Abstract
Absence of a regenerative pathway for damaged retina following injury or disease has led to experiments utilizing stem cell transplantation for retinal repair, and encouraging results have been obtained in rodents. The swine eye is a closer anatomical and physiological match to the human eye, but embryonic stem cells have not been isolated from pig, and photoreceptor differentiation has not been demonstrated with swine induced pluripotent stem cells (iPSC). Here, we subjected swine iPSC to a rod photoreceptor differentiation protocol consisting of floating culture as embryoid bodies followed by differentiation in adherent culture. Real time PCR and immunostaining of differentiated cells demonstrated loss of expression of the pluripotent genes POU5F1, NANOG and SOX2 and induction of rod photoreceptor genes RCVRN, NRL, RHO and ROM1. While these differentiated cells displayed neuronal morphology, culturing on a Matrigel substratum triggered a further morphological change resulting in concentration of RHO and ROM1 in outer segment-like projections resembling those on primary cultures of rod photoreceptors. The differentiated cells were transplanted into the subretinal space of pigs treated with iodoacetic acid to eliminate rod photoreceptors. Three weeks after transplantation, engrafted RHO+ cells were evident in the outer nuclear layer where photoreceptors normally reside. A portion of these transplanted cells had generated projections resembling outer segments. These results demonstrate that swine iPSC can differentiate into photoreceptors in culture and these cells can integrate into the damaged swine neural retina thus laying a foundation for future studies using the pig as a model for retinal stem cell transplantation.
Keywords: photoreceptor differentiation, swine retina, induced pluripotent stem cells, retinal transplantation
Introduction
The outer nuclear layer (ONL) of the retina consists of cell bodies of rod and cone photoreceptors, which convert light signals to electrical potential that is transmitted to bipolar cells in the inner nuclear layer (INL) [1]. This signal in turn is sent to ganglion cells that transmit the signal to the visual cortex. A regenerative pathway is effective in restoring damaged retina in lower vertebrates [2–3], but there is no comparable pathway with the ability to restore visual function in higher vertebrates following either retinal injury or disease, nor have adult stem cells been isolated from the retina. Therefore, therapeutic approaches to retinal damage and disease in higher vertebrates have focused on cell transplantation using embryonic stem cells (ESC) or induced pluripotent stem cells (iPSC) generated from skin fibroblasts by viral transduction of stem cell specification genes POU5F1 (OCT4), SOX2, KLF4 and c-MYC [4–13]. ESC derived from mice, humans and monkeys have been used successfully to generate cells expressing markers of retinal progenitors in culture [6–10], and iPSC derived from mouse and human skin fibroblasts can also differentiate to generate photoreceptor-like progenitors in culture [8,9,11,13].
The mouse is an attractive model for cell transplant studies because of the variety of genetic mutants available, and accordingly most retinal transplant studies have utilized rodents. Transplanted photoreceptors from neonatal mice have been shown to engraft into the damaged mouse retina, and some of these transplanted cells go on to generate outer segments [14], which consist of membranous disks containing visual pigment that are projected from the cell surface and reflect functional morphology [1]. In these studies proliferating retinal progenitors did not functionally engraft into the mouse retina, demonstrating that differentiated photoreceptors may be necessary for successful cell transplantation. Differentiated ESC have also been transplanted into the mouse retina, and these cells integrate and some of them generate outer segments, but by contrast differentiated iPSC or retinal progenitor cells have thus far failed to generate outer segments following retinal transplantation [10]. Transplantation of differentiated ESC into mouse models of rod photoreceptor loss has led to cell integration into the ONL and a modest increase in electrophysiological response to light measured by electroretinography (ERG) has been reported, but no outer segments have been detected [3–5, 9–11]. These studies in mice demonstrate the feasibility of retinal cell transplantation therapy for human retinal disease, but the mouse retina is rod-dominant, lacks a macula and is thus not an ideal anatomical and physiological model of human retinal disease. As with humans, the swine retina contains a cone-dominant central visual streak analogous to the macula with rods enriched in the peripheral retina [15]. Thus, the swine retina is a much closer anatomical and physiological match to the human retina. Use of the swine retina as an experimental model for stem cell transplantation has been hampered by the fact that ESC have not been isolated from pig [16–17], and attempts have not been made to differentiate swine iPSC into photoreceptor lineages. However, retinal progenitors derived from embryos have been injected into the swine subretinal space, and importantly they have been shown to integrate and survive [18–19], thus demonstrating the feasibility of stem cell transplantation into the swine retina. Here, we have utilized swine iPSC derived from fetal fibroblasts as a source of rod photoreceptor lineage cells for transplantation into a swine model of rod photoreceptor loss. We show that these cells can differentiate into photoreceptors in culture, and following transplantation into a model of swine rod photoreceptor loss, the cells integrate into the ONL and can generate outer segment-like projections. These results provide a foundation for future studies of retinal stem cell transplantation in a swine model.
Materials and Methods
Culture of swine iPSC
The ID6 swine iPSC line has been described previously [20]. A colony of uniform appearance at passage 24 resembling a typical human ESC colony was mechanically broken up [20] and passaged 1:10 on a feeder layer of irradiated mouse embryonic fibroblasts in media containing DMEM/F12, 20% knockout serum replacer, 1mM L-glutamine, 0.1 mM 2-mercaptoethanol, 1% non-essential amino acids, and 4 ng/ml FGF2 (Invitrogen).
Photoreceptor differentiation of swine iPSC
The swine iPSC photoreceptor differentiation protocol was modified from that of Lamba et al for human ESC [9–10]. iPSC at passages 28, 40 and 43 were used for photoreceptor differentiation with similar results. For photoreceptor differentiation, embryoid bodies were allowed to form by dissociating iPSC into small clumps with 1mg/ml type IV collagenase and the resulting embryoid bodies were cultured in 100 mm ultra-low attachment plates (VWR) in medium containing DMEM/F12, 10% knockout serum replacer, neuronal culture supplements N2 and B27 (Invitrogen), 1 ng/ml DKK1 (R&D), 1 ng/ml NOGGIN (R&D), and 1 ng/ml insulin like growth factor-1 (IGF1) (R&D) for three days. Embryoid bodies were then transferred to poly-D-lysine coated plates with undiluted Matrigel (BD), Matrigel diluted 1:10 or 1:20, or plates coated with a mixture of laminin (8 μg/cm2) and fibronectin (2 μg/cm2), after which they were cultured for 18 days in medium containing 10ng/ml DKK1, 10 ng/ml NOGGIN, 10 ng/ml IGF-1 and 5 ng/ml human recombinant FGF2 (Invitrogen). Medium was changed daily. One μM retinoic acid and 100 μM taurine (Sigma-Aldrich) were added as indicated.
Primary culture of rod photoreceptors
For primary culture of rod photoreceptors, eyes from adult pigs were obtained from the local slaughterhouse and kept in CO2 Independent Medium (Invitrogen) on ice before dissection. The protocol for harvesting photoreceptors with attached outer segments has been described previously [24]. The following procedures were performed under dim red light. The anterior segment of the eye was removed, the neural retina isolated from the eye cup, and the tissue digested with activated papain (Sigma-Aldrich) at 37ºC for 20 min. The papain solution was removed and enzymatic activity inhibited by adding media containing 2% FBS to the retina for 5 min. This media was then removed, and retinal cell culture medium containing neurobasal-A-medium, B27 and L-glutamine supplements (all from Invitrogen) was added. The digested tissue was pipetted gently several times with a P1000 Pipetman, and large tissue fragments allowed to settle for a further 5 min. Disassociated photoreceptors, which remained in suspension, were transferred to tissue chamber slides coated with poly-D-lysine and Matrigel diluted 1:20. Two days later, the cells were examined for expression of various antigens by immunostaining.
Immunostaining
Cells or swine retinas were fixed with 4 % paraformaldehyde for 15 min, and frozen sections of swine retina were dried at 37°C for 15 min. Cells and tissue sections were then washed with phosphate buffered saline (PBS) and blocking solution consisting of 0.8 % bovine serum albumin, 4 % goat serum, and 0.1 % Tween-20 in PBS was added for 1 h at 25ºC. The samples were then incubated either overnight at 4ºC or 1 h at 25ºC with primary antibody reagents in blocking solution. After primary antibodies had been removed and the samples washed, secondary antibodies were applied for 1 h at 25ºC. The primary antibodies used were: goat anti-POU5F1 (Santa Cruz 1:100), mouse anti TUBB3 (Millipore 1:500), rabbit anti-recoverin (RCVRN) (Millipore, 1:1500), mouse anti RHO (Millipore, 1:300), rabbit anti-NRL (a generous gift from Anand Swaroop, National Eye Institute, Bethesda, MD, 1:1000) [28], rabbit anti PRKCA (Sigma, 1:15,000), Rabbit anti ROM1 (Sigma, 1:400), rabbit anti red-green opsin (OPN1LW/OPN1MW) (Millipore 1:500), rat anti-ABCG2 (Abcam 1:200). Bound antibodies were visualized with either Alexa fluor 488- (Invitrogen 1:500) or Alexa Fluor 568- (Invitrogen, 1:500) conjugated secondary antibodies. Nuclei were counterstained with DAPI, and images captured with a Zeiss inverted fluorescence microscope (Axiovert 200). The immunoreactivity of each antibody was confirmed by immunostaining swine retinal tissue as a positive control, and as a negative control, primary antibody was omitted (Supplemental Fig. 1).
Statistical analysis
ANOVA with Bonferroni’s post hoc test was used for the determination of statistical significance among treatment groups, as indicated.
Real-Time PCR analysis
RNA was extracted from the cultures using the RNAeasy kit (Qiagen) and reverse transcribed with the Superscript III RT-PCR kit (Invitrogen). SYBR Green real-time PCR was performed by using a Stratagene Mx3000P Real-Time PCR system [29]. The complete coding sequence for swine genes was obtained from the EMBL and NCBI Nucleotide Sequence Database. The POU5F1 mRNA forward primer was 5′-CGAAGCTGGACAAGGAGAAG-3′, and the reverse primer 5′-GCTGAACACCTTCCCAAAGA-3′ (product 176 bp). The rhodopsin (RHO) forward primer was 5′-CTTCCCCATCAACTTCCTCA-3′ and the reverse primer was 5′-ACCACCACGTACCGTTCAAT-3′ (product 264 bp). The cone-arrestin (ARR3) forward primer was 5′-AACGGCAAGCTCTCCATCTA-3′, and the reverse primer was 5′-CAGATCTTTGCGGAATGTCA-3′ (product 195 bp). The RCVRN forward primer was 5′-GGGCTTTCTCCCTCTACGAC and the reverse primer 5′-CATCGTCCTTCTTCCCAAAG (183 bp). The RBP3 forward primer was 5′-GGCCAAGATAGCAGTCAAGC and the reverse primer 5′-CTCGAGCACGTTAGTGTGGA (product 244 bp). The NANOG forward primer was 5′-TTCCTTCCTCCATGGATCTG and the reverse primer 5′-ATCTGCTGGAGGCTGAGGTA (product 214 bp). The SOX2 forward primer was 5′-GCCCTGCAGTACAACTCCAT and the reverse primer 5′-GCTGATCATGTCCCGTAGGT (product 216 bp). The β-actin (ACTB) mRNA forward primer was 5′-GCCAACCGTGAGAAGATGAC-3′, and the reverse primer 5′-GAGTCCATCACGATGCCAGT-3′ (126 bp product).
Quantification of iPSC differentiation
Cells expressing differentiation markers were counted and compared to the total number of cells found in each field, as determined by DAPI nuclear staining. At least 2,000 cells were examined in each experiment. All sets of experiments were performed at least three times. Results are reported as means ± SE.
Lentiviral infection
RBP3-GFP (green fluorescence protein) lentiviral particles were a gift from Thomas A. Reh, University of Washington [9–10]. Differentiated iPSC were infected with the RBP3-GFP lentivirus as described [9–10],
Subretinal transplantation
All animal protocols were approved by the University of Louisville Institutional Animal Care and Use. Domestic pigs were obtained at six weeks of age (12–16 kg) from Oak Hill Genetics (Ewing, IL). Iodoacetic acid was dissolved in normal saline, and pigs were administered 12 mg/kg intravenously via a catheter placed in the ear vein. Four days later, animals were sedated with Telzol (2.0–8.8 mg/kg) and maintained under general anesthesia with 1.5% to 2% isoflurane mixed with oxygen. Intravenous access was achieved by placement of a 21-gauge catheter in an ear vein. Pupils were dilated and the ability to focus was inhibited with topical applications of 2.5% phenylephrine hydrochloride and 1% tropicamide. A three-port, 20 gauge pars plana vitrectomy was performed using a suction of 150 mm Hg and a cutting rate of 600 oscillations per minute [30]. A posterior vitreous detachment was made by suction over the optic disc and posterior retina, and a neurosensory retinal detachment (i.e., a bleb) at the visual streak was created by injecting 50 μl of BSS Plus (Alcon) into the subretinal space using a 39-gauge cannula. Differentiated iPSC (2 × 106 in 0.1 ml) were injected into the belb.
Histological Evaluation
Pigs were euthanized three weeks after cell transplant with Beuthanasia (1 ml/5 kg) administered through an ear vein catheter. Eyes were enucleated, and retinas isolated and fixed by immersion in 4 % paraformaldehyde. A strip of retina that extended from the dorsal to the ventral margin of the eyecup was bisected at the optic disc along the horizontal plane. The dorsal and ventral halves were bisected again along the horizontal plane. Each of the four pieces was notched on its dorsal edge to preserve orientation. Pieces were dehydrated and embedded in the polyvinyl alcohol, polyethylene glycol-based Optimal Cutting Temperature cutting reagent (O.C.T.). Tissues were oriented and cut to produce vertical sections along the dorsal to ventral axis. Frozen sections (14 μm) were cut for immunostaining using a cryostat.
Results
Differentiation of swine iPSC into photoreceptors
iPSC from swine (ID6) were created by lentiviral transduction of cDNAs for POU5F1, KLF4, SOX2, and c-MYC into swine fetal fibroblasts and maintained on a standard medium for human ESC containing FGF2 [20]. Although these cells were derived from a pig expressing a GFP transgene [20], colonies resembling fully reprogrammed ESC at passage 28 had silenced GFP expression (Supplemental Fig, 1). Such silencing of a GFP transgene has previously been used as an important criterion for selection of fully reprogrammed human and mouse iPSC [21]. A phase image of iPSC colonies is shown in Fig. 1A.
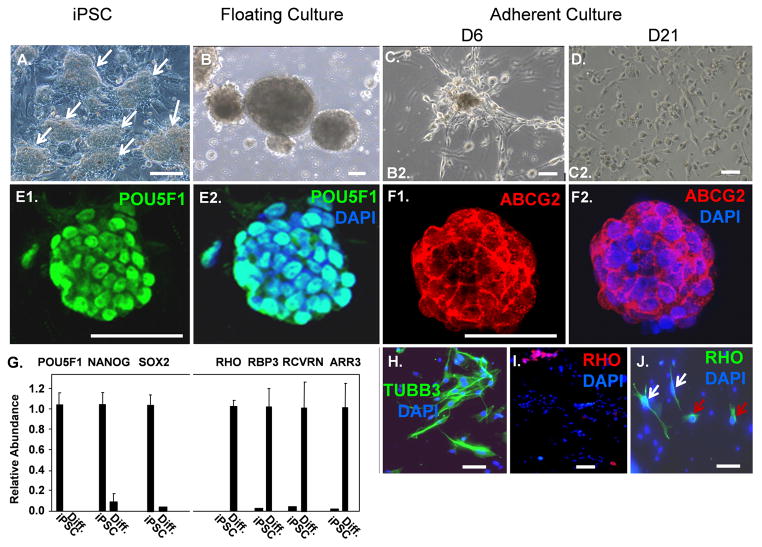
Fig 1.
Photoreceptor differentiation protocol for swine iPSC. (A): Phase image of swine iPSC colonies (arrows) cultured on an irradiated fibroblast feeder layer. (B): Phase image of embryoid bodies in floating culture. (C): Three days after embryoid bodies were allowed to adhere to matrix, cells had migrated out onto the substratum. (D): Phase image showing cells at day 21 of the differentiation protocol. (E): Immunostaining of an iPSC colony for POU5F1. (F): Immunostaining of an iPSC colony for ABCG2. (G): Real time PCR showing loss of stem cell mRNAs and induction of photoreceptor mRNAs during iPSC differentiation. Results were normalized to beta-actin (ACTB) mRNA. (H): Immunostaining for TUBB3 following differentiation. (I–J): Immunostaining for RHO following differentiation. Note the two distinct cell morphologies of RHO+ cells shown by white and red arrows in panel J (also see Fig. 4). Scale bars = 50 μm.
We utilized a two-step protocol for photoreceptor differentiation of the swine iPSC involving initial production of embryoid bodies (Fig. 1B) and subsequent outgrowth on extracellular matrix (Fig. 1C–D). Embryoid bodies in suspension on non-adherent plates were cultured with the WNT inhibitor DKK1, the BMP inhibitor NOGGIN, and insulin-like growth factor (IGF-1) for times ranging from 3 to 7 days. Then, the embryoid bodies were transferred to plates coated with poly-D-lysine and different concentrations of either Matrigel or laminin/fibronection. Culture was then continued for three more weeks with a ten-fold increase in the concentrations of DKK-1, NOGGIN, IGF1, and supplemental FGF2 in the medium. The iPSC express POU5F1 in the nucleus and the multi-drug resistance transporter ABCG2, which is expressed on stem cells and cancer stem cells, on their surface (Fig. 1E–F), and they also express mRNAs for POU5F1, NANOG, and SOX2 (Fig. 1G). Silencing of stem cell specification genes is a hallmark of ESC and iPSC differentiation, and three days of culture as embryoid bodies led to down-regulation of POU5F1, NANOG and SOX2 mRNAs (Fig. 1G; Supplemental Fig. 2A–B) implying effective differentiation, which is important to avoid the potential risk of teratoma formation following cell transplantation. Although previous studies suggested that supplementation of the medium with retinoic acid and taurine promotes differentiation of rod precursor cells from cultures of either ESC or iPSC [6,8,13], these reagents did not appear to accelerate either loss of POU5F1 mRNA expression or the process of differentiation in the swine iPSC (Supplemental Fig. 2A–F). Following differentiation, cells were immunostained for the general neuronal marker beta3 tubulin (TUBB3). Approximately 40% of the cells showed neuronal morphology and were TUBB3-positive (TUBB3+) (Fig. 1H; Fig. 3), suggesting that many of the cells had become committed to a neuronal lineage.
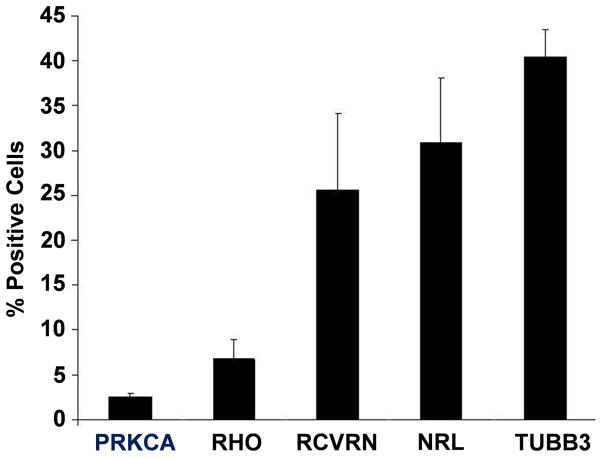
Fig. 3.
Quantification of cells expressing markers of retinal differentiation. At least 2,000 cells were examined in each experiment and results are an average of three independent experiments. The total cell number present in a field was determined by DAPI nuclear staining.
Rhodopsin (RHO) is a terminal marker of rod photoreceptor differentiation required for conversion of light to electrical signals. While earlier markers of rod photoreceptors such as neural retina leucine zipper protein (NRL), recoverin (RCVRN) and photoreceptor-specific retinol binding protein-3 (RPB3) (also known as IRBP1) can be efficiently induced during differentiation of mouse, human and monkey ESC and iPSC, few RHO+ cells have been reported [6,8,13]. We found that mRNAs for RCVRN, RBP3 and RHO as well as the cone marker, cone-arrestin (ARR3), were induced following the differentiation protocol (Fig. 1G), and approximately 6% of the cells were RHO+ by immunostaining (Fig. 1I–J; Fig. 3). These RHO+ cells showed two distinctly different morphologies. Some of the cells resembled TUBB3-expressing cells with elongated cell bodies and RHO distributed evenly throughout the cytoplasm, whereas other cells displayed rounded cell bodies with RHO concentrated in outer segment-like projections extending from the cell surface (Fig. 1H and J; Fig. 4A–G below). Three days of embryoid body culture in the absence of retinoic acid and taurine generated the greatest number of RHO+ cells (Supplemental Fig. 2C–F), and these conditions were used for all subsequent differentiation assays.
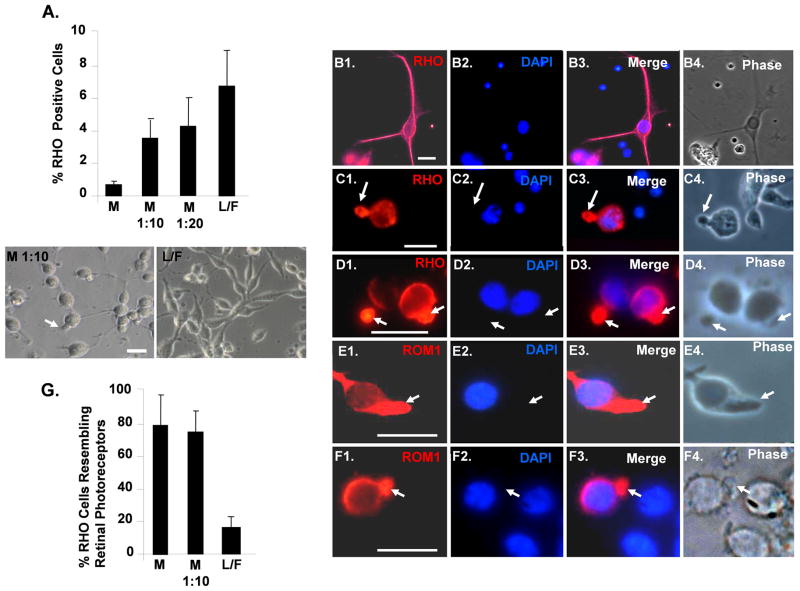
Fig 4.
Adherent culture on Matrigel enhances the number of differentiating iPSC resembling primary cultures of rod photoreceptors. (A): Quantification of RHO+ cells generated on different matrices. “L/F” laminin/fibronection, “M” Matrigel. Phase images of cells are shown below. P<0.05 for all values compare to undiluted Matrigel. (B1–B4): Differentiated iPSC plated on laminin/fibronection and immunostained for RHO. Note cells with elongated cell bodies that immunostain uniformly for RHO. (C1–C4): Differentiated iPSC cells plated on Matrigel and immunostained for RHO. Note that RHO is concentrated in projections from the main cell body. (D1–D4): Primary culture of swine retinal cells immunostained for RHO. Note that RHO is concentrated in outer segments extending from the rounded cell bodies, and that the cell morphology resembles that of the differentiated iPSC plated on Matrigel in panels C1–C4. (E1–E4): Differentiated iPSC plated on Matrigel and immunostained with the rod outer segment marker ROM1. Note that ROM1 colocalizes with RHO in projections from the rounded cell bodies. (F1–F4): Primary culture of swine retinal cells immunostained for ROM1. Note that, like RHO, ROM1 is concentrated in outer segments projected from the rounded cell bodies and that these cells resemble those in panels E1–E4. Scale bars = 20 μm. (G): Quantification of RHO+ iPSC morphologically resembling primary culture rod photoreceptors generated on different matrices. P<0.05 for L/F compared to M and M 1:10.
Differentiating swine iPSC express early markers of the rod photoreceptor lineage and a marker of rod bipolar cells
Differentiated swine iPSC were stained for the transcription factor NRL, which drives commitment to the rod lineage, and RCVRN, which is an additional marker of the early photoreceptor lineage (Fig. 2A–D). All RHO+ cells co-expressed NRL and RCVRN, but a high percentage of RHO− cells also expressed these two markers (Fig. 3), suggesting that the differentiation protocol was generating neuronal cells at early as well as later stages of rod photoreceptor lineage.
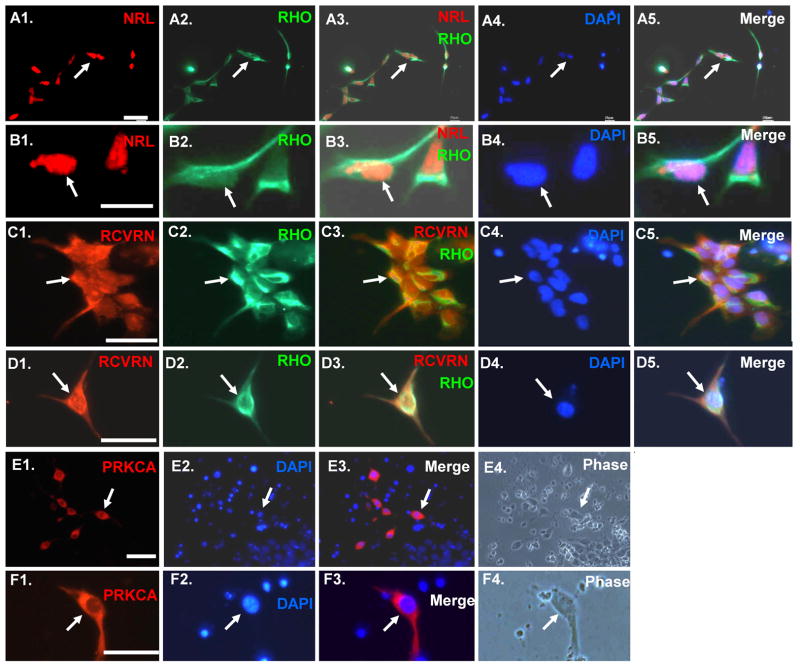
Fig 2.
RHO+ cells derived from iPSC co-express early photoreceptor lineage markers NRL and RCVRN. In addition differentiated cells expressing the rod bipolar lineage marker PRKCA are also present. (A1–B4): Cells double immunostained with RHO and NRL. (C1–D4): Cells double immunostained for RHO and RCVRN. (E1–F4): Cells immunostained for PRKCA. Scale bars = 50 μm.
Rod photoreceptors synapse with rod bipolar cells to transmit visual signals to ganglion cells [22]. Such rod bipolar cells are one of the last lineages to appear during retinal development in vivo and they are marked by expression of protein kinase C-α (PRKCA) [23]. Approximately 2.5% of the differentiated cells immunostained for PRKCA (Fig. 2E–F; Fig. 3), suggesting that the differentiation protocol also generates cells resembling rod bipolar cells.
Culture on Matrigel is required for efficient generation of cells resembling primary cultures of rods
Following embryoid body formation, we examined the effect of culturing the cells on different matrices. Culture on laminin/fibronection led to the highest percentage of RHO+ cells (Fig. 4A). However, most of these RHO+ cells on laminin/fibronection displayed elongated cell bodies resembling TUBB3+ cells seen in Fig. 1H, and RHO was distributed evenly in the cytoplasm of these cells (Fig. 4A–B). By contrast, most of the RHO+ cells on Matrigel had rounded cell bodies with thin axonal projections. RHO and rod outer segment-specific membrane protein 1 (ROM1) were concentrated in outer segment-like projections extending from the body of these cells (Fig. 4A, C and E). We then compared the morphology of the iPSC-derived RHO+ cells to primary cultures of swine rod photoreceptors generated by an isolation protocol that conserves outer segments [24]. The morphology of the rod primary cultures was similar to that of the differentiated iPSC cultured on Matrigel. In addition, RHO and ROM1 were concentrated in outer segment extensions resembling those seen on the cells cultured on Matrigel (Fig. 4D and F). Thus, even though culture on laminin/fibronectin led to a higher number of RHO+ cells, culture on Matrigel appears to be important in directing transition of RHO+ cells to a morphology with outer segment-like processes (Fig. 4G).
Iodoacetic acid treatment leads to loss of rod photoreceptors in the swine ONL
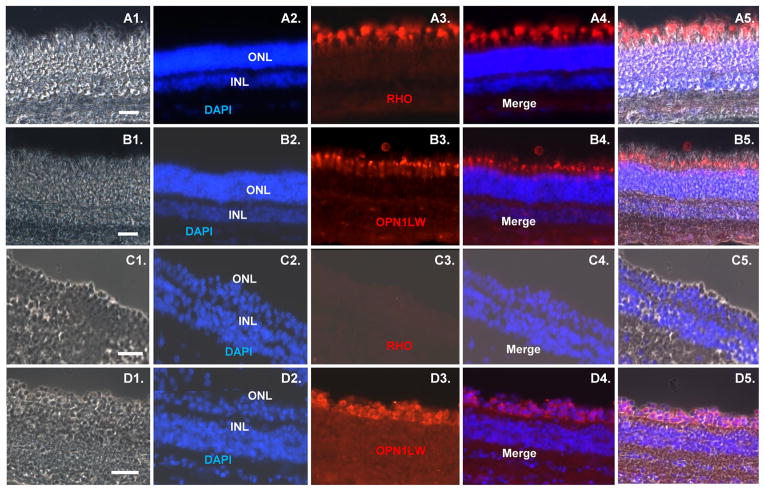
Iodoacetic acid blocks glycolysis and is thus toxic to neurons, which depend upon this pathway [25–27]. Six week old pigs were injected intravenously with 12 mg/kg of iodoacetic acid, and three weeks later retinas were removed and sectioned for immunostaining with rod and cone markers. Immunostaining for RHO was lost following iodoacetic acid treatment, demonstrating a loss of rod photoreceptors, whereas immunostaining with an antibody against cone-specific red-green, which recognizes both opsin long wavelength opsin 1 (OPN1LW) and opsin medium wavelength opsin 1 (OPN1MW), was relatively unaffected by the treatment (Fig. 5A–D). These results demonstrate that rod photoreceptors are damaged in the swine retina by iodoacetic acid.
Fig. 5.
Iodoacetic acid treatment leads to selective loss of rod photoreceptors in the swine retina. (A–B): Sections of retina from a six week old pig immunostained for rod (RHO) and cone (red-green opsin) markers. (C–D): Sections of retina from a six week old pig three weeks after iodoacetic acid treatment. Immunostaining for RHO and red-green opsin is shown. Scale bars = 50 μm.
Expression of RBP3-GFP in differentiating swine iPSC
To mark cells committed to photoreceptor lineage, differentiated swine iPSC were infected with a lentivirus vector incorporating the promoter region RPB3 driving expression of GFP [9–10]. Following infection, approximately 44 % of the cells expressed GFP (Fig. 6A–B), roughly similar to the number of neuronal cells positive for TUBB3 and slightly higher than the number expressing NRL (Fig. 3). While RBP3 is a marker of differentiating photoreceptors, it is maintained in the adult retina [9–10], and we reasoned that GFP expression from this promoter would be a useful marker to follow iPSC-derived rod precursor cells after transplantation into the eye.
Fig 6.
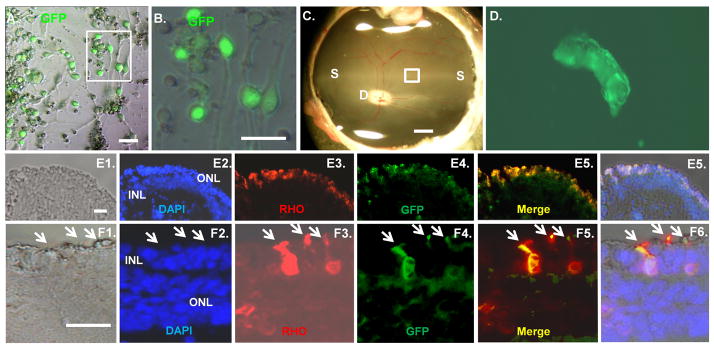
Integration of transplanted swine iPSC into the ONL. (A–B): RBP3-GFP expression in differentiated iPSC. Differentiated iPSC plated on Matrigel were infected with a lentivirus containing the photoreceptor-specific RBP3 gene promoter driving expression of GFP [9–10]. The boxed region in panel A is shown at higher magnification in panel B. Phase images are shown. (C): Fundas photograph of a six week old swine retina. The injection site is shown with a box. “D”, indicate the optic disc, and “S” indicates the visual streak. The scale bar is 2000 microns. (D): Differentiated iPSC were injected 4 days after iodoacetic treatment, and then 3 weeks later the retina was removed. A fluorescent image of GFP+ cells in the boxed area of panel C is shown in a flatmount of the retina. (E–F): Sections of retina from the region of transplanted iPSC were immunostained for RHO and GFP. In panels E and F, 1 is a Nomarski image, 2 is DAPI staining, 3 is RHO staining, 4 is a GFP image, 5 is merged DAPI, RHO and GFP image, and 6 is the merged DAPI, RHO, GFP and Nomarski image. Arrows indicate outer segment projections. Scale bars in panels A, B, E and F = 50 μm.
Integration of RHO+ transplanted cells into the swine ONL
Four days after iodoacetic acid treatment, 1 × 106 differentiated iPSC were injected subretinally into one eye (Fig. 6C), whereas DMEM/F12 medium was injected into the other control eye. After three weeks, the animals were sacrificed and their retinas removed. GFP+ cells were evident at the injection site in a flatmount of the retina (Fig. 6D). The injection site was then sectioned for immunostaining. GFP+/RHO+ transplanted cells had integrated into the ONL in this region, and some of these transplanted cells had outer segment-like projections (Fig. 6E–F). Integrated RHO+ cells were counted in six 100,000 sq. μm sections distributed throughout the injection site, which was approximately the size of the optic disk (Fig. 6C). The disk in the pig is 2 mm × 4 mm and approximately 8 × 106 sq μm. Assuming these six sections are representative of cell integration throughout the injection site, 10,414±3051 injected RHO+ cells integrated into the retina. This corresponds to approximately 1% of the injected cells. A previous study found approximately 3,000 integrated cells (about 4% of the injected cells) in the mouse retina after injection of embryonic stem cells differentiated into photoreceptors, and approximately 25% of these integrated cells expressed Rho [10].
Discussion
Here we have presented a two-step differentiation protocol for photoreceptor generation from swine iPSC that involves suspension culture as embryoid bodies followed by adherent culture on an extracellular matrix. An early event following conversion to embryoid bodies was loss of expression of the pluripotency marker POU5F1. A high percentage of the differentiating cells acquired a neuronal morphology and expressed the general neuronal marker TUBB3, and the majority of such cells also expressed the rod-specification transcription factor NRL and the early photoreceptor marker RCVRN, indicating that most of these neuronal cells were committed to the rod lineage. However, only approximately 6 % of the cells were positive for the terminal rod marker RHO. Together, these results suggested that cells at various stages of rod differentiation are present in the cultures, and the cells posed little risk of generating teratomas if injected into animals.
We noted two distinct morphologies of RHO+ cells in culture. Some cells had elongated cell bodies characteristic of neurons and RHO was distributed evenly throughout the cytoplasm, whereas other cells had rounded cell bodies with thin axonal projections and RHO and ROM1 were concentrated into outer segment-like extensions from the cell bodies. Because this latter morphology resembled primary cultures of rod photoreceptors, we suggest that following RHO expression, the cells can undergo a morphological change where RHO and ROM1 become concentrated in outer segments. Further, this morphological transition is enhanced by culture of the cells on Matrigel during the differentiation process.
Treatment of pigs with iodoacetic acid led to loss of rod photoreceptors, and this was accompanied by a diminished dark-adapted ERG (Supplemental Fig. 3). Sub-retinal injection of the differentiated iPSC after iodoacetic acid treatment led to integration of the cells into the retina. While RHO+ transplanted cells were evident in all of the retinal layers, they were most concentrated in the ONL and some of the transplanted cells in the ONL had generated outer segments. It is of note that outer segment generation has yet to be detected in cells transplanted into mouse models of rod photoreceptor damage [10]. However, despite evidence of rod outer segment generation and thus transition to functional morphology, transplantation of these cells did not lead to a significant change in ERG in the swine retina (Supplemental Fig. 3). However, it is of note that the pig retina is much larger than the mouse, and thus the injection site in these studies only represents a small region of the swine retina (Fig. 6C).
The mouse is an important model for stem cell transplant studies due to the variety of genetic models of retina disease available, but the ability to extend such experiments to the pig, because of the anatomical similarity between the pig and human retina, is likely to provide an additional model that ensures the technology is developed safety and efficiently prior to attempts at human transplantation. As an initial step toward a swine model for retinal stem cell transplant, we have developed an efficient protocol for differentiation of swine iPSC into the rod photoreceptor lineage and shown these cells are capable of integrating into the ONL of retinas depleted of rod photoreceptors.
Supplementary Material
Acknowledgments
We thank T. Reh for the RBP3-GFP lentivirus and A. Swaroop for the NRL antibody. These studies were supported in part by American Health Assistance Foundation, NIH Grants (P20 RR018733 and EY015636), Research to Prevent Blindness, and The Commonwealth of Kentucky Research Challenge. The generation of swine iPSC was supported by grants from NIH (HD 21896) and the Missouri Life Sciences Research Board (09-1018).
Footnotes
Author contributions
Liang Zhou: Conception and design, collection and assembly of data, data analysis and interpretation, manuscript writing, final approval of manuscript
Wei Wang: Conception and design, collection and assembly of data, data analysis and interpretation, final approval of manuscript
Yongqing Liu: Performed real time PCR analysis on iPSC
Juan Fernandez de Castro: Performed ERG measurements
Toshihiko Ezashi: Provision of study material, data analysis and interpretation, manuscript writing, final approval of manuscript
Bhanu Prakash V.L. Telugu: Provision of study material, data analysis and interpretation, manuscript writing, final approval of manuscript
R. Michael Roberts: Provision of study material, data analysis and interpretation, manuscript writing, final approval of manuscript
Henry Kaplan: Conception and design, data analysis and interpretation, manuscript writing, final approval of manuscript
Douglas Dean: Conception and design, data analysis and interpretation, manuscript writing, final approval of manuscript
Disclosure of potential conflicts of interest
The authors indicate no potential conflicts of interest.
References
- 1.Sung CH, Chuang JZ. The cell biology of vision. J Cell Biol. 2010;190:953–963. doi: 10.1083/jcb.201006020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Karl MO, Reh TA. Regenerative medicine for retinal diseases: activating endogenous repair mechanisms. Trends Mol Med. 2010;16:193–202. doi: 10.1016/j.molmed.2010.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lamba DA, Karl MO, Reh TA. Strategies for retinal repair: cell replacement and regeneration. Prog Brain Res. 2009;175:23–31. doi: 10.1016/S0079-6123(09)17502-7. [DOI] [PubMed] [Google Scholar]
- 4.Jin ZB, Okamoto S, Mandai M, et al. Induced pluripotent stem cells for retinal degenerative diseases: a new perspective on the challenges. J Genet. 2009;88:417–423. doi: 10.1007/s12041-009-0063-5. [DOI] [PubMed] [Google Scholar]
- 5.Bull ND, Martin KR. Using stem cells to mend the retina in ocular disease. Regen Med. 2009;4:855–864. doi: 10.2217/rme.09.59. [DOI] [PubMed] [Google Scholar]
- 6.Osakada F, Ikeda H, Mandai M, et al. Toward the generation of rod and cone photoreceptors from mouse, monkey and human embryonic stem cells. Nat Biotechnol. 2008;26:215–224. doi: 10.1038/nbt1384. [DOI] [PubMed] [Google Scholar]
- 7.Lamba DA, Karl MO, Ware CB, et al. Efficient generation of retinal progenitor cells from human embryonic stem cells. Proc Natl Acad Sci U S A. 2006;103:12769–12774. doi: 10.1073/pnas.0601990103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hirami Y, Osakada F, Takahashi K, et al. Generation of retinal cells from mouse and human induced pluripotent stem cells. Neurosci Lett. 2009;458:126–131. doi: 10.1016/j.neulet.2009.04.035. [DOI] [PubMed] [Google Scholar]
- 9.Lamba DA, McUsic A, Hirata RK, et al. Generation, purification and transplantation of photoreceptors derived from human induced pluripotent stem cells. PLoS One. 2010;5:e8763. doi: 10.1371/journal.pone.0008763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lamba DA, Gust J, Reh TA. Transplantation of human embryonic stem cell-derived photoreceptors restores some visual function in Crx-deficient mice. Cell Stem Cell. 2009;4:73–78. doi: 10.1016/j.stem.2008.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Meyer JS, Shearer RL, Capowski EE, et al. Modeling early retinal development with human embryonic and induced pluripotent stem cells. Proc Natl Acad Sci U S A. 2009;106:16698–16703. doi: 10.1073/pnas.0905245106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jaenisch R, Young R. Stem cells, the molecular circuitry of pluripotency and nuclear reprogramming. Cell. 2008;132:567–582. doi: 10.1016/j.cell.2008.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Osakada F, Ikeda H, Sasai Y, et al. Stepwise differentiation of pluripotent stem cells into retinal cells. Nature Protocols. 2009;4:811–824. doi: 10.1038/nprot.2009.51. [DOI] [PubMed] [Google Scholar]
- 14.MacLaren RE, Pearson RA, MacNeil A, et al. Retinal repair by transplantation of photoreceptor precursors. Nature. 2006;444:203–207. doi: 10.1038/nature05161. [DOI] [PubMed] [Google Scholar]
- 15.Hendrickson A, Hichs D. Distribution and density of medium- and short-wavelength selective cones in the domestic pig retina. Exp Eye Res. 2002;74:435–444. doi: 10.1006/exer.2002.1181. [DOI] [PubMed] [Google Scholar]
- 16.Roberts RM, Telugu BP, Ezashi T. Induced pluripotent stem cell from swine (sus scrofa): why they may prove to be important. Cell Cycle. 2009;8:3078–3018. doi: 10.4161/cc.8.19.9589. [DOI] [PubMed] [Google Scholar]
- 17.Telugu BP, Ezashi T, Roberts RM. The promise of stem cell research in pigs and other undulate species. Stem Cell Rev. 2010;6:31–41. doi: 10.1007/s12015-009-9101-1. [DOI] [PubMed] [Google Scholar]
- 18.Klassen H, Kiilgaard JF, Zahir T, et al. Progenitor cells from the porcine neural retina express photoreceptor markers after transplantation to the subretinal space of allorecipients. Stem Cells. 2007;25:1222–1230. doi: 10.1634/stemcells.2006-0541. [DOI] [PubMed] [Google Scholar]
- 19.Klassen H, Warfvinge K, Schwartz PH, et al. Isolation of progenitor cells from GFP-transgenic pigs and transplantation to the retina of allorecipients. Cloning Stem Cells. 2008;10:391–402. doi: 10.1089/clo.2008.0010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ezashi T, Telugu BP, Alexenko AP, et al. Derivation of induced pluripotent stem cells from pig somatic cells. Proc Natl Acad Sci U S A. 2009;106:10993–10998. doi: 10.1073/pnas.0905284106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhao Y, Yin X, Qin H, et al. Two supporting factors greatly improve the efficiency of human iPSC generation. Cell Stem Cell. 2008;3:475–479. doi: 10.1016/j.stem.2008.10.002. [DOI] [PubMed] [Google Scholar]
- 22.Dunn FA, Doan T, Sampath AP, et al. Controlling the gain of rod-mediated signals in the mammalian retina. J Neurosci. 2006;26:959–970. doi: 10.1523/JNEUROSCI.5148-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ghosh F, Arnér K. Cell Type Differentiation Dynamics in the Developing Porcine Retina. Dev Neurosci. 2010;32:47–58. doi: 10.1159/000261704. [DOI] [PubMed] [Google Scholar]
- 24.Zayas-Santiago A, Kang A, Derwent JJ. Preservation of intact adult rat photoreceptors in vitro: study of dissociation techniques and the effect of light. Mol Vis. 2009;15:1–9. [PMC free article] [PubMed] [Google Scholar]
- 25.Orzalesi N, Calabria GA, Grignolo A. Experimental degeneration of the rabbit retina induced by iodoacetic acid. A study of the ultrastructure, the rhodopsin cycle and the uptake of 14C-labeled iodoacetic acid. Exp Eye Res. 1970;9:246–253. doi: 10.1016/s0014-4835(70)80081-1. [DOI] [PubMed] [Google Scholar]
- 26.Winkler BS, Sauer MW, Starnes CA. Modulation of the Pasteur effect in retinal cells: implications for understanding compensatory metabolic mechanisms. Exp Eye Res. 2003;76:715–723. doi: 10.1016/s0014-4835(03)00052-6. [DOI] [PubMed] [Google Scholar]
- 27.Liang L, Katagiri Y, Franco LM, et al. Long-term cellular and regional specificity of the photoreceptor toxin, iodoacetic acid (IAA), in the rabbit retina. Vis Neurosci. 2008;25:167–177. doi: 10.1017/S0952523808080401. [DOI] [PubMed] [Google Scholar]
- 28.Swain PK, Hicks D, Mears AJ, et al. Multiple phosphorylated forms of NRL are expressed in rod photoreceptors. J Biol Chem. 2001;276:6152–6160. doi: 10.1074/jbc.M105855200. [DOI] [PubMed] [Google Scholar]
- 29.Liu Y, Clem B, Zuba-Surma EK, et al. Mouse Fibroblasts Lacking RB1 Function Form Spheres and Undergo Reprogramming to a Cancer Stem Cell Phenotype. Cell Stem Cell. 2009;4:336–347. doi: 10.1016/j.stem.2009.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Del Priore LV, Tezel TH, Kaplan HJ. Survival of allogenic procine retinal pigment epithelial sheets after subretinal transplantation. Invest Ophthalmol Vis Sci. 2004;45:985–992. doi: 10.1167/iovs.03-0662. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.