. F., Liu L. Z., You Y. P., Liu N., Jiang B. H. (2014) MiR-143 acts as a tumor suppressor by targeting N-RAS and enhances temozolomide-induced apoptosis in glioma. Oncotarget 5, 5416–5427 [DOI] [PMC free article] [PubMed] [Google Scholar]