Abstract
Memory impairment is often present in frontotemporal dementia (FTD) as a result of an inefficient use of learning strategies, sometimes leading to a misdiagnosis of Alzheimer's disease (AD). The Free and Cued Selective Reminding Test (FCSRT) is a memory test that controls attention and acquisition, by providing category cues in the learning process. The main goal of this study was to show the usefulness of the FCSRT in the distinction between behavioral (bv-) FTD and AD. Three matched subgroups of participants were considered: bv-FTD (n = 32), AD (n = 32), and a control group of healthy adults (n = 32). Results proved that while AD patients exhibited an overall impairment in FCSRT, bv-FTD subjects showed to benefit more from the controlled learning through category cues. AD patients were 25 times more likely to have an impaired FCSRT. The FCSRT has shown its utility in the distinction between bv-FTD and AD, therefore increasing the diagnostic accuracy.
Keywords: Frontotemporal dementia, Alzheimer's disease, Learning and memory, Assessment
Introduction
Memory impairment is one of the most common complaints in the ageing population, and one of the most prevalent symptoms in patients with neurological disorders. In the field of dementia, a deficit in memory is significant as it may indicate the onset of Alzheimer's disease (AD) or it may pose as a risk factor for the subsequent development of AD (Dubois et al., 2007; Geerlings, Jonker, Bouter, Ader, & Schmand, 1999). An accurate diagnosis of AD is primarily based on an individual's performance on cognitive tests that are designed to detect memory impairment with high sensitivity and specificity (Petersen, Smith, Ivnik, Kokmen, & Tangalos, 1994). Moreover, the design of the test is very important as an accurate assessment of memory depends on the quality of learning, which is later reflected in an effective retrieval (Buschke, Sliwinski, Kuslansky, & Lipton, 1997). However, memory impairment is not necessarily evidence of an AD-related memory disorder and can be present in other conditions (e.g., mild cognitive impairment, depression). In patients with AD, the amnestic profile is typically characterized by poor learning and rapid memory decay over relatively short periods, often concurrent with damage to the mesio-temporal structures, such as the hippocampus (Squire, Stark, & Clark, 2004). Though there is some evidence that the memory consolidation problems often observed in patients with frontotemporal dementia (FTD) may also be linked to hippocampal atrophy (Lindberg et al., 2012; Muñoz-Ruiz et al., 2012; van de Pol et al., 2006), memory deficits in FTD are more typically reflective of poor organization and lack of efficient learning strategies. Thus, this results in a defective encoding of memory leading to an inability to implement effective retrieval strategies, due to an involvement of the prefrontal cortex (Blumenfeld & Ranganath, 2007). It is of clinical importance that deficits in encoding and storage processes that are so characteristic of AD can be distinguished from non-AD memory deficits that may have a different etiology. The accurate diagnosis of the episodic memory deficit, so often observed in AD patients, may be improved upon the use of test paradigms that provide information at encoding and retrieval—encoding specificity (Buschke et al., 1997). One way of controlling the acquisition and retrieval of information is to use the same cues to direct learning and produce effective cued recall. The encoding specificity procedure has shown to promote deeper engagement with attentional and semantic processing in the encoding phase of memory, and it also controls the conditions of retrieval (Tulving & Osler, 1968; Tulving & Thomson, 1973). Furthermore, memory tests that require the ability to control acquisition and retrieval may optimize encoding specificity and thus may be more sensitive to the early signs of dementia (Buschke, 1987) than tests that use different paradigms.
The Free and Cued Selective Reminding Test (FCSRT; Buschke, 1984) is a memory test that controls attention and cognitive processing, requiring subjects to search for items in response to their category cues, in the learning process. Moreover, these same category cues are given later to participants in order to elicit the recall of the items not retrieved on the free recall trial, thus controlling acquisition and retrieval. Performance on the cued recall trial provides an estimate of the items that the subject has stored, and it has been shown that this estimate is minimally affected by guessing (Grober, Gitlin, Bang, & Buschke, 1992).
The utility of this cued selective reminding paradigm in the detection of AD-related memory dysfunction has been widely reported (Brown & Storandt, 2000; Grober & Buschke, 1987; Grober, Buschke, Crystal, Bang, & Dresner, 1988; Grober & Kawas, 1997; Grober et al., 2008; Ivanoiu et al., 2005; Vogel, Mortensen, Gade, & Waldemar, 2007). A poor performance on the FCSRT has also shown a high correlation with atrophy in the medial temporal lobe (Habert et al., 2011; Sánchez-Benavides et al., 2010; Sarazin et al., 2010) and was significantly associated with cerebrospinal fluid biomarkers of AD, thereby supporting the accuracy potential of this paradigm in the early detection of AD (Rami et al., 2011; Wagner et al., 2012).
Furthermore, the potential of the FCSRT being used as a clinical diagnostic tool is bolstered by International Working Groups proposal (Dubois et al., 2007, 2010) that it could be used to assess memory in patients with suspected AD, given its high sensitivity (Grober, Hall, Sanders, & Lipton, 2008; Grober, Sanders, Hall, & Lipton, 2010).
FTD is the second most prevalent type of dementia and typically occurs much earlier in life than AD, overcoming Lewy Body dementia (Harvey, Skelton-Robinson, & Rossor, 2003) which is more common later on. The frontal or behavioral variant (bv-FTD) is the most common subtype accounting for approximately half of all FTD cases (Johnson et al., 2005; Seelaar et al., 2008). Despite the heterogeneity in its clinical presentation, bv-FTD is characterized by an insidious onset and a progressive decline that is marked by personality and/or behavioral changes (McKhann et al., 2001; Neary et al., 1998). The cognitive deficits of bv-FTD include impairments on executive function, attention, working memory, poor abstraction and difficulty in shifting mental set leading to perseverative tendencies (Neary et al., 1998; Weder, Aziz, Wilkins, & Tampi, 2007). It is also usually associated with bilateral symmetrical frontal and anterior temporal atrophy (Neary et al., 1998; Seelaar, Rohrer, Pijnenburg, Fox, & van Swieten, 2011). Research has shown that the specific pattern of impairment of bv-FTD includes a relative sparing of memory and visuospatial functions in comparison to executive functions which are most commonly affected (Rascovsky et al. 2011). However, because impairment in executive functioning can limit effective learning in patients with bv-FTD, decrements in performance on conventional memory tests may result either from primary memory deficits or as a result of executive and attention deficits that hinder the use of learning and retrieval strategies (Wang & Miller, 2007). Therefore, the memory impaired profiles of patients with bv-FTD and AD may appear similar when memory is assessed with tests that place demands on frontal and/or attentional processes. In order to distinguish between these cognitive profiles, memory may be more accurately assessed with tests that overcome this limitation by controlling for attentional and executive processes. Consequently, bv-FTD patients should theoretically benefit from controlled learning procedures, like the category cueing FCSRT paradigm as this profile of memory impairment seems to be useful in the differentiation between bv-FTD and AD patients (Pasquier, Grymonprez, Lebert, & Van der Linden, 2001).
The main goal of this study was to show the utility of the FCSRT in the distinction between patients with bv-FTD and patients with AD, which may improve the accuracy of the diagnosis. Additionally, we aimed to characterize the memory impairment in patients with bv-FTD in comparison to AD patients. Our hypothesis was that the proposed cognitive mechanisms underlying these memory deficits would differentiate the two pathologies. This would be revealed as bv-FTD patients may benefit more from the controlled learning conditions which involves category cueing, than AD patients.
Methods
Participants
The total sample included 96 subjects divided into three subgroups: (i) 32 bv-FTD patients, (ii) 32 AD patients, and (iii) 32 cognitively healthy adults. The clinical study sample was recruited at the Neurology Department of the Coimbra University Hospital.
Study eligibility was restricted to patients with a comprehensive clinical, and neuropsychological evaluation, with a validated battery for the Portuguese population (Guerreiro, 1998), as well as a full investigation using biochemical, structural, and functional imaging (magnetic resonance imaging and single photon emission computed tomography, and/or positron emission tomography), which are essential to exclude other causes of dementia and to establish the clinical diagnosis.
The bv-FTD group included only patients with a diagnosis of the behavioral variant of FTD, established by a multidisciplinary team according to international criteria (Neary et al., 1998; Rascovsky et al., 2011). All the individuals who displayed the FTD-related Primary Progressive Aphasic syndromes (non-fluent or semantic dementia) (Gorno-Tempini et al., 2011) or mixed clinical syndromes were excluded from the study. In order to better characterize the study group, all patients with bv-FTD were further evaluated using the following instruments: the Montreal Cognitive Assessment (Freitas, Simões, Alves, & Santana, 2012), the Frontal Assessment Battery (Dubois, Slachevsky, Litvan, & Pillon, 2000), the Maze-Tracing Task (Lezak, Howieson, & Loring, 2004), the Comprehensive Affect Testing System (Schaffer, Wisniewski, Dahdah, & Froming, 2006), the Neuropsychiatric Inventory (Cummings et al., 1994), and the Frontal Behavior Inventory (Kertesz, Nadkami, Davidson, & Thomas, 2000) (data not shown).
The AD group included patients diagnosed by a multidisciplinary team consensus based on international criteria for probable AD (DSM-IV-TR; American Psychiatric Association, 2000; NINCDS-ADRDA; McKhann et al., 1984). This group was recruited to match the patients with bv-FTD by gender, age, education level, and severity of cognitive decline (mild forms), as assessed by the Clinical Dementia Rating scale (CDR; Garrett et al., 2008; Morris, 1993). Besides the comprehensive clinical/neuropsychological standard evaluation, AD patients underwent the following specific investigation: the Montreal Cognitive Assessment (Freitas, Simões, Alves, & Santana, 2012) and the Alzheimer Disease Assessment Scale (Guerreiro, Fonseca, Barreto, & Garcia, 2008; Mohs, Rosen, & Davis, 1983).
The following patient exclusion criteria were established at the outset of the study: an unstable clinical condition, with significant comorbidities; high severity dementia (only patients with CDR ≤ 1 and Mini-Mental State Examination (MMSE; Folstein, Folstein, & McHugh, 1975; Guerreiro, 1998) ≥ 15 points were included in the study); recent pharmacotherapy changes; recent psychiatric comorbidity (clinically diagnosed within 6 months prior to the current neuropsychological evaluation); and significant motor, visual or auditory deficits, all of which may influence the cognitive assessment.
The control group comprised 32 cognitively healthy adults belonging to the local community (recruited among the patients' spouses, hospital or university staff, or their relatives) that was age, education, and gender matched to the patients. They had no history of neurological or psychiatric relevant condition, including alcohol or drugs abuse or head trauma, and no significant motor, visual or auditory deficits which could influence the neuropsychological performance. All control subjects were assessed using the following instruments: a complete sociodemographic questionnaire; an inventory of current clinical health status, past habits and medical history; the MMSE; the CDR; and the Geriatric Depression Scale (GDS-30; Barreto, Leuschner, Santos, & Sobral, 2008; Yesavage et al., 1983). All control subjects had normal MMSE scores (mean 29.06), were fully autonomous, according to the information obtained through a general practitioner and/or an informant, and had no depression (depressive complaints were evaluated through a clinical interview and the GDS-30, and subjects with a score of 20 or more points were excluded).
The study was conducted in accordance with the tenets of the Declaration of Helsinki, with the approval of our local ethics committee. After obtaining an Informed consent, all the participants were submitted to the same experimental research protocol.
Demographic and clinical characteristics of the population are shown in Table 1.
Table 1.
Demographical and clinical characteristics of the population
| Control subjects (n = 32) | bv-FTD (n = 32) | AD (n = 32) | |
|---|---|---|---|
| Gender (male : female) | 22 : 10 | 22 : 10 | 17 : 15 |
| Age (years) | 68.59 (1.27) | 68.56 (1.19) | 69.72 (1.27) |
| Education level (years) | 7.06 (0.86) | 6.97 (0.84) | 6.91 (0.87) |
| MMSE (score) | 29.06 (0.20) | 26.88 (0.43)a | 21.22 (0.70)a,b |
| CDR (score) | 0 (0) | 1 (0) | 1 (0) |
Note: bv-FTD = behavioral frontotemporal dementia; AD = Alzheimer's disease; MMSE = Mini-Mental State Examination; CDR = Clinical Dementia Rating.
Data are expressed as mean (SEM).
Comparisons between Controls- bv-FTD, Controls-AD, and bv-FTD-AD patients were carried out by a one-way ANOVA with post hoc Tukey tests, Kruskal–Wallis 1-way ANOVA for k samples with pairwise comparisons, or χ2 test.
aControls versus bv-FTD: p < .05; Controls versus AD: p < .001.
bbv-FTD versus AD: p < .001.
Procedure
Subjects were assessed using the Portuguese version of the FCSRT (Lemos, Martins, Simões, & Santana, 2012). Materials and instructions of the FCSRT were provided by the original author (Buschke's FCSRT. Copyright, 2002. Albert Einstein College of Medicine of Yeshiva University, New York). The FCSRT (Buschke, 1984; Grober & Buschke, 1987) is a multi-trial memory test that uses a “selective reminding” paradigm by presenting only the words not recalled, instead of all the to-be-remembered words, thus directing the subject's attention to the words not recalled on the previous trial.
The test starts by asking subjects to identify words or pictures in response to a unique category cue. The 16 items to be learned are presented four at a time on a card, distributed by one word per quadrant. The subject is asked to search each card and point to and name aloud each item after its semantic cue was aurally presented. During this procedure, the subject is informed to learn the 16 words. There are three recall trials, each preceded by 20 s of counting backward to prevent recall from short-term memory. Each recall trial consisted of two parts. First, each subject had up to 2 min to freely recall as many items as possible. Next, aurally presented category cues were provided for items not retrieved by free immediate recall (Free IR). If subjects failed to retrieve the item with the category cue, they were reminded by presenting the cue and the item together (Cued IR). The sum of free and cued recall gives a measure of total immediate recall (Total IR). The same procedure of recalling (freely and cued) is done after a 30 min interval, during which subjects are required to perform non-verbal tasks (Delayed Recall—DR), allowing the measure of the Free DR, Cued DR, and Total DR). A percentage of retention was also computed, by comparing the total number of items recalled freely and on delayed recall to the total of items (free and cued) recalled on the third learning trial.
Besides the FCSRT, other neuropsychological tests were performed in order to attain other cognitive functions and evaluate the different performance between the two pathological groups: the Digit Span (Forward and Backward recall versions) of the WAIS-III (Wechsler, 2008) to assess immediate and working memory; the Trail Making Test (A and B) (Partington & Leiter, 1949) to measure speed of attention, working memory, sequencing and mental flexibility; the semantic verbal fluency task to evaluate the spontaneous production of words (food and animals) (Garcia, 1984); and the Brief Visuospatial Memory Test-Revised (BVMT-R; Benedict, 1997) as a measure of both visuospatial ability (copy) and visual memory (immediate and delayed recalls, learning and retention).
Statistical Analysis
Statistical analyses were performed using the Statistical Package for the Social Sciences (SPSS, version 19.0) (IBM SPSS, Inc., Chicago, IL). When data significantly deviated from normal distributions (verified using the Shapiro–Wilk normality check and Levene homogeneity tests) we did therefore choose to apply non-parametric statistical methods. Results with p < .05 were considered statistically significant. Descriptive statistics were used for sample's characterization; comparisons between means were performed with the use of the general linear model [one-way analysis of variance (ANOVA)] with post hoc Tukey for multiple comparisons or the Kruskal–Wallis one-way ANOVA for k samples with pairwise comparisons with adjusted p-value. The χ2 test was used for comparisons between categorical variables. Cronbach's alpha reliability coefficient was considered as an index of internal consistency, and analyzed separately for the immediate and delayed recalls. Non-parametric Wilcoxon test was used to compare the performance between the learning trials among each group.
A logistic regression model was fit to the data with impairment on both total immediate and total delayed recalls of the FCSRT as the outcome, and dementia subtype was a significant predictor in order to determine the sensitivity and the specificity of the FCSRT measures for distinguishing AD from bv-FTD.
Results
Sample Characterization
Demographical and clinical characteristics of the population are shown in Table 1. No statistically significant differences were found on age [F(2,93) = 0.280, p = .756], educational level (χ2KW (2) = 0.115, p = .944), or gender [χ2 (2) = 2.248, p = .325] between the three groups.
As expected, a significant effect was found for the MMSE performance (χ2KW (2) = 58.427, p = .000) among the three groups. Therefore, multiple comparisons revealed that both bv-FTD (p = .05) and AD (p < .001) performed poorly on the MMSE, when compared with control subjects and AD patients had a worst performance, when compared with bv-FTD subjects (p < .001).
Preliminary Tests
Concerning the performance on the preliminary neuropsychological tests, main effects were found in all of them, except for the Forward Digit Span. The multiple comparison analysis showed that AD patients were impaired in all other measures, whereas the bv-FTD group performed significantly worse than controls on the Trail Making Test (p < .05), Verbal Fluencies (p < .05), and BVMT recalls and learning (p < .001). Still, bv-FTD patients did not differ significantly from controls on the Backward Digit Span, BVMT-R copy and retention. The two clinical groups had a similar performance on the Backward Digit Span, Verbal Fluency (food), and BVMT-R learning (for details, see Table 2).
Table 2.
Performance on the preliminary tests
| Controls (n = 32) | bv-FTD (n = 32) | AD (n = 32) | χ2KW | Between-group comparisons | |
|---|---|---|---|---|---|
| Digit Span (forward) | 6.78 (0.32) | 6.09 (0.35) | 5.91 (0.32) | 4.366 | NS |
| Digit Span (backward) | 4.19 (0.36) | 3.16 (0.32) | 2.84 (0.24) | 7.804 | p = .02a |
| Trail Making Test A (time, s) | 89.03 (7.48) | 143.78 (16.18) | 198.75 (14.50) | 26.937 | p < .001b |
| Trail Making Test B (time, s) | 213.81 (20.86) | 374.34 (34.22) | 544.97 (19.57) | 41.723 | p < .001b |
| Verbal Fluency (food) | 15.59 (0.82) | 11.84 (0.73) | 9.47 (0.58) | 26.893 | p < .001c |
| Verbal Fluency (animals) | 13.81 (0.88) | 9.59 (0.75) | 7.06 (0.51) | 34.712 | p < .001b |
| BVMT-R Copy | 11.66 (0.12) | 11.13 (0.40) | 9.44 (0.65) | 12.603 | p = .002d |
| BVMT-R Total IR | 16.53 (1.34) | 7.06 (1.14) | 2.91 (0.48) | 51.011 | p < .001e |
| BVMT-R Learning | 4.00 (0.42) | 1.63 (0.30) | 0.72 (0.21) | 33.226 | p < .001f |
| BVMT-R Total DR | 7.00 (0.57) | 3.03 (0.56) | 0.38 (0.16) | 57.924 | p < .001g |
| BVMT-R Retention (%) | 90.50 (2.72) | 63.44 (7.42) | 14.41 (5.13) | 42.339 | p < .001h |
Notes: bv-FTD=behavioral frontotemporal dementia; AD = Alzheimer's disease; BVMT-R = Brief Visuospatial Memory Test-Revised; IR = Immediate Recall; DR = Delayed Recall.
Data are expressed as mean (SEM).
Comparisons between Controls- bv-FTD, Controls-AD, and bv-FTD-AD patients were carried out by a Kruskal–Wallis one-way ANOVA for k samples with pairwise comparisons.
aControls versus AD: p < .05.
bControls versus bv-FTD: p < .05; Controls versus AD: p < .001; bv-FTD versus AD: p < .05.
cControls versus bv-FTD: p < .05; Controls versus AD: p < .001; bv-FTD versus AD: NS.
dControls versus bv-FTD: NS; Controls versus AD: p < .05; bv-FTD versus AD: p < .05.
eControls versus bv-FTD: p < .001; Controls versus AD: p < .001; bv-FTD versus AD: p < .05.
fControls versus bv-FTD: p < .001; Controls versus AD: p < .001; bv-FTD versus AD: NS.
gControls versus bv-FTD: p < .001; Controls versus AD: p < .001; bv-FTD versus AD: p < .001.
hControls versus bv-FTD: NS; Controls versus AD: p < .001; bv-FTD versus AD: p < .001.
NS = not significant (p > .05).
Psychometric Properties—Internal Consistency Reliability of the FCSRT
Internal consistency reliability of the FCSRT was estimated using Cronbach's alpha. Within this analysis the Cronbach's alpha of the FCSRT as an index of internal consistency was 0.914 for the IR and 0.852 for the DR on the total study sample, confirming an overall good reliability of the test scores. This reliability coefficient was also computed for each clinical group [α (bv-FTD): IR = 0.813; DR =0.667 and α (AD): IR = 0.799; DR = 0.862].
Group Differences (FCSRT)
When analyzing the performance on the FCSRT selected measures (free, cued, total recalls, and retention), the bv-FTD and the AD groups were impaired relative to controls on the free and total recalls (p < .001); nevertheless, only the AD group was impaired on the two cued recalls (p < .001) and on the percentage of retention (p < .05). There was also a significant difference between the bv-FTD and AD patients in all the FCSRT selected measures (Table 3).
Table 3.
Performance on the FCSRT
| Controls (n = 32) | bv-FTD (n = 32) | AD (n = 32) | χ2KW | Between-group comparisons | |
|---|---|---|---|---|---|
| FCSRT Free IR | 21.84 (0.92) | 12.94 (1.16) | 3.41 (0.67) | 64.157 | p < .001a |
| FCSRT Cued IR | 17.41 (0.59) | 13.63 (1.18) | 7.72 (1.11) | 28.902 | p < .001b |
| FCSRT Total IR | 39.25 (0.94) | 26.56 (2.18) | 11.13 (1.61) | 55.752 | p < .001a |
| FCSRT Free DR | 8.72 (0.35) | 4.28 (0.49) | 0.72 (0.34) | 65.618 | p < .001c |
| FCSRT Cued DR | 5.34 (0.30) | 4.09 (0.42) | 2.03 (0.34) | 29.242 | p < .001b |
| FCSRT Total DR | 14.06 (0.30) | 8.38 (0.77) | 2.75 (0.55) | 62.742 | p < .001d |
| FCSRT retention (%) | −36.72 (1.98) | −37.11 (2.92) | −23.24 (2.95) | 14.835 | p = .001e |
Notes: bv-FTD=behavioral frontotemporal dementia; AD = Alzheimer's disease; FCSRT = Free and Cued Selective Reminding Test; IR = Immediate Recall; DR = Delayed Recall.
Data are expressed as mean (SEM).
Comparisons between Controls- bv-FTD, Controls-AD, and bv-FTD-AD patients were carried out by the Kruskal–Wallis one-way ANOVA for k samples with pairwise comparisons.
aControls versus bv-FTD: p < .05; Controls versus AD: p < .001; bv-FTD versus AD: p < .001.
bControls versus bv-FTD: NS; Controls versus AD: p < .001; bv-FTD versus AD: p < .05.
cControls versus bv-FTD: p < .001; Controls versus AD: p < .001; bv-FTD versus AD: p < .001.
dControls versus bv-FTD: p < .001; Controls versus AD: p < .001; bv-FTD versus AD: p < .05.
eControls versus bv-FTD: NS; Controls versus AD: p < .05; bv-FTD versus AD: p < .05.
NS = not significant (p > .05).
The learning slopes (along the three trials) among the three groups are shown in Fig. 1. Comparing the performance between the learning trials among each group, results showed a significant improvement on free recall, on both clinical groups, between trials 2 and 3 (bv-FTD: Z = −3.137; p = .002; AD: Z = −2.449; p < .05), but not between trials 1 and 2 (p > .05). The control group revealed an improvement between the three trials (Trials 2–1: Z = −3.704; p < .001; Trials 3–2: Z = −4.131; p < .001). In order to analyze the impact of cueing, the same comparisons were done for the total (free + cued) recalls along the three learning trials. Whereas the bv-FTD (Trials 2–1: Z = −3.423; p = .001; Trials 3–2: Z = −3.408; p = .001) and the control (Trials 2–1: Z = −4.610; p < .001; Trials 3–2: Z = −3.779; p < .001) groups showed a significant improvement between the three trials, the AD group only benefited from cueing between the third and second trials (Trials 2–1: Z = −0.558; p > .05; Trials 3–2: Z = −3.567; p < .001).
Fig. 1.
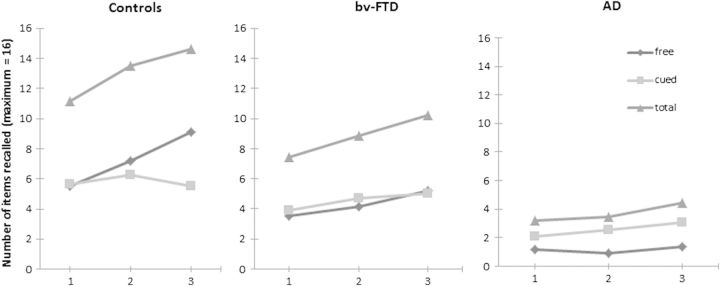
Free, cued, and total recalls (mean) in the FCSRT along the first, second, and third learning trials. bv-FTD = behavioral frontotemporal dementia; AD = Alzheimer's disease.
Established cut-off scores (Lemos, Simões, Santiago, & Santana, 2014) for AD were used to determine the sensitivity and the specificity of the total immediate and total delayed recalls of the FCSRT for patients with AD versus bv-FTD. A logistic regression model was fit to the data; impairment on both total immediate and total delayed recalls of the FCSRT were used as the outcome; dementia subtype was a significant predictor in order to determine the sensitivity and the specificity of the FCSRT measures for distinguishing AD from bv-FTD . The model showed an accuracy of 78.1% against a baseline value of 54.8% (i.e., when the classification is performed randomly) in the subjects' classification, with a specificity of 71.4%, and a sensitivity of 90.9%. Sixty-three percent (20/32) patients with bv-FTD had an intact FCSRT, whereas only 6% (2/32) of AD patients scored normally. Patients with AD were 25 times more likely to have impaired FCSRT than patients with bv-FTD.
Discussion
The main objective of this study was to show the utility of the FCSRT in distinguishing bv-FTD from AD patients. Furthermore, we aimed to contribute to the characterization of the memory impairment observed in patients with bv-FTD in comparison with AD patients.
The FCSRT showed an overall good reliability with high indexes of internal consistency for the immediate recall and for the delayed recall trials, in this sample of Portuguese participants.
Moreover, the FCSRT showed an accuracy of 78.1%, with 71.4% of specificity and 90.9% of sensitivity, allowing us to identify 95% of patients with AD and discard 63% of patients with bv-FTD, indicating that an impaired FCSRT performance emphasizes the significance of episodic memory deficits in patients with AD. Patients with AD were 25 times more likely to have impaired FCSRT performances than patients with bv-FTD.
Another aim of this study was to compare the pattern of memory impairment on FCSRT in patients with bv-FTD and AD. We observed that both groups were relatively impaired to the control group on the free and total (immediate and delayed) recalls trials. Furthermore, only the AD group showed impairment on the cued recall trial and on the percentage of items retained. Our initial hypothesis was that the mechanisms underlying the memory deficits observed in these groups would differentiate the two pathologies, as bv-FTD patients may benefit more from the controlled learning with the use of category cues, than AD patients. Our results confirmed that, although bv-FTD patients were impaired on the total recall trials, cueing was more efficient for patients with bv-FTD than AD. In addition, bv-FTD patients showed an impaired delayed recall but their ability to retain information was spared, that is, when delayed recall performance was compared with performance on the third learning trial.
The slopes of the groups' performances were compared on free recall of the three learning trials. Our results showed that the control group performance improved incrementally over the three trials while both the clinical groups' improvement was restricted to just the second and third trials. The impact of cueing resulted, overall in a significant improvement over the three trials for both the bv-FTD and control groups. However, this effect was only observed between the second and third trials in the AD group. These results showed a pattern of impairment for the AD group independently of the recall (free or total); whereas the bv-FTD group appeared to benefit from cueing which was reflected in a marked improvement in the performance on the total recall trial. The concept of cueing, inherent in the design of the FCSRT, requires subjects to search for items in response to already given category cues in the learning process, controlling for both attention and cognitive processing. This method of cueing showed an improvement of the retrieval ability in bv-FTD patients, which has not been observed with tests that employ other methods of cueing (Glosser, Gallo, Clark, & Grossman, 2002). It has been reported that learning and memory ability are based on the integrity of the temporal and frontal lobe regions of the brain. These are regions which may play different roles depending on the task demands and test characteristics. Much evidence suggests that basic learning and retrieval aspects of memory are supported by the medial temporal lobe. Specifically, it is well documented that the hippocampus plays a significant role in the formation and memorization of associations between novel non-related items (Squire, 1992). On the other hand, the frontal lobes have also been shown to be implicated in learning and memory processes, contributing to efficient working memory, conditional learning, and encoding strategies (Cummings & Miller, 2007). The FCSRT is a memory test that controls for attention and executive processing, by introducing category cues during the learning process. Additionally, the same cues are used to elicit recall of the items not retrieved on the free recall trial, and thereby control acquisition and retrieval.
Our study demonstrated memory impairment in patients within the early stages of bv-FTD. This is common in the early stages of the disease, sometimes leading to a misdiagnosis of AD (Wittenberg et al., 2008). In the FCSRT, free and cued recalls were poorer in bv-FTD patients than in controls, and free recall was as poorer in bv-FTD patients than in AD patients, which is consistent with the findings reported by Pasquier and colleagues (2001). Providing a semantic cue to bv-FTD patients showed a significant increase in recall performance, suggesting that there may be impairment in retrieval processes when encoding processes are controlled for. The rates of forgetting did not differ between bv-FTD patients and control subjects, which was assessed by comparing the retention percentages of the two groups, indicating that the bv-FTD group had a relatively intact storage processes. In sum, poor free and total recall performance rates, both in learning and after a delay, were observed in both dementia groups; low scores in cued recalls and a lower rate of retention were representative of AD, while an improvement with category cueing and spared retained items were suggestive of bv-FTD. The design characteristics of memory test paradigms may account for the distinct pattern of performance of patients with different types of dementia. Regular word list tests demand a reproduction of a list of unrelated words, thus requiring an active effort to organize information at both encoding and retrieval; however, tests with semantically organized material (such as story recall tests or the FCSRT) allow for more passive learning and implementation of less demanding retrieval strategies (Perri, Fadda, Caltagirone, & Carlesimo, 2013). Therefore, while AD patients are expected to be impaired on both paradigms, bv-FTD patients are more likely to have deficits on regular word list tests than tests which require the participant to organize semantic information (Pasquier et al., 2001; Perri et al., 2013), which is consistent with the findings from the present study. Concerning the performance on the neuropsychological tests that measure other cognitive functions, the bv-FTD group showed impairment on attention, working memory, and spontaneous production of words, and spared visuospatial ability and retention. These findings are congruent with the brain structures reported to be implicated in these abilities and often compromised in this disease. Comparison of the two clinical groups revealed that, although bv-FTD patients had better results than AD's on most neuropsychological measures, the two samples had similar performances on the Backward Digit Span, Verbal Fluency (food) and BVMT-R learning tasks. A general pattern of impairment for both bv-FTD and AD patients in the executive domain has already been reported by several authors, confirming that there was no difference of performances between these two groups (Gregory, Orrell, Sahakian, & Hodges, 1997; Hodges et al., 1999; Walker, Meares, Sachdev, & Brodaty, 2005). These findings reinforce the idea that executive functions, although expected to be impaired in cases of frontal lobe damage, should not be the only focus of attention in the differential diagnosis between AD and bv-FTD (Giovagnoli, Erbetta, Reati, & Bugiani, 2008). Nevertheless, executive measures that rely on both frontal (compromised in bv-FTD) and parietal (compromised in AD) regions comprise a similar pattern of performance in both disease groups. Thus, frontally specific executive measures are expected to inform the diagnosis of bv-FTD and therefore should be selected (Possin et al., 2013). Moreover, in our study, the main pattern of dissociation between bv-FTD and AD was the impairment of parieto-occipital-related functions which seems to be exclusive to AD. Furthermore, information regarding aspects of social cognition including personality and/or behavioral disorders was taken into consideration during the diagnosis of bv-FTD.
An accurate differential diagnosis between AD and bv-FTD is of crucial importance. Given that FTD is the second cause of primary degenerative dementia and has overlapping symptoms with AD, a misdiagnosis can occur. Deficits in memory are common in FTD patients and may be overlooked due to a greater prominence of behavioral and/or personality disturbances. For these reasons, it is more informative to use memory tests that control for the learning deficit often observed in FTD patients so as to elicit the retrieval of stored information. This may aid to isolate the memory deficit more typical in AD patients and thereby increase the accuracy of a diagnosis. The FCSRT has shown these potentialities in the present study. However, future research would benefit by including other types of dementias in order to confirm the accuracy values of the FCSRT in informing a diagnosis for AD.
Funding
This work was supported by grants from the Fundação para a Ciência e Tecnologia (FCT), Portugal: (SFRH/BD/74070/2010 to R.L. and PIC/IC/83206/2007 to I.S.).
Conflict of Interest
None declared.
Acknowledgements
We are grateful to all the participants for their contribution in this study, and Bárbara Oliveiros for her support in data analysis.
References
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders (DSM-IV-TR) 4th ed., text revision. Washington, DC: The author; 2000. [Google Scholar]
- Barreto J., Leuschner A., Santos F., Sobral M. Escala de Depressão Geriátrica [Geriatric Depression Scale] In: de Mendonça A., Garcia C., Guerreiro M., editors. escalas e Testes na Demência [Scales and tests in dementia] Grupo de Estudos de Envelhecimento Cerebral e Demência [Study Group on Brain Aging and Dementia] Lisbon: GEECD; 2008. pp. 69–72. [Google Scholar]
- Benedict R. Brief visuospatial memory test-revised. Odessa, FL: Psychological Assessment Resources, Inc; 1997. [Google Scholar]
- Blumenfeld R. S., Ranganath C. Prefrontal cortex and long-term memory encoding: An integrative review of findings from neuropsychology and neuroimaging. Neuroscientist. 2007;13:280–291. doi: 10.1177/1073858407299290. [DOI] [PubMed] [Google Scholar]
- Brown L. B., Storandt M. Sensitivity of category cued recall to very mild dementia of the Alzheimer type. Archives of Clinical Neuropsychology. 2000;15:529–534. [PubMed] [Google Scholar]
- Buschke H. Cued recall in amnesia. Journal of Clinical and Experimental Neuropsychology. 1984;6:433–440. doi: 10.1080/01688638408401233. [DOI] [PubMed] [Google Scholar]
- Buschke H. Criteria for the identification of memory deficits: Implications for the design of memory tests. In: Gorfein D. S., Hoffman R. R., editors. Memory and learning: The ebbinghaus centenial conference. Hillsdale, NJ: Lawrence Erlbaum; 1987. pp. 331–344. [Google Scholar]
- Buschke H., Sliwinski M. J., Kuslansky G., Lipton R. B. Diagnosis of early dementia by the Double Memory Test: Encoding specificity improves diagnostic sensitivity and specificity. Neurology. 1997;48:989–997. doi: 10.1212/wnl.48.4.989. [DOI] [PubMed] [Google Scholar]
- Cummings J. L., Mega M., Gray K., Rosenberg-Thompson S., Carusi D. A., Gornbein J. The Neuropsychiatric Inventory comprehensive assessment of psychopathology in dementia. Neurology. 1994;44:2308–2314. doi: 10.1212/wnl.44.12.2308. [DOI] [PubMed] [Google Scholar]
- Cummings J. L., Miller B. L. Conceptual and clinical aspects of the frontal lobes. In: Miller B. L., Cummings J. L., editors. The human frontal lobes: Functions and disorders. New York: The Guilford Press; 2007. pp. 25–43. [Google Scholar]
- Dubois B., Feldman H. H., Jacova C., Cummings J. L., Dekosky S. T., Barberger-Gateau P., et al. Revising the definition of Alzheimer's disease: A new lexicon. Lancet Neurology. 2010;9:1118–1127. doi: 10.1016/S1474-4422(10)70223-4. [DOI] [PubMed] [Google Scholar]
- Dubois B., Feldman H. H., Jacova C., Dekosky S. T., Barberger-Gateau P., Cummings J., et al. Research criteria for the diagnosis of Alzheimer's disease: Revising the NINCDS-ADRDA criteria. Lancet Neurology. 2007;6:734–746. doi: 10.1016/S1474-4422(07)70178-3. [DOI] [PubMed] [Google Scholar]
- Dubois B., Slachevsky A., Litvan I., Pillon B. The FAB: A Frontal Assessment Battery at bedside. Neurology. 2000;55:1621–1626. doi: 10.1212/wnl.55.11.1621. [DOI] [PubMed] [Google Scholar]
- Folstein M. F., Folstein S. E., McHugh P. R. Mini-Mental State: A practical method for grading the cognitive state of patients for the clinician. Journal of Psychiatric Research. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- Freitas S., Simões M. R., Alves L., Santana I. Montreal Cognitive Assessment (MoCA): Validation study for Mild Cognitive Impairment and Alzheimer's disease. Alzheimer’s Disease and Associated Disorders. 2012;27:37–43. doi: 10.1097/WAD.0b013e3182420bfe. [DOI] [PubMed] [Google Scholar]
- Garcia C. A Doença de Alzheimer: Problemas de diagnóstico clínico [Alzheimer's disease: Difficulties in clinical diagnosis] Lisbon: Faculty of Medicine, University of Lisbon; 1984. (Unpublished doctoral dissertation thesis) [Google Scholar]
- Garrett C., Santos F., Tracana I., Barreto J., Sobral M., Fonseca R. Avaliação Clínica da Demência [Clinical Dementia Rating] In: de Mendonça A., Garcia C., Guerreiro M., editors. escalas e Testes na Demência [Scales and tests in dementia] Grupo de Estudos de Envelhecimento Cerebral e Demência [Study Group on Brain Aging and Dementia] Lisbon: GEECD; 2008. pp. 18–32. [Google Scholar]
- Geerlings M. I., Jonker C., Bouter L. M., Ader H. J., Schmand B. Association between memory complaints and incident Alzheimer's disease in elderly people with normal baseline cognition. The American Journal of Psychiatry. 1999;156:531–537. doi: 10.1176/ajp.156.4.531. [DOI] [PubMed] [Google Scholar]
- Giovagnoli A. R., Erbetta A., Reati F., Bugiani O. Differential neuropsychological patterns of frontal variant frontotemporal dementia and Alzheimer's disease in a study of diagnostic concordance. Neuropsychologia. 2008;46:1495–1504. doi: 10.1016/j.neuropsychologia.2007.12.023. [DOI] [PubMed] [Google Scholar]
- Glosser G., Gallo J. L., Clark C. M., Grossman M. Memory encoding and retrieval in frontotemporal dementia and Alzheimer's disease. Neuropsychology. 2002;16:190–196. [PubMed] [Google Scholar]
- Gorno-Tempini M. L., Hillis A. E., Weintraub S., Kertesz A., Mendez M., Cappa S. F., et al. Classification of primary progressive aphasia and its variants. Neurology. 2011;76:1006–1014. doi: 10.1212/WNL.0b013e31821103e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregory C. A., Orrell M., Sahakian B., Hodges J. R. Can frontotemporal dementia and Alzheimer's disease be differentiated using a brief battery of tests? International Journal of Geriatric Psychiatry. 1997;12:375–383. doi: 10.1002/(sici)1099-1166(199703)12:3<375::aid-gps518>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- Grober E., Buschke H. Genuine memory deficits in dementia. Developmental Neuropsychology. 1987;3:13–36. [Google Scholar]
- Grober E., Buschke H., Crystal H., Bang S., Dresner R. Screening for dementia by memory testing. Neurology. 1988;38:900–903. doi: 10.1212/wnl.38.6.900. [DOI] [PubMed] [Google Scholar]
- Grober E., Gitlin H., Bang S., Buschke H. Implicit and explicit memory in young, old and demented adults. Journal of Clinical and Experimental Neuropsychology. 1992;14:298–316. doi: 10.1080/01688639208402830. [DOI] [PubMed] [Google Scholar]
- Grober E., Hall C. B., Lipton R. B., Zonderman A. B., Resnick S. M., Kawas C. Memory impairment, executive dysfunction, and intellectual decline in preclinical Alzheimer's disease. Journal of the International Neuropsychology Society. 2008;14:266–278. doi: 10.1017/S1355617708080302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grober E., Hall C., Sanders A. E., Lipton R. B. Free and cued selective reminding distinguishes Alzheimer's disease from vascular dementia. Journal of the American Geriatrics Society. 2008;56:944–946. doi: 10.1111/j.1532-5415.2008.01652.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grober E., Kawas C. Learning and retention in preclinical and early Alzheimer's disease. Psychology and Aging. 1997;12:183–188. doi: 10.1037//0882-7974.12.1.183. [DOI] [PubMed] [Google Scholar]
- Grober E., Sanders A. E., Hall C., Lipton R. B. Free and cued selective reminding identifies very mild dementia in primary care. Alzheimer’s Disease and Associated Disorders. 2010;24:284–290. doi: 10.1097/WAD.0b013e3181cfc78b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerreiro M. Contributo da neuropsicologia para o estudo das demências [contribution of neuropsychology to the study of dementia] Lisbon: University of Lisbon; 1998. (Unpublished doctoral dissertation) [Google Scholar]
- Guerreiro M., Fonseca S., Barreto J., Garcia C. Escala de Avaliação da Doença de Alzheimer – EADA [Alzheimer Disease Assessment Scale – ADAS] In: de Mendonça A., Garcia C., Guerreiro M., editors. escalas e Testes na Demência [Scales and tests in dementia] Grupo de Estudos de Envelhecimento Cerebral e Demência [Study Group on Brain Aging and Dementia] Lisbon: GEECD; 2008. pp. 42–58. [Google Scholar]
- Habert M. O., Hornn J. F., Sarazin M., Lotterie J. A., Puel M., Onen F., et al. Brain perfusion SPECT with an automated quantitative tool can identify prodromal Alzheimer's disease among patients with mild cognitive impairment. Neurobiology of Aging. 2011;32(1):15–23. doi: 10.1016/j.neurobiolaging.2009.01.013. doi:10.1016/j.neurobiolaging.2009.01.013. [DOI] [PubMed] [Google Scholar]
- Harvey R. J., Skelton-Robinson M., Rossor M. N. The prevalence and causes of dementia in people under the age of 65 years. Journal of Neurology, Neurosurgery and, Psychiatry. 2003;74:1206–1209. doi: 10.1136/jnnp.74.9.1206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodges J. R., Patterson K., Ward R., Garrard P., Bak T., Perry R., et al. The differentiation of semantic dementia and frontal lobe dementia (temporal and frontal variants of frontotemporal dementia) from early Alzheimer's disease: A comparative neuropsychological study. Neuropsychology. 1999;13:31–40. doi: 10.1037//0894-4105.13.1.31. [DOI] [PubMed] [Google Scholar]
- Ivanoiu A., Adam S., Van der Linden M., Salmon E., Juillerat A. C., Mulligan R., et al. Memory evaluation with a new cued recall test in patients with mild cognitive impairment and Alzheimer's disease. Journal of Neurology. 2005;252:47–55. doi: 10.1007/s00415-005-0597-2. [DOI] [PubMed] [Google Scholar]
- Johnson J. K., Diehl J., Mendez M. F., Neuhaus J., Shapira J. S., Forman M., et al. Frontotemporal lobar degeneration demographic characteristics of 353 patients. Archives of Neurology. 2005;62:925–930. doi: 10.1001/archneur.62.6.925. [DOI] [PubMed] [Google Scholar]
- Kertesz A., Nadkami N., Davidson W., Thomas A. W. The Frontal Behavioral Inventory in the differential diagnosis of frontotemporal dementia. Journal of the International Neuropsychological Society. 2000;6:460–468. doi: 10.1017/s1355617700644041. [DOI] [PubMed] [Google Scholar]
- Lemos R., Martins C., Simões M. R., Santana I. Estudo de adaptação do Teste de Recordação Selectiva Livre e Guiada para a população portuguesa [Adaptation Study of the Free and Cued Selective Reminding Test for the portuguese population] Avaliação Psicológica. 2012;11:49–61. [Google Scholar]
- Lemos R., Simões M. R., Santiago B., Santana I. The Free and Cued Selective Reminding Test: Validation for Mild Cognitive Impairment and Alzheimer's disease. Journal of Neuropsychology. 2014 doi: 10.1111/jnp.12048. doi:10.1111/jnp.12048. [DOI] [PubMed] [Google Scholar]
- Lezak M. D., Howieson D. B., Loring D. W. Neuropsychological assessment. New York: Oxford University Press; 2004. [Google Scholar]
- Lindberg O., Walterfang M., Looi J. C., Malykhin N., Ostberg P., Zandbelt B., et al. Hippocampal shape analysis in Alzheimer's disease and frontotemporal lobar degeneration subtypes. Journal of Alzheimer’s Disease. 2012;30(2):355–365. doi: 10.3233/JAD-2012-112210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKhann G. M., Albert M. S., Grossman M., Miller B., Dickson D., Trojanowski J. Q. Work Group on Frontotemporal Dementia and Pick's Disease. Clinical and pathological diagnosis of frontotemporal dementia: Report of the Work Group on Frontotemporal Dementia and Pick's Disease. Archives of Neurology. 2001;58:1803–1809. doi: 10.1001/archneur.58.11.1803. [DOI] [PubMed] [Google Scholar]
- McKhann G., Drachman D., Folstein M., Katzman R., Price D., Stadlan E. M. Clinical diagnosis of Alzheimer's disease: Report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer's Disease. Neurology. 1984;34:939–944. doi: 10.1212/wnl.34.7.939. [DOI] [PubMed] [Google Scholar]
- Mohs R. C., Rosen W. G., Davis K. L. The Alzheimer's Disease Assessment Scale: An instrument for assessing treatment efficacy. Psychopharmacology Bulletin. 1983;19:448–450. [PubMed] [Google Scholar]
- Morris J. C. The Clinical Dementia Rating (CDR): Current version and scoring rules. Neurology. 1993;43:2412–2414. doi: 10.1212/wnl.43.11.2412-a. [DOI] [PubMed] [Google Scholar]
- Muñoz-Ruiz M. A., Hartikainen P., Koikkalainen J., Wolz R., Julkunen V., Niskanen E., et al. Structural MRI in frontotemporal dementia: Comparisons between hippocampal volumetry, tensor-based morphometry and voxel-based morphometry. PLoS ONE. 2012;7(12):e52531. doi: 10.1371/journal.pone.0052531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neary D., Snowden J. S., Gustafson L., Passant U., Stuss D., Black S., et al. Frontotemporal lobar degeneration: A consensus on clinical diagnostic criteria. Neurology. 1998;51:1546–1554. doi: 10.1212/wnl.51.6.1546. [DOI] [PubMed] [Google Scholar]
- Partington J. E., Leiter R. G. Partington's Pathway Test. The Psychological Service Center Bulletin. 1949;1:9–20. [Google Scholar]
- Pasquier F., Grymonprez L., Lebert F., Van der Linden M. Memory impairment differs in frontotemporal dementia and Alzheimer's disease. Neurocase. 2001;7:161–171. doi: 10.1093/neucas/7.2.161. [DOI] [PubMed] [Google Scholar]
- Perri R., Fadda L., Caltagirone C., Carlesimo G. A. Word list and story recall elicit different patterns of memory deficit in patients with Alzheimer's disease, frontotemporal dementia, subcortical ischemic vascular disease, and Lewy body dementia. Journal of Alzheimer’s Disease. 2013;37:99–107. doi: 10.3233/JAD-130347. [DOI] [PubMed] [Google Scholar]
- Petersen R. C., Smith G. E., Ivnik R. J., Kokmen E., Tangalos E. G. Memory function in very early Alzheimer's disease. Neurology. 1994;44:867–872. doi: 10.1212/wnl.44.5.867. [DOI] [PubMed] [Google Scholar]
- Possin K. L., Feigenbaum D., Rankin K. P., Smith G. E., Boxer A. L., Wood K., et al. Dissociable executive functions in behavioral variant frontotemporal and Alzheimer dementias. Neurology. 2013;80:2180–2185. doi: 10.1212/WNL.0b013e318296e940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rami L., Fortea J., Bosch B., Solé-Padullés C., Lladó A., Iranzo A., et al. Cerebrospinal fluid biomarkers and memory present distinct associations along the continuum from healthy subjects to AD patients. Journal of Alzheimer’s Disease. 2011;23:319–326. doi: 10.3233/JAD-2010-101422. [DOI] [PubMed] [Google Scholar]
- Rascovsky K., Hodges J. R., Knopman D., Mendez M. F., Kramer J. H., Neuhaus J., et al. Sensitivity of revised diagnostic criteria for the behavioural variant of frontotemporal dementia. Brain. 2011;134:2456–2477. doi: 10.1093/brain/awr179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sánchez-Benavides G., Gómez-Ansón B., Molinuevo J. L., Blesa R., Monte G. C., Buschke H., et al. Medial temporal lobe correlates of memory screening measures in normal aging, MCI, and AD. Journal of Geriatric Psychiatry and Neurology. 2010;23:100–108. doi: 10.1177/0891988709355271. [DOI] [PubMed] [Google Scholar]
- Sarazin M., Chauviré V., Gerardin E., Colliot O., Kinkingnéhun S., de Souza L. C., et al. The amnestic syndrome of hippocampal type in Alzheimer's disease: An MRI study. Journal of Alzheimer's Disease. 2010;22:285–294. doi: 10.3233/JAD-2010-091150. [DOI] [PubMed] [Google Scholar]
- Schaffer S. G., Wisniewski A., Dahdah M., Froming K. B. Emotion processing: The comprehensive affect testing system. User's manual. Sanford, FL: Psychology Software Inc; 2006. [Google Scholar]
- Seelaar H., Kamphorst W., Rosso S. M., Azmani A., Masdjedi R., de Koning I., et al. Distinct genetic forms of frontotemporal dementia. Neurology. 2008;71:1220–1226. doi: 10.1212/01.wnl.0000319702.37497.72. [DOI] [PubMed] [Google Scholar]
- Seelaar H., Rohrer J. D., Pijnenburg Y. A., Fox N. C., van Swieten J. C. Clinical, genetic and pathological heterogeneity of frontotemporal dementia: A review. Journal of Neurology, Neurosurgery, and Psychiatry. 2011;82:476–486. doi: 10.1136/jnnp.2010.212225. [DOI] [PubMed] [Google Scholar]
- Squire L. R. Memory and the hippocampus: A synthesis from findings with rats, monkeys, and humans. Psychological Review. 1992;99:195–231. doi: 10.1037/0033-295x.99.2.195. [DOI] [PubMed] [Google Scholar]
- Squire L. R., Stark C. E., Clark R. E. The medial temporal lobe. Annual Review Neuroscience. 2004;27:279–306. doi: 10.1146/annurev.neuro.27.070203.144130. [DOI] [PubMed] [Google Scholar]
- Tulving E., Osler S. Effectiveness of retrieval cues in memory for words. Journal of Experimental Psychology. 1968;77:593–601. doi: 10.1037/h0026069. [DOI] [PubMed] [Google Scholar]
- Tulving E., Thomson D. M. Encoding specificity and retrieval processes in episodic memory. Psychological Review. 1973;80:352–373. [Google Scholar]
- van de Pol L. A., Hensel A., van der Flier W. M., Visser P. J., Pijnenburg Y. A., Barkhof F., et al. Hippocampal atrophy on MRI in frontotemporal lobar degeneration and Alzheimer's disease. Journal of Neurology, Neurosurgery, and Psychiatry. 2006;77:439–442. doi: 10.1136/jnnp.2005.075341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogel A., Mortensen E. L., Gade A., Waldemar G. The category cued recall test in very mild Alzheimer's disease: Discriminative validity and correlation with semantic memory functions. European Journal of Neurology. 2007;14:102–108. doi: 10.1111/j.1468-1331.2006.01568.x. [DOI] [PubMed] [Google Scholar]
- Wagner M., Wolf S., Reischies F. M., Daerr M., Wolfsgruber S., Jessen F., et al. Biomarker validation of a cued recall memory deficit in prodromal Alzheimer disease. Neurology. 2012;78:379–386. doi: 10.1212/WNL.0b013e318245f447. [DOI] [PubMed] [Google Scholar]
- Walker A. J., Meares S., Sachdev P. S., Brodaty H. The differentiation of mild frontotemporal dementia from Alzheimer's disease and healthy aging by neuropsychological tests. International Psychogeriatrics. 2005;17:57–68. doi: 10.1017/s1041610204000778. [DOI] [PubMed] [Google Scholar]
- Wang P-N., Miller B. L. Clinical aspects of frontotemporal dementia. In: Miller B. L., Cummings J. L., editors. The human frontal lobes: Functions and disorders. New York: The Guilford Press; 2007. pp. 365–381. [Google Scholar]
- Wechsler D. Escala de Inteligência de Wechsler para Adultos—3ª Ed. Lisbon: Cegoc-Tea; 2008. [Wechsler Adult Intelligence Scale—3rd ed.] [Google Scholar]
- Weder N. D., Aziz R., Wilkins K., Tampi R. R. Frontotemporal dementias: A review. Annals of General Psychiatry. 2007;6:15. doi: 10.1186/1744-859X-6-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wittenberg D., Possin K. L., Rascovsky K., Rankin K. P., Miller B. L., Kramer J. H. The early neuropsychological and behavioral characteristics of frontotemporal dementia. Neuropsychology Review. 2008;18:91–102. doi: 10.1007/s11065-008-9056-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yesavage J. A., Brink T. L., Rose T. L., Lum O., Huang V., Adey M., et al. Development and validation of a geriatric depression screening scale: A preliminary report. Journal of Psychiatric Research. 1983;17:37–49. doi: 10.1016/0022-3956(82)90033-4. [DOI] [PubMed] [Google Scholar]


