Abstract
Very few data are available on the long-term changes in the cognitive abilities of patients with loss of psychic self-activation syndrome (LPSAS). Here, we present a 25-year follow-up study on a case of LPSAS resulting from bilateral pallidal lesions caused by carbon monoxide intoxication. Typical signs of LPSAS were observed, showing no changes in severity, but Ganser syndrome (GS) gradually developed and worsened during the follow-up period. GS is generally assumed to be a psychogenic syndrome, but an organic etiology has been suspected by the authors of several case reports. Here, atypical features of GS plead against the independence of GS and LPSAS. DaTSCAN and brain 18FDG-PET were performed. Since left hippocampal hypometabolism has been previously described in patients with functional amnesia, it is possible that long periods of mental inactivity may have psychological consequences, but the atypical features of GS also suggest that an organic mechanism may be involved.
Keywords: Ganser syndrome, Loss of psychic self-activation syndrome, Functional neuroimaging, Neuropsychological assessment, CO intoxication, Pallidal lesion, Basal ganglia, Decision-making
Introduction
Loss of psychic self-activation syndrome (LPSAS) was first described by Laplane (Laplane, Baulac, & Pillon, 1982; Laplane, Baulac, Wildocher, & Dubois, 1984; Laplane, Wildocher, Pillon, Baulac, & Binoux, 1981) in patients suffering from carbon monoxide (CO) intoxication or wasp stings. These patients usually present with a persistent behavioral disorder: in the absence of external stimulation, they remain inert and devoid of spontaneous activity. When questioned, they report feelings of mental emptiness. However, their intellectual performances appear to be nearly normal when they have been stimulated by contacts with other people. Flattening of the affects and compulsive behavior are also frequently observed. The fact that bilateral pallidal lesions are detected in most cases of LPSAS suggests that the pallidum may contribute importantly to the initiation and maintenance of these patients' behavioral, affective and mental activity, probably via sub-cortico-frontal circuits (Alexander, 1986; Cummings, 1993; Levy & Dubois, 2006).
Since the long-term outcome of this condition is practically unknown, it seemed to be worth describing its evolution in order to determine what becomes of these patients, whose spontaneous cognitive activity is greatly impaired. Only two reports have been published so far to our knowledge on this topic. The neuropsychological performances of one patient with LPSAS who was followed by Piccirilli, Mazzi, Luccioli, and Sciama (1995) for 10 years remained unchanged during this period. Case 1 described by Laplane et al. (1984), who was re-examined 12 years after sustaining CO intoxication, also showed stable behavioral disorders.
Here, we report on the 25-year follow-up of Mrs PA, who sustained CO intoxication, resulting in isolated LPSAS (Ali Chérif et al., 1984). Ganser syndrome (GS) gradually developed unexpectedly, starting about 8 years after the CO intoxication. The “à côté” responses which are the main symptom of GS were first defined in 1897 (Schorer, 1965) as the inability to answer the simplest questions correctly, although the patient's comprehension is not impaired, associated with visual and auditory hallucinations and fluctuating clouding of consciousness. Since this syndrome frequently occurs in prisoners and the symptoms of this kind usually start and end quite suddenly, it may be of psychogenic origin. However, the histories of some patients with GS are consistent with central nervous involvement, as they had undergone severe head injuries (Heron, Kritchevsky, & Delis, 1991; Latcham, White, & Sims, 1978; Sigal, Altmark, Alfici, & Gelkopf, 1992; Schorer, 1965), typhoid encephalitis (Sigal et al., 1992), and diffuse micro-embolic lesions (Lee & Koenig, 2001) or presented with associated Gilles de la Tourette syndrome (Refaat, Firth, & Robertson, 2002). It has sometimes been suggested that neurological involvement may be a triggering rather than a causal factor.
Methods
Neurological Examination
The same neurologist examined Mrs PA every year from 1981 to 2006. Neurological and behavioral tests were performed, taking into account the comments made by her mother, who was living with her.
Neuropsychological Assessments
Neurological tests were performed in 1981, 1988, 1995, and 2006. The tests applied each time included tests on the patient's global intellectual performances (the Wechsler Adult Intelligence Scale Revised [WAIS] [Wechsler, 1981], the Binois–Pichot Vocabulary test [Binois & Pichot, 1956], the Wechsler Memory Scale Revised [WMS] [Wechsler, 2001]), tests on executive functions (Trail Making Test A [Reitan, 1955], the Modified Card Sorting Test [Berg, 1948], the Digit span Stroop Test [Stroop, 1935], Rey's figure copying and Verbal Fluency tests), comprehension tests (the Token test; DeRenzie & Vignolo, 1962), designation tests (in response to visual and auditory inputs including both verbal and non-verbal material), denomination tests with visual and auditory stimuli, including the Famous Face recognition test, Picture Confrontation, the oral Naming test (DO80) (Deloche & Hannequin, 1997; the Sound Lotto test, etc.), memory assessment tests (the Visual Recognition memory test [Barbeau et al., 2004], etc.), calculation tests (with verbal and written numbers), drawing and writing tests in response to instructions, etc. Because of the patient's behavioral disorders, we had to gradually adapt some of these tests to force her to choose between two possible responses.
Psychiatric Assessments
Two independent psychiatrists examined Mrs PA 25 years after the CO intoxication event, looking for psychiatric and pathological personality signs.
Cerebral Functional Imaging
Dopamine transporter SPECT imaging
Mrs PA underwent [123I]FP-CIT SPECT (DaTSCAN) 25 years after the CO intoxication event. Thyroid uptake was blocked by administering 600 mg of sodium perchlorate orally 30 min before applying the tracer. She was then given 148 MBq of FP-CIT intravenously. Data acquisition started 4 h after tracer application and lasted 35 min. Scan was performed with a dual-detector gamma camera (ECAM, Siemens, Erlangen, Germany) equipped with fan beam collimators. The imaging data were reconstructed using standard filtered backprojection methods with a Butterworth filter.
Brain 18FDG PET
PET scan was performed 25 years after the CO intoxication episode, using an integrated PET/CT camera (Discovery ST, GE Healthcare, Waukesha). She was injected intravenously with 150 MBq of 18FDG in an awake resting state with her eyes closed, in a quiet environment. Image acquisition started 30 min after the injection and ended 15 min later. Images were reconstructed using the ordered subsets’ expectation maximization algorithm with 5 iterations and 32 subsets, and corrected for attenuation using a CT transmission scan. Hypometabolism was confirmed by performing whole-brain voxel-based SPM analysis and comparing the results with those of 41 healthy subjects (mean age = 44 years; AP-HM PHRC 2007/09, registration number of the clinical trial: NCT00484523) (p < 0.005, uncorrected; k > 24), as previously described (Guedj, Aubert, McGonigal, Mundler, & Bartolomei, 2010).
Case Report
Behavioral Disorders
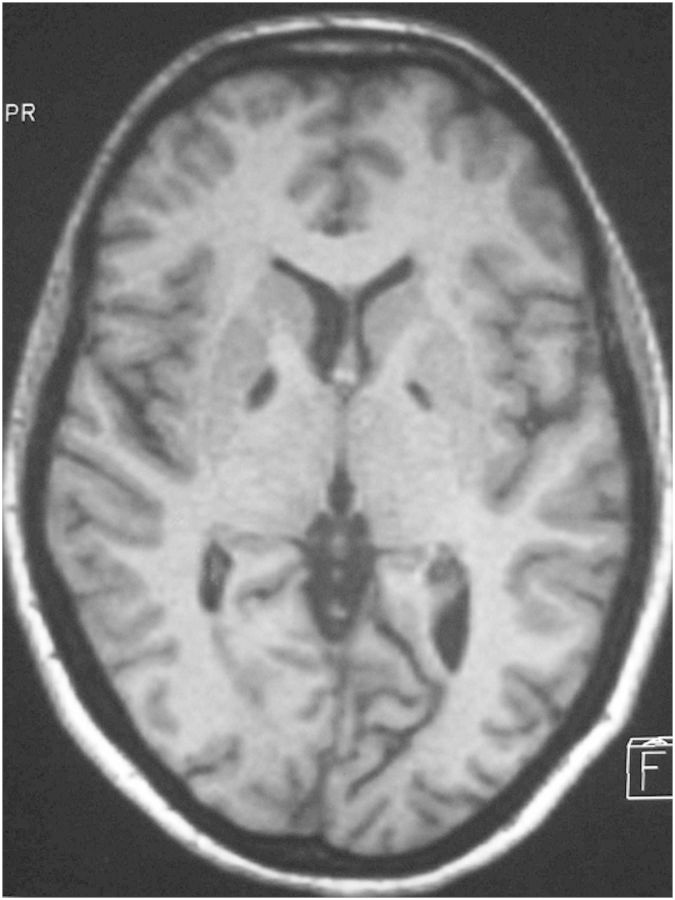
Mrs PA was born in 1962. She was taking a course in secretarial skills and accountancy when she was intoxicated in 1980 by CO and fell into a coma for 2 weeks. She was first given hyperbaric oxygenation treatment, and then had to undergo rehabilitation to recover her physical autonomy. One year after the CO intoxication event, the neurological somatic examination was normal, without any trace of Parkinsonism. It was still normal 25 years later. The brain imaging data showed the presence of isolated bilateral pallidal lesions. The anatomical MRI performed in 2004 did not bring to light any signs of cerebral atrophy or any other abnormalities apart from the bilateral internal pallidal lesions (Fig. 1). Because of the patient's persistent behavioral disorders, she was examined every year for 25 years by the same neurologist. Since the CO intoxication event, PA's spontaneous activity was affected: she never spontaneously initiated any activity: her mother had to incite her to start and pursue all her everyday activities, which she then carried out appropriately. When questioned, she reported having feelings of mental emptiness; she never expressed any affects and she never attempted to become involved in any social interactions. Her habitual state of inactivity was sometimes disrupted, however, by compulsive obsessional acts, such as the urge to clean her room, open or close a door, or collect weird objects. She would also spontaneously read books, always the same ones during the 25-year follow-up period, often working backwards (from the last to the first page), watch cartoons on TV, or play with her dog. In response to a stimulus, she could execute an appropriate activity without being able to anticipate the possible risks involved. In addition, neither internal stimuli (hunger) nor external ones (pain, cutaneous burns) induced appropriate reactions or complaints. For example, she once lay in the sun for a long time without changing position. When she was discovered several hours later, she had sustained second grade cutaneous burns on all the exposed parts of her body. On another occasion, she was knocked down by a car, but stood up immediately and walked away without reacting at all. Contrasting with this lack of appropriate reactions to real stimuli, she often complained of headaches or abdominal pain, although no organic causes could be detected. She rarely expressed any affects. These behavioral disorders, which were previously described by Ali Chérif et al. (1984)), remained unchanged during the whole 25-year follow-up period.
Fig. 1.
T1 weighted Cerebral Magnetic Resonance Image of Mrs PA in 2005: bilateral internal pallidal lesions without any cerebral atrophy, nor any brain lesion elsewhere.
Neuropsychological Assessments
The patient's performances in the neuropsychological tests evolved, however, during the 25-year follow-up period. The initial neuropsychological assessment showed the presence of a dysexecutive syndrome, including slowness of thought, conceptualization and programming deficits, and a lack of attention and working memory. Her ability to recall information was impaired, but there was no anterograde amnesia. In 2006, her comprehension was still satisfactory: she always participated fully in the tests and never showed any difficulty in understanding the instructions, which never had to be repeated. She very occasionally took part spontaneously in discussions and talked about something she had done, forming fairly long sentences and using an appropriate choice of vocabulary.
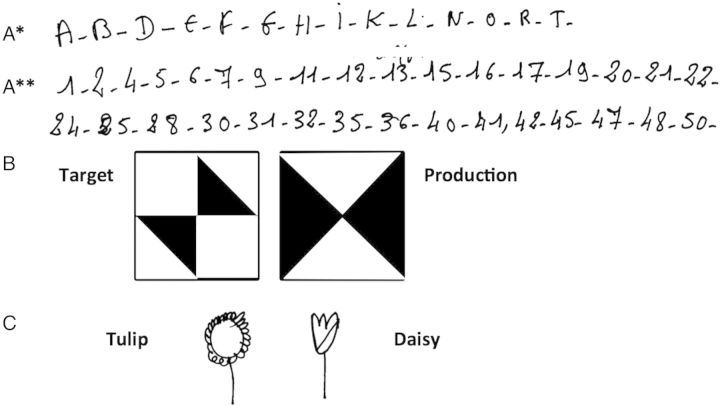
However, during the 25-year follow-up period, the patient's scores in tests such as the WAIS, WSM, verbal fluency, Famous Face recognition, denomination and Modified Card Sorting tests gradually worsened considerably (Table 1). These low scores were largely due to the frequent use of “I don't know” answers, even to very simple questions such as “what's your name?.” It is worth noting that the patients' comprehension and designation performances did not deteriorate at all. She continued to perform the Trail Making Test and copy Rey's Figure correctly. Because of the patient's tendency to answer “I don't know,” we had to force her to choose an answer in several tests, and she then gradually developed the habit of giving “à côté” answers to even the simplest questions. The fact that these wrong answers fell just short of the correct answers showed that the question had been understood and that the patient probably knew the right answer. When asked, for example, whether a dog is an object or an animal, she would answer “an object.” “A côté” answers also occurred strikingly frequently in the calculation tests (“7+1=?, 9;” “9–6=?, 4;” “4 × 3=?, 10”…). Her wrong responses in the visual and verbal recognition memory tasks scored as high as 70%–80%, whereas the probability of wrong responses was only 50% (Barbeau et al., 2004). This habit was also adopted in most of the neuropsychological tasks, with both verbal and visual modes of presentation (Fig. 2). Intriguingly, these “à côté” answers were produced easily, quickly and without any apparent effort. They were initially absent in 1981. They began to appear in 1988, but not very frequently. They became clinically meaningful in 1995 and their frequency gradually increased from then on.
Table 1.
Evolution of the patient's performances in several tests during the 25-year follow-up period
| 1981 | 1988 | 1995 | 2006 | |
|---|---|---|---|---|
| Overall performances | ||||
| Verbal IQ (WAIS) | 88 | 70* | na | <47* |
| Performance IQ (WAIS) | 86 | 75* | na | 52* |
| Total IQ (WAIS) | 87 | 75* | na | 47* |
| Binois–Pichot Vocabulary test | 102 | na | na | Variable (74–91) |
| Mnesic quotient (WMS) | 92 | na | na | 57* |
| Executive functions | ||||
| Trail Making test A (s) | na | na | 80 | 77 |
| Modified Card Sorting test (categories) | 4 | 4 | na | 0* |
| Digit span (forward/backward) | 4/3* | 5/5* | 3/3* | 3/3* |
| Stroop test | Failure in the interfering condition | |||
| Copying Rey's figure | Type 1 | Type 1 | ||
| Verbal fluency (animals) (Nb/1 min) | 18 | 5* | na | 2* |
| Comprehension, designation, and denomination | ||||
| Token test (score/120) | na | na | na | 115 |
| Designation (right answers) | >90% | |||
| Denomination (right answers) | >90% | |||
| Famous face recognition test | 28/30 | na | 15/30* | 0/30* |
| Picture confrontation oral naming (DO80) (% of right answers/% of “I don't know” answers) | 75% | na | na | 30%*/57% |
| Sound lotto (% of right answers/% of “I don't know” answers) | 25%*/50% | |||
| Forced choice between two possible answers (B2) about categories in auditory material (e.g., “a dog: is it an object or an animal?”) (% of wrong answers) | 100% | |||
| Forced B2 lexical decisions (% of wrong answers to auditory/visual stimuli) | 75%*/66%* | |||
| Forced choice B2 when naming plastic objects (% of wrong answers) | 80% | |||
Note: na = not available; * = impaired; B2 = between 2 possible answers.
Fig. 2.
Illustrations of GS. (A) Omissions made, without any errors in the ranks, when asked to write alphabetic letters (*) or the numbers from 1 to 50 (**). (B) Mirror-image errors in the WAIS “Cube” sub-test. (C) Pen drawing in response to instructions: inversions between tulip and daisy.
Psychiatric Assessments
The two psychiatrists who examined Mrs PA independently 25 years after the CO intoxication event concluded that she showed no signs of hysterical or psychotic personality, anxiety or aggressiveness, no intention to retreat from reality, no effective secondary benefits and no affective depressive symptoms.
Functional Imaging
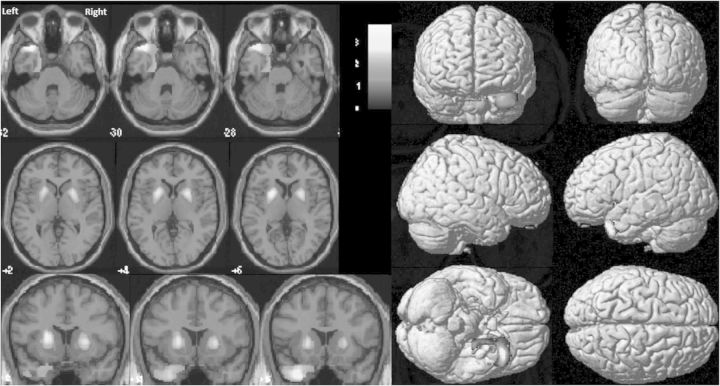
The dopamine transporter images (Fig. 3) showed a greatly decreased bilateral striatal uptake, a conserved fixing pattern in the left caudate nucleus, and impairments in the right caudate nucleus and right and left putamen.
Fig. 3.
DAT-scan (25 years after CO intoxication event): markedly decreased symmetrical bilateral striatal uptake, both at the head of the caudate nucleus and the putamen.
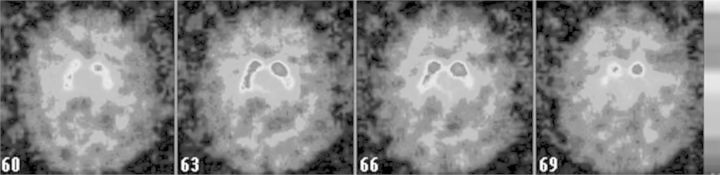
The brain 18FDG PET analysis (Fig. 4) showed the presence of a strong bilateral striatal hypometabolism, as well as hypometabolism in the left sub-hippocampal structures and temporal pole (p < 0.005, uncorrected; k > 24).
Fig. 4.
18FDG PET (25 years after CO intoxication event): marked hypometabolism of bilateral putamen and external pallidum (accumbens nucleus and caudate nucleus seem to be relatively spared), and of left entorhinal (28 Broadman area) and perirhinal (36 Broadman area) cortex and temporal pole (38 Broadman area).
Discussion
The typical LPSAS symptoms observed in patient PA showed no change during the whole 25-year follow-up period. These findings are in agreement with those made by other authors, who observed no changes in their patients after 10 and 12 years of follow-up (Laplane et al., 1982; Levy & Dubois, 2006). However, the increasing occurrence of “I don't know” and “à côté” responses, which accounted largely for the worsening of the patient's cognitive performances, has never been described so far in patients with LPSAS. These symptoms developed ∼8 years after the intoxication occurred, became more obvious ∼15 years later, and worsened consistently up to our last examination, 25 years after the CO intoxication event. These symptoms therefore evolved gradually during a period of ∼17 years, starting at a fairly late stage. As there exist few reports on the long-term follow-up of patients with LPSAS syndrome, and none on follow-up periods of >12 years, it is possible that these symptoms may correspond to the usual evolution of LPSAS, but that they have been overlooked so far. These symptoms are the main ones associated with symptoms of GS, as initially described in 1897 (Schorer, 1965). In the case of our patient, who complained of somatic pains corresponding to no visible organic lesions (whereas she made no complaints after sustaining a real injury) strongly suggest that a psychogenic etiology may be involved, although complaints of this kind are so frequent in the general population that it cannot be definitely concluded that they are conversive symptoms. In addition, our patient had a rather low socio-educational level, which may increase the risk of developing GS (Sigal et al., 1992; Tsoi, 1973). All these features are consistent with the diagnosis of GS, but the gradual onset and the very long course of the condition are not typical features of GS, although one progressive persistent (7-year) case of GS has been previously described (Hampel, Berger, & Müller, 1996). We did not identify any factor (including psychological factors), that might have triggered the GS. The patient's LPAS meant, however, that we had little access to any suffering or anxiety (because of the brevity of her mode of expression): the existence of emotional factors of this kind cannot therefore be ruled out. We also noted that the patient's answers were consistently correct and included no “à côté” responses in some tests, such as the Trail Making Test, the Token Test, copying Rey's figure, etc. However, since very few neuropsychological assessments on GS patients are available in the literature, we have no evidence that GS patients tend to consistently produce “à côté” answers in neuropsychological tests of all kinds. In addition, from 1981 to 2005, no clouding of consciousness or hallucinations, which are classical features of GS, were ever present, PA always took part readily in the medical investigations and the neuropsychological tests, and the psychiatrists did not detect any signs of hysterical or psychotic personality. Although these features are not typical of GS, we have to remember that LPAS induces mental emptiness and reduces the spontaneous expression of affects and thought, which is liable to make psychiatric disorders particularly difficult to detect.
The main question that arises her is whether this may be a malingering disease. The “à côté” and “I don't know” answers occurred consistently, regardless of the questioner (doctors, nurses, neuropsychologists, family members) and the location (at hospital or at home) and their rate of occurrence increased from year to year. In view of the long follow-up time, the many weeks of hospitalization, and the long hours devoted to neuropsychological assessments, any malingering would surely have been detected. None of the important family events which occurred, such as the death of her father, or that of her dog, of which she was very fond, or the holidays she spent with her brother, affected the patient's symptoms in any way. Nor were any secondary benefits identified. The social status of a disabled person was already acquired because of the patient's LPAS. Since the behavioral disorders associated with LPAS had already made her dependent on her mother's assistance with her everyday activities her “à côté” and “I don't know” answers had no effect on her mother's attitude: she continued to perform her daily tasks as usual. Lastly, although the patient expressed little emotion, she seemed to be very briefly disappointed when she was told that she had failed a neuropsychological test, and briefly please when she had succeeded. She therefore seems unlikely to have indulged in malingering. However, no performance validity test was given, which is acknowledged as a limitation to the present study and further work need to investigate this issue more thoroughly.
Interpreting the functional imaging findings was also a complex task. The dopamine transporter imaging data showed that the whole striatum was affected, apart from the left putamen, which was partly spared. Presynaptic dopaminergic pathway denervation was therefore present. It is worth noting here that Mrs PA did not show any extrapyramidal motor symptoms, although extensive pallidal lesions affecting the motor pathways were present. It is therefore possible that the patient's bipallidal lesions may have prevented the occurrence of the extrapyramidal disorders, which often result from over-activation of Alexander's (1986) cortico-striato-pallido-thalamo-cortical output pathways due to the dopamine deficiency. Pallidotomy or inhibiting stimulations, performed for the surgical treatment of Parkinson's disease (PD), induce the same process. The main differences are that PD surgery focuses mainly on motor pathways, and that Mrs PA's dopaminergic denervation was severe but not degenerative, as occurs in PD. The finding that self-initiated activities are not impaired in PD patients during bipallidal stimulation (Lozachmeur & Drapier, 2012) was probably facilitated because it is possible to stimulate specific motor pathways in this context. Apathy has often been described in patients with PD after applying inhibitory stimulation to the sub-thalamic nucleus (STN). The size and anatomical location of the STN make it harder to stimulate single motor pathways, and limbic and associative pathways are therefore also often involved. The apathy induced by stimulating the STN seems to be secondary to the predominantly dopaminergic denervation of the mesolimbic pathways, especially on the left side, which is sometimes unmasked at post-operative drug withdrawal (Thobois, Ardouin, Lhommée, Klinger, & Lagrange, 2010). A similar mechanism may have been at work in our patient, whose self-initiated activity deficits may have been due to the denervation of the cognitive and limbic dopaminergic pathways.
The 18FDG PET showed the presence of severe hypometabolism in the left sub-hippocampal and temporal poles. Hypometabolism of the left temporal lobe obviously does not point to any specific pathology. But the question of a possibly psychogenic or organic origin had to be addressed in this case. Several case reports have been published in the literature in which functional imaging has been performed on patients with psychogenic symptoms. Some authors have reported the existence of temporal hypometabolism in patients with psychogenic amnesia (Markowitsch et al., 1998 [left hippocampal formation and the right one to a lesser extent, right temporo-basal cortex, left temporo-mesial and left insular cortex, bilateral fronto-basal and parietal cortex]; Antherion-Thomas, Guedj, Decousus, & Laurent, 2010 [left hippocampal hypometabolism]; Hennig-Fast et al., 2008 [right temporo-mesial hypometabolism]). Other authors have reported the existence, again in patients with psychogenic amnesia, of right prefrontal hypometabolism, based on PET-scan findings (Brand et al., 2009; Piolino et al., 2005) or MR-spectroscopy and magnetization transfer imaging (Tramoni et al., 2009). Although these abnormalities are not always specifically associated with psychogenic symptoms, the left sub-hippocampal hypometabolism observed in Mrs PA does seem to be consistent in this case with the existence of a psychogenic disorder.
A closer analysis of the PETscan findings showed that the temporal hypometabolism observed corresponded to the left Broadman areas 28 (the entorhinal cortex), 36 (the perirhinal cortex), and 38 (the temporal pole), whereas the basal ganglia hypometabolism involved the bilateral putamen. The accumbens nucleus and caudate nucleus seemed to be relatively spared bilaterally on the PETscan images (Lucas-Neto et al., 2013). Basal ganglia pathways are involved in decision-making, which requires estimating reward prediction errors, selecting/amplifying the most rewarding decision, and learning to improve future decision-making. Based on recent models for basal ganglia circuits, it has been suggested that baseline dopaminergic levels and phasic dopamine responses to rewards or stimuli predicting rewards may contribute to estimate reward prediction error and decision optimization (Hsiao & Lo, 2013). The present patient's ability to estimate reward prediction errors and optimize decisions was probably impaired by the denervation of the dopaminergic pathways, which may have affected either the accuracy and/or the velocity of the decision-making processes. The dorsal caudate nucleus and internal pallidum, which are involved here, are the main cognitive relays in the basal ganglia (Levy & Dubois, 2006). Learning to improve future decision-making is therefore less effective because of the lack of enhanced dopamine secretion due to reward. The left temporal pole and the entorhinal and perirhinal cortex probably play a role in this part of the mechanism because they are connected to the limbic circuits and the hippocampus. This might explain temporal hypometabolism observed in Mrs PA's PETscan.
As mentioned previously, the GS was atypical because discordant results were obtained between the tests performed. Some of the tests, including the Trail making Test, the Token Test, and copying Rey's figure, were performed accurately but rather slowly. Unlike some of the other tests used, these tests do not require the use of episodic or semantic knowledge or reasoning abilities, since they are based mainly on comprehension and execution. The tasks showing the changes in the patient's performances induced by GS were therefore those mobilizing the associative and cognitive basal ganglia loops. This suggests that an organic mechanism may be responsible for GS. The basal ganglia circuits can certainly not be assumed to be static systems. Neuronal plasticity is probably induced by repetitive external or internal stimulations and a process of learning via decision-making. The absence of the basal ganglia circuits' activation and output may have led to changes in the neuronal connections or the neurotransmitter receptors' concentrations year after year, which might account for the progressive evolution of GS.
Applying external stimulation is generally thought to induce an appropriate response in patients with LPSAS. But the results obtained may depend on the type of stimulation used, as in the case of GS. Responses were obtained only when the patient was performing “simple” tasks, that is, implementation tasks requiring little if any semantic or episodic knowledge or reasoning abilities. The complete inability to make any decision in response to an internal or external emotional/affective or cognitive stimulus will therefore presumably suffice to constitute what is known as an auto-activation deficit.
Conclusion
LPSAS was not previously known to be associated with GS, but no data have been published so far on follow-up periods longer than 12 years. It seemed possible at first sight that this patient's GS might be a primitive syndrome which was independent of LPSAS. But the atypical features of this GS argue against the idea that these are two independent syndromes. The present functional imaging data are consistent with the existence of a psychogenic disorder, but they also support the possible involvement of an organic mechanism. LPSAS can be said to be a state of “psychological confinement,” which makes the patients incapable of expressing affective feelings or intellectual thoughts, as occurs in the case of prisoners. Although this parallel does not constitute scientific evidence, it shows that the ability to make decisions can be altered because of basal ganglia pathway lesions. The fact that the causal mechanism (permanent basal ganglia lesions) is irreversible may explain the irreversibility of the GS.
One of the most unusual characteristics of the present patient was the fact that the CO intoxication responsible for the onset of her condition occurred at an early age (18 years). According to Yakovlev and Lecours (1967), the frontal myelination process has not been completed at this age, and the gray matter continues to evolve at puberty and thereafter, reflecting a process of synaptic reorganization (Huttenlocher, 1979). Brain lesions occurring at this age and the resulting process of functional reorganization may worsen, or at least change the symptoms.
Conflict of Interest
None declared.
References
- Alexander G. E. Parallel organization of functionally segregated circuits linking basal ganglia and cortex. Annual Review of Neuroscience. 1986;9:357–381. doi: 10.1146/annurev.ne.09.030186.002041. [DOI] [PubMed] [Google Scholar]
- Ali Chérif A., Royere M. L., Gosset A., Poncet M., Salamon G., Khalil R. Trouble du comportement et de l'activité mentale après intoxication oxycarbonée. Revue Neurologique. 1984;140(6–7):401–405. [PubMed] [Google Scholar]
- Antherion-Thomas C., Guedj E., Decousus M., Laurent B. Can we see personal identity loss? A functional imaging study of typical “hysterical amnesia.”. Journal of Neurology, Neurosurgery, and Psychiatry. 2010;81:468–469. doi: 10.1136/jnnp.2008.163808. [DOI] [PubMed] [Google Scholar]
- Barbeau E., Didic M., Tramoni E., Felician O., Joubert S., Sontheimer A., et al. Evaluation of visual recognition memory in MCI patients. Neurology. 2004;62(8):1317–1322. doi: 10.1212/01.wnl.0000120548.24298.db. [DOI] [PubMed] [Google Scholar]
- Berg E. A. A simple objective technique for measuring flexibility in thinking. The Journal of General Psychology. 1948;39:15–22. doi: 10.1080/00221309.1948.9918159. [DOI] [PubMed] [Google Scholar]
- Binois R., Pichot P. Test de vocabulaire: Manuel d'application. Centre de psychologie appliqué; 1956. [Google Scholar]
- Brand M., Eggers C., Reinhold N., Fujiwara E., Kessler J., Heiss W. D., et al. Functional brain imaging in 14 patients with dissociative amnesia reveals right inferolateral prefrontal hypometabolism. Psychiatry Research: Neuroimaging. 2009;174:32–39. doi: 10.1016/j.pscychresns.2009.03.008. [DOI] [PubMed] [Google Scholar]
- Cummings J. L. Frontal-subcortical circuits and human behavior. Archives of Neurology. 1993;50:873–880. doi: 10.1001/archneur.1993.00540080076020. [DOI] [PubMed] [Google Scholar]
- Deloche G., Hannequin D. Epreuve de denomination orale d'images. ECPA Ed; 1997. [Google Scholar]
- DeRenzie E., Vignolo L. A. The Token test: A sensitive test to detect receptive disturbances in aphasics. Brain. 1962;85:665–678. doi: 10.1093/brain/85.4.665. [DOI] [PubMed] [Google Scholar]
- Guedj E., Aubert S., McGonigal A., Mundler O., Bartolomei F. Déjà-vu temporal lobe epilepsy: Metabolic pattern of cortical involvement in patients with normal brain MRI. Neuropsychologia. 2010;48(7):2174–2181. doi: 10.1016/j.neuropsychologia.2010.04.009. [DOI] [PubMed] [Google Scholar]
- Hampel H., Berger C., Müller N. A case of Ganser's state presenting as a dementia syndrome. Psychopathology. 1996;29:236–241. doi: 10.1159/000284999. [DOI] [PubMed] [Google Scholar]
- Hennig-Fast K., Meister F., Frodl T., Beraldi A., Padberg F., Engel R. R., et al. A case of persistant retrograde amnesia following dissociative fugue: Neuropsychological and neurofunctional underpinnings of loss of autobiographical memory and self-awareness. Neuropsychologia. 2008;46:2993–3005. doi: 10.1016/j.neuropsychologia.2008.06.014. [DOI] [PubMed] [Google Scholar]
- Heron E. A., Kritchevsky M., Delis D. C. Neuropsychological presentation of Ganser symptoms. Journal of Clinical and Experimental Neuropsychology. 1991;13(5):652–666. doi: 10.1080/01688639108401080. [DOI] [PubMed] [Google Scholar]
- Hsiao P. Y., Lo C. C. A plastic corticostriatal circuit model of adaptation in perceptual decision making. Frontiers in Computational Neuroscience. 2013;7(178):1–16. doi: 10.3389/fncom.2013.00178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huttenlocher P. R. Synaptic density in human frontal cortex – Developmental changes and effects of aging. Brain Research. 1979;163:195–205. doi: 10.1016/0006-8993(79)90349-4. [DOI] [PubMed] [Google Scholar]
- Ishii K., Hosaka K., Mori T., Mori E. Comparison of FDG-PET and IMP-SPECT in patients with dementia with Lewy bodies. Annals of Nuclear Medicine. 2004;18(5):447–451. doi: 10.1007/BF02984489. [DOI] [PubMed] [Google Scholar]
- Laplane D., Baulac M., Pillon B., Panayotopoulou-Achimatos I. Perte d'autoactivation psychique. Activité compulsive d'allure obsessionnelle. Lésion lenticulaire bilatérale. Revue Neurologique. 1982;138:137–141. [PubMed] [Google Scholar]
- Laplane D., Baulac M., Widlöcher D., Dubois B. Pure psychic akinesia with bilateral lesions of basal ganglia. Journal of Neurology, Neurosurgery, and Psychiatry. 1984;47:377–385. doi: 10.1136/jnnp.47.4.377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laplane D., Wildocher D., Pillon B., Baulac M., Binoux F. Comportement compulsif d'allure obsessionnelle par nécrose circonscrite bilatérale pallido-striatale. Encéphalopathie par piqûre de guêpe. Revue Neurologique. 1981;137:269–276. [PubMed] [Google Scholar]
- Latcham R., White A., Sims A. Ganser syndrome: The aetiological argument. Journal of Neurology, Neurosurgery, and Psychiatry. 1978;41:851–854. doi: 10.1136/jnnp.41.9.851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee H., Koenig M. D. A case of Ganser syndrome: Organic or hysterical? General Hospital Psychiatry. 2001;23:230–231. doi: 10.1016/s0163-8343(01)00147-5. [DOI] [PubMed] [Google Scholar]
- Levy R., Dubois B. Apathy and the functional anatomy of the prefrontal cortex-basal ganglia circuits. Cerebral Cortex. 2006;16:916–928. doi: 10.1093/cercor/bhj043. [DOI] [PubMed] [Google Scholar]
- Lozachmeur C., Drapier D. Apathy and deep brain stimulation in Parkinson's disease. Revue Neurologique. 2012;168:620–623. doi: 10.1016/j.neurol.2012.06.011. [DOI] [PubMed] [Google Scholar]
- Lucas-Neto L., Neto D., Oliveira E., Martins H., Mourato B., Correia F., et al. Three dimensional anatomy of the human nucleux accumbens. Acta Neurochirurgica. 2013;155:2389–2398. doi: 10.1007/s00701-013-1820-z. [DOI] [PubMed] [Google Scholar]
- Markowitsch H., Kessler J., Van Der Ven C., Weber-Luxenburger G., Albers M., Heiss W. D. Psychic trauma causing grossly reduced brain metabolism and cognitive deterioration. Neuropsychologia. 1998;36(1):77–82. doi: 10.1016/s0028-3932(97)00093-6. [DOI] [PubMed] [Google Scholar]
- Piccirilli M., Mazzi P., Luccioli R., Sciarma T. Selective bilateral lesion of the globus pallidus: Ten-year follow-up of memory impairment and frontal symptomatology. Italian Journal of Neurological Sciences. 1995;16:635–640. doi: 10.1007/BF02230914. [DOI] [PubMed] [Google Scholar]
- Piolino P., Hannequin D., Desgranges B., Girard C., Beaunieux H., Giffard B., et al. Right ventral frontal hypometabolism and abnormal sense of self in a case of disproportionate retrograde amnesia. Cognitive Neuropsychology. 2005;22(8):1005–1034. doi: 10.1080/02643290442000428. [DOI] [PubMed] [Google Scholar]
- Refaat R., Firth D. A., Robertson M. M. Uncomplicated Gilles de la Tourette syndrome and probable Ganser syndrome. A case report and review of the literature. European Child & Adolescent Psychiatry. 2002;11(5):234–239. doi: 10.1007/s00787-002-0280-4. [DOI] [PubMed] [Google Scholar]
- Reitan R. M. The relation of the trail making test to organic brain damage. Journal of Consulting Psychology. 1955;19(5):393–394. doi: 10.1037/h0044509. [DOI] [PubMed] [Google Scholar]
- Schorer C. E. The Ganser syndrome. British Journal of Criminology. 1965;5(2):120–131. [Google Scholar]
- Sigal M., Altmark D., Alfici S., Gelkopf M. Ganser syndrome: A review of 15 cases. Comprehensive Psychiatry. 1992;33(2):134–138. doi: 10.1016/0010-440x(92)90011-e. [DOI] [PubMed] [Google Scholar]
- Stroop J. R. Studies of interference in serial verbal reactions. Journal of Experimental Psychology. 1935;18:643–662. [Google Scholar]
- Thobois S., Ardouin C., Lhommée E., Klinger H., Lagrange C. Non-motor dopamine withdrawal syndrome after surgery for Parkinson's disease: Predictors and underlying mesolimbic denervation. Brain. 2010;133:1111–1127. doi: 10.1093/brain/awq032. [DOI] [PubMed] [Google Scholar]
- Tramoni E., Aubert-Khalfa S., Guye M., Ranjeva J. P., Felician O., Ceccaldi M. Hypo-retrieval and hyper-suppression mechanisms in functional amnesia. Neuropsychologia. 2009;47:611–624. doi: 10.1016/j.neuropsychologia.2008.11.012. [DOI] [PubMed] [Google Scholar]
- Tsoi W. F. The Ganser syndrome in Singapore: A report of ten cases. The British Journal of Psychiatry: The Journal of Mental Science. 1973;123:567–572. doi: 10.1192/bjp.123.5.567. [DOI] [PubMed] [Google Scholar]
- Wechsler D. Echelle d'intelligence de l'Adulte de Wechsler. ECPA Ed; 1981. [Google Scholar]
- Wechsler D. Echelle clinique de mémoire, Troisième edition. ECPA Ed; 2001. [Google Scholar]
- Yakovlev P. A., Lecours I. R. The myelogenetic cycles of regional maturation of the brain. In: Minkowski A., editor. Regional development of the brain in early life. Oxford: Blackwell; 1967. pp. 3–70. [Google Scholar]