Abstract
Pain is a primary symptom driving patients to seek physical therapy, and its attenuation commonly defines a successful outcome. A large body of evidence is dedicated to elucidating the relationship between chronic stress and pain; however, stress is rarely addressed in pain rehabilitation. A physiologic stress response may be evoked by fear or perceived threat to safety, status, or well-being and elicits the secretion of sympathetic catecholamines (epinephrine and norepinepherine) and neuroendocrine hormones (cortisol) to promote survival and motivate success. Cortisol is a potent anti-inflammatory that functions to mobilize glucose reserves for energy and modulate inflammation. Cortisol also may facilitate the consolidation of fear-based memories for future survival and avoidance of danger. Although short-term stress may be adaptive, maladaptive responses (eg, magnification, rumination, helplessness) to pain or non–pain-related stressors may intensify cortisol secretion and condition a sensitized physiologic stress response that is readily recruited. Ultimately, a prolonged or exaggerated stress response may perpetuate cortisol dysfunction, widespread inflammation, and pain. Stress may be unavoidable in life, and challenges are inherent to success; however, humans have the capability to modify what they perceive as stressful and how they respond to it. Exaggerated psychological responses (eg, catastrophizing) following maladaptive cognitive appraisals of potential stressors as threatening may exacerbate cortisol secretion and facilitate the consolidation of fear-based memories of pain or non–pain-related stressors; however, coping, cognitive reappraisal, or confrontation of stressors may minimize cortisol secretion and prevent chronic, recurrent pain. Given the parallel mechanisms underlying the physiologic effects of a maladaptive response to pain and non–pain-related stressors, physical therapists should consider screening for non–pain-related stress to facilitate treatment, prevent chronic disability, and improve quality of life.
Pain is a leading cause of disability, decreases in work productivity, poor quality of life, and rising medical expenses.1,2 Pain is also a primary symptom driving patients to seek physical therapy, and its attenuation commonly defines a successful outcome.3,4 Patient evaluation and differential diagnosis require clinicians to differentiate among a broad range of potential sources of pain. To simplify this process, physical therapists are trained to identify and rule out “red flags” and “yellow flags” prior to initiating treatment. Although red flags generally refer to symptoms of nonmusculoskeletal origin (eg, visceral pain), yellow flags are used to signify psychosocial components such as fear and catastrophizing behaviors.5,6 Similarly, although red flags warrant referral, yellow flags are meant to warn clinicians that treatment is likely to be complicated by psychological variables and prognosis may be fair.5,6 Physical therapists are becoming increasingly proficient in the identification and treatment of yellow flags; however, outcomes vary by individual, and the influence of non–pain-related stress is worthy of consideration.
A pain-induced stress response is elicited by a magnified perception of pain as threatening or dangerous (catastrophizing) and often manifests as fear and avoidance of pain-provoking stimuli.2,7,8 The overall literature suggests that exaggerated psychosocial responses to acute pain are maladaptive and likely to intensify the pain experience and impede recovery.2,7–10 A large number of studies examining risk factors for pain report an association between musculoskeletal pain and pain-related psychosocial stress, such as fear, catastrophizing, and negative coping.1,2,7–9 The fear-avoidance model (FAM) describes 2 alternative responses to the experience of pain; a fear-avoidance or catastrophizing response may prolong the pain experience and augment a cycle of chronic pain and disability, whereas confrontation may break the pain-fear-avoidance cycle and promote recovery.2,7–9 This approach to chronic pain management is occasionally used by physical therapists with techniques such as graded exercise and graded exposure.11 Although these methods may be used to promote confrontation of pain-related fears, similar exaggerated responses to non–pain-related stressors may initiate, exacerbate, or prolong the pain experience. A greater understanding of the detrimental role of an exaggerated response to pain or non–pain-related stressors in perpetuating chronic pain and disability requires a broad understanding of the underlying sympathetic and neuroendocrine mechanisms involved.
The first aim of the current article is to review the physiologic stress response and convey the parallel mechanisms underpinning maladaptive responses to pain and non–pain-related stressors. The second aim is to underscore the role of the physiologic stress response in exacerbation of the pain experience and the development of chronic symptoms. Finally, the overarching goal of this article is to highlight the importance of addressing and managing the fear-based perception of potential stressors as threatening for the effective and long-term treatment of pain. Although life-threatening psychological symptoms may warrant referral, patient education regarding the juxtaposed psychological and physiologic relationship between pain and stress, in addition to coping skills, may be utilized to minimize pain and disability and maximize quality of life.
The Stress Response—the Hypothalamic-Pituitary-Adrenal (HPA) Axis and Cortisol
Neurologically, humans function on a continuum between sympathetic (fight or flight) and parasympathetic (rest and digest). The sympathetic nervous system promotes catabolic tissue breakdown and fat metabolism to mobilize glucose for energy and promote arousal, alertness, motivation, and goal-directed behavior.12 At the other end of the spectrum, the parasympathetic nervous system promotes healing, repair, immunity, and the anabolic growth required for restored energy reserves and longevity.12 Needless to say, a delicate balance between sympathetic and parasympathetic activity is critical for long-term physical and psychological health.
Cortisol is a vital catabolic hormone produced by the adrenal cortex of the kidney.13 It is released in a diurnal fashion, with blood levels peaking in the morning to facilitate arousal and steadily declining thereafter.14,15 Throughout the day, cortisol maintains blood glucose and suppresses nonvital organ systems to provide energy to an actively functioning brain and neuromuscular system.16 Cortisol is also a potent anti-inflammatory hormone; it prevents the widespread tissue and nerve damage associated with inflammation.16
In addition to its paramount role in normal daily function, cortisol is a key player in the stress response. In the presence of a physical or psychological threat, cortisol levels surge to provide the energy and substrate necessary to cope with stress-provoking stimuli or escape from danger.13,17 However, although a stress-induced increase in cortisol secretion is adaptive in the short-term, excessive or prolonged cortisol secretion may have crippling effects, both physically and psychologically.16–18
The Acute Stress Response
A “stressor” is any stimulus or event that evokes a physiologic stress response, commonly referred to as a state of “stress” or “anxiety.” A stressor may be a physical or psychological threat to safety, status, or well-being; physical or psychological demands that exceed available resources; an unpredictable change in environment; or an inconsistency between expectations and outcomes.7,19,20 Whether the stressor is pain or non–pain-related (eg, work overload, financial troubles, social embarrassment), the perception of uncontrollable or unpredictable environmental demands that exceed coping resources is likely to evoke a physiologic stress response, manifesting as a feeling of uneasiness or impending doom, rumination or worry, and avoidance of stress-provoking stimuli.1,2,7,20 However, the perception of environmental stimuli as threatening or frightening varies by individual; therefore, the same fear-based stressor that evokes a stress response in one individual may be innocuous to another. Fear of the worst possible outcome (eg, unemployment, bankruptcy), fear of social embarrassment, fear of pain, or fear of failure activates the amygdala, a portion of the brain's limbic system.21 The amygdala responds to fear or danger by initiating an immediate sympathetic response, followed shortly thereafter by a neuroendocrine response, in an instinctive attempt to restore homeostasis and promote survival.13,15,22,23 Maladaptive cognitive appraisals or beliefs regarding the threatening nature of potential stressors may promote an exaggerated physiologic stress response that is likely to initiate, exacerbate, or prolong the pain experience.20,24,25 Importantly, pain itself is a potential stressor, and a maladaptive perception of pain as threatening or frightening may evoke an exaggerated physiologic stress response, thereby perpetuating chronic pain and disability.
In the initial stage of the acute stress response, the amygdala signals the brain stem to release sympathetic adrenergic catecholamines, norepinephrine and epinephrine.15,26 Once released into the blood flow, catecholamine neurotransmitters increase heart rate, blood pressure, and respiration; vasoconstrict arterioles; and stimulate sweat secretion and pupillary dilation.12 Importantly, this short-term sympathetic response is proinflammatory, functioning to destroy antigens, pathogens, or foreign invaders; adrenoreceptor antagonists have been shown to inhibit stress-induced inflammation and cytokine production by blocking the proinflammatory effects of norepinepherine.13,26,27 Although the role of the sympathoadrenal medullary response in chronic pain is important to consider, the details are beyond the scope of this article.
While sympathetic neurotransmitters regulate the initial stage of the acute stress response, a neuroendocrine response follows in a delayed but prolonged and gene-mediated fashion.26,28 Upon the perception of stress, the amygdala activates the HPA axis by signaling the hypothalamus to release corticotropin-releasing hormone (CRH).17 This hormone then triggers the release of adrenocorticotropic hormone (ACTH) from the anterior pituitary, and ACTH stimulates the release of cortisol from the adrenal cortex.14 Approximately 15 minutes after the onset of stress, cortisol levels rise systemically and remain elevated for several hours.13,22 Increased levels of cortisol mobilize glucose and tissue substrates for fuel, suppress nonvital organ systems, and decrease inflammation to allow for the effective management of stress.16 The critical anti-inflammatory role of cortisol may be emphasized by the countless inflammatory disorders commonly treated with its synthetic pharmaceutical replacement, corticosteroids.29
The Chronic Stress Response
Stressful events are inevitable in daily life, and overcoming obstacles is inherent to success. Although it may not be realistic to live and work in a world free of stressors, humans have the capacity to control what they perceive as stressful and how they respond to it.20 A short-term stress response to pain or non–pain-related stressors may serve an adaptive function to promote survival or motivate success; however, a chronic stress response may be catastrophic.15,17 Exaggerated or recurrent negative cognitions, rumination or worry, magnification, and helplessness are all maladaptive catastrophizing responses to pain or non–pain-related stress that may prolong cortisol secretion.30–33 Similar alterations in cortisol levels have been reported following laboratory-induced stress, self-reported stress, and a catastrophizing response to pain.20,30,31,34,35 Whether the stress-provoking stimulus is pain or non–pain-related, chronic reactivation of the stress response and repeated surges of cortisol result in cortisol dysfunction.
The overall literature suggests several possible neuroendocrine alterations underlying cortisol dysfunction: depletion of cortisol, insufficient free (unbound) cortisol, impaired cortisol secretion or CRH function, glucocorticoid receptor (GR) resistance or down-regulation, or hypersensitivity of the negative feedback system.18,36–39 Therefore, although cortisol depletion following prolonged or excessive secretion may contribute to its dysfunction, there are additional explanations to consider.
Under normal conditions, cortisol binds to the GR and acts as an anti-inflammatory.15,36 However, prolonged or excessive cortisol secretion may result in a compensatory down-regulation or resistance of the GR that blocks cortisol binding, similar to the mechanism underlying insulin-resistant diabetes.28,36 It also has been suggested that extreme surges in cortisol may increase its affinity for the mineralocorticoid receptor (MR), and when bound to the MR, cortisol has proinflammatory effects.26 In either case, elevated inflammatory by-products may damage the GR, thereby compounding cortisol dysfunction.39 Additionally, impaired binding to the GR may disrupt the negative feedback mechanism by which cortisol normally inhibits the continued release of CRH when its levels are sufficient.36 Corticotropin-releasing hormone has been reported to activate inflammatory mast cells, stimulate the release of norepinepherine (a proinflammatory sympathetic neurotransmitter) from the locus coeruleus, and up-regulate glutamate and N-methyl-D-aspartate (NMDA) in the amygdala to condition a fear-based stress response.15,36,38,40 It also has been reported that non–pain-related activation of CRH receptors in the amygdala may trigger pain in the absence of tissue damage and increased excitatory input may hyperpolarize postsynaptic potentials, rendering the amygdala resistant to inhibitory input from the prefrontal cortex.35,40 Although research has yet to identify exactly which mechanisms underlie the process of stress-induced cortisol dysfunction, its etiology is likely a combined, multifactorial, and cyclical process that may be tissue specific and vary by individual and environmental factors.
Regardless of the neuroendocrine mechanisms involved, the long-term effect of chronic stress remains the same: cortisol fails to function. In an animal model, blunted corticosterone (equivalent to cortisol in a rat) levels were observed after 2 weeks of repeated restraint stress, and the constant stress of morphine withdrawal produced hypocortisolism after 8 days.36 In humans, stress-induced inflammation has been implicated in diseases such as osteoporosis, rheumatoid arthritis, myopathy, fibromyalgia, chronic fatigue syndrome, chronic pelvic pain, temporomandibular joint dysfunction, chronic low back pain, sciatica, and more.14,31,36,41,42 Cortisol is a potent anti-inflammatory, and its failure to function results in an unmodulated inflammatory response to physical pathogens, unrecognized proteins, or psychological stressors.15,36 Inflammation induces oxidative and nitrosative stress, free radical damage, cellular death, aging, and systemic tissue degeneration.43,44 Signs and symptoms of stress-induced cortisol dysfunction include bone and muscle breakdown, fatigue, depression, pain, memory impairments, sodium-potassium dysregulation, orthostatic hypotension, and impaired pupillary light reflex.36 Furthermore, stress-induced widespread inflammation may be the final straw in a multifactorial chain of events contributing to hundreds of idiopathic inflammatory autoimmune diseases.15,36
The HPA Axis and Cortisol: Influences on Pain
A large body of evidence is dedicated to elucidating the relationship between pain and stress. Numerous prospective studies have reported baseline anxiety scores to be significant predictors of pain, depression, and a reduced quality of life.1,41,45–48 Importantly, pain itself is a stressor, and a maladaptive response to acute pain may intensify the pain experience and condition a sensitized physiologic stress response to pain-provoking stimuli.21,24,25,49 Exaggerated, prolonged, or recurrent activation of a sensitized stress response to pain or non–pain-related stressors may initiate or exacerbate pain and disability.16,41,45 Although the relationship between pain and stress is widely accepted, the underlying neuroendocrine mechanisms involved are less understood.
The Acute Stress Response: Influences on Pain
Under normal conditions, cortisol secretion during an acute stress response serves to mobilize glucose reserves for energy, inhibit pain and non–vital organ systems, and promote an adaptive fight-or-flight response.16 Additionally, stress-induced cortisol secretion may facilitate the formation of a fear-based memory conditioned to elicit a sensitized fight-or-flight response to promote survival and avoidance of future threat.50 However, these effects may be specific to the fear or stress-provoking stimuli. For example, whereas cortisol secretion during a non–pain-related stress response (eg, public speaking) may distract attention from a concurrent painful stimulus, thereby inhibiting pain, cortisol secretion in response to pain (where pain is the stressor) may intensify its experience and condition a fear-based memory of pain.24,25,49 Whatever the perceived threat or focus of attention may be, excessive cortisol secretion is likely to intensify its experience, facilitate the formation of a fear-based memory, and condition a sensitized physiologic stress response.20
The amygdala regulates the perception of fear and the conditioning of a learned physiologic stress response to negatively appraised emotional stimuli.21,50,51 Numerous studies have shown associations between cortisol and increased levels of activity in the amygdala during states of anxiety and fear.23,35,40,51–54 Animal studies have demonstrated that cortisol promotes dendritic growth and strengthens synaptic connectivity in the amygdala to facilitate the formation of fear-based emotional memories by increasing glutamate levels, up-regulating NMDA receptors, potentiating prolonged calcium uptake, and increasing levels of brain-derived neurotrophic factor (BDNF).23,35,40,51,55 It also has been reported that lesions of the amygdala block the conditioning of a fear-based stress response and inhibit the progression from acute to chronic pain (ie, chronic reactivation of a fear-based memory of pain).23,40,56
In a systematic review of 48 human functional magnetic resonance imaging (fMRI) studies examining brain regions involved in fear conditioning, the amygdala was reported to be the most consistently activated across all studies.54 Although the role of the amygdala in the fear response is widely accepted, the additional brain regions involved are less agreed upon. The hippocampus, cingulate cortex, insula, caudate, and dentate gyrus have all been considered to play a role in the formation of fear-based memories.22,52–54 However, some fMRI studies in humans have shown that during an acute stress response, a decrease in amygdala-hippocampal connectivity was associated with elevated salivary cortisol.53,57 While the hippocampus is a key player in the encoding and retrieval of long-term memory under normal conditions, recent studies hypothesized that humans utilize alternative pathways to consolidate emotional fear-based memories during episodes of acute stress.23 Furthermore, under normal conditions, the hippocampus may tonically inhibit the amygdala and hypothalamus, thereby reducing hyperactivity of the HPA axis.15,17 It has been suggested that decreased hippocampal activity during acute stress, and long-term hippocampal atrophy following chronic stress, may disinhibit the amygdala and disrupt the balance between hippocampal and amygdaloid regulation of the HPA axis.22,51 To summarize, the amygdala interprets a potential stressor as threatening or frightening and elicits cortisol secretion; cortisol facilitates the formation of a fear-based memory by conditioning maladaptive emotional responses in the amygdala, thereby increasing HPA axis activation.24,25,49 Importantly, cortisol-induced memory formation may be specific to the focus of attention during an acute stress response, and the prefrontal cortex may exacerbate or attenuate amygdala activity and cortisol secretion.17,20
Human studies have demonstrated that cortisol levels rise in anticipation of a stressful event and elevations vary by magnitude of perceived threat.13,20,22 A verbally induced nocebo effect (whereby negative expectation enhances the pain experience) was shown to elicit an increase in pain intensity and cortisol secretion during laboratory-induced ischemic pain, and nocebo-induced pain (but not ischemic pain alone) was successfully reversed with diazepam, a common antianxiety medication.24 Similarly, attention to a stressor before or during its occurrence is likely to elevate cortisol secretion and condition a sensitized, fear-based stress response.20,58 Magnification, helplessness, and rumination are 3 catastrophizing responses whereby attention to pain as a stressor, in addition to worry and passive coping, prolong the stress response and elevate cortisol secretion.30–33 Similar alterations in cortisol have been demonstrated in studies examining a catastrophizing response to pain, negative cognitive appraisals or pessimistic beliefs about life events, pre-examination stress in medical students, and work overload in hospital employees.20,30–32,58 Whether the stressor is pain or non–pain-related, cortisol secretion is likely to contribute to the consolidation of fear-based emotional memories that are readily recruited by nonthreatening stimuli and conditioned to reactivate the stress response.
The Chronic Stress Response: Influences on Pain
Chronic reactivation of a sensitized stress response exhausts the HPA axis, and cortisol dysfunction is commonly implicated in idiopathic pain and inflammation.14,16,32,36,41 Chronic stress-induced hypocortisolism has been well documented and linked to pain somatization disorders, such as fibromyalgia, chronic fatigue syndrome, chronic pelvic pain, and temporomandibular disorder.14,15,41 Long-term stress has been shown to attenuate the cortisol awakening response and contribute to morning fatigue, pain, and inflammation.59,60 In a study of 121 middle-aged adults, a blunted cortisol awakening response was predictive of pain and fatigue later that day.41 Similarly, hypocortisolism has been associated with low back pain, and a low cortisol awakening response was associated with leg pain intensity, pain catastrophizing, and low coping scores in 42 patients diagnosed with lumbar disk herniations.33,61 Furthermore, long-term follow-up investigations have suggested a predictive relationship between hypocortisolism and new-onset musculoskeletal pain.38,41
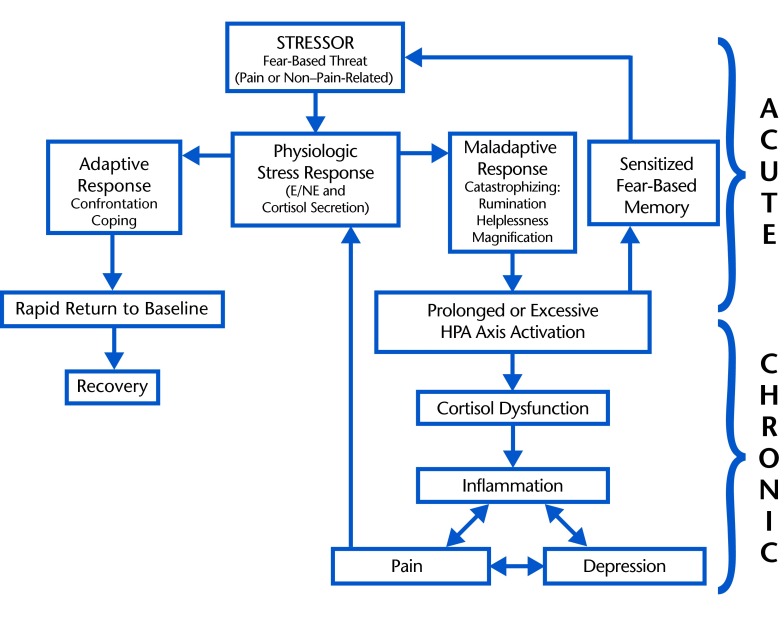
Acute pain is a stressful stimulus that is likely to elicit cortisol secretion and is commonly associated with hypercortisolism, whereas repeated or magnified cortisol secretion following maladaptive responses to acute pain or a non–pain-related stressor is likely to perpetuate hypocortisolism and chronic, recurrent pain (Figure).14,15,36,41 Although the overall evidence suggests an association between hypocortisolism and chronic pain, several studies have reported hypercortisolism in chronic pain conditions, and the temporal aspects of cortisol dysfunction may depend on the magnitude and duration of perceived threat.13,20,22,25 For example, short-term exaggerated responses to daily stressors may cause repeated short-term surges of cortisol secretion that rapidly exhaust cortisol levels, presenting as recurrent episodes of hypercortisolism followed by hypocortisolism and pain.13,16,36 Alternatively, a prolonged or constant low-amplitude stress response may perpetuate constant pain and prolonged hypocortisolism.62 Additionally, the varying temporal aspects of the relationship between cortisol dysfunction and pain may be attributed to different underlying mechanisms of cortisol dysfunction (previously discussed). For example, depletion of circulating free (unbound) cortisol may cause short-term hypocortisolism, whereas inflammatory damage to the GR receptor may cause more long-term effects. To summarize, the temporal aspects of the relationship between pain and cortisol dysfunction may vary based on the specific parameters of the individualized stress response (duration and magnitude of perceived threat), the varying mechanisms of cortisol dysfunction (eg, sufficient free cortisol levels, GR receptor dysfunction), and countless environmental or situation-specific factors. Although these inconclusive hypotheses require clarification by future study, it is reasonable to conclude that cortisol dysfunction is likely to contribute to the development of chronic pain.
Figure.
Proposed role of stress-related hypothalamic-pituitary-adrenal (HPA) axis activation in the transition from acute to chronic pain. Acute stress response: pain or non–pain-related stressor activates a normal physiologic stress response (short-term sympathetic release of epinepherine and norepinepherine [E/NE] followed by secretion of the anti-inflammatory hormone, cortisol). An adaptive coping response permits the return to normal levels of E/NE, cortisol, and inflammation; a maladaptive response causes excessive or prolonged cortisol secretion and creates a fear-based memory of the stressful stimulus that is sensitized and readily reactivated by future stressors. Chronic stress response: prolonged cortisol secretion (due to maladaptive coping response to acute stress) results in cortisol dysfunction. Cortisol dysfunction results in unmodulated inflammation following reactivation of the stress response, which may contribute to a cycle of inflammation, depression, and pain; pain is a stressor that may reactivate a proinflammatory stress response, now unmodulated due to cortisol dysfunction.
Cortisol is a potent anti-inflammatory hormone, and its dysfunction is likely to result in widespread inflammation following the reactivation of an acute proinflammatory stress response. Studies have shown associations among inflammatory cytokines, stress-related chronic pain, and salivary hypocortisolism.26,63 There are countless mechanisms by which widespread inflammation may contribute to pain, as pain is a definitive component of the inflammatory response. Reactivation of a sensitized stress response in the presence of physical or psychological threat or fear elicits the release of proinflammatory sympathetic catecholamines, and the impaired anti-inflammatory function of cortisol may intensify and prolong a formerly short-term inflammatory response.17 Similarly, following physical injury under normal conditions, localized secretion of inflammatory cytokines initiates the healing process and lowers nociceptor thresholds to elicit a protective pain response. However, physical injury during a state of stress-induced hypocortisolism may result in a persistent inflammatory response that impairs healing. Additionally, prolonged elevation of inflammatory cytokines sensitizes nociceptors, manifesting as an increase in pain sensitivity. Furthermore, continuous reactivation of the stress response by unmodulated inflammatory mediators and conditioned emotional hyper-responsiveness may compound the effects of inflammation, reinforce a conditioned stress response, and amplify the cycle of stress, inflammation, and pain.15,36
Inflammation produces free radical byproducts, and oxidative and nitrosative stress damage healthy tissue.43,44 Accumulation of free radicals over time underlies the aging process, and oxidative stress may be responsible for widespread tissue degeneration. Osteoporosis, myopathies, and idiopathic neuropathies are common manifestations of widespread inflammation, and pain is a common side effect of these conditions.13–16 To complicate the process, inflammation widens gap junctions in the blood brain barrier and intestinal lining, allowing for harmful toxins and large foreign bodies (unrecognized by the immune system) to breach the protective barriers and exacerbate the inflammatory response.26 Furthermore, widespread inflammatory hypersensitivities to unrecognized proteins may give rise to autoimmunity, whereby the immune system mistakenly attacks healthy tissue.15,16 Additionally, widespread inflammation and free radical binding to healthy cells may create abnormal growths or cancerous cells.64 Low cortisol awakening responses have been associated with poor overall health, acquired immunodeficiency syndrome, and cancer.64 Ultimately, the damaging effects of widespread inflammation may contribute to the multifactorial etiology of countless pathologies.43,64,65 Although genetics and environmental exposures may be unavoidable, a conditioned physiologic response to a maladaptive psychological perception of threat may be a modifiable contributing factor.
Low levels of serotonin have been widely implicated in the etiology of pain and impaired inhibition of nociceptive transmission in the spinal cord.66 Selective serotonin reuptake inhibitor antidepressant medications are frequently prescribed for pain relief.66 Serotonin is synthesized from the amino acid, tryptophan, and inflammatory activation of the indoleamine 2,3-dioxygenase enzyme activates tryptophan breakdown into kyneurine and quinolinic acid (tryptophan catabolites [TRYCATs], or by-products of tryptophan catabolism).43,44 Activation of the TRYCAT pathway depletes tryptophan availability for serotonin synthesis and has been associated with depression, anxiety, and pain.43,44 Additionally, quinolinic acid is a strong NMDA agonist with neurotoxic effects that may exacerbate pain via hippocampal degeneration.43,44 Reduced hippocampal volume has been reported to be directly correlated with self-reported pain intensity and chronic stress.55,67 Therefore, chronic stress and inflammation may contribute to pain and depression by TRYCAT-induced serotonin depletion and hippocampal degeneration.
Chronic stress and lack of control over life experiences when outcomes fail to meet expectations may manifest as a sense of helplessness or hopelessness.19 After repeated disappointments and failures to achieve success, humans are likely to give up when they feel a loss of control. Although chronic fears, challenges, and unexpected events define anxiety, repeated failures, giving up, helplessness, and hopelessness are all characteristics of depression.68 Similarly, chronic pain and repeated failures in pain management are likely to be perceived as a lack of control over health and may manifest as depression. In either case, the transition to depressive symptoms should be addressed and prevented by modifying the way pain or non–pain-related stressors are perceived and managed.
Clinical Implications: Assessment and Treatment
Pain is a leading cause of disability and the number one symptom driving patients to seek physical therapy. Although faulty ergonomic practices, postural strain, and repetitive overuse microtrauma must be addressed for the effective treatment of musculoskeletal pain, individuals in the same environment with equivalent ergonomic and postural demands do not all experience pain, and a dysfunctional HPA axis may mediate this discrepancy. Although the factor initiating treatment may be musculoskeletal in origin, a chronic stress response (to pain or non–pain-related stressors), cortisol dysfunction, and widespread inflammation may initiate, exacerbate, or prolong the pain experience, impair healing, and perpetuate chronic disability. Screening tools such as the Pain Catastrophizing Scale and the Fear-Avoidance Beliefs Questionnaire are becoming increasingly common in clinical practice and utilized to identify maladaptive responses to pain.5,6,9 However, in the physical therapy setting, there is less emphasis on the role of non–pain-related stress in the pain experience.
Prior to addressing non–pain-related stress in pain management, it is important to identify patients most likely to benefit from stress management. Objective measures of cortisol may be obtained from blood, saliva, urine, or hair; however, laboratory tests may not be appropriate for the physical therapy setting, and each test has specific limitations. Alternatively, there are a multitude of subjective measures of self-reported stress that may be easily integrated into the screening process. The Perceived Stress Scale, the Impact of Events Scale, the Daily Stress Inventory, and the State-Trait Anxiety Inventory are reliable and valid measures of stress that have demonstrated varying associations with objective measures of cortisol.69–72 However, different scales measure different components of stress; for example, the Perceived Stress Scale is a measure of chronic stress (within the previous 30 days), and the State-Trait Anxiety Inventory is a measure of current stress-related symptoms (state) and characteristics of an anxious personality type (trait).70,72 Coping scales also have been created to identify patients with poor stress management skills. The Connor-Davidson Resilience Scale, the Resilience Scale for Adults, and the Brief Resilience Scale received the best psychometric ratings in a review of resilience scales for adaptive coping styles.73 There is a plethora of stress-related outcome measures and screening tools, each with unique advantages and disadvantages.
Following the identification of stress or maladaptive coping skills during initial screening, educating patients about the role of stress in the pain experience may allow for cortical inhibition of emotional fear-based responses to nonthreatening stimuli.20,24,25 Additionally, awareness of the influence of non–pain-related stress in the pain experience may allow patients to address non–pain-related stressors, thereby facilitating pain rehabilitation. Physical therapists also may address musculoskeletal or ergonomic contributions to pain that may be exacerbated by aberrant posture associated with psychological stress (eg, stress-related postural dysfunction with prolonged computer use at work). However, in cases of severe stress or mental illness, the physical therapist should refer patients to the proper health care provider for a multidisciplinary team approach to rehabilitation. In a recent case report of multidisciplinary stress management interventions, biofeedback administered by a physical therapist and psychotherapy administered by a clinical psychologist were successfully implemented to facilitate the long-term resolution of neck pain and disability.74
Mindfulness-based interventions and cognitive-behavioral therapy have been suggested for pain or stress management, and improvements are thought to be mediated by prefrontal modulation of amygdala activity with reappraisal of faulty beliefs and restructuring of negative cognitions.75 However, although correcting maladaptive responses to nonthreatening stimuli is important to minimize stress, it may only apply if individuals are falsely appraising nonthreatening stimuli as threatening. The function of the physiologic stress response is to promote survival, and when a real threat exists, fear induces a stress response to motivate survival, success, or goal achievement. Therefore, if the stressor or threat is real, such as a quarrelsome coworker or financial troubles, the optimal solution may be confrontation to address the underlying cause of stress (rather than reappraisal). Although the achievement of success and avoidance of danger are not always possible or optimal, managing failure and unexpected events, accepting challenges, and confronting fears may drive success and prevent an overactive stress response. Ultimately, to effectively manage chronic stress and prevent its debilitating long-term effects, individuals must identify the fear that underlies the stress response (be it physical, psychological, social, or environmental), assess its rationality (threatening or nonthreatening), and address it (confrontation or cognitive reappraisal).
Conclusion
Although stressful events may be an inevitable part life, a prolonged or exaggerated response to pain or non–pain-related stressors may intensify sympathetic and neuroendocrine activity, exhaust cortisol, and perpetuate widespread pain and inflammation. Elevated cortisol levels following acute stress may facilitate the consolidation of fear-based emotional memories and condition a sensitized physiologic stress response. Negative appraisals or maladaptive beliefs, a hypervigilant response to stressful stimuli, cognitive inflexibility, and passive or avoidant coping may contribute to prolonged or frequent activations of the HPA axis. Following chronic reactivation of the HPA axis, cortisol dysfunction and inflammation may directly facilitate pain transmission via impaired modulation or repeated nociceptor activation by inflammatory mediators. Secondary effects of widespread inflammation may include autoimmune hypersensitivities, inflammation-induced widespread oxidative or free radical damage, and idiopathic inflammatory tissue degeneration. Serotonin depletion and hippocampal degeneration are likely to compound the effects of inflammation on pain and depression. However, there are a multitude of mechanisms by which inflammation, stress, and pain are related and the underlying processes are likely multidirectional and cyclical in nature. Future study is needed to identify patients most likely to benefit from stress management and those most appropriate for referral. Ultimately, the early identification of stress and the incorporation of stress management education into pain rehabilitation may facilitate effective pain management, prevent the transition to chronic and depressive symptoms, minimize disability, and improve quality of life.
Footnotes
Dr Hannibal provided concept/idea and writing. Dr Bishop provided fund procurement (NIH, NCAAM, AT006334) and assisted with concept development.
References
- 1. Linton SJ. A review of psychological risk factors in back and neck pain. Spine (Phila Pa 1976). 2000;25:1148–1156. [DOI] [PubMed] [Google Scholar]
- 2. Lucchetti G, Oliveira AB, Mercante JP, Peres MF. Anxiety and fear-avoidance in musculoskeletal pain. Curr Pain Headache Rep. 2012;16:399–406. [DOI] [PubMed] [Google Scholar]
- 3. Ariëns GA, van Mechelen W, Bongers PM, et al. Psychosocial risk factors for neck pain: a systematic review. Am J Ind Med. 2001;39:180–193. [DOI] [PubMed] [Google Scholar]
- 4. Steglitz J, Buscemi J, Ferguson MJ. The future of pain research, education, and treatment: a summary of the IOM report “Relieving pain in America: a blueprint for transforming prevention, care, education, and research.” Transl Behav Med. 2012;2:6–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Main CJ, George SZ. Psychologically informed practice for management of low back pain: future directions in practice and research. Phys Ther. 2011;91:820–824. [DOI] [PubMed] [Google Scholar]
- 6. Nicholas MK, Linton SJ, Watson PJ, et al. Early identification and management of psychological risk factors (“yellow flags”) in patients with low back pain: a reappraisal. Phys Ther. 2011;91:737–753. [DOI] [PubMed] [Google Scholar]
- 7. Lumley MA, Cohen JL, Borszcz GS, et al. Pain and emotion: a biopsychosocial review of recent research. J Clin Psychol. 2011;67:942–968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Crombez G, Eccleston C, Van Damme S, et al. Fear-avoidance model of chronic pain: the next generation. Clin J Pain. 2012;28:475–483. [DOI] [PubMed] [Google Scholar]
- 9. George SZ, Stryker SE. Fear-avoidance beliefs and clinical outcomes for patients seeking outpatient physical therapy for musculoskeletal pain conditions. J Orthop Sports Phys Ther. 2011;41:249–259. [DOI] [PubMed] [Google Scholar]
- 10. George SZ, Hirsh AT. Psychologic influence on experimental pain sensitivity and clinical pain intensity for patients with shoulder pain. J Pain. 2009;10:293–299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. George SZ, Wittmer VT, Fillingim RB, Robinson ME. Comparison of graded exercise and graded exposure clinical outcomes for patients with chronic low back pain. J Orthop Sports Phys Ther. 2010;40:694–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Vinik AI, Maser RE, Ziegler D. Autonomic imbalance: prophet of doom or scope for hope? Diabet Med. 2011;28:643–651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Blackburn-Munro G, Blackburn-Munro R. Pain in the brain: are hormones to blame? Trends Endocrinol Metab. 2003;14:20–27. [DOI] [PubMed] [Google Scholar]
- 14. Ehlert U, Gaab J, Heinrichs M. Psychoneuroendocrinological contributions to the etiology of depression, posttraumatic stress disorder, and stress-related bodily disorders: the role of the hypothalamus-pituitary-adrenal axis. Biol Psychol. 2001;57:141–152. [DOI] [PubMed] [Google Scholar]
- 15. Tsigos C, Chrousos GP. Hypothalamic-pituitary-adrenal axis, neuroendocrine factors and stress. J Psychosom Res. 2002;53:865–871. [DOI] [PubMed] [Google Scholar]
- 16. Heim C, Ehlert U, Hellhammer DH. The potential role of hypocortisolism in the pathophysiology of stress-related bodily disorders. Psychoneuroendocrinology. 2000;25:1–35. [DOI] [PubMed] [Google Scholar]
- 17. Jankord R, Herman JP. Limbic regulation of hypothalamo-pituitary-adrenocortical function during acute and chronic stress. Ann N Y Acad Sci. 2008;1148:64–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. McEwen BS. Central effects of stress hormones in health and disease: understanding the protective and damaging effects of stress and stress mediators. Eur J Pharmacol. 2008;583:174–185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Ursin H, Eriksen HR. Cognitive activation theory of stress (CATS). Neurosci Biobehav Rev. 2010;34:877–881. [DOI] [PubMed] [Google Scholar]
- 20. Denson TF, Spanovic M, Miller N. Cognitive appraisals and emotions predict cortisol and immune responses: a meta-analysis of acute laboratory social stressors and emotion inductions. Psychol Bull. 2009;135:823–853. [DOI] [PubMed] [Google Scholar]
- 21. Beckers T, Krypotos AM, Boddez Y, et al. What's wrong with fear conditioning? Biol Psychol. 2013;92:90–96. [DOI] [PubMed] [Google Scholar]
- 22. Dedovic K, Duchesne A, Andrews J, et al. The brain and the stress axis: the neural correlates of cortisol regulation in response to stress. Neuroimage. 2009;47:864–871. [DOI] [PubMed] [Google Scholar]
- 23. Mora F, Segovia G, Del Arco A, et al. Stress, neurotransmitters, corticosterone and body-brain integration. Brain Res. 2012;1476:71–85. [DOI] [PubMed] [Google Scholar]
- 24. Benedetti F, Amanzio M, Vighetti S, Asteggiano G. The biochemical and neuroendocrine bases of the hyperalgesic nocebo effect. J Neurosci. 2006;26:12014–12022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Colloca L, Benedetti F. Nocebo hyperalgesia: how anxiety is turned into pain. Curr Opin Anaesthesiol. 2007;20:435–439. [DOI] [PubMed] [Google Scholar]
- 26. Sorrells SF, Caso JR, Munhoz CD, Sapolsky RM. The stressed CNS: when glucocorticoids aggravate inflammation. Neuron. 2009;64:33–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Gosain A, Jones SB, Shankar R, et al. Norepinephrine modulates the inflammatory and proliferative phases of wound healing. J Trauma. 2006;60:736–744. [DOI] [PubMed] [Google Scholar]
- 28. Norman M, Hearing SD. Glucocorticoid resistance: what is known? Curr Opin Pharmacol. 2002;2:723–729. [DOI] [PubMed] [Google Scholar]
- 29. Barnes PJ. Mechanisms and resistance in glucocorticoid control of inflammation. J Steroid Biochem Mol Biol. 2010;120:76–85. [DOI] [PubMed] [Google Scholar]
- 30. Müller MJ. Helplessness and perceived pain intensity: relations to cortisol concentrations after electrocutaneous stimulation in healthy young men. Biopsychosoc Med. 2011;5:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Quartana PJ, Buenaver LF, Edwards RR, et al. Pain catastrophizing and salivary cortisol responses to laboratory pain testing in temporomandibular disorder and healthy participants. J Pain. 2010;11:186–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Edwards RR, Kronfli T, Haythornthwaite JA, et al. Association of catastrophizing with interleukin-6 responses to acute pain. Pain. 2008;140:135–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Johansson AC, Gunnarsson LG, Linton SJ, et al. Pain, disability and coping reflected in the diurnal cortisol variability in patients scheduled for lumbar disc surgery. Eur J Pain. 2008;12:633–640. [DOI] [PubMed] [Google Scholar]
- 34. Baig A, Siddiqui I, Naqvi H, et al. Correlation of serum cortisol levels and stress among medical doctors working in emergency departments. J Coll Physicians Surg. 2006;16:576–580. [PubMed] [Google Scholar]
- 35. Ji G, Fu Y, Adwanikar H, Neugebauer V. Non–pain-related CRF1 activation in the amygdala facilitates synaptic transmission and pain responses. Mol Pain. 2013;9:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Fries E, Hesse J, Hellhammer J, Hellhammer DH. A new view on hypocortisolism. Psychoneuroendocrinology. 2005;30:1010–1016. [DOI] [PubMed] [Google Scholar]
- 37. Joëls M. Impact of glucocorticoids on brain function: relevance for mood disorders. Psychoneuroendocrinology. 2011;36:406–414. [DOI] [PubMed] [Google Scholar]
- 38. McEwen BS, Kalia M. The role of corticosteroids and stress in chronic pain conditions. Metabolism. 2010;59(suppl 1):S9–S15. [DOI] [PubMed] [Google Scholar]
- 39. Yang N, Ray DW, Matthews LC. Current concepts in glucocorticoid resistance. Steroids. 2012;77:1041–1049. [DOI] [PubMed] [Google Scholar]
- 40. Shekhar A, Truitt W, Rainnie D, Sajdyk T. Role of stress, corticotrophin releasing factor (CRF) and amygdala plasticity in chronic anxiety. Stress. 2005;8:209–219. [DOI] [PubMed] [Google Scholar]
- 41. Tak LM, Rosmalen JG. Dysfunction of stress responsive systems as a risk factor for functional somatic syndromes. J Psychosom Res. 2010;68:461–468. [DOI] [PubMed] [Google Scholar]
- 42. Tak LM, Cleare AJ, Ormel J, et al. Meta-analysis and meta-regression of hypothalamic-pituitary-adrenal axis activity in functional somatic disorders. Biol Psychol. 2011;87:183–194. [DOI] [PubMed] [Google Scholar]
- 43. Maes M, Galecki P, Chang YS, Berk M. A review on the oxidative and nitrosative stress (O&NS) pathways in major depression and their possible contribution to the (neuro)degenerative processes in that illness. Prog Neuropsychopharmacol Biol Psychiatry. 2011;35:676–692. [DOI] [PubMed] [Google Scholar]
- 44. Zunszain PA, Anacker C, Cattaneo A, et al. Glucocorticoids, cytokines and brain abnormalities in depression. Prog Neuropsychopharmacol Biol Psychiatry. 2011;35:722–729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Hall AM, Kamper SJ, Maher CG, et al. Symptoms of depression and stress mediate the effect of pain on disability. Pain. 2011;152:1044–1051. [DOI] [PubMed] [Google Scholar]
- 46. Orenius T, Koskela T, Koho P, et al. Anxiety and depression are independent predictors of quality of life of patients with chronic musculoskeletal pain. J Health Psychol. 2013;18:167–175. [DOI] [PubMed] [Google Scholar]
- 47. Bair MJ, Poleshuck EL, Wu J, et al. Anxiety but not social stressors predict 12-month depression and pain severity. Clin J Pain. 2013;29:95–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Pincus T, Burton AK, Vogel S, Field AP. A systematic review of psychological factors as predictors of chronicity/disability in prospective cohorts of low back pain. Spine (Phila Pa 1976). 2002;27:E109–E120. [DOI] [PubMed] [Google Scholar]
- 49. Keltner JR, Furst A, Fan C, et al. Isolating the modulatory effect of expectation on pain transmission: a functional magnetic resonance imaging study. J Neurosci. 2006;26:4437–4443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Hartley CA, Phelps EA. Changing fear: the neurocircuitry of emotion regulation. Neuropsychopharmacology. 2010;35:136–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Boyle LM. A neuroplasticity hypothesis of chronic stress in the basolateral amygdala. Yale J Biol Med. 2013;86:117–125. [PMC free article] [PubMed] [Google Scholar]
- 52. Vachon-Presseau E, Martel MO, Roy M, et al. Acute stress contributes to individual differences in pain and pain-related brain activity in healthy and chronic pain patients. J Neurosci. 2013;33:6826–6833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Vaisvaser S, Lin T, Admon R, et al. Neural traces of stress: cortisol related sustained enhancement of amygdala-hippocampal functional connectivity. Front Hum Neurosci. 2013;7:313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Sehlmeyer C, Schöning S, Zwitserlood P, et al. Human fear conditioning and extinction in neuroimaging: a systematic review. PloS One. 2009;4:e5865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Lakshminarasimhan H, Chattarji S. Stress leads to contrasting effects on the levels of brain derived neurotrophic factor in the hippocampus and amygdala. PloS One. 2012;7:e30481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Li Z, Wang J, Chen L, et al. Basolateral amygdala lesion inhibits the development of pain chronicity in neuropathic pain rats. PloS One. 2013;8:e70921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Veer IM, Oei NY, Spinhoven P, et al. Beyond acute social stress: increased functional connectivity between amygdala and cortical midline structures. Neuroimage. 2011;57:1534–1541. [DOI] [PubMed] [Google Scholar]
- 58. Chan SC, Chan CC, Kwan AS, et al. Orienting attention modulates pain perception: an ERP study. PloS One. 2012;7:e40215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Riva R, Mork PJ, Westgaard RH, Lundberg U. Comparison of the cortisol awakening response in women with shoulder and neck pain and women with fibromyalgia [erratum in: Psychoneuroendocrinology. 2012;37:587]. Psychoneuroendocrinology. 2012;37:299–306. [DOI] [PubMed] [Google Scholar]
- 60. Sudhaus S, Fricke B, Stachon A, et al. Salivary cortisol and psychological mechanisms in patients with acute versus chronic low back pain. Psychoneuroendocrinology. 2009;34:513–522. [DOI] [PubMed] [Google Scholar]
- 61. Muhtz C, Rodriguez-Raecke R, Hinkelmann K, et al. Cortisol response to experimental pain in patients with chronic low back pain and patients with major depression. Pain Med. 2013;14:498–503. [DOI] [PubMed] [Google Scholar]
- 62. Riva R, Mork PJ, Westgaard RH, et al. Fibromyalgia syndrome is associated with hypocortisolism. Int J Behav Med. 2010;17:223–233. [DOI] [PubMed] [Google Scholar]
- 63. Brydon L, Walker C, Wawrzyniak A, et al. Synergistic effects of psychological and immune stressors on inflammatory cytokine and sickness responses in humans. Brain Behav Immun. 2009;23:217–224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Morrison WB. Inflammation and cancer: a comparative view. J Vet Intern Med. 2012;26:18–31. [DOI] [PubMed] [Google Scholar]
- 65. Pei C, Barbour M, Fairlie-Clarke KJ, et al. Emerging role of interleukin-33 in autoimmune diseases. Immunology. 2014;141:9–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Stahl SM, Lee-Zimmerman C, Cartwright S, Morrissette DA. Serotonergic drugs for depression and beyond. Curr Drug Targets. 2013;14:578–585. [DOI] [PubMed] [Google Scholar]
- 67. Kiem SA, Andrade KC, Spoormaker VI, et al. Resting state functional MRI connectivity predicts hypothalamus-pituitary-axis status in healthy males. Psychoneuroendocrinology. 2013;38:1338–1348. [DOI] [PubMed] [Google Scholar]
- 68. Pryce CR, Azzinnari D, Spinelli S, et al. Helplessness: a systematic translational review of theory and evidence for its relevance to understanding and treating depression. Pharmacol Ther. 2011;132:242–267. [DOI] [PubMed] [Google Scholar]
- 69. Brantley PJ, Waggoner CD, Jones GN, Rappaport NB. A Daily Stress Inventory: development, reliability, and validity. J Behav Med. 1987;10:61–74. [DOI] [PubMed] [Google Scholar]
- 70. Tluczek A, Henriques JB, Brown RL. Support for the reliability and validity of a six-item state anxiety scale derived from the State-Trait Anxiety Inventory. J Nurs Meas. 2009;17:19–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Briere J, Elliott DM. Clinical utility of the impact of event scale: psychometrics in the general population. Assessment. 1998;5:171–180. [DOI] [PubMed] [Google Scholar]
- 72. Levenstein S, Prantera C, Varvo V, et al. Development of the Perceived Stress Questionnaire: a new tool for psychosomatic research. J Psychosom Res. 1993;37:19–32. [DOI] [PubMed] [Google Scholar]
- 73. Windle G, Bennett KM, Noyes J. A methodological review of resilience measurement scales. Health Qual Life Outcomes. 2011;9:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Bruflat AK, Balter JE, McGuire D, et al. Stress management as an adjunct to physical therapy for chronic neck pain. Phys Ther. 2012;92:1348–1359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Cramer H, Haller H, Lauche R, Dobos G. Mindfulness-based stress reduction for low back pain: a systematic review. BMC Complement Altern Med. 2012;12:162. [DOI] [PMC free article] [PubMed] [Google Scholar]