Abstract
BACKGROUND
Blood pressure (BP) and low-density lipoprotein cholesterol (LDL-C) are risk factors for coronary artery disease (CAD) and ischemic stroke. However, the hazards of their coexistence are not fully understood in Asian populations. We investigated whether the relationship between BP and cardiovascular disease (CVD) outcomes are modified by LDL-C level in a Japanese population.
METHODS
Individuals aged 30–79 years (n = 5,151) were classified into 6 groups according to LDL-C levels (<140 and ≥140mg/dL or lipid medication) and BP levels (optimal BP, prehypertension, and hypertension; reference: low LDL-C and optimal BP). Hazard ratios (HRs) were calculated after adjusting for age, high-density lipoprotein cholesterol, diabetes, smoking status, and alcohol consumption. The effect modification of LDL-C on BP–CVD association was assessed using likelihood ratio tests.
RESULTS
There were 264 CAD and 215 ischemic stroke events during 13 years of follow-up. With low LDL-C, the HRs of prehypertension and hypertension for CAD were 2.01 and 4.71, respectively. Similar trends of HRs were observed with high LDL-C (optimal BP = 2.09, prehypertension = 3.45, hypertension = 5.94). However, the HRs for ischemic stroke did not differ between normal and high LDL-C levels at the same BP level. The apparent effect modification of LDL-C was not observed in the BP–CVD association in either CAD (P = 0.48) or ischemic stroke (P = 0.39).
CONCLUSIONS
The HRs for CAD in prehypertensive and hypertensive groups were higher than those in the optimal BP group at the same LDL-C levels in a Japanese population; however, there was no statistical effect modification of LDL-C on the BP–CAD association.
Keywords: Asian, blood pressure, cohort study, coronary artery disease, hypertension, incidence, ischemic stroke, low-density lipoprotein cholesterol, Suita Study.
Cardiovascular disease is a leading cause of mortality and morbidity in Asian countries.1 Elevated blood pressure (BP)1–5 and hypercholesterolemia1,6–10 are well-established independent cardiovascular risk factors. Moreover, the combination of these risk factors is a better predictor of the risk of cardiovascular disease in Western populations.11,12 In Japan, the Japan Lipid Intervention Trial (J-LIT) study showed that Japanese hypercholesterolemia patients with high systolic BP (SBP; ≥130mm Hg) and high total cholesterol levels (≥220mg/dl) treated with low-dose simvastatin had an increased risk of cardiovascular disease events.13 In Asia, the Asia Pacific Cohort Studies Collaborations (APCSC) demonstrated that the combination of high SBP (≥130mm Hg) and high total cholesterol (≥212mg/dl) increased the risks of fatal and nonfatal cardiovascular disease among both Western and Asian populations.14 However, the J-LIT and APCSC studies have some drawbacks, including relatively short follow-up periods (mean follow-up period of approximately 6 years) and lipid profiles based on total cholesterol and not low-density lipoprotein cholesterol (LDL-C). Furthermore, the J-LIT study was a patient-based clinical trial,13 and the APCSC study14 did not exclusively involve Asian populations, which have a higher incidence of stroke and lower incidence of coronary artery disease (CAD) than Western populations.1
The purpose of our study was to examine whether the relationship between BP and CVD outcomes (CAD and ischemic stroke) is modified by LDL-C levels in a community-based cohort study in a Japanese population.
METHODS
Population
The Suita Study, a cohort study evaluating cardiovascular disease risk in an urban Japanese population, was established in 1989. This cohort study has been extensively used to evaluate risk factors associated with the incidences of CAD and stroke.4,15–18 The details of this study have been described previously.4,15–18 Briefly, 6,483 men and women aged 30–79 years underwent a baseline survey at the National Cerebral and Cardiovascular Centre (Japan) between September 1989 and March 1994. Subjects older than 80 years were excluded because it remains unconfirmed whether LDL-C is a risk factor for cardiovascular disease in the elderly population (aged ≥80 years).19 A total of 1,332 participants were excluded for the following reasons: history of CAD or stroke (n = 208); loss to follow-up (n = 535); lack of participation in the baseline survey (n = 78); nonfasting visit (n = 239); triglyceride level >400mg/dl (n = 86); LDL-C ≤0 (n = 1); missing total cholesterol, high-density lipoprotein cholesterol (HDL-C), or triglyceride data (n = 30); aged ≥80 years (n = 12); and other missing data (n = 145). Therefore, data from the remaining 5,151 participants (men: n = 2,399; women: n = 2,752) were included in our analysis.
This study was approved by the institutional review board of the National Cerebral and Cardiovascular Centre. Informed consent was obtained from all participants by health professions at the baseline examination. The collected data were anonymized.
Baseline examination
Blood samples were collected at the National Cerebral and Cardiovascular Centre after the participants had fasted for at least 10 hours. The samples were immediately centrifuged, and a routine blood examination that included serum total cholesterol, HDL-C, triglyceride, and glucose levels was performed. LDL-C was estimated for both men and women using the Friedewald formula.20 Participants with triglyceride levels >400mg/dl were excluded because LDL-C estimates are inaccurate among such persons.19,21,22
BP was measured by well-trained physicians using a standard mercury sphygmomanometer. After the participant had been in the seated position for 5 minutes, BP was measured 3 times on the right arm, and the average of the second and third measurements was used in the analyses to avoid bias due to white coat hypertension. Because HbA1c data from before 1995 were unavailable, diabetes was defined according to the American Diabetes Association 2013 guidelines as a fasting serum glucose level ≥126mg/dl, the use of diabetes medication, or both.23,24 Height and weight were measured while the subjects wore socks and light clothing. Public health nurses obtained information about smoking status, alcohol consumption, and medical history of the participants.
The information about smoking and alcohol consumption have been reported previously.4,15–18 Well-trained nurses obtained information on smoking and alcohol consumption. Smoking status was classified as never, ex-smoker, or current smoker. If a participant responded yes to “current smoker,” the number of cigarettes smoked per day was ascertained. Alcohol consumption was categorized as never drinker, ex-drinker, or current drinker (i.e., >1 time per week).
Endpoint determination
The endpoint determination in the Suita Study has been described previously.4,15–18 The Suita Study is an ongoing cohort study, and the latest endpoint determination was performed on 31December 2007. The endpoints in our follow-up study were as follows: (i) the date of the first CAD or stroke event, (ii) the date of death, (iii) the date of leaving Suita City, and (iv) 31 December 2007.
The first step in the CAD and stroke survey involved checking the health status of all participants at biennial clinical visits every 2 years and through annual questionnaires sent by mail or administered by telephone. The second step involved the review of the in-hospital medical records of participants suspected of having had CAD or stroke; the reviews were performed by registered hospital physicians or research physicians blinded to the baseline information. The diagnosis of stroke was based on the US National Survey of Stroke criteria.25 Stroke subtypes, including ischemic stroke, intracerebral hemorrhage, and subarachnoid hemorrhage, were diagnosed on the basis of computed tomography, magnetic resonance imaging, or autopsy results. Definite and probable acute myocardial infarction were defined according to the criteria of the Monitoring Trends and Determinants of Cardiovascular Disease (MONICA) Project.26 In addition to acute myocardial infarction, the criteria for CAD diagnosis included sudden cardiac death within 24 hours from symptom onset or CAD followed by coronary artery bypass or angioplasty. Furthermore, myocardial infarction and stroke fatalities were recorded by searching for systematic death certificates.
Statistical analysis
BP was categorized into 3 groups; optimal BP (SBP <120mm Hg and diastolic blood pressure (DBP) <80mm Hg), prehypertension (SBP = 120–139mm Hg, DBP = 80–89mm Hg, or both), and hypertension (SBP ≥140mm Hg, or DBP ≥90mm Hg, or the use of antihypertensive agents). LDL-C was categorized into 2 groups; normal (LDL-C <140mg/dl) or high (LDL-C ≥140mg/dl or lipid medication) according to the diagnostic criteria for screening of the Japan Atherosclerosis Society Guidelines for Prevention of Atherosclerotic Cardiovascular Diseases.19 This guideline has set the cutoff point of LDL-C to 140 mg/dl as diagnostic criteria of dyslipidemia. Using the abovementioned BP and LDL-C categories, combinations of BP and LDL-C (3×2 groups) were made to estimate hazard ratios (HRs) with optimal BP and normal LDL-C as the reference group. Analysis of variance and the Bonferroni test were used to compare continuous variables, and the χ2 test was used to compare dichotomous variables.
Sex-stratified Cox proportional hazard models, accounting for different baseline hazards in men and women, were used to estimate the HRs of the combination of BP and LDL-C on cardiovascular disease outcomes. Age, HDL-C, smoking status, alcohol consumption, and diabetes were included in the models as confounders. For the primary analysis, HRs and 95% confidence intervals (CIs) were estimated for CAD and stroke events by analyzing BP as a categorical variable within each LDL-C group. Moreover, the association between BP and LDL-C was examined by comparing the HRs for CAD and stroke events across the 6 groups, adjusting for age, HDL-C, diabetes, smoking status, and alcohol consumption. The interaction between BP and LDL-C on cardiovascular outcomes was assessed using likelihood ratio tests.27 The level of significance was set at P < 0.05. All statistical analyses were performed using STATA release 12 (Stata Corp LP, College Station, TX).
RESULTS
Overall, 5,151 individuals (men: n = 2,399; women: n = 2,752) were analyzed. Table 1 shows the baseline characteristics of groups with BP and LDL-C combination in men. In both LDL-C groups, men with hypertension had the highest mean age, body mass index, and fasting blood glucose, as well as the most cases of diabetes and medications. There were fewer current drinkers in the high LDL-C group than the normal LDL-C group (P < 0.001). Moreover, there were more current smokers in the normal LDL-C group than the high LDL-C group (P < 0.001).
Table 1.
Baseline characteristics of men with respect to blood pressure and serum low-density lipoprotein categories
| Normal LDL-C | High LDL-C | P value | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Optimal BP | Prehypertension | Hypertension | Optimal BP | Prehypertension | Hypertension | ||||||||
| No. | 543 | 593 | 491 | 232 | 269 | 271 | |||||||
| Age, y | 49.5 | (13.2) | 54.8 | (13.2)** | 61.3 | (11.4)** | 52.4 | (12.5)* | 56.2 | (11.5)** | 60.7 | (10.8)** | <0.001 |
| Systolic blood pressure, mm Hg | 107.6 | (7.5) | 126.1 | (7.3)** | 150.8 | (17.6)** | 108.3 | (7.4)* | 126.3 | (7.7)** | 149.3 | (16)** | <0.001 |
| Diastolic blood pressure, mm Hg | 67.9 | (7) | 67.9 | (7)** | 90.1 | (10.6)** | 69.3 | (6.3) | 79.5 | (6.8)** | 90.1 | (10.3)** | <0.001 |
| LDL-C, mg/dl | 108.7 | (20.5) | 109.2 | (20.7)* | 108.6 | (21.6)** | 159.9 | (18.5)** | 159.8 | (19.4)** | 161.5 | (22.5)** | <0.001 |
| Fasting blood glucose, mg/dl | 96.5 | (13) | 100.6 | (17.8)* | 102.6 | (19.4)** | 98.0 | (20.7)** | 102.9 | (22.7)** | 104.2 | (21.2)** | <0.001 |
| Body mass index, kg/m2 | 21.8 | (2.7) | 22.7 | (2.7)** | 23.1 | (3)** | 22.6 | (2.5)* | 23.3 | (2.6)** | 23.8 | (2.7)** | <0.001 |
| Diabetes, % | 3.5 | 5.4 | 7.9 | 3.4 | 4.8 | 10 | 0.004 | ||||||
| Medication for hypertension, % | 0 | 0 | 49.7 | 0 | 0 | 34.3 | |||||||
| Medication for hypercholesterolemia, % | 0 | 0 | 0 | 2.6 | 5.6 | 8.5 | |||||||
| Medication for diabetes, % | 0.9 | 2 | 2 | 1.3 | 1.9 | 5.2 | 0.001 | ||||||
| Smoking, % | <0.001 | ||||||||||||
| Current smoker | 60.6 | 49.7 | 43.6 | 32.8 | 44.2 | 41.7 | |||||||
| Ex-smoker | 22.7 | 29.3 | 36.9 | 67.8 | 32.7 | 40.6 | |||||||
| Never smoker | 16.8 | 20.9 | 19.6 | 43.3 | 23 | 17.7 | |||||||
| Alcohol consumption, % | <0.001 | ||||||||||||
| Current drinker | 74.2 | 78.8 | 79.6 | 65.1 | 72.9 | 73.1 | |||||||
| Ex-drinker | 2.2 | 3.9 | 3.1 | 3.9 | 4.8 | 6.6 | |||||||
| Never drinker | 23.6 | 17.4 | 17.3 | 31 | 22.3 | 20.3 | |||||||
Age, systolic blood pressure, diastolic blood pressure, low-density lipoprotein cholesterol (LDL-C), fasting blood glucose, and body mass index were analyzed by analysis of variance. The percentages of diabetes, medication for hypertension, medication for hypercholesterolemia, medication for diabetes, smoking, and alcohol consumption were analyzed by the χ 2 test. Data are expressed as mean (SD) or percentages. Optimal BP: systolic blood pressure <120mm Hg and diastolic blood pressure <80mm Hg. Prehypertension: systolic blood pressure 120–139mm Hg or diastolic blood pressure 80–89mm Hg. Hypertension: systolic blood pressure ≥140mm Hg, diastolic blood pressure ≥90mm Hg, or the use of antihypertensive medication. Normal LDL-C: fasting LDL-C <140mg/dl. High LDL-C: fasting LDL-C ≥140mg/dl or the use of medication for hypercholesterolemia. Diabetes: fasting plasma glucose ≥126mg/dl or the use of antidiabetic medication.
Abbreviations: BP, blood pressure; LDL-C, low-density lipoprotein cholesterol.
*P < 0.05, **P < 0.001: Bonferroni test (with normal LDL-C and optimal BP as the reference).
Table 2 shows the means and prevalence of baseline characteristics with respect to BP and LDL-C groups in women. As with the men, in both LDL-C groups, women with hypertension had the highest mean age, body mass index, and fasting blood glucose, as well as the most cases of diabetes and medications. There were fewer current drinkers in the high LDL-C group than the normal LDL-C group (P < 0.001). Moreover, there were more current smokers in the normal LDL-C group than in high LDL-C group (P < 0.001).
Table 2.
Baseline characteristics of women with respect to blood pressure and serum low-density lipoprotein categories
| Normal LDL-C | High LDL-C | P value | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Optimal BP | Prehypertension | Hypertension | Optimal BP | Prehypertension | Hypertension | ||||||||
| No. | 837 | 427 | 311 | 364 | 403 | 410 | |||||||
| Age, y | 44.9 | (11.3) | 53.2 | (12.4)** | 61.9 | (10.4)** | 53.3 | (10.8)** | 57.9 | (10.1)** | 62.5 | (8.9)** | <0.001 |
| Systolic blood pressure, mm Hg | 104.8 | (8.1) | 126.3 | (7.1)** | 151.8 | (16.9)** | 106.9 | (7.5) | 127.5 | (6.8)** | 151.4 | (16.6)** | <0.001 |
| Diastolic blood pressure, mm Hg | 66 | (6.6) | 77.4 | (6.8)** | 86.3 | (11.2)** | 67 | (6.8) | 77.7 | (7)** | 86.8 | (11.3)** | <0.001 |
| LDL-C, mg/dl | 108.9 | (19.4) | 114.2 | (18.2) | 115.4 | (18.1) | 163.3 | (23.6)** | 167.2 | (26.6)** | 168 | (26.3)** | <0.001 |
| Fasting blood glucose, mg/dl | 91.3 | (8.3) | 96.2 | (14.7)** | 100.1 | (20.5)** | 93.5 | (11.4) | 98.6 | (22.7)** | 101.9 | (18.3)** | <0.001 |
| Body mass index, kg/m2 | 20.9 | (2.65) | 22.2 | (3.03)** | 23.2 | (3.81)** | 21.7 | (2.82)* | 22.9 | (3.08)** | 23.8 | (3.43)** | <0.001 |
| Diabetes, % | 1.2 | 2.3 | 6.1 | 1.4 | 4.5 | 6.3 | <0.001 | ||||||
| Medication for hypertension, % | 0 | 0 | 37.6 | 0 | 0 | 39.3 | |||||||
| Medication for hypercholesterolemia, % | 0 | 0 | 0 | 3.6 | 5.2 | 7.6 | |||||||
| Medication for diabetes, % | 0.6 | 0.9 | 2.3 | 0.5 | 1.5 | 2 | 0.09 | ||||||
| Smoking, % | <0.001 | ||||||||||||
| Current smoker | 16.4 | 9.8 | 8.7 | 13.7 | 10.2 | 7.6 | |||||||
| Ex-smoker | 3.8 | 3.5 | 2.3 | 2.7 | 3.2 | 5.6 | |||||||
| Never smoker | 79.8 | 86.7 | 89.1 | 83.5 | 86.6 | 86.8 | |||||||
| Alcohol consumption, % | <0.001 | ||||||||||||
| Current drinker | 41.1 | 33 | 29.3 | 26.1 | 29.3 | 27.8 | |||||||
| Ex-drinker | 2 | 1.6 | 1.9 | 1.6 | 1.7 | 1.7 | |||||||
| Never drinker | 56.9 | 65.3 | 68.8 | 72.3 | 69 | 70.5 | |||||||
Age, systolic blood pressure, diastolic blood pressure, low-density lipoprotein cholesterol (LDL-C), fasting blood glucose, and body mass index were analyzed by analysis of variance. The percentages of diabetes, medication for hypertension, medication for hypercholesterolemia, medication for diabetes, smoking, and alcohol consumption were analyzed by the χ 2 test. Data are expressed as mean (SD) or percentages. Optimal BP: systolic blood pressure <120mm Hg and diastolic blood pressure <80mm Hg. Prehypertension: systolic blood pressure 120–139mm Hg or diastolic blood pressure 80–89mm Hg. Hypertension: systolic blood pressure ≥140mm Hg, diastolic blood pressure ≥90mm Hg, or the use of antihypertensive medication. Normal LDL-C: fasting LDL-C <140mg/dl. High LDL-C: fasting LDL-C ≥140mg/dl or the use of medication for hypercholesterolemia. Diabetes: fasting plasma glucose ≥126mg/dl or the use of antidiabetic medication. When mean body mass index was examined in women, there were 836 women with normal blood pressure and normal LDL-C.
Abbreviation: BP, blood pressure.
*P < 0.05, **P < 0.001: Bonferroni test (with normal LDL-C and optimal BP as the reference).
The associations of alcohol consumption and smoking status with LDL-C levels were similar in both sexes. In addition, the rates of current smokers and drinkers were obviously higher in men than women (Tables 1 and 2).
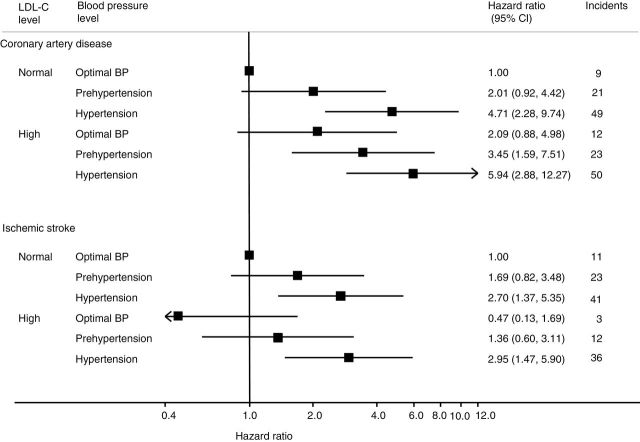
During the 13-year follow-up (total = 67,287 person-years), 164 CAD cases (men: n = 110; women: n = 54) and 215 stroke cases (ischemic: n = 126; hemorrhagic: n = 48; subarachnoid hemorrhage: n = 22; and unclassified stroke: n = 19) were documented. The adjusted HRs for CAD in hypertension were highest in both the normal and the high LDL-C groups. The high HRs were observed as both BP and LDL-C upgraded in CAD (Figure 1). In the high LDL-C group, the HRs of CAD with optimal BP, prehypertension, and hypertension were 2.09 (95% CI = 0.88–4.98), 3.45 (95% CI = 1.59–7.51), and 5.94 (95% CI = 2.88–12.27), respectively. In the normal LDL-C group, the HR of CAD with prehypertension (2.01; 95% CI = 0.92–4.42) was almost the same as those with optimal BP and high LDL-C, whereas the HR with hypertension (2.95; 95% CI = 1.45–5.9) was significantly higher (Figure 1). The HR for ischemic stroke was 2.70 (95% CI = 1.37–5.35) in the hypertension and normal LDL-C group and 2.95 (95% CI = 1.47–5.90) in the hypertension and high LDL-C group. No apparent interaction between BP and LDL-C was detected with either CAD (P = 0.48) or ischemic stroke (P = 0.39).
Figure 1.
Hazard ratios (HRs) for coronary artery disease and stroke by blood pressure (BP) group with respect to low-density lipoprotein cholesterol (LDL-C; mg/dl) categories adjusted for age, high-density lipoprotein cholesterol, diabetes, smoking status, and alcohol consumption.  indicate HR estimates:
indicate HR estimates:  indicate 95% confidence intervals (CIs) by Cox proportional hazard model stratified by sex. The reference group had optimal BP (systolic BP <120mm Hg and diastolic BP <80mm Hg) and normal LDL-C levels (LDL-C <140mg/dl). P = 0.48 for CAD; P = 0.39 for ischemic stroke.
indicate 95% confidence intervals (CIs) by Cox proportional hazard model stratified by sex. The reference group had optimal BP (systolic BP <120mm Hg and diastolic BP <80mm Hg) and normal LDL-C levels (LDL-C <140mg/dl). P = 0.48 for CAD; P = 0.39 for ischemic stroke.
These results have almost no differences when medication use (lipid-lowering medicine and BP-lowering medicine) is not considered the BP and LDL classification.
DISCUSSION
This study showed that the HRs for CAD in prehypertensive and hypertensive groups were higher than those in the optimal BP group at the same LDL-C levels in the Japanese population; however, 95% CIs for these groups almost overlapped, and no apparent modification by LDL-C was observed in the BP–CVD relationship. Furthermore, the HRs for ischemic stroke were not different between normal and higher LDL-C levels at the same BP levels.
Our results support the results from the APCSC, which demonstrated that the combination of elevated BP and elevated total cholesterol increases the risks of fatal and nonfatal cardiovascular disease in Asian, Australian, and New Zealand populations.14 The APCSC showed that cardiovascular disease events are particularly increased in individuals with SBP ≥130mm Hg and the highest total cholesterol levels (≥212mg/dl). Furthermore, the relative risk of cardiovascular disease events in individuals with an SBP of 130–144mm Hg and total cholesterol levels of 212–241mg/dl is similar to that of individuals with an SBP of 145–159mm Hg and total cholesterol levels <212mg/dl. The J-LIT study, which was an observational study among Japanese patients that investigated the relationship between total cholesterol and BP on cardiovascular disease, also found that the relative risk of cardiovascular disease events was significantly higher in patients with poorly controlled hypercholesterolemia patients (total cholesterol >220mg/dl), prehypertension (SBP = 130–139mm Hg; DBP = 80–89mm Hg), and hypertension (SBP >140mm Hg; DBP >90mm Hg) compared with the reference group (SBP <130mm Hg; DBP <80mm Hg).13 Thus, our findings are concordant with those of the APCSC and J-LIT studies. However, it should be emphasized that our study cohort consisted exclusively of a general Asian population in contrast with the APCSC. In addition, hypercholesterolemia patients in the J-LIT study were treated with low-dose simvastatin.
The HRs for ischemic stroke did not differ between the normal and high LDL-C groups at the same BP levels. However, most cohort studies in Japan report that total cholesterol and LDL-C are not risk factors for total stroke28 despite their weak association with ischemic stroke.29 Given these contradictory results between clinical trials and cohort studies, the effects of statins on the prevention of stroke should be interpreted cautiously. Several studies report cholesterol-lowering statins are beneficial for the prevention of stroke in hypertensive patients.30,31 The post hoc analysis of the Management of Elevated Cholesterol in the Primary Prevention Group of Adult Japanese study revealed that pravastatin effectively reduced the incidence of cardiovascular disease, particularly ischemic stroke, in individuals with both hypertension and mildly elevated cholesterol.30 Meanwhile, the Anglo–Scandinavian Cardiac Outcomes Trial–Lipid Lowering Arm showed that atorvastatin significantly reduces CAD, stroke, and cardiovascular disease even in hypertensive patients without dyslipidemia.31 Although there have been numerous clinical trials for statin therapy, the primary endpoint of these studies was CAD, with ischemic stroke as the secondary endpoint. Furthermore, statins have pleiotropic effects that help prevent cardiovascular disease, including anti-inflammatory effects, improved vascular endothelial function, and plaque stabilization. These factors may explain the significant discrepancy between cohort studies and clinical trials with respect to the efficacy of statins in stroke prevention. Therefore, further investigation is required to clarify the role of statin therapy in stroke prevention. However, BP management should be the first priority in ischemic stroke prevention irrespective of LDL-C level.
Our study used a stratified Cox model that included 3 BP and 2 LDL-C categories, as well as their interaction terms and confounders. This combined model is statistically appropriate for the investigation of interactions between risk factors and disease outcomes. Furthermore, LDL-C and hypertension, which were the main targets of this study, and the abovementioned confounding factors encompass all major risk factors in the Framingham risk score,22 the European SCORE chart,21 and the Japanese Atherosclerosis Society risk chart19 for predicting future coronary events.
We found that the HRs for CAD in high LDL-C group were higher than those in normal LDL-C at the same BP levels in the Japanese population. However, the apparent effect modification of LDL-C was not detected in the relation between BP and CAD. There are 3 possibilities to explain these results; no interaction, low statistical power, and bias. Our results suggested that BP and LDL-C were mutually independent risk factors and no interaction exist between them. This result was not as similar to other previous studies, and explanations were needed to claim the independence. A second possibility is that lack of statistical power induces the results; a very small number of events was assigned in each category. The third possibility is that the single assessment of BP and LDL-C at the baseline survey and the fact that we did not evaluate the longitudinal trend for each risk factor may have underestimated the relationship between these conditions and CAD because of regression dilution bias.32 All of these misclassification diluted the HRs and made the effect modification obscure. We are not quite sure which is the correct answer for this issue, but we believe our description of the BP, LDL-C, and CAD relationship among an Asian population gives important insight to future epidemiological studies.
In conclusion, to the best of our knowledge, this study is the first epidemiological study to examine the combined association between BP and LDL-C on the incidences of cardiovascular disease subtypes in an Asian population only. The results show that risk for CAD due to hypertension or prehypertension in the high LDL-C group was higher than those in the normal LDL-C group, although there were no statistical interaction between BP and LDL-C. Accordingly, BP and LDL-C should be managed for the early prevention of CAD in Japanese and other Asian individuals with hypertension, prehypertension, and high LDL-C levels. Furthermore, large-scale epidemiological studies should carefully assess the association between BP and LDL-C with the incidence of cardiovascular disease subtypes among Asian populations.
DISCLOSURE
The authors declared no conflict of interest.
ACKNOWLEDGMENTS
This work was supported by the Intramural Research Fund of the National Cerebral and Cardiovascular Center (22-4-5). This work was also supported by a Health and Labour Sciences Research Grant (H23–Junkankitou (Seisyu)–Ippan–005) from the Ministry of Health, Labour, and Welfare, Japan.
REFERENCES
- 1. Ueshima H, Sekikawa A, Miura K, Turin TC, Takashima N, Kita Y, Watanabe M, Kadota A, Okuda N, Kadowaki T, Nakamura Y, Okamura T. Cardiovascular disease and risk factors in Asia: a selected review. Circulation 2008; 118:2702–2709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Lewington S, Clarke R, Qizilbash N, Peto R, Collins R; Prospective Studies Collaboration. Age-specific relevance of usual blood pressure to vascular mortality: a meta-analysis of individual data for one million adults in 61 prospective studies. Lancet 2002; 360:1903–1913. [DOI] [PubMed] [Google Scholar]
- 3. Lawes CM, Bennett DA, Parag V, Woodward M, Whitlock G, Lam TH, Suh I, Rodgers A; Asia Pacific Cohort Studies Collaboration. Blood pressure indices and cardiovascular disease in the Asia Pacific region: a pooled analysis. Hypertension 2003; 42:69–75. [DOI] [PubMed] [Google Scholar]
- 4. Kokubo Y, Kamide K, Okamura T, Watanabe M, Higashiyama A, Kawanishi K, Okayama A, Kawano Y. Impact of high-normal blood pressure on the risk of cardiovascular disease in a Japanese urban cohort: the Suita study. Hypertension 2008; 52:652–659. [DOI] [PubMed] [Google Scholar]
- 5. Fujiyoshi A, Ohkubo T, Miura K, Murakami Y, Nagasawa SY, Okamura T, Ueshima H; Evidence for Cardiovascular Prevention from Observational Cohorts in Japan (EPOCH-JAPAN) Research Group. Blood pressure categories and long-term risk of cardiovascular disease according to age group in Japanese men and women. Hypertens Res 2012; 35:947–953. [DOI] [PubMed] [Google Scholar]
- 6. Lewington S, Whitlock G, Clarke R, Sherliker P, Emberson J, Halsey J, Qizilbash N, Peto R, Collins R; Prospective Studies Collaboration. Blood cholesterol and vascular mortality by age, sex, and blood pressure: a meta-analysis of individual data from 61 prospective studies with 55,000 vascular deaths. Lancet 2007; 370:1829–1839. [DOI] [PubMed] [Google Scholar]
- 7. Okamura T. Dyslipidemia and cardiovascular disease: a series of epidemiologic studies in Japanese populations. J Epidemiol 2010; 20:259–265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Zhang X, Patel A, Horibe H, Wu Z, Barzi F, Rodgers A, MacMahon S, Woodward M; Asia Pacific Cohort Studies Collaboration. Cholesterol, coronary heart disease, and stroke in the Asia Pacific region. Int J Epidemiol 2003; 32:563–572. [DOI] [PubMed] [Google Scholar]
- 9. Patel A, Woodward M, Campbell DJ, Sullivan DR, Colman S, Chalmers J, Neal B, MacMahon S. Plasma lipids predict myocardial infarction, but not stroke, in patients with established cerebrovascular disease. Eur Heart J 2005; 26:1910–1915. [DOI] [PubMed] [Google Scholar]
- 10. Nagasawa SY, Okamura T, Iso H, Tamakoshi A, Yamada M, Watanabe M, Murakami Y, Miura K, Ueshima H; Evidence for Cardiovascular Prevention from Observational Cohorts in Japan (EPOCH-JAPAN) Research Group. Relation between serum total cholesterol level and cardiovascular disease stratified by sex and age group: a pooled analysis of 65 594 individuals from 10 cohort studies in Japan. J Am Heart Assoc 2012; 1:e001974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Neaton JD, Wentworth D. Serum cholesterol, blood pressure, cigarette smoking, and death from coronary heart disease. Overall findings and differences by age for 316,099 white men. Multiple Risk Factor Intervention Trial Research Group. Arch Intern Med 1992; 152:56–64. [PubMed] [Google Scholar]
- 12. Marmot M, Elliott P. Coronary Heart Disease Epidemiology. 2nd ed. Oxford University Press: New York, 2005. [Google Scholar]
- 13. Shimamoto K, Kita T, Mabuchi H, Matsuzaki M, Matsuzawa Y, Nakaya N, Oikawa S, Saito Y, Sasaki J, Itakura H; J-LIT Study Group. The risk of cardiovascular events in Japanese hypertensive patients with hypercholesterolemia: sub-analysis of the Japan Lipid Intervention Trial (J-LIT) Study, a large-scale observational cohort study. Hypertens Res 2005; 28:879–887. [DOI] [PubMed] [Google Scholar]
- 14. Asia Pacific Cohort Studies Collaboration. Joint effects of systolic blood pressure and serum cholesterol on cardiovascular disease in the Asia Pacific region. Circulation 2005; 112:3384–3390. [DOI] [PubMed] [Google Scholar]
- 15. Higashiyama A, Wakabayashi I, Ono Y, Watanabe M, Kokubo Y, Okayama A, Miyamoto Y, Okamura T. Association with serum gamma-glutamyltransferase levels and alcohol consumption on stroke and coronary artery disease: the Suita study. Stroke 2011; 42:1764–1767. [DOI] [PubMed] [Google Scholar]
- 16. Okamura T, Kokubo Y, Watanabe M, Higashiyama A, Ono Y, Nishimura K, Okayama A, Miyamoto Y. A revised definition of the metabolic syndrome predicts coronary artery disease and ischemic stroke after adjusting for low density lipoprotein cholesterol in a 13-year cohort study of Japanese: the suita study. Atherosclerosis 2011; 217:201–206. [DOI] [PubMed] [Google Scholar]
- 17. Watanabe M, Okamura T, Kokubo Y, Higashiyama A, Okayama A. Elevated serum creatine kinase predicts first-ever myocardial infarction: a 12-year population-based cohort study in Japan, the Suita study. Int J Epidemiol 2009; 38:1571–1579. [DOI] [PubMed] [Google Scholar]
- 18. Kokubo Y, Okamura T, Watanabe M, Higashiyama A, Ono Y, Miyamoto Y, Furukawa Y, Kamide K, Kawanishi K, Okayama A, Yoshimasa Y. The combined impact of blood pressure category and glucose abnormality on the incidence of cardiovascular diseases in a Japanese urban cohort: the suita study. Hypertens Res 2010; 33:1238–1243. [DOI] [PubMed] [Google Scholar]
- 19. Teramoto T, Sasaki J, Ishibashi S, Birou S, Daida H, Dohi S, Egusa G, Hiro T, Hirobe K, Iida M, Kihara S, Kinoshita M, Maruyama C, Ohta T, Okamura T, Yamashita S, Yokode M, Yokote K; Japan Atherosclerosis Society. Executive summary of the Japan Atherosclerosis Society (JAS) guidelines for the diagnosis and prevention of atherosclerotic cardiovascular diseases in Japan—2012 version. J Atheroscler Thromb 2013; 20:517–523. [DOI] [PubMed] [Google Scholar]
- 20. Friedewald W, Levy R, Fredrickson D. Estimation of the concentration of low density lipoprotein cholesterol in plasma without use of the ultracentrifuge. Clin Chem 1972; 18:499–502. [PubMed] [Google Scholar]
- 21. European Association for Cardiovascular Prevention & Rehabilitation1. Reiner Z, Catapano AL, De Backer G, Graham I, Taskinen MR, Wiklund O, Agewall S, Alegria E, Chapman MJ, Durrington P, Erdine S, Halcox J, Hobbs R, Kjekshus J, Filardi PP, Riccardi G, Storey RF, Wood D; ESC Committee for Practice Guidelines (CPG) 2008–2010 and 2010–2012 Committees. ESC/EAS Guidelines for the management of dyslipidaemias: the Task Force for the Management of Dyslipidaemias of the European Society of Cardiology (ESC) and the European Atherosclerosis Society (EAS). Eur Heart J 2011; 32:1769–1818. [DOI] [PubMed] [Google Scholar]
- 22. National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III). Third report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) final report. Circulation 2002; 106:3143–3421. [PubMed] [Google Scholar]
- 23. American Diabetes Association. Executive summary: standards of medical care in diabetes—2013. Diabetes Care 2013; 36:S4–S10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. American Diabetes Association. Diagnosis and classification of diabetes mellitus. Diabetes Care 2013; 36:S67–S74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Walker AE, Robins M, Weinfeld FD. The National Survey of Stroke. Clinical Findings. Stroke 1981; 12:I13–I44. [PubMed] [Google Scholar]
- 26. World Health Organization. Document for Meeting of MONICA Principal Investigators. WHO: Geneva, Switzerland, 1986. [Google Scholar]
- 27. Woodward M. Epidemiology: Study Design and Data Analysis. 2nd ed. Chapman & Hall/CRC Press, Boca Raton, FL, 2005. [Google Scholar]
- 28. Tanaka T, Okamura T. Blood cholesterol level and risk of stroke in community-based or worksite cohort studies: a review of Japanese cohort studies in the past 20 years. Keio J Med 2012; 61:79–88. [DOI] [PubMed] [Google Scholar]
- 29. Amarenco P, Steg PG. The paradox of cholesterol and stroke. Lancet 2007; 370:1803–1804. [DOI] [PubMed] [Google Scholar]
- 30. Kushiro T, Mizuno K, Nakaya N, Ohashi Y, Tajima N, Teramoto T, Uchiyama S, Nakamura H; Management of Elevated Cholesterol in the Primary Prevention Group of Adult Japanese Study Group. Pravastatin for cardiovascular event primary prevention in patients with mild-to-moderate hypertension in the Management of Elevated Cholesterol in the Primary Prevention Group of Adult Japanese (MEGA) Study. Hypertension 2009; 53:135–141. [DOI] [PubMed] [Google Scholar]
- 31. Sever PS, Dahlöf B, Poulter NR, Wedel H, Beevers G, Caulfield M, Collins R, Kjeldsen SE, Kristinsson A, McInnes GT, Mehlsen J, Nieminen M, O’Brien E, Ostergren J; ASCOT investigators. Prevention of coronary and stroke events with atorvastatin in hypertensive patients who have average or lower-than-average cholesterol concentrations, in the Anglo-Scandinavian Cardiac Outcomes Trial-Lipid Lowering Arm (ASCOT-LLA): a multicentre randomised controlled trial. Lancet 2003; 361:1149–1158. [DOI] [PubMed] [Google Scholar]
- 32. MacMahon S, Peto R, Cutler J, Collins R, Sorlie P, Neaton J, Abbott R, Godwin J, Dyer A, Stamler J. Blood pressure, stroke, and coronary heart disease. Part 1, prolonged differences in blood pressure: prospective observational studies corrected for the regression dilution bias. Lancet 1990; 335:765–774. [DOI] [PubMed] [Google Scholar]