Background: Malaria parasites require heme for growth.
Results: Genetic disruption of the P. falciparum heme biosynthesis pathway ablated growth in mosquitoes but had no effect on blood-stage growth.
Conclusion: The heme biosynthesis pathway is only essential for exoerythrocytic parasite growth and transmission to mosquitoes.
Significance: Pathway inhibition is unlikely to be an effective antimalarial drug strategy. Heme salvage mechanisms likely exist in blood stages.
Keywords: Heme, Insect, Malaria, Mitochondria, Mitochondrial Metabolism
Abstract
Heme is an essential cofactor for aerobic organisms. Its redox chemistry is central to a variety of biological functions mediated by hemoproteins. In blood stages, malaria parasites consume most of the hemoglobin inside the infected erythrocytes, forming nontoxic hemozoin crystals from large quantities of heme released during digestion. At the same time, the parasites possess a heme de novo biosynthetic pathway. This pathway in the human malaria parasite Plasmodium falciparum has been considered essential and is proposed as a potential drug target. However, we successfully disrupted the first and last genes of the pathway, individually and in combination. These knock-out parasite lines, lacking 5-aminolevulinic acid synthase and/or ferrochelatase (FC), grew normally in blood-stage culture and exhibited no changes in sensitivity to heme-related antimalarial drugs. We developed a sensitive LC-MS/MS assay to monitor stable isotope incorporation into heme from its precursor 5-[13C4]aminolevulinic acid, and this assay confirmed that de novo heme synthesis was ablated in FC knock-out parasites. Disrupting the FC gene also caused no defects in gametocyte generation or maturation but resulted in a greater than 70% reduction in male gamete formation and completely prevented oocyst formation in female Anopheles stephensi mosquitoes. Our data demonstrate that the heme biosynthesis pathway is not essential for asexual blood-stage growth of P. falciparum parasites but is required for mosquito transmission. Drug inhibition of pathway activity is therefore unlikely to provide successful antimalarial therapy. These data also suggest the existence of a parasite mechanism for scavenging host heme to meet metabolic needs.
Introduction
Heme is a prosthetic group consisting of a large heterocyclic protoporphyrin IX ring with an iron atom coordinated at its center. Numerous hemoproteins exploit the redox properties of heme to fulfill a variety of biological functions, such as electron transport, gas synthesis and transport, drug detoxification, and defense against reactive oxygen species (1–3). Because of its ubiquitous presence in living organisms and its pivotal biological roles, heme is therefore an essential molecule for almost all life forms. Conversely, free heme can be toxic to cells, as its redox-active iron can catalyze the formation of peroxide. Consequently, acquisition of an adequate, but not excessive, amount of heme is a challenge for all heme-requiring organisms.
Malaria remains a huge public health problem in the tropical and subtropical regions of the world, causing ∼300 million clinical cases and about 600,000 deaths each year (4). Plasmodium falciparum causes the most severe type of malaria in humans. Although malaria parasites appear to have fewer heme-requiring proteins than other higher eukaryotes (5), heme is a cofactor for cytochromes in the essential mitochondrial electron transport chain (mtETC)5 (6, 7) and it is therefore indispensable for parasite growth.
While growing within erythrocytes, malaria parasites digest up to 80% of the host cell hemoglobin (Hb). This massive catabolic process liberates enormous amounts of the chemically reactive heme cofactor, which parasites must neutralize to avoid the toxic side effects of its accumulation. It has been estimated that the concentration of free heme inside the parasite food vacuole would reach about 400 mm, if it were allowed to accumulate (8). To overcome the toxicity of free heme, malaria parasites crystallize heme by forming iron-carboxylate bonds between two heme molecules in a repeating arrangement, yielding inert hemozoin crystals. Hemozoin formation seems to be the dominant process used by malaria parasites to detoxify heme, as >95% of the heme released from Hb digestion is estimated to end up in hemozoin (9). Although a heme oxygenase (HO) homologue was found in P. falciparum, it lacks key catalytic residues and does not degrade heme (10). Interference with hemozoin formation appears to be the mechanism by which many antimalarials, such as chloroquine, exert their antiparasitic activities (11, 12). This mode of action may suggest that generation of substantial free heme is detrimental to parasite survival.
The Plasmodium genome encodes all enzymes to comprise a complete heme biosynthetic pathway capable of making heme using glycine and succinyl-CoA as initial substrates (Fig. 1). This pathway in Apicomplexan parasites appears to be the evolutionary condensation of the two well known heme biosynthesis pathways (5) as follows: the Shemin pathway spanning the mitochondrion and cytosol (13–15), and the C5 pathway common in plastids (16). In Apicomplexans, 5-aminolevulinic acid (5-ALA) is exclusively synthesized in the mitochondria by the Shemin pathway and not by the C5 pathway. In P. falciparum, a total of eight enzymes in this pathway are located in three different compartments as follows: the mitochondrion (three enzymes) (17–19), the cytoplasm (one enzyme) (20), and the apicoplast (four enzymes) (21–23). ALAS is the first enzyme of the pathway, and in many organisms its activity is rate-limiting and tightly regulated. FC is the last enzyme and inserts a ferrous ion into the protoporphyrin ring to form the final heme product. Both ALAS and FC have been localized to the parasite mitochondrion (17, 19). Because hemozoin formation in the food vacuole appears to be the dominant fate of host heme, it has been assumed that heme released by Hb digestion is not available for scavenge and metabolic utilization by parasites within its own hemoproteins. Rather, blood-stage P. falciparum parasites have been assumed to synthesize heme de novo to satisfy critical metabolic needs. This heme biosynthesis pathway has therefore been considered essential and a potential drug target (24–27).
FIGURE 1.
Hybrid heme biosynthesis pathway in P. falciparum. This pathway is composed of eight enzymes that have been localized to the mitochondrion, the cytosol, and the apicoplast. The substrates and products of the pathway are in black. The enzymes involved are in red. The fraction of the pathway labeled by [13C]ALA is in blue, and the compounds detected by LC-MS/MS are boxed. The molecular structures of ALA, CPP III, PPIX, and heme are shown. ALAS, 5-aminolevulinic synthase; ALAD, 5-aminolevulinic acid dehydratase; PBGD, porphobilinogen deaminase; UROS, uroporphyrinogen III synthase; UROD, uroporphyrinogen decarboxylase; CPO, coproporphyrinogen oxidase; PPO, protoporphyrinogen oxidase; FC, ferrochelatase. This figure was adapted from Ref. 44.
A recent study reported successful ablation of the ALAS and FC genes in the rodent malaria parasite, Plasmodium berghei (28). Unlike P. falciparum, P. berghei parasites preferentially invade metabolically active reticulocytes, which also synthesize heme (29, 30). Therefore, Nagaraj et al. (28), who assessed heme biosynthesis by monitoring incorporation of [14C]ALA into heme by autoradiography, were unable to show that de novo heme synthesis was disrupted in the knock-out parasites due to host cell background. This complication prevented a definitive conclusion regarding the function of these genes and the essentiality of heme biosynthesis for blood-stage parasite growth.
Here, we report the successful knock-out of the ALAS and FC genes in the human malaria parasite, P. falciparum. Using 13C labeling and tandem mass spectrometry, we confirmed that heme biosynthesis is ablated in the FC KO parasites. Despite the absence of de novo heme synthesis, these KO parasites displayed no detectable defects in blood-stage growth but failed to progress through mosquito stages. We conclude that heme biosynthesis in P. falciparum is dispensable during blood-stage infection but plays a critical role within the insect host.
EXPERIMENTAL PROCEDURES
Plasmid Construction
Strategies of gene KO by double crossover recombination for ALAS (PF3D7_1246100) and FC (PF3D7_1364900) are illustrated in Fig. 2, A and B. For knock-out of ALAS, two flanking homologous sequences were cloned into the pCC1 plasmid, which contains human dihydrofolate reductase and confers resistance to WR99210 (5 nm) (31). For knock-out of FC, the pCC4 plasmid was used, which possesses blasticidin-S deaminase and confers resistance to blasticidin (2.5 μg/ml) (31). Both pCC1 and pCC4 share the same negative selection marker, the yFCU gene (the fusion gene of yeast cytosine deaminase/uracil phosphoribosyltransferase), which confers sensitivity to 5-fluorocytosine (5-FC, 2.0 μm). All PCR products used for making KO constructs were sequenced (GeneWiz) prior to final cloning steps. Primers for PCR amplifications are shown in Table 1.
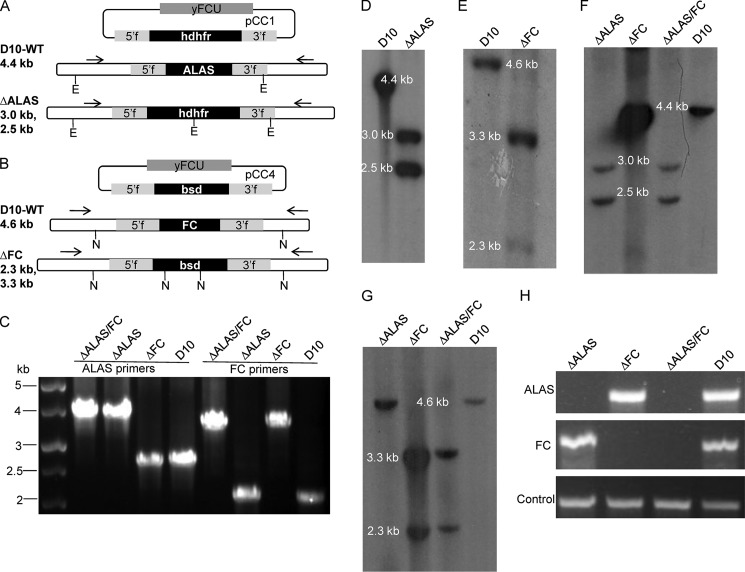
FIGURE 2.
Disruption of ALAS and FC in P. falciparum. A, genotypes of D10 WT and ΔALAS parasites. The 5′-homologous sequence is 851 bp long (−243 to +607) and the 3′-homologous sequence is 750 bp long (+1489 to +2238). B, genotypes of D10 WT and FC KO parasites. The 5′-homologous sequence has 553 nucleotides (−162 to +390) and the 3′-homologous sequence has 805 nucleotides (+944 to +1748). In both A and B, arrows indicate primers used for diagnostic PCRs in C. E, EcoRI; N, NdeI. C, diagnostic PCRs assessing the genotypes of ΔALAS, ΔFC, and ΔALAS/FC KO parasites. Results of PCRs using ALAS primers in A are shown in lanes 1–4, and FC primers in B are shown in lanes 5–8. Lanes 1 and 5, ΔALAS/FC; lanes 2 and 6, ΔALAS; lanes 3 and 7, ΔFC; lanes 4 and 8, D10 WT control. D–G, Southern blot analyses confirm the genotypes for ΔALAS (D), ΔFC (E), and ΔALAS/FC (F and G) parasites. DNA digestion pattern are illustrated in A and B. The full-length genes of ALAS or FC were used for synthesizing 32P probes. H, RT-PCR analyses reveal that ALAS or FC transcripts are absent in the corresponding KO parasites. Primers are shown in Table 1.
TABLE 1.
Primers used in this study
| Primer ID | Primer sequencea | Purpose |
|---|---|---|
| PfALAS5fNcoI | TccatggGTAGACTAATATTTAACAATTTGTATTTAC | KO construct for ALAS |
| PfALAS5fEcoRIR | CTgaattcCATAAAATGAAGGTAACATTA | |
| PfALAS3fSpeIF | GTactagtACTCCAGTTAATATTAATACGTCTG | |
| PfALAS3fSacIIR | AccgcggTGAATTTATTAAATCCATCTACCATG | |
| PfALASKO_F | GGCATACCAAATTAATCTCCACTTG | Diagnostic PCR for ALAS |
| PfALASKO_R | CAAAGATTATTAAAATTATTTTGTCATCTTGTTC | |
| 5fFFCNcoI | ATccatggCAATTTGTTTTGCTCCTCTCC | KO construct for FC |
| 5fRFCEcoRI | ATgaattcTATTTGTACCACTCCTGAAAGG | |
| 3fFFCSpeI | CTactagtGGTACAATAACAAAGTTGTC | |
| 3fRFCSacII | ATccgcggCGAAGGGAATATATAAAACC | |
| 5foutFC | CGGATATTTGCTCTTCTTGT | Diagnostic PCR for FC |
| 3foutFC | TTATCATACGATGACATATATGAATG | |
| PfALASRT01 | AAAAACGGTTGTGTGGTGTTC | RT-PCR for ALAS |
| PfALASRT02 | CGATCATAATTTTTCGGTTTTCA | |
| PfFCRT01 | CAAATTTAGGAAGCCCAGAAAA | RT-PCR for FC |
| PfFCRT02 | TGGACCTTTCTCCATATCTCA |
a Restriction endonuclease sites are shown in lowercase letters.
Parasite Line, Parasite Culture, and Transfection
P. falciparum strains D10 and NF54 were the parental lines used for gene KO studies. Parasites were cultured in human O+ erythrocytes (5% hematocrit) in RPMI 1640 medium containing 0.5% AlbuMAX® (Invitrogen) and incubated at 37 °C in an incubator filled with a low oxygen gas mixture (89% N2, 5% CO2, 6% O2). The human red blood cells used for parasite culture were purchased from Interstate Blood Bank, Inc. (Memphis, TN). Transfections were carried out using the standard method (32). Briefly, ring-stage parasites at 5% parasitemia were washed three times with warm Cytomix, and then the parasitized RBC pellet was resuspended in an equal volume of ice-cold Cytomix to 50% hematocrit. Each 250 μl of parasitized RBC suspension was mixed with 50 μg of plasmid in a 0.2-cm cuvette and electroporated using a Bio-Rad Genepulser set at 0.31 kV, 960 microfarads. Cultures were exposed to appropriate drugs 48 h post-transfection.
Gene Knock-out
We transfected D10 and NF54 parasites with each KO construct and performed positive and negative selections to generate resistant parasites. Following transfection and positive drug selection, a portion of the transgenic culture was treated with 5-FC (2.0 μm) to isolate potential KO parasites. If initial treatment with 5-FC was unsuccessful, multiple drug off-and-on cycles followed by 5-FC selection were undertaken until double crossover recombination was observed.
DNA and RNA Isolation
DNA was isolated from mixed-stage parasites using the QIAamp DNA blood mini kit (Qiagen). RNA was isolated from mixed-stage parasites using the RNAgents RNA isolation kit (Promega). Purified RNA was treated with RNase-free DNase I (New England Biolabs) in the presence of RNase inhibitor (Promega) for 30 min at 37 °C to eliminate any contaminating DNA. The treated RNA was then recovered using an RNeasy mini kit (Qiagen). cDNA was synthesized from 2 μg of RNA using avian myeloblastosis virus reverse transcriptase (Promega) using oligo(dT)15 as primers. Two μl of cDNA were used as template for one PCR. Primers for PCR and RT-PCR are shown in Table 1.
Southern Blot Analysis
DNA was isolated as described above. For each parasite line, 3 μg of genomic DNA was digested overnight using the specific restriction endonucleases (New England Biolabs). The DNAs were separated on a 0.8% agarose gel and transferred to Gene Screen Plus membrane (PerkinElmer Life Sciences) using the high salt capillary transfer method. Probes were PCR-amplified, cleaned, and labeled with [α-32P]dATP (PerkinElmer Life Sciences) using a Prime-It II random primer labeling kit (Stratagene). The blots were hybridized with the labeled probes, washed, and exposed to film at −80 °C in a cassette overnight.
Growth Curves
WT and KO parasites were synchronized with 0.5 m alanine, 10 mm HEPES (pH 7.5) once. On day 0, the parasitemias of WT and KO cultures (mostly at ring stages) were adjusted to 1% by diluting with uninfected RBCs. Parasitemias were monitored daily from at least 1000 RBCs on thin blood smears over 8 days (four intraerythrocytic development cycles). On days 2, 4, and 6, cultures were split 1:5.
Growth Inhibition Assays
All growth inhibition assays were performed using the conventional [3H]hypoxanthine incorporation method in 96-well plates (33). Briefly, parasites at 1% parasitemia and 2% hematocrit were exposed to inhibitors at various concentrations for 24 h. After 24 h, each well was pulsed with 0.5 μCi of [3H]hypoxanthine and incubated for another 24 h. Parasites were lysed by freeze/thaw. Nucleic acids were collected on filters using a cell harvester (PerkinElmer Life Sciences). After addition of MicroScint O (PerkinElmer Life Sciences), incorporation of [3H]hypoxanthine was quantified using a TopCount scintillation counter (PerkinElmer Life Sciences).
Stable Isotope Labeling and LC-MS/MS Detection of Heme and Porphyrin Intermediates
5-[13C4]ALA (only position 4 is labeled) (Cambridge Isotope Laboratories, Inc.) was added at a final concentration of 200 μm to cultures of WT and KO parasites (24 ml of culture volume, 3% hematocrit, 10% asynchronous parasitemia). Cultures were harvested 24 h later by centrifugation, and pellets were resuspended in 0.05% saponin and agitated on ice for 5 min to lyse RBC membranes. The saponin-released parasites were harvested by centrifugation, washed in PBS, and stored at −20 °C. For studies involving succinylacetone (Sigma), WT cultures were incubated 12 h in 50 μm (final concentration) succinylacetone prior to addition of 5-[13C4]aminolevulinic acid and maintained in succinylacetone until cultures were harvested as above. Each harvested parasite pellet was resuspended in 900 μl of DMSO, and 1 nmol of deuteroporphyrin (Frontier Scientific) was added as an internal standard. Samples were sonicated three times for 10 s (50% duty cycle, 40% power) on a Brandon Ultrasonic micro-tip sonicator, vortexed three times for 1 min, and centrifuged 10 min at 16,000 relative centrifugal force, and the clarified supernatant was transferred to a clean tube. Three or more biological replicates were prepared for each sample.
Liquid chromatography-tandem mass spectrometry (LC-MS/MS) analyses were carried out using an AB Sciex API-4000 QTRAP tandem mass spectrometer running Analyst version 1.5.1 coupled to a Shimadzu UFLC and operated in the positive ion mode using the Turbo V ESI ion source. 2 μl of each sample was injected onto an Ascentis Express phenyl-hexyl column (10 × 2.1 mm × 2.7 μm) and eluted via a linear gradient of 5–90% acetonitrile (including 0.1% formic acid). Blank runs were included between each sample to rule out carry-over between injections. The ion spray voltage was set to 4500 V. The heater temperature was 400 °C. The declustering potential, nebulizer gas (G1), and auxiliary gas (G2) were set at 110, 40, and 35 V, respectively. Fragmentation profiling of analytes was carried out using multiple reaction monitoring in the positive ion mode with precursor and product ions at their experimentally determined optimal values of m/z 511.2 and 452.0 for deuteroporphyrin, 616.4 and 557.6 for heme, 624.4 and 565.4 for [13C]heme, 563.3 and 503.8 for protoporphyrin IX, 571.4 and 511.8 for [13C]protoporphyrin IX, 655.2 and 597.0 for coproporphyrin III, and 663.8 and 605.0 for [13C]coproporphyrin III. Isolation widths for precursor and product ions were 0.7 and 1.1 atomic mass units, respectively. Collision energies were set at 55, 55, 65, and 70 for the deuteroporphyrins, hemes, protoporphyrins, and coproporphyrins, respectively. Analyte ion intensities were quantified by peak integration using the Analyst software and normalized to the peak area for the deuteroporphyrin internal standard in each sample.
Gametocyte Culture and Exflagellation
On day 0, NF54 lines (WT and ΔFC, asynchronous) were established at 0.5% parasitemia with 2.5% hematocrit in a 50-ml volume. The standard RPMI 1640 medium was supplemented with 10% human serum (type O, from Key Biologics, LLC (Memphis, TN)) and 0.25% AlbuMAX. The cultures were fed daily without adding new blood. On days 14–20, for each line, the total number of erythrocytes was determined by a hemocytometer. The total number of gametocytes in each culture was then calculated by multiplying gametocytemia by the total number of erythrocytes. On each of these days, 1 ml of the entire bulk culture was removed, centrifuged quickly, and resuspended in 200 μl of exflagellation medium containing 25% human serum (type O) and 50 μm xanthurenic acid (34) at pH 8.4 at room temperature. After 10 min of incubation at room temperature, a 10-μl cell suspension was pipetted onto a hemocytometer, and the number of exflagellating centers was counted using a standard optical microscope (×20 objective) over the next 20 min. The total number of exflagellating centers in each culture was then calculated, and the percentage of exflagellation was determined by dividing the total number of exflagellating centers by the total number of gametocytes in each culture.
Standard Membrane Feeding Assay
P. falciparum sexual stage cultures were generated, and the membrane feeding assays were performed as described previously (35). In brief, P. falciparum parasites were cultured for 16–18 days to induce mature gametocytes in vitro. The culture of 50% hematocrit in human serum was fed to ∼50 female Anopheles stephensi (3–6 days old) through a membrane-feeding apparatus. Mosquitoes were kept for 8 days and dissected (n = 20 per sample) to enumerate oocysts in their midguts. Only midguts from mosquitoes with any eggs at the time of dissection were analyzed.
RESULTS
The Heme Biosynthesis Pathway Is Dispensable in the Asexual Blood Stage
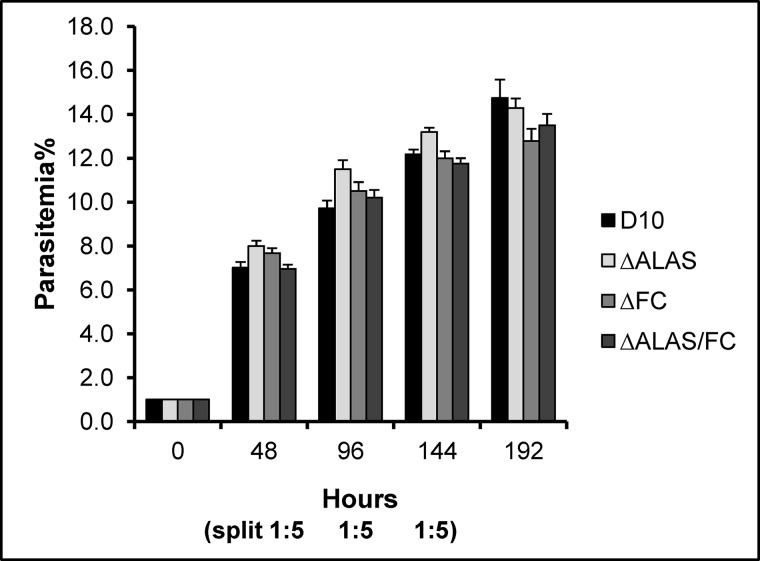
To study the heme synthesis pathway in P. falciparum, we carried out genetic disruptions of ALAS and FC via double crossover recombination and successfully obtained KO lines in the D10 WT strain (Fig. 2). To achieve a double KO line, the FC KO construct in pCC4 (containing blasticidin-S deaminase as the selectable marker) was transfected into the ΔALAS KO line, which retains the human dihydrofolate reductase marker from pCC1 (see “Experimental Procedures”). Diagnostic PCRs revealed that the three KO lines have the expected genotypes (Fig. 2C). The results from Southern blot analyses further confirmed the correct gene deletions in each individual KO line (Fig. 2, D–G). RT-PCR analyses using specific primers did not detect corresponding mRNA transcripts in the three KO lines (Fig. 2H). To examine the growth rate of these KO lines, we synchronized the cultures and monitored growth for four intraerythrocytic development cycles (see “Experimental Procedures”). The observed growth rates of the KO and WT parasite line were indistinguishable (Fig. 3), indicating that these enzymes are not essential in asexual blood stages of P. falciparum.
FIGURE 3.
The Heme biosynthesis pathway in P. falciparum is not essential in asexual blood stages. The WT and KO cultures were synchronized once and monitored for four intraerythrocytic development cycles. On days 2, 4, and 6, all cultures were split 1:5. Parasitemias of various parasite lines were determined by counting 1000 RBCs on thin blood smears independently three times at each time point. Data of parasitemias every 48 h are shown.
Direct Detection of Heme Biosynthesis in WT and KO Parasites Using Stable Isotope Labeling and LC-MS/MS
To probe heme biosynthesis activity in WT and KO parasites, we developed a sensitive liquid chromatography-tandem mass spectrometry (LC-MS/MS) assay to selectively monitor stable isotope labeling of heme and the upstream porphyrins, PPIX and CPP (see “Experimental Procedures”). Isotopic labeling was achieved by culturing parasites in 200 μm 5-[13C4]ALA. ALA is the first committed precursor in heme biosynthesis (Fig. 1). The metabolic condensation of eight 5-[13C4]ALA molecules to form the porphyrin macrocycle results in a stable 8-Da increase in the molecular mass of heme, PPIX, and CPP relative to unlabeled masses for these analytes, because no carbon atoms derived from the C4 carbon of ALA are lost from decarboxylations en route to heme (36). This mass increase can be used to specifically detect and distinguish newly synthesized heme from the huge background of host-derived unlabeled heme. CPP, although not a direct pathway intermediate of heme biosynthesis (Fig. 1), is produced from oxidation of the coproporphyrinogen III intermediate during sample extraction (37). CPP detection therefore serves as a biomarker for the labile coproporphyrinogen III intermediate.
The levels of heme, PPIX, and CPP in various samples were quantified based upon their collisionally induced dissociation ion fragmentation pathways (see “Experimental Procedures”). Each compound's product ion spectrum was determined using commercially available standards, and the dominant peaks corresponded to previously published values (data not shown) (37, 38). The [13C]porphyrins displayed LC retention times identical to the unlabeled commercial standards, with precursor and product ions shifted by the expected m/z values due to 13C labeling (data not shown). The dominant product ion for all three porphyrins, resulting from dissociation of an ethylcarboxyl side chain, was used to construct a targeted LC-multiple reaction monitoring analysis. Each porphyrin was then quantified by determining the ratio of its LC-multiple reaction monitoring peak area to that of the deuteroporphyrin internal standard.
As shown in Fig. 4A, growth of WT parasites in 200 μm 5-[13C4]ALA resulted in robust [13C]PPIX and -CPP signals and comparatively smaller levels of [13C]heme. Accumulation of PPIX and CPP relative to heme is consistent with prior reports that FC activity becomes rate-limiting with exogenously added ALA (39, 40), because this precursor is downstream of the normally rate-limiting initial reaction catalyzed by ALAS. No [13C]porphyrins were detected in the absence of 5-[13C4]ALA (data not shown).
FIGURE 4.
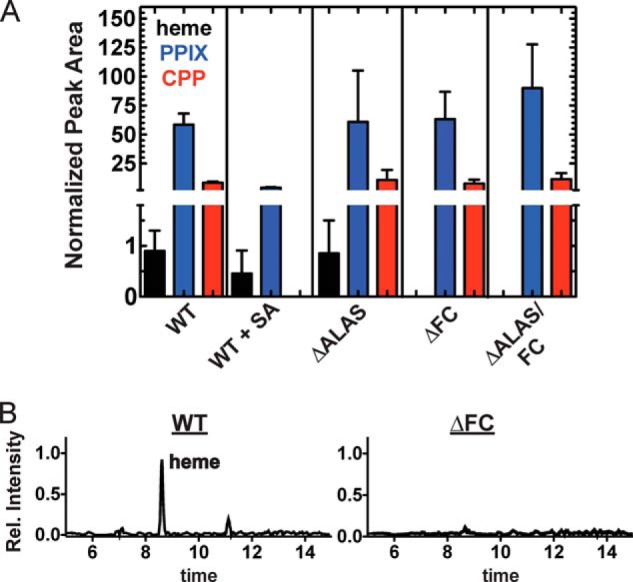
LC-MS/MS detection of [13C]heme, -PPIX, and -CPP in WT and KO P. falciparum parasites. Parasites were cultured in 200 μm 5-[4-13C]ALA in the absence or presence of 50 μm SA. Parasite samples were extracted in DMSO, supplemented with deuteroporphyrin as an internal standard, and analyzed by LC-MS/MS. Integrated analyte peak areas were normalized to the peak area measured for the internal standard in each sample. The average normalized peak area and the standard error from three or more independent replicates are shown in A. As explained in the text, CPP serves as a biomarker for detecting the labile coproporphyrinogen III, which rapidly oxidizes to CPP upon cell lysis and extraction. Based on unpaired t test, the detected levels of [13C]PPIX (p = 0.0016) and -CPP (p < 0.0001) in WT + SA parasites and [13C]heme (p < 0.01) in ΔFC and ΔALAS/FC parasites were significantly different from WT. B, representative mass spectra for detection of [13C]heme in WT and ΔFC samples. Spectra are normalized to the intensity of the heme peak in the WT spectrum.
We next tested the previously reported ability of 50 μm succinylacetone (SA) to inhibit heme biosynthesis in WT P. falciparum parasites (28). SA is a specific inhibitor of aminolevulinic acid dehydratase, the second enzyme in the heme biosynthesis pathway. WT parasites were incubated in 50 μm SA for 12 h prior to addition of 5-[13C4]ALA and maintained in SA until harvest. Although 50 μm SA treatment resulted in a substantial (>15-fold) reduction in labeled PPIX levels and no detectable CPP labeling, we observed residual labeling of heme (Fig. 4A), indicating that this inhibitor concentration substantially reduces but does not completely eliminate heme biosynthesis in parasites.
Finally, we probed the heme biosynthesis activity of the ΔALAS, ΔFC, and ΔALAS/FC parasites. Levels of labeled heme, PPIX, and CPP in the ΔALAS parasites were indistinguishable from WT parasites (Fig. 4A), as expected because 5-[13C4]ALA incorporation is downstream of ALAS (Fig. 1). In the case of the ΔFC and ΔALAS/FC parasites, however, we detected [13C]PPIX and -CPP but were unable to detect [13C]heme in three biological replicates of each sample (Fig. 4A), as expected if FC activity is absent. Fig. 4B shows representative mass spectra of [13C]heme in WT and ΔFC samples. We conclude that de novo heme synthesis is completely ablated in the ΔFC and ΔALAS/FC parasites.
WT and KO Parasites Are Equally Susceptible to Antimalarial Drugs That Potentially Alter Heme Metabolism
Several major classes of antimalarial drugs may affect heme metabolism, including chloroquine and artemisinin. Chloroquine is proposed to inhibit ferriprotoporphyrin IX crystallization by binding to heme directly (41) or to the growing hemozoin chains (42). Although the mode of action of artemisinin is still debated (43), one proposed mechanism involves the formation of toxic heme-artemisinin adducts by alkylation (44, 45). In addition to inhibiting heme crystallization, these drug-heme complexes might also limit the amount of free heme available for potential salvage mechanisms. We therefore investigated the possibility that heme biosynthesis of KO parasites, which presumably depend on the salvage pathway(s) to supply heme, might be hypersensitive to chloroquine and/or artemisinin. The results of standard 48-h growth inhibition assays are shown in Table 2. All three KO lines and D10 WT parasites were equally susceptible to chloroquine and artemisinin. These results suggest that chloroquine and artemisinin have no impact on the heme salvage capacity of P. falciparum.
TABLE 2.
The EC50 values of antimalarial drugs in the heme biosynthesis KO lines of P. falciparum
Data are the average of three independent [3H]hypoxanthine incorporation assays carried out according to the standard methods. For each compound, values of average ± S.D. are shown. EC50 indicates half-maximal effective concentration.
| Compound | Parasite line, EC50 (nm) |
|||
|---|---|---|---|---|
| D10 | ΔALAS | ΔFC | ΔALAS/FC | |
| Artemisinin | 8.9 ± 0.3 | 7.4 ± 0.3 | 8.6 ± 0.7 | 9.3 ± 0.9 |
| Chloroquine | 37.0 ± 4.0 | 33.3 ± 3.3 | 39.7 ± 1.5 | 29.9 ± 0.6 |
| Atovaquone | 0.8 ± 0.2 | 0.7 ± 0.2 | 0.9 ± 0.3 | 0.8 ± 0.4 |
In malaria parasites, heme is thought to be primarily required to serve as the prosthetic group of the cytochromes in the mtETC (5). To assess whether the ALAS and FC KO lines were deficient in their ability to incorporate heme into mitochondrial cytochromes, we tested whether these parasites had an increased sensitivity to atovaquone, a specific inhibitor of the mtETC cytochrome bc1 complex (6). As shown in Table 2, all three KO lines as well as WT parasites were equally sensitive to atovaquone (within experimental error), suggesting that the cytochrome bc1 complex remains functionally intact despite ablation of the parasite heme biosynthesis pathway. These data further support the argument that malaria parasites are able to salvage heme from the host.
Heme Biosynthesis Pathway Is Critical for Mosquito Transmission of Malaria Parasites
Transmission of malaria parasites from the asexual blood stage in humans to the mosquito vector requires parasite differentiation into male and female gametocytes, which get taken up by mosquitoes during a blood meal, differentiate into gametes, mate to form a zygote, develop into oocysts, and finally form sporozoites within the mosquito.
To test whether a functional heme biosynthesis pathway is required to form gametocytes, the FC gene was knocked out in the NF54 line of P. falciparum. Unlike the D10 line, the NF54 line is fully capable of forming gametocytes in vitro. NF54-ΔFC formed healthy gametocytes at all stages, with morphologies indistinguishable from their WT counterparts under light microscopy (Fig. 5A). As shown in Fig. 5B, the gametocytemia in the NF54-ΔFC line was quantitatively indistinguishable from that of the NF54 WT line, suggesting that the gametocytes in the knock-out line progressed normally over time. Importantly, the NF54-ΔFC line also produced the same number of mature stage V gametocytes as the WT parasites (Fig. 5C). These stage V gametocytes in NF54-ΔFC line appeared to be healthy in their morphologies. The male-to-female sex ratio of mature stage V gametocytes in NF54-ΔFC line was similar to that of its WT counterpart (data not shown). Although detailed biochemical characterizations of these stage V gametocytes (male and female) in the ΔFC line were not conducted in this study, they appeared to be mature based on their morphology and looked normal compared with the equivalent stage in the WT line. We then assessed male gamete formation in vitro by examining exflagellation centers following xanthurenic acid induction (34). Interestingly, we found that there was >70% reduction in exflagellation centers in NF54-ΔFC relative to NF54 WT parasites (Fig. 5D). The biochemical basis for this reduced exflagellation ability in the ΔFC line remains unknown at this point. Nevertheless, these data strongly suggest that the heme biosynthesis pathway is not required for gametocyte development and maturation, but it appears to be critical for male gamete formation. This conclusion will be tested in future studies that explore the mechanistic basis for the observed defect in male gamete formation and the steps in which that occurs.
FIGURE 5.
Effect of heme biosynthesis knock-out on gametocyte development in P. falciparum. A, representative Giemsa-stained images of NF54 WT (top row) and NF54-ΔFC (bottom row) gametocytes at various developmental stages are shown. EX, exflagellation. B, NF54-ΔFC parasites show a normal development of gametocytes in comparison with NF54 WT control. Gametocyte percentage is the fraction of gametocytes of all stages determined by counting 1000 RBCs. C, NF54-ΔFC line generates mature stage V gametocytes. Stage V percentage is the fraction of stage V gametocytes determined by counting 1000 RBCs. D, NF54-ΔFC line has a defect in male gamete formation. Exflagellation percentage is the fraction of exflagellating gametocytes determined from 1000 gametocytes of all stages. Blue bars, NF54 WT. Orange bars, NF54-ΔFC. B–D, results averaged from three independent experiments are shown (error bars = ±S.D.).
Although asexual parasites develop within the heme-rich environment of human erythrocytes, heme may be less accessible during development within mosquitoes. To assess the requirement for a heme biosynthesis pathway in mosquito stage parasites, mature gametocytes from NF54-ΔFC and WT parasites were fed to female A. stephensi mosquitoes. Oocyst numbers per mosquito midgut were determined 1 week after feeding. As shown in Fig. 6A, WT parasites produced 16 ± 11.7 oocysts per mosquito, but parasites of the NF54-ΔFC line failed to generate any oocysts. Representative images of mosquito midguts infected with WT or ΔFC parasites are shown in Fig. 6B. These data strongly suggest that a functional heme biosynthesis pathway is required for full parasite development within the insect vector.
FIGURE 6.

Heme de novo synthesis pathway is essential for P. falciparum transmission. A, number of oocysts per mosquito infected with NF54-WT or NF54-ΔFC. Data are derived from two independent feeding experiments. B, representative images of the midgut of a mosquito infected with NF54-WT (left) or NF54-ΔFC (right) parasites, respectively. Oocysts were stained using 0.05% mercurochrome and appear as red dots.
DISCUSSION
The de novo heme synthesis pathway in P. falciparum has been considered essential and a prospective antimalarial drug target for more than 20 years (24–27). This view has been based predominantly on prior reports that 1–2 mm succinylacetone, an inhibitor of aminolevulinic acid dehydratase (the second enzyme in the pathway), was lethal to the parasites (27, 46). The most direct test of the essentiality of a metabolic pathway, however, is to genetically knock out key enzymes in that pathway, confirm that metabolic flux through that pathway has been ablated by deletion of the targeted genes, and then assess the effect of these disruptions on cellular growth and viability. No prior knockouts of heme biosynthesis genes in P. falciparum have been reported. Our study is thus the first direct, comprehensive test of the heme biosynthesis pathway in human malaria parasites.
A recent study reported successful KO of the ALAS and FC genes in the related rodent malaria parasite, P. berghei, but the authors were unable to show that these mutations ablated de novo heme synthesis (28), most likely due to background heme biosynthetic activity from the metabolically active host reticulocytes (29, 30). This same study also revisited the effects of succinylacetone on the growth and heme biosynthesis activity of blood-stage P. falciparum. Whereas treatment of parasites with 1–2 mm drug was lethal, at just 50 μm parasites grew normally despite the apparent loss of heme biosynthesis, as assessed using autoradiography to monitor [14C]ALA incorporation into heme (28). In contrast, using the sensitive LC-MS/MS assay reported herein, we observed that residual [13C]ALA incorporation into heme and upstream porphyrin intermediates persisted at 50 μm succinylacetone despite substantial overall reduction in pathway flux (Fig. 4). Because available evidence suggests that parasites may only require trace amounts of heme to satisfy metabolic needs (5), it remained possible that the residual heme biosynthesis observed at 50 μm inhibitor might be sufficient to sustain parasite growth if pathway activities were essential. Because of these complexities, the requirement of parasite heme biosynthesis for P. falciparum blood-stage growth has remained unresolved.
As reported herein, we generated three heme biosynthesis KO lines in P. falciparum, disrupting ALAS and FC genes individually and in combination (Fig. 2). In asexual blood stages, the growth of these KO lines was indistinguishable from that of WT parasites (Fig. 3). Tandem mass spectrometry confirmed the absence of heme biosynthesis in the ΔFC and ΔALAS/FC parasites (Fig. 4). Thus, our results provide unequivocal evidence that the heme biosynthesis pathway is not essential for P. falciparum parasites in the asexual blood stage.
Our results further suggest that the previous parasite growth inhibition observed at 1–2 mm succinylacetone (27, 46) was due to off-target activity, rather than inhibition of heme biosynthesis. Loss of de novo heme synthesis in the ΔFC parasites also establishes the parasite-encoded FC enzyme as the sole source of FC activity in parasites, contradicting prior proposals that parasites might maintain a complete and parallel heme biosynthesis pathway composed of imported host enzymes (26, 47, 48). Indeed, mature erythrocytes lack mitochondria and thus are missing the host ferrochelatase (29).
Our results and those from prior studies using exogenous ALA provide strong evidence that the parasite heme biosynthesis pathway has the capacity to be active. However, addition of exogenous ALA bypasses the rate-limiting ALAS and artificially stimulates pathway activity. Under normal flux conditions, ALA is produced in the mitochondrion by ALAS-catalyzed condensation of glycine and succinyl-CoA (Fig. 1). Prior metabolic studies indicate a low level of tricarboxylic acid (TCA) cycle activity and thus limited production of succinyl CoA in blood-stage parasites (49),6 which may substantially restrict pathway activity during this stage. Also, the amount of cytochromes in mitochondria of asexual stages appears to be rather low (50, 51). Thus, the basal level of pathway flux under normal growth conditions in vitro and in vivo remains unclear.
Whereas heme biosynthesis in the asexual blood stage appears to be nonessential, heme molecules themselves are clearly essential, because they are critical components of the mtETC. The ability to ablate heme biosynthesis strongly suggests that P. falciparum parasites have mechanisms to scavenge host-derived heme. We propose two possible sources for host heme salvage. First, a small amount of heme released from Hb digestion in the food vacuole may be sequestered by heme-binding proteins and subsequently transported to the mitochondrion or other cellular locations where heme is required. Second, a low (submicromolar) concentration of free heme has been identified in the cytosol of erythrocytes (52, 53). This free heme might be taken up by parasites and be incorporated into heme-requiring proteins via unknown mechanisms. It is interesting to note that some Apicomplexan parasites, such as Theileria and Babesia species, have lost the heme biosynthesis genes and must rely entirely on scavenging mechanisms to acquire heme molecules (5, 54).
The heme biosynthesis pathway was also lost among nematode worms, such as Caenorhabditis elegans, and parasitic helminthes (55). Moreover, Trypanosoma spp., have lost all of the enzymes involved in heme biosynthesis (56), whereas Leishmania spp. retain only three enzymes for the last steps of heme biosynthesis (56). These organisms are either heme auxotrophs or require heme intermediates for growth. Through a genetic screen, Hamza and co-workers (57) discovered that C. elegans utilized heme-responsive genes (HRG) to uptake heme and regulate heme homeostasis; hrg-1 orthologues were also present in vertebrates with a relatively high sequence identity. A Leishmania heme response-1 (lhr-1) gene was later identified in Leishmania parasites, which shared homology to C. elegans hrg-4 (58). These HRG-related proteins are membrane-bound transporters with four transmembrane domains with their topologically conserved amino acid residues being critical for heme-transport functions (59). Bioinformatic searches for HRG-like proteins in Plasmodium spp. using sequences of C. elegans hrg-1, hrg-4, or Leishmania lhr-1 failed to find any homologues. Thus, malaria parasites may utilize heme transporters with low sequence homology to the known HRGs or rely on novel heme transport proteins.
In the gametocyte stages of P. falciparum, the de novo heme synthesis pathway appears to be nonessential and thus dispensable until gamete formation (male) (Fig. 5). Because gametocytes develop inside erythrocytes, they might also be able to scavenge sufficient heme from the host. For reasons that remain unknown, male gamete formation was significantly reduced in the ΔFC gametocytes (Fig. 5), although the ΔFC line did form an equal number of mature stage V gametocytes with a similar male-to-female ratio, compared with the WT line (Fig. 5). Detailed biochemical and phenotypic analyses to explore the biochemical basis for this defect will be the subject of future investigations. Defective male gamete formation in ΔFC parasites is not likely to be due to malfunction of the mtETC, however, because the mtETC can be inhibited by atovaquone in mature gametocytes with no effect on mature gametocyte exflagellation.6
Although the heme biosynthesis pathway is not essential in blood stages, it is absolutely required for parasite transmission to mosquitoes. ΔFC parasites failed to develop into oocysts in the mosquitoes (Fig. 6). Our observation in P. falciparum is consistent with the prior study of P. berghei (28), which also reported a defect in oocyst formation in ΔFC parasites. The failure of oocyst formation could be attributed to failure of mosquito-stage progression at any of the following three steps: fertilization in the mosquito midgut; ookinete development; or oocyst development. Although it remains unclear at which step the parasite fails to develop, this developmental defect reveals that malaria parasites are more reliant on heme biosynthesis in mosquitoes than they are in the human host. Once they leave the heme-rich erythrocytes, malaria parasites are confronted with a comparatively heme-poor environment within mosquitoes. We propose that the heme salvage pathway is either not sufficient or absent in the insect stages, which forces parasites to rely on heme biosynthesis. The inability of the ΔFC parasites to produce heme within mosquitoes would then severely affect the mtETC and subsequent ATP generation through oxidative phosphorylation. Although blood-stage malaria parasites obtain energy mainly from glycolysis, they appear to have an enhanced reliance on the TCA cycle,6 mtETC (60, 61), and oxidative phosphorylation for ATP generation during growth within mosquitoes. This up-regulation of oxidative energy metabolism may result in a greater demand for heme in mosquito stages compared with human blood stages that cannot be adequately met by heme salvage alone.
In conclusion, our study demonstrates that the heme biosynthesis pathway is not essential in the asexual form of P. falciparum, but it is essential in mosquito stages. Inhibitors targeting this pathway are unlikely to be effective against the asexual stages of the parasite but might interfere with parasite transmission to the mosquito vector.
Acknowledgments
The mosquito feeding experiments were supported by the Intramural Research Program of the NIAID, National Institutes of Health, and also by the Path Malaria Vaccine Initiative.
This work was supported, in whole or in part, by National Institutes of Health Grants R01 AI028398 (to A. B. V.) and R01 DK099534 (to J. P. H.).
H. Ke, I. A. Lewis, J. M. Morrisey, K. J. McLean, S. M. Ganesan, H. J. Painter, M. W. Mather, M. Jacobs-Lorena, M. Llinas, and A. B. Vaidya, submitted for publication.
- mtETC
- mitochondrial electron transport chain
- 5-ALA
- 5-aminolevulinic acid
- ALAS
- 5-aminolevulinic acid synthase
- FC
- ferrochelatase
- CPP
- coproporphyrin III
- PPIX
- protoporphyrin IX
- HRG
- heme-responsive gene
- SA
- succinylacetone.
REFERENCES
- 1. Hamza I., Dailey H. A. (2012) One ring to rule them all: trafficking of heme and heme synthesis intermediates in the metazoans. Biochim. Biophys. Acta 1823, 1617–1632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Khan A. A., Quigley J. G. (2011) Control of intracellular heme levels: heme transporters and heme oxygenases. Biochim. Biophys. Acta 1813, 668–682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Chiabrando D., Vinchi F., Fiorito V., Mercurio S., Tolosano E. (2014) Heme in pathophysiology: a matter of scavenging, metabolism, and trafficking across cell membranes. Front. Pharmacol. 5, 61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. World Health Organization (2013) World Malaria Report, Geneva, Switzerland: (http://b.3cdn.net/malaria/2fd5b0ab91df7d9176_mlbr81ua8.pdf) [Google Scholar]
- 5. van Dooren G. G., Kennedy A. T., McFadden G. I. (2012) The use and abuse of heme in apicomplexan parasites. Antioxid. Redox Signal. 17, 634–656 [DOI] [PubMed] [Google Scholar]
- 6. Fry M., Pudney M. (1992) Site of action of the antimalarial hydroxynaphthoquinone, 2-[trans-4-(4′-chlorophenyl) cyclohexyl]-3-hydroxy-1,4-naphthoquinone (566C80). Biochem. Pharmacol. 43, 1545–1553 [DOI] [PubMed] [Google Scholar]
- 7. Srivastava I. K., Rottenberg H., Vaidya A. B. (1997) Atovaquone, a broad spectrum antiparasitic drug, collapses mitochondrial membrane potential in a malarial parasite. J. Biol. Chem. 272, 3961–3966 [DOI] [PubMed] [Google Scholar]
- 8. Francis S. E., Sullivan D. J., Jr., Goldberg D. E. (1997) Hemoglobin metabolism in the malaria parasite Plasmodium falciparum. Annu. Rev. Microbiol. 51, 97–123 [DOI] [PubMed] [Google Scholar]
- 9. Egan T. J., Combrinck J. M., Egan J., Hearne G. R., Marques H. M., Ntenteni S., Sewell B. T., Smith P. J., Taylor D., van Schalkwyk D. A., Walden J. C. (2002) Fate of haem iron in the malaria parasite Plasmodium falciparum. Biochem. J. 365, 343–347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Sigala P. A., Crowley J. R., Hsieh S., Henderson J. P., Goldberg D. E. (2012) Direct tests of enzymatic heme degradation by the malaria parasite Plasmodium falciparum. J. Biol. Chem. 287, 37793–37807 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Fitch C. D. (1998) Involvement of heme in the antimalarial action of chloroquine. Trans. Am. Clin. Climatol. Assoc. 109, 97–105 [PMC free article] [PubMed] [Google Scholar]
- 12. Ginsburg H., Ward S. A., Bray P. G. (1999) An integrated model of chloroquine action. Parasitol. Today 15, 357–360 [DOI] [PubMed] [Google Scholar]
- 13. Wriston J. C., Jr., Lack L., Shemin D. (1955) The mechanism of porphyrin formation; further evidence on the relationship of the citric acid cycle and porphyrin formation. J. Biol. Chem. 215, 603–611 [PubMed] [Google Scholar]
- 14. Shemin D., Russell C. S., Abramsky T. (1955) The succinate-glycine cycle. I. The mechanism of pyrrole synthesis. J. Biol. Chem. 215, 613–626 [PubMed] [Google Scholar]
- 15. Kresge N., Simoni R. D., Hill R. L. (2006) A pathway for heme biosynthesis: the work of David Shemin. J. Biol. Chem. 281, e28–e29 [Google Scholar]
- 16. Porra R. J., Klein O., Wright P. E. (1983) The proof by 13C-NMR spectroscopy of the predominance of the C5 pathway over the Shemin pathway in chlorophyll biosynthesis in higher plants and of the formation of the methyl ester group of chlorophyll from glycine. Eur. J. Biochem. 130, 509–516 [DOI] [PubMed] [Google Scholar]
- 17. Varadharajan S., Dhanasekaran S., Bonday Z. Q., Rangarajan P. N., Padmanaban G. (2002) Involvement of δ-aminolaevulinate synthase encoded by the parasite gene in de novo haem synthesis by Plasmodium falciparum. Biochem. J. 367, 321–327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Nagaraj V. A., Arumugam R., Prasad D., Rangarajan P. N., Padmanaban G. (2010) Protoporphyrinogen IX oxidase from Plasmodium falciparum is anaerobic and is localized to the mitochondrion. Mol. Biochem. Parasitol. 174, 44–52 [DOI] [PubMed] [Google Scholar]
- 19. Nagaraj V. A., Prasad D., Rangarajan P. N., Padmanaban G. (2009) Mitochondrial localization of functional ferrochelatase from Plasmodium falciparum. Mol. Biochem. Parasitol. 168, 109–112 [DOI] [PubMed] [Google Scholar]
- 20. Nagaraj V. A., Prasad D., Arumugam R., Rangarajan P. N., Padmanaban G. (2010) Characterization of coproporphyrinogen III oxidase in Plasmodium falciparum cytosol. Parasitol. Int. 59, 121–127 [DOI] [PubMed] [Google Scholar]
- 21. Dhanasekaran S., Chandra N. R., Chandrasekhar Sagar B. K., Rangarajan P. N., Padmanaban G. (2004) δ-Aminolevulinic acid dehydratase from Plasmodium falciparum: indigenous versus imported. J. Biol. Chem. 279, 6934–6942 [DOI] [PubMed] [Google Scholar]
- 22. Nagaraj V. A., Arumugam R., Gopalakrishnan B., Jyothsna Y. S., Rangarajan P. N., Padmanaban G. (2008) Unique properties of Plasmodium falciparum porphobilinogen deaminase. J. Biol. Chem. 283, 437–444 [DOI] [PubMed] [Google Scholar]
- 23. Nagaraj V. A., Arumugam R., Chandra N. R., Prasad D., Rangarajan P. N., Padmanaban G. (2009) Localisation of Plasmodium falciparum uroporphyrinogen III decarboxylase of the heme-biosynthetic pathway in the apicoplast and characterisation of its catalytic properties. Int. J. Parasitol. 39, 559–568 [DOI] [PubMed] [Google Scholar]
- 24. Padmanaban G., Rangarajan P. N. (2000) Heme metabolism of Plasmodium is a major antimalarial target. Biochem. Biophys. Res. Commun. 268, 665–668 [DOI] [PubMed] [Google Scholar]
- 25. Padmanaban G., Rangarajan P. N. (2001) Emerging targets for antimalarial drugs. Expert Opin. Ther. Targets 5, 423–441 [DOI] [PubMed] [Google Scholar]
- 26. Padmanaban G., Nagaraj V. A., Rangarajan P. N. (2007) An alternative model for heme biosynthesis in the malarial parasite. Trends Biochem. Sci. 32, 443–449 [DOI] [PubMed] [Google Scholar]
- 27. Surolia N., Padmanaban G. (1992) De novo biosynthesis of heme offers a new chemotherapeutic target in the human malarial parasite. Biochem. Biophys. Res. Commun. 187, 744–750 [DOI] [PubMed] [Google Scholar]
- 28. Nagaraj V. A., Sundaram B., Varadarajan N. M., Subramani P. A., Kalappa D. M., Ghosh S. K., Padmanaban G. (2013) Malaria parasite-synthesized heme is essential in the mosquito and liver stages and complements host heme in the blood stages of infection. PLoS Pathog. 9, e1003522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Ponka P. (1997) Tissue-specific regulation of iron metabolism and heme synthesis: distinct control mechanisms in erythroid cells. Blood 89, 1–25 [PubMed] [Google Scholar]
- 30. Chan R. Y., Schulman H. M., Ponka P. (1993) Expression of ferrochelatase mRNA in erythroid and non-erythroid cells. Biochem. J. 292, 343–349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Maier A. G., Braks J. A., Waters A. P., Cowman A. F. (2006) Negative selection using yeast cytosine deaminase/uracil phosphoribosyl transferase in Plasmodium falciparum for targeted gene deletion by double crossover recombination. Mol. Biochem. Parasitol. 150, 118–121 [DOI] [PubMed] [Google Scholar]
- 32. Fidock D. A., Wellems T. E. (1997) Transformation with human dihydrofolate reductase renders malaria parasites insensitive to WR99210 but does not affect the intrinsic activity of proguanil. Proc. Natl. Acad. Sci. U.S.A. 94, 10931–10936 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Desjardins R. E., Canfield C. J., Haynes J. D., Chulay J. D. (1979) Quantitative assessment of antimalarial activity in vitro by a semiautomated microdilution technique. Antimicrob. Agents Chemother. 16, 710–718 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Billker O., Lindo V., Panico M., Etienne A. E., Paxton T., Dell A., Rogers M., Sinden R. E., Morris H. R. (1998) Identification of xanthurenic acid as the putative inducer of malaria development in the mosquito. Nature 392, 289–292 [DOI] [PubMed] [Google Scholar]
- 35. Miura K., Deng B., Tullo G., Diouf A., Moretz S. E., Locke E., Morin M., Fay M. P., Long C. A. (2013) Qualification of standard membrane-feeding assay with Plasmodium falciparum malaria and potential improvements for future assays. PLoS One 8, e57909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Rivera M., Caignan G. A. (2004) Recent developments in the 13C NMR spectroscopic analysis of paramagnetic hemes and heme proteins. Anal. Bioanal. Chem. 378, 1464–1483 [DOI] [PubMed] [Google Scholar]
- 37. Wang Y., Gatti P., Sadílek M., Scott C. R., Turecek F., Gelb M. H. (2008) Direct assay of enzymes in heme biosynthesis for the detection of porphyrias by tandem mass spectrometry. Uroporphyrinogen decarboxylase and coproporphyrinogen III oxidase. Anal. Chem. 80, 2599–2605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Pashynska V. A., Van den Heuvel H., Claeys M., Kosevich M. V. (2004) Characterization of noncovalent complexes of antimalarial agents of the artemisinin-type and FE(III)-heme by electrospray mass spectrometry and collisional activation tandem mass spectrometry. J. Am. Soc. Mass Spectrom. 15, 1181–1190 [DOI] [PubMed] [Google Scholar]
- 39. Rutherford T., Thompson G. G., Moore M. R. (1979) Heme biosynthesis in Friend erythroleukemia cells: control by ferrochelatase. Proc. Natl. Acad. Sci. U.S.A. 76, 833–836 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Celli J. P., Spring B. Q., Rizvi I., Evans C. L., Samkoe K. S., Verma S., Pogue B. W., Hasan T. (2010) Imaging and photodynamic therapy: mechanisms, monitoring, and optimization. Chem. Rev. 110, 2795–2838 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Slater A. F., Cerami A. (1992) Inhibition by chloroquine of a novel haem polymerase enzyme activity in malaria trophozoites. Nature 355, 167–169 [DOI] [PubMed] [Google Scholar]
- 42. Sullivan D. J., Jr., Gluzman I. Y., Russell D. G., Goldberg D. E. (1996) On the molecular mechanism of chloroquine's antimalarial action. Proc. Natl. Acad. Sci. U.S.A. 93, 11865–11870 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Meunier B., Robert A. (2010) Heme as trigger and target for trioxane-containing antimalarial drugs. Acc. Chem. Res. 43, 1444–1451 [DOI] [PubMed] [Google Scholar]
- 44. Sigala P. A., Goldberg D. E. (2014) The peculiarities and paradoxes of Plasmodium heme metabolism. Annu. Rev. Microbiol. 68, 259–278 [DOI] [PubMed] [Google Scholar]
- 45. Robert A., Claparols C., Witkowski B., Benoit-Vical F. (2013) Correlation between Plasmodium yoelii nigeriensis susceptibility to artemisinin and alkylation of heme by the drug. Antimicrob. Agents Chemother. 57, 3998–4000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Ramya T. N., Mishra S., Karmodiya K., Surolia N., Surolia A. (2007) Inhibitors of nonhousekeeping functions of the apicoplast defy delayed death in Plasmodium falciparum. Antimicrob. Agents Chemother. 51, 307–316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Bonday Z. Q., Taketani S., Gupta P. D., Padmanaban G. (1997) Heme biosynthesis by the malarial parasite. Import of δ-aminolevulinate dehydrase from the host red cell. J. Biol. Chem. 272, 21839–21846 [DOI] [PubMed] [Google Scholar]
- 48. Bonday Z. Q., Dhanasekaran S., Rangarajan P. N., Padmanaban G. (2000) Import of host δ-aminolevulinate dehydratase into the malarial parasite: identification of a new drug target. Nat. Med. 6, 898–903 [DOI] [PubMed] [Google Scholar]
- 49. MacRae J. I., Dixon M. W., Dearnley M. K., Chua H. H., Chambers J. M., Kenny S., Bottova I., Tilley L., McConville M. J. (2013) Mitochondrial metabolism of sexual and asexual blood stages of the malaria parasite Plasmodium falciparum. BMC Biol. 11, 67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Fry M., Beesley J. E. (1991) Mitochondria of mammalian Plasmodium spp. Parasitology 102, 17–26 [DOI] [PubMed] [Google Scholar]
- 51. Mather M. W., Morrisey J. M., Vaidya A. B. (2010) Hemozoin-free Plasmodium falciparum mitochondria for physiological and drug susceptibility studies. Mol. Biochem. Parasitol. 174, 150–153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Liu S. C., Zhai S., Palek J. (1988) Detection of hemin release during hemoglobin S denaturation. Blood 71, 1755–1758 [PubMed] [Google Scholar]
- 53. Atamna H., Ginsburg H. (1995) Heme degradation in the presence of glutathione. A proposed mechanism to account for the high levels of non-heme iron found in the membranes of hemoglobinopathic red blood cells. J. Biol. Chem. 270, 24876–24883 [DOI] [PubMed] [Google Scholar]
- 54. Lau A. O. (2009) An overview of the Babesia, Plasmodium and Theileria genomes: a comparative perspective. Mol. Biochem. Parasitol. 164, 1–8 [DOI] [PubMed] [Google Scholar]
- 55. Rao A. U., Carta L. K., Lesuisse E., Hamza I. (2005) Lack of heme synthesis in a free-living eukaryote. Proc. Natl. Acad. Sci. U.S.A. 102, 4270–4275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Korený L., Lukes J., Oborník M. (2010) Evolution of the haem synthetic pathway in kinetoplastid flagellates: an essential pathway that is not essential after all? Int. J. Parasitol. 40, 149–156 [DOI] [PubMed] [Google Scholar]
- 57. Rajagopal A., Rao A. U., Amigo J., Tian M., Upadhyay S. K., Hall C., Uhm S., Mathew M. K., Fleming M. D., Paw B. H., Krause M., Hamza I. (2008) Haem homeostasis is regulated by the conserved and concerted functions of HRG-1 proteins. Nature 453, 1127–1131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Huynh C., Yuan X., Miguel D. C., Renberg R. L., Protchenko O., Philpott C. C., Hamza I., Andrews N. W. (2012) Heme uptake by Leishmania amazonensis is mediated by the transmembrane protein LHR1. PLoS Pathog. 8, e1002795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Yuan X., Protchenko O., Philpott C. C., Hamza I. (2012) Topologically conserved residues direct heme transport in HRG-1-related proteins. J. Biol. Chem. 287, 4914–4924 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Boysen K. E., Matuschewski K. (2011) Arrested oocyst maturation in Plasmodium parasites lacking type II NADH:ubiquinone dehydrogenase. J. Biol. Chem. 286, 32661–32671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Hino A., Hirai M., Tanaka T. Q., Watanabe Y., Matsuoka H., Kita K. (2012) Critical roles of the mitochondrial complex II in oocyst formation of rodent malaria parasite Plasmodium berghei. J. Biochem. 152, 259–268 [DOI] [PubMed] [Google Scholar]