Abstract
BACKGROUND
Systolic blood pressure (SBP) goal for chronic kidney disease (CKD) patients is ≤140mm Hg. However, the SBP target provides no suggested lower limit, and some studies indicate that a lower SBP target may be harmful. We aimed to investigate the J-shaped relationship between SBP and clinical outcomes in CKD patients and the factors that modify this relationship.
METHODS
This prospective observational study enrolled 2,144 CKD stage 3–4 patients between November 2002 and May 2009 and followed them until July 2010 or death. Patients included were also enrolled within the Integrated CKD Care Program for Delaying Dialysis in a medical center and its branch hospital. Demographic, clinical, laboratory, and disease variables at baseline and end of observation were measured.
RESULTS
In diabetic CKD patients, the hazard ratio (HR) at SBP 96–110mm Hg vs. 111–120mm Hg was 2.52 (95% confidence interval (CI) = 1.13–5.58) for cardiovascular outcomes and was 3.14 (95% CI = 1.16–8.49) for renal outcomes. In nondiabetic CKD patients, this J-shaped relationship was not seen. Heavy proteinuria was further found to modify the J-shaped relationship in diabetic CKD patients. The HR for renal outcomes at SBP 96–110mm Hg vs. 111–120mm Hg was 4.07 (95% CI = 1.18–13.99) in diabetic CKD patients with heavy proteinuria vs. 1.72 (95% CI = 0.13–22.5) in those without heavy proteinuria.
CONCLUSIONS
Diabetic CKD patients have a J-shaped relationship between SBP and cardiovascular or renal outcomes, but nondiabetic CKD patients do not. The optimal SBP range might be narrower in the diabetic CKD patients.
Keywords: blood pressure, chronic kidney disease, hypertension, J-shaped relationship, outcome assessment, systolic blood pressure.
Hypertension is a leading cause of mortality, renal dysfunction, and cardiovascular events in the general population1,2 and in patients with chronic kidney disease (CKD).3–5 Blood pressure (BP) components have been investigated, and systolic BP (SBP) has the strongest association with rapid decline in kidney function in community-living older adults,6 and SBP is a much stronger predictor of end-stage renal disease (ESRD) compared with diastolic BP in CKD patients.7 The SBP goal for CKD patients is ≤140mm Hg.8,9 Guidelines suggest stricter BP targets in patients with proteinuria >1g/day10 or with urine albumin excretion ≥30mg/day.9
However, lower BP targets may involve risks. Clinical trials using a randomized, intention-to-treat design testing lower SBP (<120mm Hg) vs. traditional SBP (<140mm Hg) are ongoing.11 The adverse effects of low SBP are widely reported. Several observational studies or secondary analyses have demonstrated a J-shaped relationship between SBP and cardiovascular outcomes3,5,12 and all-cause mortality7,13 in CKD patients or in subgroups with heavy proteinuria. One study showed a J-shaped relationship between SBP and ESRD14 in CKD patients. Whether the lower limit of SBP is dangerous in all or only certain subgroups of CKD patients remains unclear. Delineation of the lower limit of SBP is important for both future clinical trials and clinical practice, particularly when executing lower SBP target strategies.11
The discrepancies in studies regarding the J-shaped relationship between SBP and clinical outcomes may be the result of effect modifiers. Previous studies have demonstrated that heavy proteinuria modifies the association between SBP <110mm Hg and worse outcomes.14 We also observed an increased risk of clinical outcomes in the lowest SBP group or intensive BP control group in CKD patients with diabetes mellitus (DM)12,13 but not in those without DM.15 However, whether DM modifies this relationship is not demonstrated.
Thus, we investigated the J-shaped relationship between SBP and clinical outcomes in CKD patients and the factors modifying this relationship in an observational cohort study.
METHODS
Ethics statement
The study protocol was approved by the institutional review board of Kaohsiung Medical University Hospital (KMUH-IRB-990198). Written, informed consent was obtained from all patients, and all clinical investigations were conducted according to the principles of the Declaration of Helsinki.
Participants and measurements
Between November 2002 and May 2009, 3,749 patients from the Integrated CKD Care Program in Kaohsiung for Delaying Dialysis in 2 hospitals in Southern Taiwan were included. All were followed until 31 July 2010, or death. In the first 3 months, 90 patients were lost to follow-up. To be compatible with other studies, we included only the 2,144 patients with CKD stages 3 and 4. Standardized BP measurements were obtained by a standard mercury sphygmomanometer or a validated automated device in seated patients after a 10-minute rest. The average of the first 2 BP readings was recorded each visit. All participants had 3–7 BP records based on the frequency of the follow-ups. All BP records of each patient were averaged as the baseline BP for the analysis. We excluded 13 patients with a baseline mean SBP of ≤95mm Hg, for a final 2,131 CKD stage 3 and 4 patients. The median follow-up time was 2.91 years. To better analyze the relationship between SBP and clinical outcomes for SBP ≤140mm Hg, we categorized SBP into 4 groups: 96–110, 111–120, 121–140, and >140mm Hg.
CKD was defined in accordance with the National Kidney Foundation–Kidney Disease Outcomes Quality Initiative guidelines. Baseline variables included demographic features, medical history, physical examinations, laboratory data, and medication history. Medical history was obtained from chart review. DM and hypertension were defined by clinical diagnosis. The laboratory data and BP for the 3 months before and after enrollment in the CKD care system were averaged and analyzed.
Quantification of renal function and progression
Kidney function was quantified using estimated glomerular filtration rate (eGFR) derived from the simplified Modification of Diet in Renal Disease Study equation. The equation was eGFR ml/min/1.73 m2 = 186 × serum creatinine−1.154 × age−0.203 × 0.742 (if female) or × 1.212 (if black). The average eGFR slope (ml/min/1.73 m2/year) for each patient was calculated with a varying-intercept and a varying-slope equation without covariables to estimate the annual change in eGFR.
Outcomes
Four outcomes were assessed: all-cause mortality, cardiovascular events, need for renal replacement therapy (RRT), and rapid renal function decline. Survival status and cause of death were ascertained from review of medical charts or the National Death Index. Cardiovascular events were ascertained by chart review and defined as hospitalization for acute coronary syndrome (Deyo’s modified Charlson score, International Classification of Diseases, Clinical Modification (ICD-9-CM): 410.x–412.x), acute cerebrovascular disease (430.x–438.x), congestive heart failure (428.x), peripheral arterial occlusion disease (443.9, 441.x, 785.4, V43.4, procedure 38.48), and death from the aforementioned conditions. A need for RRT was defined as initiation of maintenance hemodialysis, peritoneal dialysis, or renal transplantation. The RRT was ascertained from chart review and national database. RRT was begun according to the Bureau of National Health Insurance of Taiwan standards for laboratory data, nutritional status, uremic status, and eGFR. All eGFR values and at least 3 measurements in each individual were included for calculating eGFR slope. Rapid renal function decline was defined as eGFR slope <−5ml/min/1.73m2/year based on the Kidney Disease Improving Global Outcomes (KDIGO) guideline.16
Statistical analysis
Summary statistical results of baseline characteristics of all patients and stratification by diabetes status were expressed as percentages for categorical data, mean ± SD for continuous variables with approximately normal distribution, and median and interquartile ranges for continuous variables with skewed distribution.
Cox proportional hazards analysis was used to evaluate the relationship between SBP and all-cause mortality, cardiovascular events, and a need for RRT. Multivariable logistic regression analysis was used to evaluate the relationship between SBP and rapid renal function decline. Skewed-distributed continuous variables were log-transformed to reduce the skewness. The analysis was performed on all patients, then in subgroups of patients stratified by diabetes, urine protein–creatinine ratio (UPCR > 1g/g), and usage of antihypertensive agents. Interactions between subgroups were tested by adding interaction terms into the Cox regression model.
Models for all-cause mortality, including patients who reached RRT, were censored only at death or end of follow-up. Models for cardiovascular events were censored at the development of these events, death, or end of follow-up. Models for RRT were censored at the commencement of RRT, death, or end of follow-up.
Our variable selection is based on previous literatures in this population and the medications that could be confounding factors. The adjusted covariables were age, sex, eGFR, UPCR, hemoglobin, albumin, phosphorus, total cholesterol, C-reactive protein, body mass index, smoking status, baseline congestive heart failure, myocardial infarction, cerebral vascular disease, and the use of angiotensin-converting enzyme inhibitors, angiotensin receptor blockers, calcium channel blockers, beta-blockers, alpha-blockers, and diuretics.
RESULTS
Characteristics of study populations by diabetes and baseline SBP
In total, of 2,131 CKD stage 3 and 4 patients, 939 had DM. The mean age was 64.2±13.5 years, 64.6% were men, and 63.5% had hypertension at baseline (Table 1). In the diabetic group, those at SBP 96–110 and SBP 111–120mm Hg did not differ in UPCR, serum albumin, glycosylated hemoglobin, eGFR, and percentage of patients taking antihypertensive agents. In the non-DM group, those at SBP 96–110 and SBP 111–120mm Hg did not differ in UPCR and serum albumin. The percentage of patients taking antihypertensive agents at SBP 96–110mm Hg was significantly lower than for those at SBP 111–120mm Hg (50.6% vs. 64.6%; P = 0.02).
Table 1.
Characteristics stratified by diabetes mellitus status and baseline systolic blood pressure level in chronic kidney disease stage 3–4 patients
| Variables | All patients | Non-DM | P value | DM | P value | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| SBP 96–110mm Hg | SBP 111–120mm Hg | SBP 121–140mm Hg | SBP >140mm Hg | SBP 96–110mm Hg | SBP 111–120mm Hg | SBP 121–140mm Hg | SBP >140mm Hg | ||||
| Patients, no. | 2,131 | 87 | 178 | 505 | 422 | 66 | 104 | 327 | 442 | ||
| Demographic data and medical history | |||||||||||
| Age, mean (SD), y | 64.2 (13.5) | 63.3 (15.1) | 63.2 (14.9) | 63.0 (14.1) | 63.5 (14.6) | 0.97 | 62.7 (12.4) | 65.3 (12.1) | 65.7 (12.2) | 65.8 (11.7) | 0.25 |
| Male, no. (%) | 1,377 (64.6) | 58 (66.7) | 119 (66.9) | 337 (66.7) | 283 (67.1) | 1 | 43 (65.2) | 75 (72.1) | 216 (66.1) | 246 (55.7) | <0.01 |
| Smoker, no. (%) | 277 (13.0) | 12 (13.8) | 23 (12.9) | 63 (12.5) | 54 (12.8) | 0.99 | 13 (19.7) | 18 (17.3) | 36 (11.0) | 58 (13.1) | 0.15 |
| Stroke, no. (%) | 377 (17.7) | 14 (16.1) | 20 (11.2) | 62 (12.3) | 53 (12.6) | 0.73 | 10 (15.2) | 25 (24.0) | 82 (25.1) | 111 (25.1) | 0.35 |
| MI, no. (%) | 53 (2.5) | 3 (3.4) | 2 (1.1) | 11 (2.2) | 6 (1.4) | 0.48 | 4 (6.1) | 5 (4.8) | 8 (2.4) | 14 (3.2) | 0.38 |
| CHF, no. (%) | 214 (10.0) | 11 (12.6) | 8 (4.5) | 40 (7.9) | 27 (6.4) | 0.09 | 8 (12.1) | 14 (13.5) | 48 (14.7) | 58 (13.1) | 0.91 |
| SBP, mean (SD), mm Hg | 138.9 (19.8) | 105.5 (4.8) | 118.3 (2.6) | 132.2 (5.7) | 157.2 (13.0) | <0.01 | 106.2 (4.0) | 118.1 (2.7) | 132.1 (5.5) | 158.6 (14.8) | <0.01 |
| BMI, mean (SD), kg/m2 | 25.1 (3.9) | 23.1 (3.4) | 24.4 (3.9) | 25.0 (4.0) | 25.1 (3.8) | <0.01 | 24.5 (3.7) | 24.9 (4.0) | 25.3 (3.9) | 25.7 (4.0) | 0.04 |
| Renal function and proteinuria | |||||||||||
| eGFR, mean (SD), ml/min/1.73m2a | 33.2 (11.9) | 36.0 (12.4) | 35.1 (11.8) | 34.2 (12.1) | 33.2 (12.4) | 0.14 | 33.1 (11.4) | 34.4 (12.7) | 32.5 (11.4) | 30.9 (10.9) | 0.02 |
| Urine PCR, median (IQR), mg/g | 706 (263–1,709) | 389 (148–1,047) | 398 (175–974) | 529 (221–1,161) | 732 (254–1,578) | <0.01 | 380 (197–1,020) | 582 (239–1,703) | 739 (281–2,173) | 1,462 (549–3,622) | <0.01 |
| Biochemistry | |||||||||||
| Hemoglobin, mean (SD), g/dl | 11.9 (2.1) | 12.2 (2.2) | 12.1 (2.1) | 12.3 (2.1) | 12.2 (2.0) | 0.69 | 12.1 (2.2) | 11.8 (2.3) | 11.7 (2.1) | 11.4 (2.0) | 0.01 |
| Albumin, mean (SD), g/dl | 3.9 (0.5) | 3.9 (0.5) | 3.9 (0.5) | 4.0 (0.4) | 4.0 (0.5) | 0.02 | 3.9 (0.4) | 3.8 (0.6) | 3.8 (0.5) | 3.7 (0.6) | 0.03 |
| Hba1c, mean (SD),% | 6.6 (1.7) | 5.8 (0.9) | 5.8 (1.0) | 5.9 (1.0) | 6.1 (1.3) | 0.01 | 7.0 (1.8) | 7.3 (1.7) | 7.3 (1.9) | 7.7 (1.9) | <0.01 |
| Phosphorus, mean (SD), mg/dl | 3.9 (0.8) | 3.7 (0.8) | 3.8 (0.8) | 3.8 (0.8) | 3.9 (0.8) | 0.06 | 4.0 (0.9) | 3.9 (0.7) | 3.9 (0.9) | 4.2 (0.9) | <0.01 |
| CRP, median (IQR), mg/L | 1.0 (0.4–4.6) | 1.3 (0.5–4.8) | 0.8 (0.3–2.3) | 0.9 (0.4–3.5) | 1.1 (0.3–5.3) | 0.09 | 0.7 (0.3–3.2) | 0.8 (0.3–5.4) | 1.4 (0.4–6.3) | 1.2 (0.4–5.6) | 0.27 |
| Cholesterol, median (IQR), mg/dL | 192.0 (165.0–224.0) | 190.0 (167.0–222.0) | 189.0 (158.8–216.0) | 192.0 (165.8–219.5) | 195.3 (170.9–229.0) | 0.03 | 174.6 (145.5–204.3) | 180.5 (152.8–208.0) | 194.0 (164.0–224.0) | 196.0 (167.0–233.0) | <0.01 |
| LDL, median (IQR), mg/dL | 111.0 (91.0–136.0) | 114.7 (88.5–135.5) | 109.1 (89.2–133.7) | 111.0 (93.0–134.0) | 111.0 (90.8–136.1) | 0.91 | 98.9 (79.8–122.8) | 102.3 (87.3–131.9) | 110.6 (89.4–138.0) | 113.4 (91.0–137.7) | 0.02 |
| Medications, no. (%) | |||||||||||
| CCB | 711 (34.0) | 7 (8.0) | 42 (24.0) | 126 (25.4) | 165 (40.0) | <0.01 | 11 (16.9) | 19 (18.4) | 115 (35.9) | 226 (51.8) | <0.01 |
| Beta blockers | 435 (20.8) | 10 (11.5) | 19 (10.9) | 85 (17.1) | 92 (22.3) | <0.01 | 12 (18.5) | 23 (22.3) | 76 (23.8) | 118 (27.1) | <0.01 |
| Alpha blockers | 208 (9.9) | 3 (3.4) | 13 (7.4) | 49 (9.9) | 46 (11.2) | 0.11 | 5 (7.7) | 10 (9.7) | 35 (10.9) | 47 (10.8) | 0.87 |
| ACEI | 644 (30.6) | 26 (29.9) | 49 (27.7) | 155 (31.2) | 98 (23.7) | 0.09 | 21 (32.3) | 34 (33.0) | 106 (32.9) | 155 (35.3) | 0.89 |
| ARB | 907 (43.1) | 27 (31.0) | 57 (32.2) | 159 (32.0) | 173 (41.9) | <0.01 | 24 (36.9) | 57 (55.3) | 168 (52.2) | 242 (55.1) | 0.05 |
| Diuretics | 340 (16.0) | 7 (8.0) | 14 (7.9) | 46 (9.1) | 42 (10.0) | 0.85 | 12 (18.2) | 18 (17.3) | 67 (20.6) | 134 (30.3) | 0.85 |
| Anti-HT | 1,606 (75.4) | 44 (50.6) | 115 (64.6) | 354 (70.1) | 339 (80.3) | <0.01 | 44 (66.7) | 74 (71.2) | 264 (80.7) | 372 (84.2) | <0.01 |
| Statins | 756 (35.9) | 16 (18.4) | 40 (22.6) | 128 (25.8) | 120 (29.1) | 0.13 | 29 (44.6) | 43 (41.7) | 163 (50.6) | 217 (49.4) | 0.39 |
Abbreviations: ACEI, angiotensin-converting enzyme inhibitor; anti-HT, antihypertension agents; ARB, angiotensin II receptor blocker; BMI, body mass index; CCB, calcium channel blocker; CHF, congestive heart failure; CRP, C-reactive protein; DM, diabetes mellitus; eGFR, estimated glomerular filtration rate; Hba1c, glycosylated hemoglobin; IQR, interquartile range; LDL, low-density lipoprotein; MI, myocardial infarction; PCR, protein to creatinine ratio; SBP, systolic blood pressure; SD, standard deviation.
aObtained by using the Modification of Diet in Renal Disease study equation.
Relationship between baseline SBP and cardiovascular outcome
The crude event rate of clinical outcomes by baseline SBP and DM is shown in Table 2. Cardiovascular outcome occurred in 101 non-DM patients and in 181 DM patients. The crude event rate per 100 patient-years was 2.937 in the non-DM group and 7.596 in the DM group.
Table 2.
Crude event rate and estimated glomerular filtration rate slope per year by diabetes mellitus status and baseline systolic blood pressure in patients with stage 3 or 4 chronic kidney disease during the observation period
| Non-DM | DM | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| SBP 96–110mm Hg | SBP 111–120mm Hg | SBP 121–140mm Hg | SBP >140mm Hg | P value | SBP 96–110mm Hg | SBP 111–120mm Hg | SBP 121–140mm Hg | SBP >140mm Hg | P value | |
| Patients, no. | 87 | 178 | 505 | 422 | 66 | 104 | 327 | 442 | ||
| Crude event rate, per 100 patient-yearsa | ||||||||||
| Cardiovascular outcome | 1.75 | 2.75 | 2.92 | 3.28 | NA | 7.51 | 4.16 | 7.08 | 8.80 | NA |
| Renal outcome | 2.81 | 2.72 | 2.53 | 4.36 | NA | 3.74 | 3.78 | 5.42 | 9.86 | NA |
| All-cause mortality | 3.89 | 2.46 | 3.29 | 3.86 | NA | 4.78 | 4.63 | 4.25 | 5.14 | NA |
| eGFR slope per year, median (IQR), ml/min/1.73 m2/yb | −0.6 (−2.1 to 1.9) | −0.9 (−3.7 to 1.0) | −1.1 (−4.3 to 1.0) | −2.2 (−5.1 to 0.1) | <0.01 | −0.9 (−4.2 to 0.8) | −1.1 (−5.0 to 1.4) | −2.1 (−5.7 to 0.1) | −3.8 (−8.4 to -0.8) | <0.01 |
Abbreviations: DM, diabetes mellitus; eGFR, estimated glomerular filtration rate; IQR, interquartile range; NA, not applicable; SBP, systolic blood pressure.
aThe number of events is assessed by diagnostic codes. This method underestimates the true event rate because these codes are specific but not sensitive.
bCalculated with a varying intercept and a varying slope without covariables for estimation of the annual change of eGFR.
The Cox survival analysis of clinical outcomes by baseline SBP and DM was shown in Tables 3–5 and in Supplementary Table S1 with a different reference group. In fully adjusted Cox regression, no statistically significant J-shaped relationship existed between SBP and cardiovascular outcomes: the hazard ratio (HR) of SBP 96–110mm Hg vs 111–120mm Hg was 1.22 (95% confidence interval (CI) = 0.67–2.23), and the HR of SBP >140mm Hg vs. 111–120mm Hg was 1.29 (95% CI = 0.84–1.98). Because of significant interactions between SBP and DM on cardiovascular outcomes (P < 0.05), we further divided these CKD patients by DM. In diabetic CKD patients, the HR of cardiovascular outcomes at SBP 96–110mm Hg vs. SBP 111–120 mmHg was 2.52 (95% CI = 1.13–5.58). However, in non-DM CKD patients, the HR of cardiovascular outcomes at SBP 96–110mm Hg vs. SBP 111–120mm Hg was 0.53 (95% CI = 0.19–1.49) (Tables 3–5; Figure 1; Supplementary Table S1).
Table 3.
Unadjusted and adjusted hazard ratio of clinical outcome by diabetes mellitus status and baseline systolic blood pressure in patients with stage 3 or 4 chronic kidney disease
| Total | Non-DM | DM | ||||
|---|---|---|---|---|---|---|
| Unadjusted HR (95% CI) | Adjusted HRa (95% CI) | Unadjusted HR (95% CI) | Adjusted HRa (95% CI) | Unadjusted HR (95% CI) | Adjusted HRa (95% CI) | |
| CV outcomes SBP, mm Hg | ||||||
| 96–110 | 1.22 (0.67–2.22) | 1.22 (0.67–2.23) | 0.65 (0.24–1.78) | 0.53 (0.19–1.49) | 1.78 (0.81–3.91) | 2.52 (1.13–5.58) |
| 111–120 | Reference group | Reference group | Reference group | Reference group | Reference group | Reference group |
| 121–140 | 1.32 (0.86–2.02) | 1.20 (0.78–1.84) | 1.02 (0.56–1.84) | 1.02 (0.55–1.91) | 1.64 (0.88–3.05) | 1.45 (0.77–2.72) |
| >140 | 1.76 (1.17–2.67) | 1.29 (0.84–1.98) | 1.16 (0.64–2.11) | 1.04 (0.56–1.95) | 2.03 (1.11–3.70) | 1.62 (0.87–3.00) |
| Renal outcomes SBP, mm Hg | ||||||
| 96–110 | 1.01 (0.54–1.91) | 1.41 (0.73–2.74) | 0.99 (0.42–2.34) | 0.65 (0.26–1.64) | 0.99 (0.38–2.55) | 3.14 (1.16–8.49) |
| 111–120 | Reference group | Reference group | Reference group | Reference group | Reference group | Reference group |
| 121–140 | 1.19 (0.77–1.85) | 1.27 (0.80–2.01) | 0.95 (0.52–1.74) | 0.83 (0.43–1.58) | 1.42 (0.74–2.72) | 1.64 (0.82–3.29) |
| >140 | 2.49 (1.64–3.76) | 1.75 (1.13–2.71) | 1.68 (0.95–2.98) | 0.89 (0.48–1.69) | 2.85 (1.54–5.30) | 2.92 (1.51–5.66) |
| All-cause mortality SBP, mm Hg | ||||||
| 96–110 | 1.32 (0.76–2.28) | 1.18 (0.68–2.07) | 1.57 (0.73–3.35) | 1.40 (0.63–3.10) | 1.03 (0.46–2.29) | 1.37 (0.60–3.08) |
| 111–120 | Reference group | Reference group | Reference group | Reference group | Reference group | Reference group |
| 121–140 | 1.13 (0.75–1.71) | 1.15 (0.75–1.74) | 1.34 (0.76–2.38) | 1.58 (0.86–2.91) | 0.90 (0.50–1.62) | 0.85 (0.46–1.55) |
| >140 | 1.42 (0.95–2.11) | 1.25 (0.82–1.90) | 1.59 (0.89–2.82) | 1.60 (0.87–2.94) | 1.12 (0.64–1.95) | 1.03 (0.58–1.86) |
Abbreviations: CI, confidence interval; CV, cardiovascular; DM, diabetes mellitus; HR, hazard ratio; SBP, systolic blood pressure.
aFully adjusted model included age, sex, estimated glomerular filtration rate, urine protein–creatinine ratio, hemoglobin, albumin, phosphorus, total cholesterol, C-reactive protein, body mass index, smoking status, baseline congestive heart failure, myocardial infarction, cerebral vascular disease, and the use of angiotensin-converting enzyme inhibitors, angiotensin receptor blockers, calcium channel blockers, beta-blockers, alpha-blockers, and diuretics.
Table 5.
Unadjusted and adjusted hazard ratio for clinical outcome by diabetes mellitus status, proteinuria, and baseline systolic blood pressure in chronic kidney disease stage 3 or 4
| Non-DM | P interaction b | DM | P interaction b | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| UPCR < 1g/g (n = 812) | UPCR ≥1g/g (n= 380) | UPCR <1g/g (n = 459) | UPCR ≥ 1g/g (n = 480) | |||||||
| Unadjusted HR (95% CI) | Adjusted HRa (95% CI) | Unadjusted HR (95% CI) | Adjusted HRa (95% CI) | Unadjusted HR (95% CI) | Adjusted HRa (95% CI) | Unadjusted HR (95% CI) | Adjusted HRa (95% CI) | |||
| CV outcomes SBP, mm Hg | 0.92 | 0.45 | ||||||||
| 96–110 | 0.56 (0.12–2.69) | 0.42 (0.08–2.09) | 0.73 (0.19–2.78) | 0.59 (0.14–2.50) | 2.93 (0.90–9.50) | 4.40 (1.29–14.99) | 1.47 (0.44–4.89) | 1.90 (0.55–6.58) | ||
| 111–120 | Reference group | Reference group | Reference group | Reference group | Reference group | Reference group | Reference group | Reference group | ||
| 121–140 | 1.42 (0.61–3.29) | 1.33 (0.56–3.19) | 0.56 (0.24–1.31) | 0.62 (0.24–1.64) | 2.50 (0.87–7.14) | 2.70 (0.92–7.95) | 1.17 (0.54–2.54) | 0.99 (0.44–2.20) | ||
| >140 | 1.43 (0.59–3.45) | 1.49 (0.59–3.78) | 0.67 (0.30–1.51) | 0.62 (0.25–1.51) | 2.17 (0.74–6.32) | 1.87 (0.62–5.63) | 1.48 (0.71–3.07) | 1.44 (0.68–3.07) | ||
| Renal outcomes SBP, mm Hg | 0.59 | <0.01 | ||||||||
| 96–110 | 2.56 (0.69–9.55) | 1.90 (0.41–8.79) | 0.37 (0.10–1.34) | 0.27 (0.06–1.10) | 1.73 (0.29–0.33) | 1.72 (0.13–22.5) | 1.30 (0.40–4.22) | 4.07 (1.18–13.99) | ||
| 111–120 | Reference group | Reference group | Reference group | Reference group | Reference group | Reference group | Reference group | Reference group | ||
| 121–140 | 1.25 (0.40–3.92) | 1.23 (0.34–4.42) | 0.60 (0.30–1.22) | 0.74 (0.33–1.67) | 0.78 (0.15–4.04) | 0.68 (0.07–6.73) | 1.36 (0.66–2.78) | 1.90 (0.89–4.06) | ||
| >140 | 2.16 (0.70–6.62) | 2.04 (0.57–7.32) | 0.82 (0.42–1.59) | 0.74 (0.33–1.65) | 1.63 (0.35–7.62) | 3.69 (0.41–3.38) | 2.05 (1.04–4.05) | 3.27 (1.58–6.74) | ||
| Mortality SBP, mm Hg | 0.65 | 0.69 | ||||||||
| 96–110 | 1.97 (0.69–5.63) | 1.22 (0.40–3.71) | 1.21 (0.40–3.71) | 2.28 (0.61–8.58) | 1.05 (0.38–2.89) | 1.75 (0.58–5.26) | 1.12 (0.29–4.32) | 1.82 (0.45–7.41) | ||
| 111–120 | Reference group | Reference group | Reference group | Reference group | Reference group | Reference group | Reference group | Reference group | ||
| 121–140 | 1.79 (0.79–4.08) | 1.62 (0.68–3.84) | 0.87 (0.39–1.96) | 2.33 (0.87–6.23) | 0.73 (0.32–1.70) | 0.80 (0.32–1.99) | 1.03 (0.45–7.41) | 1.10 (0.45–2.70) | ||
| >140 | 1.71 (0.72–4.07) | 1.46 (0.58–3.73) | 1.04 (0.48–2.26) | 2.36 (0.94–5.93) | 0.76 (0.33–1.79) | 0.84 (0.33–2.14) | 1.19 (0.54–2.62) | 1.59 (0.68–3.72) | ||
Abbreviations: CI, confidence interval; DM, diabetes mellitus; HR, hazard ratio; SBP, systolic blood pressure; UPCR, urine protein–creatinine ratio
aFully adjusted model included age, sex, estimated glomerular filtration rate, urine protein–creatinine ratio, hemoglobin, albumin, phosphorus, total cholesterol, C-reactive protein, body mass index, smoking status, baseline congestive heart failure, myocardial infarction, cerebral vascular disease, and the use of angiotensin-converting enzyme inhibitors, angiotensin receptor blockers, calcium channel blockers, beta-blockers, alpha-blockers, and diuretics.
bDefined as categorized SBP × UPCR ≥1g/g interaction P.
Figure 1.
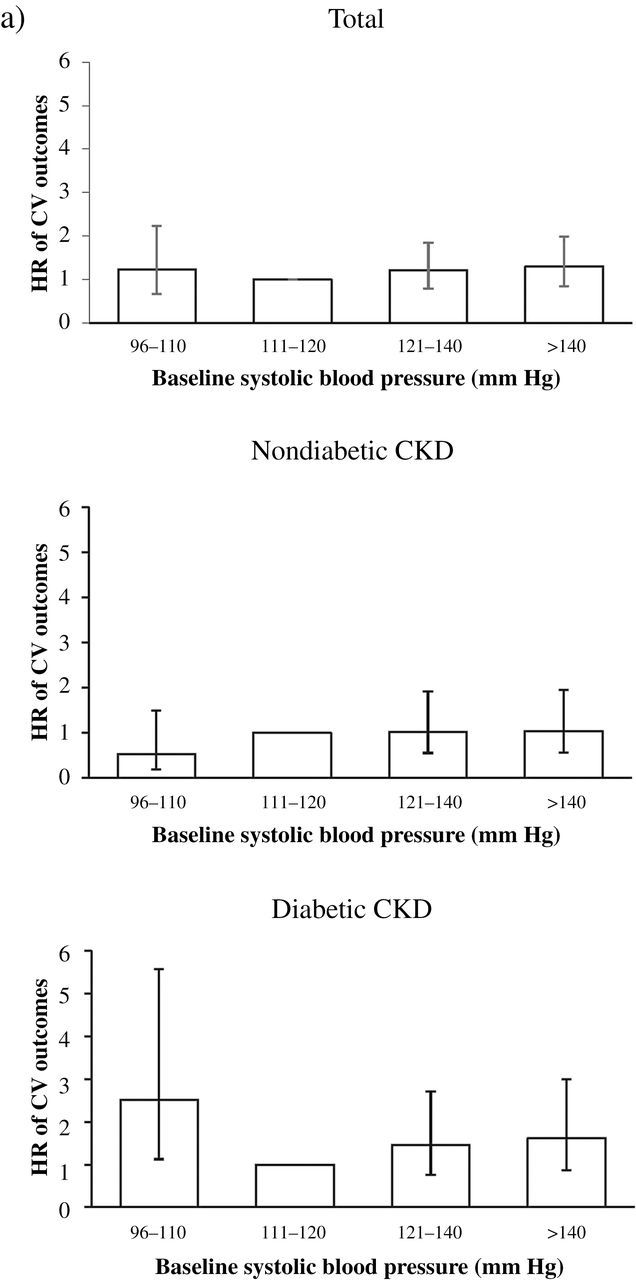
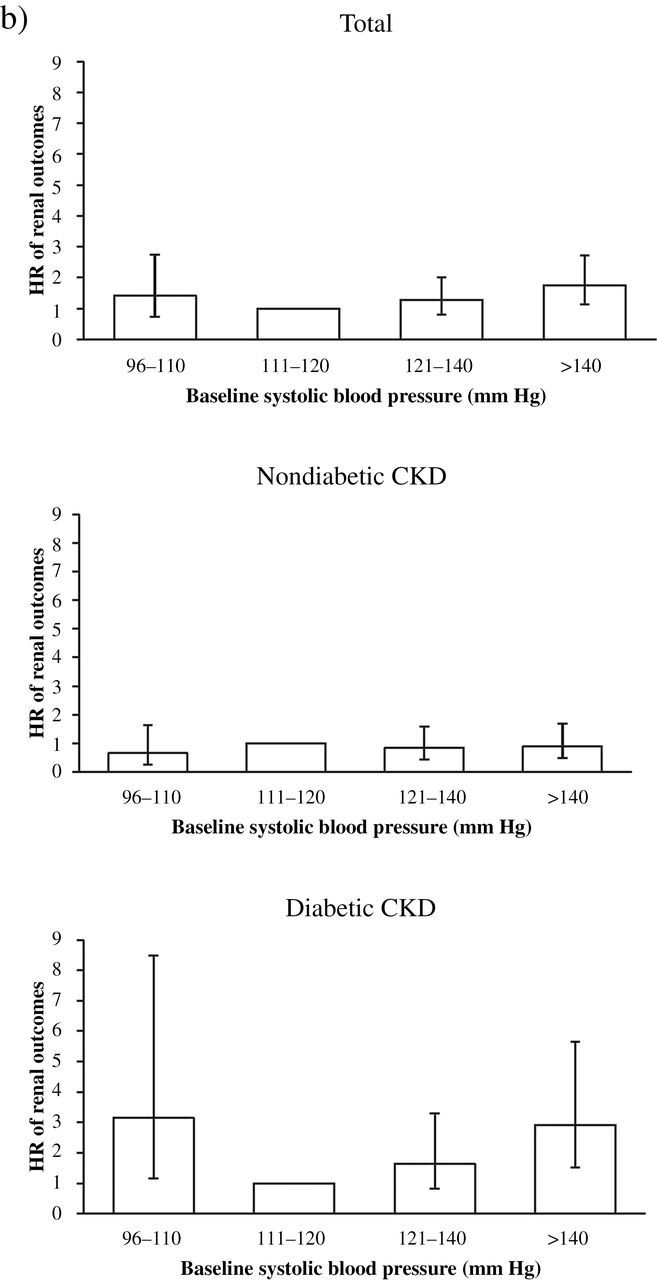
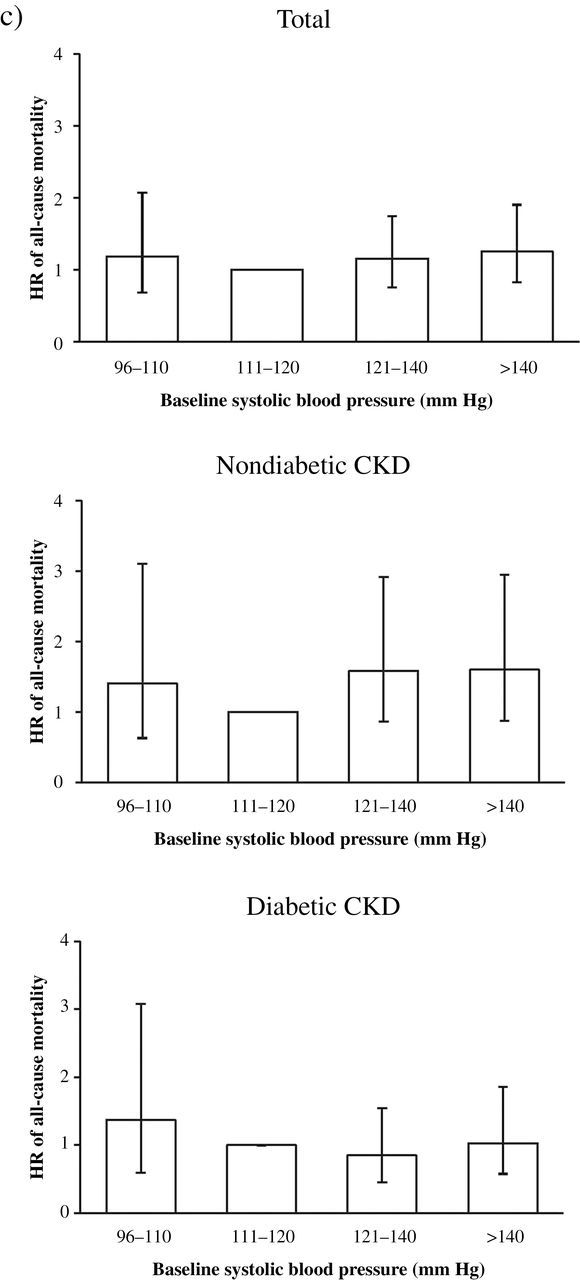
Adjusted hazard ratio (HR) of clinical outcomes associated with diabetes mellitus and systolic blood pressure (SBP). (a) HR of cardiovascular (CV) outcome in total, nondiabetic, and diabetic stage 3–4 chronic kidney disease (CKD) patients. (b) HR of renal outcome in total, nondiabetic, and diabetic stage 3–4 CKD patients. (c) HR of all-cause mortality in total, nondiabetic, or diabetic stage 3–to 4 CKD patients. Significantly increased HR of CV outcome and renal outcome is observed at SBP 96–110mm Hg in diabetic patients with stage 3–4 CKD.
Relationship between baseline SBP and ESRD
ESRD occurred in 114 non-DM patients and in 185 DM patients. The crude event rate per 100 patient-years was 3.296 in the non-DM group and 7.208 in the DM group (Table 2).
In fully adjusted Cox regression, no statistically significant J-shaped relationship existed between SBP and renal outcome: HR of SBP 96–110mm Hg vs. 111–120mm Hg was 1.41 (95% CI = 0.73–2.74), and HR of SBP >140mm Hg vs 111–120mm Hg was 1.75 (95% CI = 1.13–2.71). Because of significant interactions between SBP and DM on renal outcomes (P < 0.05), we further divided patients by DM. In diabetic CKD patients, the HR of renal outcomes at SBP 96–110mm Hg vs. SBP 111–120mm Hg was 2.92 (95% CI = 1.51–5.66). However, in nondiabetic CKD patients, the HR of renal outcomes at SBP 96–110mm Hg vs. SBP 110–120mm Hg was 0.65 (95% CI = 0.26–1.64) (Tables 3–5; Figure 1; Supplementary Table S1).
Relationship between baseline SBP and all-cause mortality
All-cause mortality occurred in 131 non-DM patients and in 138 DM patients. The crude event rate per 100 patient-years was 3.413 in the non-DM group and 4.749 in the DM group (Table 2).
In fully adjusted Cox regression, no statistically significant J-shaped relationship existed between SBP and all-cause mortality: HR of SBP 96–110 mmHg vs. 111–120mm Hg was 1.18 (95% CI = 0.68–2.07), and HR of SBP >140mm Hg vs. 111–120mm Hg was 1.25 (95% CI = 0.82–1.90). Unlike cardiovascular and renal outcomes, the interaction between SBP and DM on all-cause mortality was not statistically significant (P > 0.05). The role of DM was studied in this group for comparison. Both DM and non-DM group had no statistically significant J-shaped relationship between SBP and all-cause mortality (Tables 3–5; Figure 1; Supplementary Table S1).
Relationship between baseline SBP and rapid renal function decline
The multivariable logistic regression analysis of rapid renal function decline by baseline SBP and DM is shown in Table 4. Rapid renal function decline was defined as eGFR slope <−5ml/min/1.73 m2/year based on Kidney Disease Improving Global Outcomes (KDIGO) guidelines.16
Table 4.
Unadjusted and adjusted odds ratio of rapid renal function progression by diabetes mellitus status and baseline systolic blood pressure in stage 3 or 4 chronic kidney disease
| Total | Non-DM | DM | ||||
|---|---|---|---|---|---|---|
| Unadjusted OR (95% CI) | Adjusted ORa (95% CI) | Unadjusted OR (95% CI) | Adjusted ORa (95% CI) | Unadjusted OR (95% CI) | Adjusted ORa (95% CI) | |
| Rapid renal progressionb SBP, mm Hg | ||||||
| 96–110 | 0.58 (0.40–1.00) | 0.56 (0.30–1.03) | 0.50 (0.23–1.09) | 0.42 (0.18–0.99) | 0.64 (0.30–1.37) | 0.83 (0.33–2.04) |
| 111–120 | Reference group | Reference group | Reference group | Reference group | Reference group | Reference group |
| 121–140 | 1.16 (0.83–1.60) | 1.15 (0.79–1.67) | 1.06 (0.68–1.64) | 1.00 (0.62–1.61) | 1.26 (0.77–2.08) | 1.37 (0.76–2.47) |
| >140 | 1.84 (1.34–2.53) | 1.30 (0.89–1.89) | 1.43 (0.92–2.23) | 1.04 (0.64–1.71) | 2.02 (1.25–3.25) | 1.67 (0.93–2.98) |
Abbreviations: CI, confidence interval; DM, diabetes mellitus; OR, odds ratio; SBP, systolic blood pressure.
aFully adjusted model included age, sex, estimated glomerular filtration rate, urine protein–creatinine ratio, hemoglobin, albumin, phosphorus, total cholesterol, C-reactive protein, body mass index, smoking status, baseline congestive heart failure, myocardial infarction, cerebral vascular disease, and the use of angiotensin-converting enzyme inhibitors, angiotensin receptor blockers, calcium channel blockers, beta-blockers, alpha-blockers, and diuretics.
bDefined as an estimated glomerular filtration rate slope <−5ml/min/1.73 m2/year.
In nondiabetic CKD patients, the odds ratio (OR) of rapid renal function decline at SBP 96–110mm Hg vs. SBP 111–120mm Hg was 0.42 (95% CI = 0.18–0.99) in the fully adjusted model. However, in diabetic CKD patients, the OR of rapid renal function decline at SBP 96–110mm Hg vs. SBP 111–120mm Hg was 0.83 (95% CI = 0.33–2.04) in the fully adjusted model.
Modification by proteinuria of the J-shaped relationship between SBP and clinical outcomes in diabetic CKD patients
We performed subgroup analysis to evaluate the impact of proteinuria. Significant interaction between categorized SBP and UPCR ≥1g/g on renal outcomes in the diabetic CKD patients was noted. We further divided diabetic CKD patients into UPCR ≥1g/g and UPCR <1g/g. In diabetic CKD patients with UPCR ≥1g/g, HR of SBP 96–110mm Hg vs. SBP 111–120mm Hg was 4.07 (95% CI = 1.18–13.99) for renal outcomes in the fully adjusted model. However, in diabetic CKD patients with UPCR <1g/g, HR of SBP 96–110mm Hg vs. SBP 111–120mm Hg was 1.72 (95% CI = 0.13 to 22.5) for renal outcomes in the fully adjusted model (Table 5).
Subgroup analysis of those taking regular antihypertensive agents
Of those taking regular antihypertensive agents (n = 852 of 1192 non-DM and 754 of 939 DM CKD patients), only the DM group showed a J-shaped relationship between SBP and cardiovascular or renal outcomes (HR of SBP 96–110mm Hg vs. SBP 111–120mm Hg = 5.01 (95% CI = 1.85–13.56) for cardiovascular outcome and 2.85 (95% CI = 0.98–8.30) for renal outcomes). No statistically significant J-shaped relationship between SBP and all-cause mortality existed in either diabetic CKD patients (HR of SBP 96–110mm Hg = 1.40; 95% CI = 0.37–5.35) or nondiabetic CKD patients (HR of SBP 96–110mm Hg = 2.87; 95% CI = 0.78–10.62) (Table 6; Supplementary Table S2).
Table 6.
Adjusted hazard ratios of clinical outcome by diabetes mellitus status and baseline systolic blood pressure in patients with stage 3 or 4 chronic kidney disease taking regular antihypertensive agents
| Total Adjusted HR (95% CI)a | Non-DM Adjusted HR (95% CI)a | DM Adjusted HR (95% CI)a | |
|---|---|---|---|
| Cardiovascular outcomes: SBP, mm Hg | |||
| 96–110 | 2.76 (1.26–6.02) | 0.78 (0.15–4.12) | 5.01 (1.85–13.56) |
| 111–120 | Reference group | Reference group | Reference group |
| 121–140 | 1.82 (0.98–3.38) | 1.27 (0.51–3.19) | 2.28 (0.96–5.38) |
| >140 | 1.93 (1.05–3.55) | 1.31 (0.53–3.24) | 2.34 (1.005–5.46) |
| Renal outcomes: SBP, mm Hg | |||
| 96–110 | 1.69 (0.78–3.67) | 0.70 (0.21–2.32) | 2.85 (0.98–8.30) |
| 111–120 | Reference group | Reference group | Reference group |
| 121–140 | 1.30 (0.76–2.22) | 0.85 (0.39–1.87) | 1.49 (0.71–3.12) |
| >140 | 1.84 (1.11–3.04) | 0.86 (0.40–1.89) | 2.60 (1.29–5.26) |
| All-cause mortality: SBP, mm Hg | |||
| 96–110 | 1.84 (0.73–4.59) | 2.87 (0.78–10.62) | 1.40 (0.37–5.35) |
| 111–120 | Reference group | Reference group | Reference group |
| 121–140 | 1.65 (0.83–3.27) | 1.87 (0.71–4.94) | 1.41 (0.52–3.80) |
| >140 | 1.89 (0.96–3.71) | 2.12 (0.81–5.54) | 1.75 (0.66–4.61) |
Abbreviations: CI, confidence interval; DM, diabetes mellitus; HR, hazard ratio; SBP, systolic blood pressure.
aFully adjusted model included age, sex, estimated glomerular filtration rate, urine protein–creatinine ratio, hemoglobin, albumin, phosphorus, total cholesterol, C-reactive protein, body mass index, smoking status, baseline congestive heart failure, myocardial infarction, cerebral vascular disease, and the use of angiotensin-converting enzyme inhibitors, angiotensin receptor blockers, calcium channel blockers, beta-blockers, alpha-blockers, and diuretics.
DISCUSSION
We demonstrated, for the first time, that DM modified the J-shaped relationship of SBP with cardiovascular and renal outcomes in stage 3 and 4 CKD patients. Diabetic CKD patients had 2.5-fold and 3.1-fold increased risk for cardiovascular and renal outcomes, respectively, at SBP 96–110mm Hg compared with SBP 111–120mm Hg, but the J-shaped relationship was not observed in nondiabetic CKD patients (Tables 3–5; Figure 1). Also, we found that heavy proteinuria modified the J-shaped relationship between SBP and renal outcomes in diabetic CKD patients (Table 5).
In diabetic CKD patients, our study showed a J-shaped relationship of SBP with renal outcomes (Tables 3–5; Figure 1). The J-shaped trend was also noted in the RENAAL study, in which all participants had DM and nephropathy and the lowest SBP group was <130mm Hg.17 However, no J-shaped relationship between SBP and ESRD was observed in a prospective cohort study of 218 CKD veterans, 48% with diabetes.7 These results imply that DM may be a key modifier of the J-shaped relationship in CKD patients. Future large-scale observational research or randomized controlled trials should consider diabetes.
In nondiabetic CKD patients, we found no J-shaped relationship of SBP with renal outcomes and rapid renal function decline (Tables 3–5; Figure 1). The risk for renal outcomes was not different between heavy proteinuria and nonheavy proteinuria groups of nondiabetic CKD patients (Table 5). Several randomized controlled trials tested whether intensive BP targets slow the progression of CKD in nondiabetic CKD patients. No J-shaped relationship was observed in nondiabetic CKD patients, regardless of the severity of proteinuria in the African American Study of Kidney Disease18 and the Ramipril Efficacy in Nephropathy Study,19 although the SBP target might have been insufficiently low to demonstrate a J-curve. However, Jafar et al. did find a J-shaped relationship between SBP and renal outcomes in nondiabetic CKD patients with proteinuria in a meta-analysis of 11 randomized controlled trials.14 The relationship between SBP and renal outcomes in nondiabetic CKD patients with proteinuria is controversial, and further analysis is necessary to clarify the lower end of safe SBP limits.
For the relationship between SBP and cardiovascular outcomes, our results showed diabetic CKD patients had significantly increased risk of cardiovascular outcomes at SBP 96–110mm Hg compared with SBP 111–120mm Hg (Figure 1). This result is compatible with that of the Irbesartan Diabetic Nephropathy Trial (IDNT), in which the lowest SBP group, SBP ≤120mm Hg, was associated with a significantly increased risk of cardiovascular deaths (4.06-fold) and congestive heart failure events (1.8-fold).12 Comparatively, nondiabetic CKD patients had no increased risk for cardiovascular outcome at SBP 96–110mm Hg compared with SBP 111–120mm Hg (Tables 3–5). In the African American Study of Kidney Disease trial with nondiabetic CKD patients, the HR for cardiovascular mortality did not increase in the low BP target group (HR = 0.98; P = 0.96), although the SBP target might have been insufficiently low to reveal a J-shaped relationship.20
Our study found no statistically significant J-shaped relationship of SBP with all-cause mortality in either diabetic or nondiabetic CKD patients (Tables 3–5; Figure 1). This result is compatible with several randomized control trials,5,12,21–24 prospective cohort studies,7 and retrospective studies.25 Agarwal found increased HR of all-cause mortality at SBP <130mm Hg in a prospective cohort study of 218 CKD veterans, 48% with DM.7 Kovesdy et al. noted increased HR of all-cause mortality at SBP <133mm Hg in a prospective cohort of 860 CKD stage 3–5 patients, approximately half with DM.5 In diabetic CKD patients, SBP ≤120mm Hg significantly increases the relative risk of all-cause mortality (3.05-fold) in the Irbesartan Diabetic Nephropathy Trial (IDNT).12 In nondiabetic CKD patients, the evidence is limited. Further studies of such patients are necessary to clarify the safest lower limit of SBP.
Previous studies focused separately on diabetic and nondiabetic CKD patients. This study is the first to analyze the impact of diabetes in the relationship between SBP and clinical outcomes of CKD patients within the same study design. Rapid renal function decline associates with higher risk for the renal outcomes, and for the CV outcomes as well.26 Based on our analyses, nondiabetic CKD patients had significantly less rapid renal function decline at the lowest SBP group compared with the reference group; however, diabetic CKD patients did not. Rapid renal function decline might associate with the modification of the J-shaped relationship between SBP and cardiovascular or renal outcomes in diabetic CKD patients. Microvascular and macrovascular complications of diabetes are well known. In animal studies, ischemia delays recovery of renal regional blood flow in diabetic mice27 and aggravates chronic inflammation and vasculopathy, accelerating renal failure in diabetic rats.28 Impaired myogenic responsiveness of the afferent arteriole in diabetic rats had been observed. The deranged renal microcirculatory response to pressure may contribute to the progression of diabetic nephropathy.29 A complex interplay of oxidative stress, endothelial dysfunction, profibrogenic cytokines, and inflammatory mediators due to ischemia and renal hypoperfusion had been proposed.30 Therefore, although the mechanism of the changing relationship between SBP and cardiovascular or renal outcomes remains obscure, microvascular and macrovascular complications related to hypoperfusion in diabetic CKD patients may be a contributor.28,31
This study has several limitations. First, this is an observational study, unable to delineate causal relationships. However, potential risks allow for only observational studies to establish the lower safe limits of SBP. Second, the follow-up duration was short (median = 2.91 years), and the rates of mortality and cardiovascular events were relatively low. Third, the analyzed BP was not the time-averaged BP during the whole study but that during the 3 months before and after enrollment. Fourth, the recorded BP was office BP, not ambulatory-monitored BP, which would better correlate with outcomes in both non-CKD32,33 and CKD patients.34
In conclusion, DM modifies the J-shaped relationship of SBP with cardiovascular and renal outcomes in stage 3 and 4 CKD patients. Diabetic CKD patients are at 2.5-fold and 3.1-fold increased risk for cardiovascular and renal outcomes, respectively, at SBP 96–110mm Hg compared with SBP 111–120mm Hg, but the J-shaped relationship is not observed in nondiabetic CKD patients. These findings suggest that the optimal SBP range may be narrower in diabetic CKD patients than in nondiabetic ones. Further analysis is warranted to determine the lower safe limits of SBP in these patients.
SUPPLEMENTARY MATERIAL
Supplementary materials are available at American Journal of Hypertension (http://ajh.oxfordjournals.org).
DISCLOSURE
The authors declared no conflict of interest.
Supplementary Material
ACKNOWLEDGMENTS
We thank the Statistical Analysis Laboratory, Department of Medical Research, Kaohsiung Medical University Hospital, Kaohsiung Medical University for assistance with this work. Heng-Pin Chiang and Jia-Jung Lee contributed equally to this work.
REFERENCES
- 1. Klag MJ, Whelton PK, Randall BL, Neaton JD, Brancati FL, Ford CE, Shulman NB, Stamler J. Blood pressure and end-stage renal disease in men. N Engl J Med 1996; 334:13–18. [DOI] [PubMed] [Google Scholar]
- 2. Lewington S, Clarke R, Qizilbash N, Peto R, Collins R. Age-specific relevance of usual blood pressure to vascular mortality: a meta-analysis of individual data for one million adults in 61 prospective studies. Lancet 2002; 360:1903–1913. [DOI] [PubMed] [Google Scholar]
- 3. Weiner DE, Tighiouart H, Levey AS, Elsayed E, Griffith JL, Salem DN, Sarnak MJ. Lowest systolic blood pressure is associated with stroke in stages 3 to 4 chronic kidney disease. J Am Soc Nephrol 2007; 18:960–966. [DOI] [PubMed] [Google Scholar]
- 4. de Galan BE, Perkovic V, Ninomiya T, Pillai A, Patel A, Cass A, Neal B, Poulter N, Harrap S, Mogensen CE, Cooper M, Marre M, Williams B, Hamet P, Mancia G, Woodward M, Glasziou P, Grobbee DE, MacMahon S, Chalmers J. Lowering blood pressure reduces renal events in type 2 diabetes. J Am Soc Nephrol 2009; 20:883–892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Kovesdy CP, Trivedi BK, Kalantar-Zadeh K, Anderson JE. Association of low blood pressure with increased mortality in patients with moderate to severe chronic kidney disease. Nephrol Dial Transplant 2006; 21:1257–1262. [DOI] [PubMed] [Google Scholar]
- 6. Rifkin DE, Katz R, Chonchol M, Shlipak MG, Sarnak MJ, Fried LF, Newman AB, Siscovick DS, Peralta CA. Blood pressure components and decline in kidney function in community-living older adults: the cardiovascular health study. Am J Hypertens 2013; 26:1037–1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Agarwal R. Blood pressure components and the risk for end-stage renal disease and death in chronic kidney disease. Clin J Am Soc Nephrol 2009; 4:830–837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Mancia G, Laurent S, Agabiti-Rosei E, Ambrosioni E, Burnier M, Caulfield MJ, Cifkova R, Clement D, Coca A, Dominiczak A, Erdine S, Fagard R, Farsang C, Grassi G, Haller H, Heagerty A, Kjeldsen SE, Kiowski W, Mallion JM, Manolis A, Narkiewicz K, Nilsson P, Olsen MH, Rahn KH, Redon J, Rodicio J, Ruilope L, Schmieder RE, Struijker-Boudier HA, van Zwieten PA, Viigimaa M, Zanchetti A. Reappraisal of European guidelines on hypertension management: a european society of hypertension task force document. J Hypertens 2009; 27:2121–2158. [DOI] [PubMed] [Google Scholar]
- 9. Wheeler DC, Becker GJ. Summary of KDIGO guideline. What do we really know about management of blood pressure in patients with chronic kidney disease? Kidney Int 2013; 83:377–383. [DOI] [PubMed] [Google Scholar]
- 10. K/DOQI Clinical Practice Guidelines Work Group. K/DOQI clinical practice guidelines on hypertension and antihypertensive agents in chronic kidney disease. Am J Kidney Dis. 2004; 43:S1–290. [PubMed] [Google Scholar]
- 11. Lewis JB. Blood pressure control in chronic kidney disease: is less really more? J Am Soc Nephrol 2010; 21:1086–1092. [DOI] [PubMed] [Google Scholar]
- 12. Berl T, Hunsicker LG, Lewis JB, Pfeffer MA, Porush JG, Rouleau JL, Drury PL, Esmatjes E, Hricik D, Pohl M, Raz I, Vanhille P, Wiegmann TB, Wolfe BM, Locatelli F, Goldhaber SZ, Lewis EJ. Impact of achieved blood pressure on cardiovascular outcomes in the irbesartan diabetic nephropathy trial. J Am Soc Nephrol 2005; 16:2170–2179. [DOI] [PubMed] [Google Scholar]
- 13. Pohl MA, Blumenthal S, Cordonnier DJ, De Alvaro F, Deferrari G, Eisner G, Esmatjes E, Gilbert RE, Hunsicker LG, de Faria JB, Mangili R, Moore J, Jr, Reisin E, Ritz E, Schernthaner G, Spitalewitz S, Tindall H, Rodby RA, Lewis EJ. Independent and additive impact of blood pressure control and angiotensin II receptor blockade on renal outcomes in the Irbesartan Diabetic Nephropathy Trial: clinical implications and limitations. J Am Soc Nephrol 2005; 16:3027–3037. [DOI] [PubMed] [Google Scholar]
- 14. Jafar TH, Stark PC, Schmid CH, Landa M, Maschio G, de Jong PE, de Zeeuw D, Shahinfar S, Toto R, Levey AS. Progression of chronic kidney disease: the role of blood pressure control, proteinuria, and angiotensin-converting enzyme inhibition: a patient-level meta-analysis. Ann Intern Med 2003; 139:244–252. [DOI] [PubMed] [Google Scholar]
- 15. Appel LJ, Wright JT, Jr., Greene T, Agodoa LY, Astor BC, Bakris GL, Cleveland WH, Charleston J, Contreras G, Faulkner ML, Gabbai FB, Gassman JJ, Hebert LA, Jamerson KA, Kopple JD, Kusek JW, Lash JP, Lea JP, Lewis JB, Lipkowitz MS, Massry SG, Miller ER, Norris K, Phillips RA, Pogue VA, Randall OS, Rostand SG, Smogorzewski MJ, Toto RD, Wang X. Intensive blood-pressure control in hypertensive chronic kidney disease. N Engl J Med 2010; 363:918–929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kidney Disease: Improving Global Outcomes (KDIGO) CKD Work Group. KDIGO 2012 Clinical Practice Guideline for the Evaluation and Management of Chronic Kidney Disease. Chapter 2: definition, identification, and prediction of CKD progression. Kidney Inter. Suppl. 2013; 3:63–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Bakris GL, Weir MR, Shanifar S, Zhang Z, Douglas J, van Dijk DJ, Brenner BM. Effects of blood pressure level on progression of diabetic nephropathy: results from the RENAAL study. Arch Intern Med 2003; 163:1555–1565. [DOI] [PubMed] [Google Scholar]
- 18. Sarafidis PA, Khosla N, Bakris GL. Antihypertensive therapy in the presence of proteinuria. Am J Kidney Dis 2007; 49:12–26. [DOI] [PubMed] [Google Scholar]
- 19. Ruggenenti P, Perna A, Loriga G, Ganeva M, Ene-Iordache B, Turturro M, Lesti M, Perticucci E, Chakarski IN, Leonardis D, Garini G, Sessa A, Basile C, Alpa M, Scanziani R, Sorba G, Zoccali C, Remuzzi G. Blood-pressure control for renoprotection in patients with non-diabetic chronic renal disease (REIN-2): multicentre, randomised controlled trial. Lancet 2005; 365:939–946. [DOI] [PubMed] [Google Scholar]
- 20. Upadhyay A, Earley A, Haynes SM, Uhlig K. Systematic review: blood pressure target in chronic kidney disease and proteinuria as an effect modifier. Ann Intern Med 2011; 154:541–548. [DOI] [PubMed] [Google Scholar]
- 21. Cushman WC, Evans GW, Byington RP, Goff DC, Jr, Grimm RH, Jr, Cutler JA, Simons-Morton DG, Basile JN, Corson MA, Probstfield JL, Katz L, Peterson KA, Friedewald WT, Buse JB, Bigger JT, Gerstein HC, Ismail-Beigi F. Effects of intensive blood-pressure control in type 2 diabetes mellitus. N Engl J Med 2010; 362:1575–1585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Klahr S, Levey AS, Beck GJ, Caggiula AW, Hunsicker L, Kusek JW, Striker G. The effects of dietary protein restriction and blood-pressure control on the progression of chronic renal disease. Modification of Diet in Renal Disease study group. N Engl J Med 1994; 330:877–884. [DOI] [PubMed] [Google Scholar]
- 23. Peterson JC, Adler S, Burkart JM, Greene T, Hebert LA, Hunsicker LG, King AJ, Klahr S, Massry SG, Seifter JL. Blood pressure control, proteinuria, and the progression of renal disease. The Modification of Diet in Renal Disease study. Ann Intern Med 1995; 123:754–762. [DOI] [PubMed] [Google Scholar]
- 24. Lazarus JM, Bourgoignie JJ, Buckalew VM, Greene T, Levey AS, Milas NC, Paranandi L, Peterson JC, Porush JG, Rauch S, Soucie JM, Stollar C. Achievement and safety of a low blood pressure goal in chronic renal disease. The Modification of Diet in Renal Disease study group. Hypertension 1997; 29:641–650. [DOI] [PubMed] [Google Scholar]
- 25. Weiss JW, Johnson ES, Petrik A, Smith DH, Yang X, Thorp ML. Systolic blood pressure and mortality among older community-dwelling adults with ckd. Am J Kidney Dis. 2010; 56:1062–1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Shlipak MG, Katz R, Kestenbaum B, Siscovick D, Fried L, Newman A, Rifkin D, Sarnak MJ. Rapid decline of kidney function increases cardiovascular risk in the elderly. J Am Soc Nephrol 2009; 20:2625–2630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Shi H, Patschan D, Epstein T, Goligorsky MS, Winaver J. Delayed recovery of renal regional blood flow in diabetic mice subjected to acute ischemic kidney injury. Am J Physiol Renal Physiol 2007; 293:F1512–F1517. [DOI] [PubMed] [Google Scholar]
- 28. Kelly KJ, Burford JL, Dominguez JH. Postischemic inflammatory syndrome: a critical mechanism of progression in diabetic nephropathy. Am J Physiol Renal Physiol 2009; 297:F923–F931. [DOI] [PubMed] [Google Scholar]
- 29. Hayashi K, Epstein M, Loutzenhiser R, Forster H. Impaired myogenic responsiveness of the afferent arteriole in streptozotocin-induced diabetic rats: role of eicosanoid derangements. J Am Soc Nephrol 1992; 2:1578–1586. [DOI] [PubMed] [Google Scholar]
- 30. Textor SC. Ischemic nephropathy: where are we now? J Am Soc Nephrol 2004; 15:1974–1982. [DOI] [PubMed] [Google Scholar]
- 31. Last D, Alsop DC, Abduljalil AM, Marquis RP, de Bazelaire C, Hu K, Cavallerano J, Novak V. Global and regional effects of type 2 diabetes on brain tissue volumes and cerebral vasoreactivity. Diabetes Care 2007; 30:1193–1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Dolan E, Stanton A, Thijs L, Hinedi K, Atkins N, McClory S, Den Hond E, McCormack P, Staessen JA, O’Brien E. Superiority of ambulatory over clinic blood pressure measurement in predicting mortality: the Dublin Outcome Study. Hypertension 2005; 46:156–161. [DOI] [PubMed] [Google Scholar]
- 33. Fan HQ, Li Y, Thijs L, Hansen TW, Boggia J, Kikuya M, Bjorklund-Bodegard K, Richart T, Ohkubo T, Jeppesen J, Torp-Pedersen C, Dolan E, Kuznetsova T, Stolarz-Skrzypek K, Tikhonoff V, Malyutina S, Casiglia E, Nikitin Y, Lind L, Sandoya E, Kawecka-Jaszcz K, Imai Y, Ibsen H, O’Brien E, Wang J, Staessen JA. Prognostic value of isolated nocturnal hypertension on ambulatory measurement in 8711 individuals from 10 populations. J Hypertens 2010; 28:2036–2045. [DOI] [PubMed] [Google Scholar]
- 34. Sarafidis PA, Rumjon A, Macdougall IC. Ambulatory blood pressure monitoring: an invaluable tool comes of age for patients with chronic kidney disease? Am J Nephrol 2012; 35:238–241. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


