Abstract
BACKGROUND
Insulin resistance has been related to elevated blood pressure (BP) in obese children and may adversely affect the vasculature by arterial stiffening. The objective was to investigate whether daytime and nighttime BP were elevated and related to insulin resistance and arterial stiffness in obese children and adolescents.
METHODS
Ninety-two obese patients aged 10–18 years were compared with 49 healthy control individuals. Insulin resistance was measured as the homeostatic assessment model (HOMA), and arterial stiffness was measured as carotid–femoral pulse wave velocity (cfPWV).
RESULTS
Mean ± SD daytime systolic BP (SBP) (obese: 125±8.3mm Hg; control: 121±10.1mm Hg; P = 0.03) and nighttime SBP (obese: 108±10.7mm Hg; control: 102±8.2mm Hg; P = 0.0001) were higher in the obese group when compared with the control group. No difference was found in daytime diastolic BP (DBP), whereas nighttime DBP (obese: 60±6.6mm Hg; control: 57±4.8mm Hg; P = 0.001) and night-to-day BP ratios were higher in the obese group. Nighttime SBP was related to BMI z score (β = 6.0; 95% confidence interval (CI) = 2.9–9.1; P = 0.0002) and waist/height ratio (β = 36.7; 95% CI = 5.6–67.9; P = 0.02) in the obese group. HOMA index (obese: median = 3.7, interquartile range (IQR) = 2.3–6.0; control: median = 2.6, IQR = 1.8–3.4; P = 0.002) was higher, whereas cfPWV (obese: 4.8±0.8 m/s; control: 5.1±0.6 m/s; P = 0.03) was lower in the obese group. CfPWV was not related to logHOMA index. In multiple regression analyses, the higher nighttime BP in the obese group was independent of logHOMA and cfPWV.
CONCLUSIONS
Obese children had a higher nighttime BP when compared with the control group independently of insulin resistance and arterial stiffness. No relationship was found between insulin resistance and arterial stiffness.
CLINICAL TRIAL REGISTRATION
Clinicaltrials.gov identifier NCT01310088
Keywords: adolescence, ambulatory blood pressure monitoring, arterial stiffness, blood pressure, children, hypertension, microalbuminuria, nighttime, obesity, pulse wave velocity.
Obesity-related elevated blood pressure (BP) has been linked to insulin resistance in children and adolescents.1–3 In this respect, insulin resistance may impact the cardiovascular system, contributing to the obesity-related elevated BP.4 The adverse effect associated with insulin resistance could be arterial wall stiffening (arterial stiffness)5,6 and universal microvascular damage.7–9 Carotid–femoral pulse wave velocity (cfPWV) is the gold standard for measuring arterial stiffness.10 Microalbminuria (i.e., slightly elevated excretion of urinary albumin) is a marker of glomerular damage, as well as a marker of universal microvascular damage.11,12
Ambulatory BP monitoring (ABPM) is regarded as the most precise measure of the BP burden,13 and focus on nighttime BP is growing because of its significant prognostic role.14 The focus on nighttime BP is also anticipated to be relevant among children and adolescents.15 The relationships between insulin resistance, microalbuminuria, arterial stiffness, and their influence on ABPM are unclear in obese children and adolescents.
The objective of this study was to investigate whether daytime and nighttime BP are elevated in obese children and adolescents when compared with a nonobese control group and, if so, whether these ambulatory BP levels are related to insulin resistance, arterial stiffness, and/or microalbuminuria.
METHODS
Design and participants
Our cross-sectional study design has been described in a recent publication.16 Briefly, severely obese white patients aged 10–18 years were recruited at inclusion to the Children’s Obesity Clinic, Department of Pediatrics, Holbaek University Hospital.17 Age- and sex-matched white control individuals were recruited from the local area. All measures were performed on 2 consecutive days. Blood sampling was performed in proximity to inclusion in the Children’s Obesity Clinic as part of the treatment protocol.17
Data in this study is constrained to 92 patients in the obese group (89% of the initial included patients) and 49 individuals in the control group (98% of the control individuals) with valid ABPM and no signs of secondary hypertension.
Use of medication (yes vs. no) was recorded in 5 obese and 4 control individuals with a history of asthma or allergy symptoms, 3 obese individuals with gastrointestinal symptoms, 3 obese individuals and 1 control individual with hormonal supplementation, 3 obese individuals on birth control medication, and 3 obese and 5 control individuals receiving other not specified medications.
The study was declared to ClinicalTrials.gov (NCT01310088) and the Danish Data Agency and approved by the Scientific Ethical Committee of Region Zealand. Written informed consent was obtained from parents and individuals aged 18 according to the Helsinki Declaration.
Obesity measures
Height was measured to the nearest 0.1cm and weight to the nearest 0.1kg wearing light indoor clothes without shoes using an integrated calibrated weight and stadiometer (Model MZ10023, ADE, Hamburg, Germany). Body mass index (BMI; kg/m2) was calculated into BMI z scores in respect to a Danish standard population with the same age and sex.18 Waist circumference was measured to the nearest 0.1cm with subjects standing using a stretch-resistant tape.19 Waist/height ratio (WHR) was calculated.
Clinic and ambulatory BP
Brachial clinic BP was measured after a rest of 10 minutes in supine position with the oscillometric device Omron 705IT (Omron Healthcare Europe, Gl Hoofddorp, The Netherlands) using cuff sizes as recommended by the manufacturer: small (arm circumference <22cm), medium (22–32cm), and large (≥32cm).20 Mean of the last 2 of 3 BP measurements was reported and calculated into z scores according to an American standard population based on individuals’ sex, age, and height.21
Ambulatory BP was measured with the oscillometric device Boso TM-2430 (Bosch + Sohn GmbH u. Co. KG, Jungingen, Germany).22 The device was mounted on the upper brachial arm using cuff size as recommended by the manufacturer: small (arm circumference <22cm), medium (22–32cm), and large (≥32cm). The device was programmed to measure with 15-minute intervals during the day (7 am to 10 pm) and 30-minute intervals during the night. Individuals were asked to keep a diary of their sleep time interval to differentiate awake (daytime) from sleep (nighttime) in the BP analyses. Mean values of ambulatory BP and heart rate (HR) were calculated into z scores according to a German standard population based on individuals’ sex and height.15,23 Only individuals with valid ABPM with at least 20 valid BP measurements during the day and at least 7 at night were included in the analysis. Night-to-day BP ratio was calculated as nighttime BP divided by daytime BP.14
The BP classification was based on cutoff levels for both clinic and 24-hour systolic and diastolic BP:15,21,23 normotension (clinic and 24-hour BP <95th percentile), white-coat hypertension (clinic BP ≥95th percentile and 24-hour BP <95th percentile), masked hypertension (clinic BP <95th percentile and 24-hour BP ≥95th percentile), and hypertension (clinic and 24-hour BP ≥95th percentile). The 95th percentile equals a z score of 1.645.
Subclinical organ markers
Arterial stiffness was measured as cfPWV by noninvasive applanation tonometry using the SphygmoCor 9.0 device (AtCor Medical, Sydney, Australia).10 CfPWV was computed as pulse wave travel distance divided by pulse wave transit time. Travel distance was measured with a caliper, being 80% of the direct distance from the carotid artery to the femoral artery. The transit time was determined from the carotid and femoral arterial waveforms recorded consecutively with an electrocardiogram gated signal.16 The day-to-day variation (repeatability) of cfPWV was 0.03±0.36 m/s (mean difference ± SD), and the measurements did not differ 2 days in between (P = 0.64) in a subsample of 25 of the obese patients.
Individuals were asked to refrain from smoking at least 3 hours before the cfPWV and clinic BP measurements. The corresponding author performed all anthropometric, clinic BP, and cfPWV measurements.
Venous blood samples were drawn early morning after overnight fasting. Biochemical plasma concentrations were measured by an enzymatic colorimetric method (Cobas 6000; F. Hoffmann-La Roche AG, Basel, Switzerland). However, plasma insulin in 6 of the obese blood samples was measured with the former laboratory method (Immulite 2000; Siemens Healthcare, Erlangen, Germany). Insulin resistance was determined as the homeostatic model assessment (HOMA) index calculated as glucose (mg/dl) multiplied by insulin (µIU/ml) and divided by 405.24 Plasma creatinine was measured by a colorimetric reaction with alkaline picrate (the Jaffe method) and automatically corrected by −26 µmol/L (Cobas 6000). Estimated glomerular filtration rate was calculated using the updated Schwartz’s formula applied for Jaffe methods: 0.55 × height (cm) / (plasma creatinine (µmol/L)/ 76.26).25,26
Microvascular damage was assessed as mean urine-albumin-creatinine ratio (UACR; mg/g) from 2 overnight urine spot samples. Urine albumin concentration (mg/L) was measured by an immunoturbidimetric precipitation method (Konelab 30i, Thermo Fischer Scientific, Waltham, MA), whereas creatinine (mmol/L) was measured by an enzymatic method (Cobas 6000). Urine albumin and UACR was set to 0.1 (mg/L and mg/g) when albumin was below the detectable level of 1.0mg/L. Microalbuminuria was defined as an UACR between 30 and 299mg/g.12
Statistics
Statistical analyses were performed using SAS software (version 9.2; SAS Institute, Cary, NC). Differences between the obese and the control group were assessed by unpaired (2 sample) Student t tests for normally distributed continuous variables, otherwise by Wilcoxon rank sum tests, χ2 tests for categorical variables, or Fischer exact tests when appropriate. Differences in ambulatory BP z scores between the obese and the control group were adjusted for age in multiple regression analyses. Cochran–Armitage trend test was used to test for a potential difference in the BP classification between the obese and the control group, whereas paired Student t tests were used for comparison of clinic and ambulatory BP.
In linear regression analyses, relationships between explanatory and dependent variables were investigated separately for the obese and the control group. Explanatory variables skewed to the right were log-transformed to fit models; otherwise Pearson correlation coefficient (rP) was used. Potential sex differences in these potential relationships were investigated in multiple regression analyses.
Daytime and nighttime BP were related to a group variable (obese vs control individuals) in pooled multiple regression analyses when adjusting for subclinical organ markers (logHOMA index, cfPWV, and logUACR), as well as relevant confounders (sex, age, height, and period-dependent HR). Because of the design of the recruitment, the group variable encompasses the differences between the 2 groups in obesity measures (BMI z score and WHR). Avoiding overadjustment, these measures were not included in the analyses. To pool data from the obese and the control group, the regression models were tested for possible interaction of the group variable with the other explanatory variables. Furthermore, to pool data from male and female subjects, the multiple regression models were tested for possible interaction of the sex variable with the other explanatory variables. Finally, analyses comparing ambulatory BP between the obese and the control group were repeated when excluding smokers and individuals receiving medication.
RESULTS
Study design: obesity measures
The obese and the control groups were matched for age, sex, and height (Table 1). As expected because of the design of the recruitment, the obese group had higher weight, BMI, BMI z score, waist circumference, and WHR as compared with the control group.
Table 1.
Body composition
| Variable | Obese group (n = 92) | Control group (n = 49) | P value |
|---|---|---|---|
| Male/female sex, no. | 42/50 | 22/27 | 0.93 |
| Age, y | 12.7 (11.4–14.9) | 13.5 (11.7–14.9) | 0.45 |
| Height, cm | 160.2±11.6 | 163.3±12.2 | 0.13 |
| Weight, kg | 67.2 (58.3–90.7) | 50.7 (41.3–58.4) | <0.0001 |
| BMI, kg/m2 | 27.3 (24.1–32.2) | 18.9 (16.7–20.1) | <0.0001 |
| BMI z score | 2.73±0.66 | 0.07±0.85 | <0.0001 |
| Waist circumference, cm | 94.7 (84.9–106.8) | 66.3 (62.7–69.6) | <0.0001 |
| WHR | 0.60 (0.56–0.64) | 0.40 (0.38–0.42) | <0.0001 |
Data are mean ± SD or median (interquartile range) unless otherwise specified.
Abbreviations: BMI, body mass index; WHR, waist/height ratio.
Clinic and ambulatory BP
The obese group had higher levels of clinic systolic and diastolic BP when compared with the control group (Table 2).
Table 2.
Clinic and ambulatory blood pressure
| Variable | Obese group (n = 92) | Control group (n = 49) | P value |
|---|---|---|---|
| Clinic systolic BP, mm Hg | 111±9 | 107±8 | 0.01 |
| Clinic diastolic BP, mm Hg | 62±6 | 59±5 | 0.006 |
| 24-hour systolic BP, mm Hg | 121±8 | 117±9 | 0.002 |
| 24-hour diastolic BP, mm Hg | 70±5 | 69±6 | 0.11 |
| 24-hour MAP, mm Hg | 87±5 | 85±6 | 0.01 |
| 24-hour PP, mm Hg | 51±6 | 48±6 | 0.002 |
| 24-hour HR, bpm | 80±8 | 75±9 | 0.002 |
| Daytime systolic BP, mm Hg | 125±8 | 121±10 | 0.03 |
| Daytime diastolic BP, mm Hg | 73±6 | 73±7 | 0.67 |
| Daytime MAP, mm Hg | 90±6 | 89±7 | 0.22 |
| Daytime PP, mm Hg | 52±6 | 49±7 | 0.005 |
| Daytime HR, bpm | 82±8 | 78±10 | 0.02 |
| Nighttime systolic BP, mm Hg | 108±11 | 102±8 | 0.0001 |
| Nighttime diastolic BP, mm Hg | 60±7 | 57±5 | 0.001 |
| Nighttime MAP, mm Hg | 76±7 | 72±6 | <0.0001 |
| Nighttime PP, mm Hg | 48±7 | 45±6 | 0.004 |
| Nighttime HR, bpm | 70±9 | 64±9 | 0.0002 |
Data are mean ± SD.
Abbreviations: BP, blood pressure; HR, heart rate; MAP, mean arterial pressure; PP, pulse pressure.
Twenty-four-hour, daytime, and nighttime systolic BP, pulse pressure, and HR were consistently higher in the obese group when compared with the control group. No differences were found in 24-hour or daytime diastolic BP, whereas nighttime diastolic BP was higher in the obese group. Twenty-four-hour mean arterial pressure was higher in the obese group and apparently driven by a higher nighttime mean arterial pressure because no difference was found in daytime mean arterial pressure.
Differences in ambulatory and clinic BP z scores between the obese and the control group did not differ from differences in BPs in millimeters of mercury (Supplementary Tables S1 and S2). However, clinic systolic BP z scores were not significantly higher in the obese group. Ambulatory BP z scores were not related to age (Supplementary Table S2).
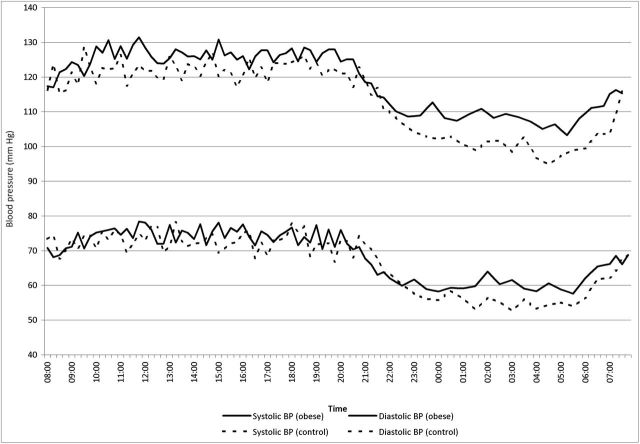
The variation of systolic and diastolic BP throughout the day in the 2 groups is plotted in Figure 1. The figure displays the relatively higher nighttime vs. daytime BP in the obese group when compared with the control group, also demonstrated by higher night-to-day BP ratios in the obese group (systolic BP: 0.864±0.074 for obese vs. 0.835±0.062 for control, P = 0.02; diastolic BP: 0.820±0.103 for obese vs. 0.781±0.082 for control, P = 0.02).
Figure 1.
Circadian variation of the ambulatory blood pressure (BP). Mean values of ambulatory systolic and diastolic BP for a given time plotted thoughout the day in the obese and the control group. Time interval between BP readings was every 15 minutes during the daytime (7 am to 10 pm) and every 30 minutes during the nighttime.
Twenty-four-hour BP was consistently higher than clinic BP in the obese group (Δsystolic BP = 10.1±8.0mm Hg; Δdiastolic BP = 8.4±6.2mm Hg; P < 0.0001 for both) and the control group (Δsystolic BP = 9.3±10.7mm Hg; Δdiastolic BP = 9.8±6.6mm Hg; P < 0.0001 for both). Twenty (22%) obese vs. 12 (24%) control individuals were classified as white-coat hypertensive. No overall difference was found in the BP classification as found by the Cochran–Armitage trend test (P = 0.18): 15 (16%) obese vs. 3 (6%) control individuals were hypertensive, 10 (11%) obese vs. 6 (12%) control individuals were masked hypertensive, and 47 (51%) obese vs. 28 (57%) controls individuals were normotensive.
Metabolic factors and markers of subclinical organ damage
CfPWV and fasting glucose were lower in the obese group (Table 3). Metabolic measures, including insulin, HOMA index, and lipids, were higher in the obese group vs. the control group, except for high-density lipoprotein cholesterol.
Table 3.
Metabolic factors and markers of subclinical organ damage
| Variable | Obese group (n = 92) | Control group (n = 49) | P value |
|---|---|---|---|
| CfPWV, m/s | 4.84±0.57 | 5.08±0.63 | 0.03 |
| Fasting glucose, mg/dl | 95.5±10.8 | 100.9±10.8 | 0.03 |
| Insulin, µIU/ml | 15.9 (9.5–26.0) | 10.0 (7.1–14.1) | 0.0001 |
| HOMA index | 3.7 (2.3–6.0) | 2.6 (1.8–3.4) | 0.002 |
| Total cholesterol, mg/dl | 162.2 (142.9–181.5) | 142.9 (139.0–158.3) | 0.005 |
| LDL cholesterol, mg/dl | 92.7±27.0 | 77.2±23.2 | 0.0002 |
| HDL cholesterol, mg/dl | 50.2±11.6 | 57.9±11.6 | <0.0001 |
| Triglycerides, mg/dl | 79.6 (53.1–123.9) | 61.9 (44.2–70.8) | 0.0004 |
| Creatinine, µmol/L | 53.3±9.3 | 61.2±11.4 | <0.0001 |
| eGFR, ml/min/1.73m2 | 128.9±18.7 | 114.8±15.9 | <0.0001 |
| Urine albumin, mg/l | 2.1 (0.1–9.5) | 5.1 (0.1–10.0) | 0.33 |
| UACR, mg/g | 1.6 (0.1–6.1) | 2.8 (0.1–5.6) | 0.68 |
Data are mean + SD or median (interquartile range). Because of either hemolysis of blood samples, no shows, or visit delay exceeding 60 days, the total number of blood samples were 79 (86%) in the obese and 47 (96%) in the control group, and the number of urine samples were 88 (96%) in the obese and 46 (94%) in the control group.
Abbreviations: CfPWV, carotid–femoral pulse wave velocity; eGFR, estimated glomerular filtration rate; HOMA index, homeostatic model assessment index; HDL, high-density lipoprotein; LDL, low-density lipoprotein; UACR, urine-albumin-creatinine ratio.
No differences were found in UACR, urine albumin, or in the prevalence of microalbuminuria (obese: 2 (2%); control: 1 (2%); P = 1.00) between the 2 groups. Urine albumin was undetectable low in more than half of the urine samples in both groups (obese: 103 (61%); control: 51 (56%); P = 0.44). Estimated glomerular filtration rate was higher in the obese group, whereas plasma creatinine was lower, when compared with the control group.
Relationship between obesity measures and markers of subclinical organ damage
LogHOMA index was related to BMI z score (β = 0.25, 95% confidence interval (CI) = 0.13–0.37; P < 0.0001) and WHR (β = 2.52; 95% CI = 1.39–3.65; P < 0.0001) in the obese group, whereas these relationships were not found in the control group. No significant sex differences were found in these relationships.
In the obese group, logUACR was related to logHOMA (rP = 0.36; P = 0.002), whereas cfPWV was not (β = −0.04; 95% CI = −0.42 to 0.33; P = 0.82). In the control group, neither logUACR nor cfPWV was related to logHOMA index. No sex differences were found when cfPWV was related to logHOMA.
Relationship between obesity measures and daytime and nighttime BP
In the obese group, no relationship was found between BMI z score or WHR and daytime systolic or diastolic BP. Daytime systolic BP tended to be related to BMI z score (β = 2.3; 95% CI = −0.2 to 4.9; P = 0.08).
Nighttime systolic BP was related to BMI z score (β = 6.0; 95% CI = 2.9–9.1; P = 0.0002) and WHR (β = 36.7; 95% CI = 5.6–67.9; P = 0.02) in the obese group. Nighttime diastolic BP was related to BMI z score (β = 2.4; 95% CI = 0.3–4.4; P = 0.02) but not to WHR.
In the control group, only nighttime systolic BP tended to be related to BMI z score (β = 2.3; 95% CI = −0.4 to 5.1; P = 0.10).
Multiple regression analyses of daytime and nighttime BP
Nighttime systolic BP was 7.9mm Hg higher in the obese group when compared with the control group, independent of cfPWV, logHOMA, and relevant confounders (Table 4). Nighttime systolic BP was related to cfPWV and tended to be related to logHOMA (P = 0.06).
Table 4.
Multiple regression models of nighttime systolic and diastolic blood pressure
| Nighttime systolic BP | Nighttime diastolic BP | |||
|---|---|---|---|---|
| β | 95% CI | β | 95% CI | |
| Group, obese vs. control | 7.9*** | 4.1 to 11.6 | 2.9* | 0.4 to 5.4 |
| Age, y | −0.7 | −1.9 to 0.4 | 0.1 | −0.7 to 0.8 |
| Height, cm | 0.3* | 0.06 to 0.5 | 0.01 | −0.1 to 0.1 |
| Gender, male vs. female | 4.8** | 1.4 to 8.1 | 3.3** | 0.4 to 5.4 |
| Period dependent HR, bpm | 0.1 | −0.1 to 0.3 | 0.2** | 0.07 to 0.3 |
| CfPWV, m/s | 3.8* | 0.7 to 6.8 | 1.5 | −0.5 to 3.5 |
| LogHOMA index | 5.5**** | −0.1 to 11.1 | 0.8 | −2.9 to 4.5 |
| Model, r 2 | *** | 0.355 | *** | 0.236 |
The number of individuals (n = 115) was reduced in the models because of missing blood sample values. No interactions existed between group and sex with the other explanatory variables.
Abbreviations: BP, blood pressure; CI, confidence interval; CfPWV, carotid–femoral pulse wave velocity; HOMA, homeostatic model assessment; HR, heart rate.
*P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.10.
Nighttime diastolic BP was 2.9mm Hg higher in the obese group when compared with the control group but was not related to logHOMA index or cfPWV when adjusted for relevant confounders.
The analysis of daytime systolic BP was restricted to the obese group (n = 74) because of interactions of the group variable with other explanatory variables: group × daytime HR (P = 0.02) and group × sex (P = 0.04). In the obese group, daytime systolic BP was related to logHOMA (β = 8.0; 95% CI = 2.6–13.5; P = 0.004) and tended to be related to cfPWV (β = 3.3; 95% CI = −0.05 to 6.7; P = 0.053) when adjusted for relevant confounders (model: r 2 = 0.288; P = 0.0007; no interactions).
The daytime diastolic BP model was also restricted to the obese group because of an interaction of group × heart rate (P = 0.02) in pooled analysis. However, the daytime diastolic BP model including only the obese group was inconclusive (P = 0.52; r 2 = 0.07). The number of individuals was reduced in the models because of missing blood sample values.
In additional analyses, triglycerides, low-density lipoprotein cholesterol, high-density lipoprotein cholesterol, estimated glomerular filtration rate, and logUrine-albumin did not enter the multiple regression models for daytime or nighttime systolic BP.
No differences were found between the 2 groups in the prevalence of smoking (5 (5%) obese vs. 0 control individuals; P = 0.16) and use of medication (14 (15%) obese vs. 9 (18%) control individuals; P = 0.63). Ambulatory BP differences between the 2 groups were reproducible when restricted to nonsmokers and individuals not receiving medication. However, the relationship between nighttime systolic BP and logHOMA (P = 0.21), sex (P = 0.11), and height (P = 0.06) became insignificant.
DISCUSSION
The main finding of our study was that the obese group had a relatively higher nighttime than daytime BP when compared with the control group. The obesity-related elevated nighttime systolic and diastolic BP were independent of insulin resistance and arterial stiffness. Although nighttime systolic BP was related to arterial stiffness and tended to be related to insulin resistance, insulin resistance and arterial stiffness were not related.
The obesity-related elevated BP was primarily driven by the elevated nighttime BP, as also found by Aguilar et al.27 This was supported by our findings of increased night-to-day BP ratios in obese subjects and the association between the degree of obesity (BMI z score) and nighttime BP in the obese group.
The 24-hour BP was markedly higher than the clinic BP. Although obesity might increase the chances of masked hypertension,15 we also found higher out-of-office BP in the control group. The observed higher out-of-office BP pattern may be because the young age of the participants.28 Alternatively, it is likely that BP measured after 10 minutes rest in supine position is the most relaxing moment during a day for active children, leading to relatively higher ambulatory daytime BP measurements. Part of it might be because of other differences in methodology (e.g., oscillometric algorithms or cuff bladder sizes). The method used was chosen to ensure the best suitable brachial BP measure for the noninvasive central hemodynamic measurements.16
Nighttime systolic BP has been related to insulin resistance independent of BMI z score in children and adolescents in a study by Lurbe et al..1 In our study, nighttime systolic BP only tended to be related to insulin resistance when adjusted for obesity status. This could be because of a type II error following limited sample size. However, in other studies using multiple regression analysis, nighttime systolic BP was related to BMI z score but not to HOMA index or other metabolic measures.27,29 In adults, it has been shown that the relationship between insulin resistance and BP is largely, if not entirely, explained by waist circumference.30 In our study design, the group variable (obese vs. control) encompasses the differences in obesity measures, which is why we could not include BMI z score or WHR in the multiple regression models.
The prevalence of microalbuminuria was equally low in the obese and the control group, suggesting that the obese children and adolescents in this study had not developed microvascular damage.31 However, the estimated glomerular filtration rate was higher in the obese group, and this might indicate an early stage of renal hyperperfusion and hyperfiltration, as can be found in nondiabetic obese children.32
In some studies, increased arterial stiffness (cfPWV) has been related to insulin resistance5,6 but not in all.33 However, both groups5,34 found, contrary to our findings, higher cfPWV in obese individuals and even higher cfPWV in obese type 2 diabetics when compared with nonobese control individuals. This fundamental difference could be because of differences in age, ethnicity, the methodology of cfPWV,16,35 and the fact that we had no patients with type 2 diabetes in our obese group. The lower cfPWV in the obese group might be a compensatory mechanism to a hyperkinetic circulation in obese children and adolescents with a supposed higher stroke volume, cardiac output, and a higher circulating blood volume.16.36,37 Despite the lower cfPWV in the obese patients in our study, we found a positive relationship between cfPWV and nighttime systolic BP, as also seen in other studies.38,39 The findings suggest that the obese children in our study, despite their higher BP, might have had a too short duration or magnitude of obesity to develop subclinical organ damage (i.e., elevated cfPWV or UACR).6
The lack of a relationship between cfPWV and nighttime diastolic BP may be explained by the low distending pressure on the arterial wall in the diastole, where tension is borne by elastin-distensible fibers.40 Contrary, a high distending pressure exists in the systole, where the tension on the arterial wall is mainly transferred to and borne by less extensible collagen fibers, making the arterial wall becomes stiffer.40
We can only speculate on the possible mechanisms involved in the higher nighttime BP in the obese group, such as inferior sleep quality because of snoring or obstructive sleep apnea,41 a changed autonomic function,42 or an impaired ability to excrete sodium.43
Our study has several limitations. First, HOMA index reflects insulin resistance in a fasting situation predominantly determined by hepatic insulin sensitivity and not peripheral (muscle) insulin sensitivity, whereas it does not provide information on glucose tolerance.44 However, HOMA index is simpler, less time consuming, and more acceptable for participants when compared with the hyperinsulinimic euglycemic clamp, which is the gold standard of assessing insulin resistance.24,44,45 Second, no puberty measures were collected, and these can potentially affect BP and all other measures. However, no differences in age, sex, or height were identified between the obese and the control group, suggesting a similar development in these characteristics. Third, it was difficult to recruit control individuals from the same social class as the obese group because overweight is more often seen in lower socioeconomic groups. Fourth, we cannot infer on pathophysiological mechanisms because of the cross-sectional study design.
In conclusion, the obese children and adolescents had a relatively higher nighttime than daytime BP when compared with a nonobese control group. The obesity-related elevated nighttime BP was independent of insulin resistance and arterial stiffness. Notably, the obese children had not developed subclinical organ damage as assessed by cfPWV or UACR.
In perspective of known tracking in BP, the adverse nighttime BP pattern of the obese children might contribute in the future to the adverse cardiovascular risk profile of adult obese patients. Therefore, early treatment and prevention of childhood obesity are important because they may prevent irreversible damage to the cardiovascular system.
SUPPLEMENTARY MATERIAL
Supplementary materials are available at American Journal of Hypertension (http://ajh.oxfordjournals.org).
DISCLOSURE
K. N. Hvidt received LEO Pharma’s Travel Grant during data collection of this study. All authors declare that there is no conflict of interests in respect to executing, analyzing, or reporting the present research project.
Supplementary Material
ACKNOWLEDGMENTS
We would like to thank the participants and the staff at the Children’s Obesity Clinic, in particularly laboratory technicians Oda Troest and Birgitte Holloese, Secretary Dorte Jensen, and database manager Arne Lykke Nielsen. For financial support we would like to thank the Health Sciences Research Foundation of Region Zealand, the Danish Heart Foundation, Kathrine og Vigo Skovgaards Fond, Det Medicinske Selskab i København, Edith og Henrik Henriksens Mindelegat, and LEO Pharma’s Travel Grant. The research activities are part of the Danish Childhood Obesity Biobank (ClinicalTrials.gov: NCT00928473) and related to TARGET (The impact of our genomes on individual treatment response in obese children) and BIOCHILD (Genetics and systems biology of childhood obesity in India and Denmark). Part of this work was presented orally in abstract form at the 23rd Annual Scientific Meeting of the European Society of Hypertension, 15 June 2013, Milan, Italy.
REFERENCES
- 1. Lurbe E, Torro I, Aguilar F, Alvarez J, Alcon J, Pascual JM, Redon J. Added impact of obesity and insulin resistance in nocturnal blood pressure elevation in children and adolescents. Hypertension 2008; 51:635–641. [DOI] [PubMed] [Google Scholar]
- 2. Westerståhl M, Marcus C. Association between nocturnal blood pressure dipping and insulin metabolism in obese adolescents. Int J Obes (Lond) 2010; 34:472–477. [DOI] [PubMed] [Google Scholar]
- 3. Marcovecchio ML, Patricelli L, Zito M, Capanna R, Ciampani M, Chiarelli F, Mohn A. Ambulatory blood pressure monitoring in obese children: role of insulin resistance. J Hypertens 2006; 24:2431–2436. [DOI] [PubMed] [Google Scholar]
- 4. Poirier P, Giles TD, Bray GA, Hong Y, Stern JS, Pi-Sunyer FX, Eckel RH. Obesity and cardiovascular disease: pathophysiology, evaluation, and effect of weight loss: an update of the 1997 American Heart Association Scientific Statement on Obesity and Heart Disease from the Obesity Committee of the Council on Nutrition, Physical. Circulation 2006; 113:898–918. [DOI] [PubMed] [Google Scholar]
- 5. Gungor N, Thompson T, Sutton-Tyrrell K, Janosky J, Arslanian S. Early signs of cardiovascular disease in youth with obesity and type 2 diabetes. Diabetes Care 2005; 28:1219–1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Webb DR, Khunti K, Silverman R, Gray LJ, Srinivasan B, Lacy PS, Williams B, Davies MJ. Impact of metabolic indices on central artery stiffness: independent association of insulin resistance and glucose with aortic pulse wave velocity. Diabetologia 2010; 53:1190–1198. [DOI] [PubMed] [Google Scholar]
- 7. Sanad M, Gharib A. Evaluation of microalbuminuria in obese children and its relation to metabolic syndrome. Pediatr Nephrol 2011; 26:2193–2199. [DOI] [PubMed] [Google Scholar]
- 8. Di Bonito P, Moio N, Scilla C, Cavuto L, Sibilio G, Forziato C, Sanguignoc E, Saittac F, Iardinod MR, Capaldo B. Preclinical manifestations of organ damage associated with the metabolic syndrome and its factors in outpatient children. Atherosclerosis 2010; 213:611–615. [DOI] [PubMed] [Google Scholar]
- 9. Invitti C, Maffeis C, Gilardini L, Pontiggia B, Mazzilli G, Girola A, Sartorio A, Morabito F, Viberti GC. Metabolic syndrome in obese Caucasian children: prevalence using WHO-derived criteria and association with nontraditional cardiovascular risk factors. Int J Obes (Lond) 2006; 30:627–633. [DOI] [PubMed] [Google Scholar]
- 10. Van Bortel LM, Laurent S, Boutouyrie P, Chowienczyk P, Cruickshank JK, De Backer T, Filipovsky J, Huybrechts S, Mattace-Raso FUS, Protogerou AD, Schillaci G, Segers P, Vermeersch S, Weber T; on behalf of the Artery Society, the European Society of Hypertension Working Group on Vascular Structure and Function and the European Network for Noninvasive Investigation of Large Arteries. Expert consensus document on the measurement of aortic stiffness in daily practice using carotid-femoral pulse wave velocity. J Hypertens 2012; 30:445–448. [DOI] [PubMed] [Google Scholar]
- 11. Jensen JS, Borch-Johnsen K, Jensen G, Feldt-Rasmussen B. Microalbuminuria reflects a generalized transvascular albumin leakiness in clinically healthy subjects. Clin Sci (Lond) 1995; 88:629–633. [DOI] [PubMed] [Google Scholar]
- 12. Rademacher ER, Sinaiko AR. Albuminuria in children. Curr Opin Nephrol Hypertens 2009; 18:246–251. [DOI] [PubMed] [Google Scholar]
- 13. Pickering TG, Hall JE, Appel LJ, Falkner BE, Graves J, Hill MN, Jones DW, Kurtz T, Sheps SG, Roccella EJ. Recommendations for blood pressure measurement in humans and experimental animals: part 1: blood pressure measurement in humans: a statement for professionals from the Subcommittee of Professional and Public Education of the American Heart Association Council on High Blood Pressure Research. Circulation 2005; 111:697–716. [DOI] [PubMed] [Google Scholar]
- 14. Hansen TW, Li Y, Boggia J, Thijs L, Richart T, Staessen JA. Predictive role of the nighttime blood pressure. Hypertension 2011; 57:3–10. [DOI] [PubMed] [Google Scholar]
- 15. Flynn JT, Urbina EM. Pediatric ambulatory blood pressure monitoring: indications and interpretations. J Clin Hypertens (Greenwich) 2012; 14:372–382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Hvidt KN, Olsen MH, Holm J-C, Ibsen H. Aortic stiffness in obese children and adolescents: comparison of two distance measures of carotid-femoral pulse wave velocity. Artery Res 2013; 7 :186–193. [Google Scholar]
- 17. Holm J-C, Gamborg M, Bille DS, Grønbæk HN, Ward LC, Faerk J. Chronic care treatment of obese children and adolescents. Int J Pediatr Obes 2011; 6:188–196. [DOI] [PubMed] [Google Scholar]
- 18. Nysom K, Mølgaard C, Hutchings B, Michaelsen KF. Body mass index of 0 to 45-y-old Danes: reference values and comparison with published European reference values. Int J Obes Relat Metab Disord 2001; 25:177–184. [DOI] [PubMed] [Google Scholar]
- 19. World Health Organization Expert Consultation. Waist Circumference and Waist-Hip Ratio: Report of a WHO Expert Consultation. WHO: Geneva, Switzerland, 2008. [Google Scholar]
- 20. Stergiou GS, Yiannes NG, Rarra VC. Validation of the Omron 705 IT oscillometric device for home blood pressure measurement in children and adolescents: the arsakion school study. Blood Press Monit 2006; 11:229–234. [DOI] [PubMed] [Google Scholar]
- 21. National High Blood Pressure Education Program Working Group on High Blood Pressure in Children and Adolescents. The fourth report on the diagnosis, evaluation, and treatment of high blood pressure in children and adolescents. Pediatrics 2004; 114:555–576. [PubMed] [Google Scholar]
- 22. Yip GW-K, So H-K, Li AM, Tomlinson B, Wong S-N, Sung RY-T. Validation of A&D TM-2430 upper-arm blood pressure monitor for ambulatory blood pressure monitoring in children and adolescents, according to the British Hypertension Society protocol. Blood Press Monit 2012; 17:76–79. [DOI] [PubMed] [Google Scholar]
- 23. Wühl E, Witte K, Soergel M, Mehls O, Schaefer F. Distribution of 24-h ambulatory blood pressure in children: normalized reference values and role of body dimensions. J Hypertens 2002; 20:1995–2007. [DOI] [PubMed] [Google Scholar]
- 24. Quon MJ. Limitations of the fasting glucose to insulin ratio as an index of insulin sensitivity. J Clin Endocrinol Metab 2001; 86:4615–4617. [DOI] [PubMed] [Google Scholar]
- 25. Schwartz GJ, Work DF. Measurement and estimation of GFR in children and adolescents. Clin J Am Soc Nephrol 2009; 4:1832–1843. [DOI] [PubMed] [Google Scholar]
- 26. National Institutes of Health. Glomerular Filtration Rate (GFR)—National Kidney Disease Education Program (NKDEP). http://nkdep.nih.gov/lab-evaluation/gfr.shtml.
- 27. Aguilar A, Ostrow V, De Luca F, Suarez E. Elevated ambulatory blood pressure in a multi-ethnic population of obese children and adolescents. J Pediatr 2010; 156:930–935. [DOI] [PubMed] [Google Scholar]
- 28. Stergiou GS, Yiannes NJ, Rarra VC, Alamara CV. White-coat hypertension and masked hypertension in children. Blood Press Monit 2005; 10:297–300. [DOI] [PubMed] [Google Scholar]
- 29. Gilardini L, Parati G, Sartorio A, Mazzilli G, Pontiggia B, Invitti C. Sympathoadrenergic and metabolic factors are involved in ambulatory blood pressure rise in childhood obesity. J Hum Hypertens 2008; 22:75–82. [DOI] [PubMed] [Google Scholar]
- 30. Poirier P, Lemieux I, Mauriège P, Dewailly E, Blanchet C, Bergeron J, Després J-P. Impact of waist circumference on the relationship between blood pressure and insulin: the quebec health survey. Hypertension 2005; 45:363–367. [DOI] [PubMed] [Google Scholar]
- 31. Nguyen S, McCulloch C, Brakeman P, Portale A, Hsu C. Being overweight modifies the association between cardiovascular risk factors and microalbuminuria in adolescents. Pediatrics 2008; 121:37–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Srivastava T. Nondiabetic consequences of obesity on kidney. Pediatr Nephrol 2006; 21:463–70. [DOI] [PubMed] [Google Scholar]
- 33. Urbina EM, Kimball TR, Khoury PR, Daniels SR, Dolan LM. Increased arterial stiffness is found in adolescents with obesity or obesity-related type 2 diabetes mellitus. J Hypertens 2010; 28:1692–1698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Urbina EM, Gao Z, Khoury PR, Martin LJ, Dolan LM. Insulin resistance and arterial stiffness in healthy adolescents and young adults. Diabetologia 2012; 55:625–631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Hvidt KN, Olsen MH, Holm J-C, Ibsen H. Carotid–femoral pulse wave velocity in obese children and adolescents: the potential bias of tape distance measuring. Artery Res 2013; 7:234–237. [Google Scholar]
- 36. De Simone G, Devereux RB, Daniels SR, Mureddu G, Roman MJ, Kimball TR, Greco R, Witt S, Contaldo F. Stroke volume and cardiac output in normotensive children and adults. Assessment of relations with body size and impact of overweight. Circulation 1997; 95:1837–1843. [DOI] [PubMed] [Google Scholar]
- 37. Alpert MA. Obesity cardiomyopathy: pathophysiology and evolution of the clinical syndrome. Am J Med Sci 2001; 321:225–236. [DOI] [PubMed] [Google Scholar]
- 38. Lurbe E, Torro I, Garcia-Vicent C, Alvarez J, Fernández-Fornoso JA, Redon J. Blood pressure and obesity exert independent influences on pulse wave velocity in youth. Hypertension 2012; 60:550–555. [DOI] [PubMed] [Google Scholar]
- 39. Corden B, Keenan NG, de Marvao ASM, Dawes TJW, Decesare A, Diamond T, Durighel G, Hughes AD, Cook SA, O’Regan DP. Body fat is associated with reduced aortic stiffness until middle age. Hypertension 2013; 61:1322–1327. [DOI] [PubMed] [Google Scholar]
- 40. London GM, Pannier B. Arterial functions: how to interpret the complex physiology. Nephrol Dial Transplant 2010; 25:3815–3823. [DOI] [PubMed] [Google Scholar]
- 41. Leung LCK, Ng DK, Lau MW, Chan C-H, Kwok K-L, Chow P-Y, Cheung JMY. Twenty-four-hour ambulatory BP in snoring children with obstructive sleep apnea syndrome. Chest 2006; 130:1009–1017. [DOI] [PubMed] [Google Scholar]
- 42. Lurbe E, Invitti C, Torro I, Maronati A, Aguilar F, Sartorio A, Redon J, Parati G. The impact of the degree of obesity on the discrepancies between office and ambulatory blood pressure values in youth. J Hypertens 2006; 24:1557–1564. [DOI] [PubMed] [Google Scholar]
- 43. Lurbe E, Alvarez V, Liao Y, Torro I, Cremades B, Redón J, Cooper R. Obesity modifies the relationship between ambulatory blood pressure and natriuresis in children. Blood Press Monit 2000; 5:275–280. [DOI] [PubMed] [Google Scholar]
- 44. Henderson M, Rabasa-Lhoret R, Bastard J-P, Chiasson J-L, Baillargeon J-P, Hanley JA, Lambert M. Measuring insulin sensitivity in youth: how do the different indices compare with the gold-standard method? Diabetes Metab 2011; 37:72–78. [DOI] [PubMed] [Google Scholar]
- 45. Conwell LS, Trost SG, Brown WJ, Batch JA. Indexes of insulin resistance and secretion in obese children and adolescents: a validation study. Diabetes Care 2004; 27:314–319. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.