Abstract
Background
Antimitotic agents are essential components for curative therapy of pediatric acute leukemias and many solid tumors. Eribulin is a novel agent that differs from both Vinca alkaloids and taxanes in its mode of binding to tubulin polymers.
Procedures
Eribulin was tested against the PPTP in vitro cell line panel at concentrations from 0.1 nM to 1.0 μM and against the PPTP in vivo xenograft panels at a dose of 1 mg/kg (solid tumors) or 1.5 mg/kg (ALL models) using a q4dx3 schedule repeated at Day 21.
Results
In vitro eribulin demonstrated cytotoxic activity, with a median relative IC50 value of 0.27 nM, (range <0.1–14.8 nM). Eribulin was well tolerated in vivo, and all 43 xenograft models were considered evaluable for efficacy. Eribulin induced significant differences in event-free survival (EFS) distribution compared to control in 29 of 35 (83%) of the solid tumors and in 8 of 8 (100%) of the ALL xenografts. Objective responses were observed in 18 of 35 (51%) solid tumor xenografts. Complete responses (CR) or maintained CR were observed in panels of Wilms tumor, Ewing sarcoma, rhabdomyosarcoma, glioblastoma, and osteosarcoma xenografts. All eight ALL xenografts achieved CR or MCR.
Conclusions
The high level of activity observed for eribulin against the PPTP preclinical models makes this an interesting agent to consider for pediatric evaluation. The activity pattern observed for eribulin in the solid tumor panels is equal or superior to that observed previously for vincristine.
Keywords: developmental therapeutics, PI3K inhibitor, preclinical testing
INTRODUCTION
Drugs that inhibit microtubules include the vinca alkaloids (e.g., vincristine), the taxanes (e.g., paclitaxel), and the epothilones (e.g., ixabepilone). The taxanes and epothilones stabilize microtubules reducing the dynamics necessary for cell movement and division. Vinca alkaloids inhibit microtubule growth through depolymerization. Halichondrin B analogs are a relatively new class of microtubule targeted agents with a novel mechanism of action. Eribulin is a fully synthetic macrocyclic ketone analogue of halichondrin B, a natural product derived from the marine sponge Halichondria okadai [1,2]. Halichondrin B and eribulin are capable of inducing irreversible mitotic blockade and apoptosis by inhibiting microtubule dynamic instability [3]. Dynamic instability applies to the growth and shortening of microtubules required for mitosis. Eribulin inhibits microtubule growth by binding with high affinity at the plus ends [4]. The mechanism of inhibition of microtubule dynamic instability by eribulin is distinctive from that of other tubulin-binding antimitotic agents in that eribulin suppresses the growth parameters at microtubule plus ends without affecting microtubule shortening parameters [4,5]. With a novel mechanism of inhibition of microtubule dynamic instability coupled with prolonged cellular retention [6], eribulin has promise as an antineoplastic for multiple cancer histologies [7].
In 2010, the Food and Drug Administration approved eribulin for patients with metastatic breast cancer previously treated with at least two chemotherapeutic regimens for advanced disease and where patients had been treated with both an anthracycline and a taxane at some point in their treatment history. Initial Phase 1 trials of eribulin evaluated several different dosing schedules: once every 21 days [8,9], Days 1 and 8 of 21-day cycles [10], and Days 1, 8, and 15 of 28-day cycles [11,12]. In patients with previously treated metastatic breast cancer, the 21-day cycle was tolerable while the 28-day cycle was associated with a higher incidence of neutropenia necessitating dose delays and reductions [13]. The current recommended dosing for eribulin mesylate is 1.4 mg/m2 as an intravenous bolus on Days 1 and 8 of a 21-day cycle [14,15]. The most common dose limiting toxicities reported to date include febrile neutropenia, fatigue, anorexia, and peripheral neuropathy [11,14,15]. With a serum half-life of 37.8 hours and prolonged cellular retention [11], the pharmacokinetic and pharmacodynamic profile of eribulin is favorable in comparison to other microtubule inhibitors. Eribulin has shown evidence of activity in several adult cancers in addition to breast cancer [15], including soft tissue sarcomas based on favorable progression-free survival at 12 weeks for patients with leiomyosarcoma and adipocytic sarcoma [16].
We report the results of eribulin evaluated in vitro and in vivo by the NCI-funded Pediatric Preclinical Testing Program (PPTP). Given that microtubule inhibitors are commonly used in pediatric tumors and that eribulin has a novel mechanism of inhibiting tubulin action with a tolerable toxicity profile in adults, it was reasonable to consider testing eribulin against childhood cancer preclinical models. Our results show that eribulin compares favorably to vincristine in several histologies and demonstrates activity in xenograft models of osteosarcoma, a histology for which microtubule inhibitors are not commonly used.
MATERIALS AND METHODS
In Vitro Testing
In vitro testing was performed using DIMSCAN [20], as previously described in a characterized panel of 24 cell lines [17]. Cells were incubated in the presence of eribulin for 96 hours at concentrations from 0.1 nM to 1 μM and analyzed as previously described [18].
In Vivo Tumor Growth Inhibition Studies
CB17SC scid−/− female mice (Taconic Farms, Germantown, NY), were used to propagate subcutaneously implanted kidney/rhabdoid tumors, sarcomas (Ewing sarcoma, osteosarcoma, rhabdomyosarcoma), neuroblastoma, and non-glioblastoma brain tumors, while BALB/c nu/nu mice were used for glioma models, as previously described [19]. Human leukemia cells were propagated by intravenous inoculation in female non-obese diabetic (NOD)/scid−/− mice as described previously [20]. Female mice were used irrespective of the patient gender from which the original tumor was derived. All mice were maintained under barrier conditions and experiments were conducted using protocols and conditions approved by the institutional animal care and use committee of the appropriate consortium member. Eight to 10 mice were used in each control or treatment group. Tumor volumes (cm3) [solid tumor xenografts] or percentages of human CD45-positive [%hCD45+] cells [ALL xenografts] were determined and responses were determined using three activity measures as previously described [19]. An in-depth description of the analysis methods is included in the Supplemental Response Definitions section.
Statistical Methods
The exact log-rank test, as implemented using Proc StatXact for SAS, was used to compare event-free survival (EFS) distributions between treatment and control groups. P-values were two-sided and were not adjusted for multiple comparisons given the exploratory nature of the studies.
Drugs and Formulation
Eribulin was provided to the Pediatric Preclinical Testing Program by the Esai Company, through the Cancer Therapy Evaluation Program (NCI). Eribulin was formulated in ethanol: water(5:95) and diluted with sterile saline and stored for up to 7 days at 4°C, protected from light. Eribulin was administered intraperitoneally (IP) at 1 mg/kg (solid tumors) or 1.5 mg/kg (ALL models) to mice using a q 4 days × 3 schedule repeated at Day 21. Eribulin was provided to each consortium investigator in coded vials for blinded testing.
RESULTS
In Vitro Testing
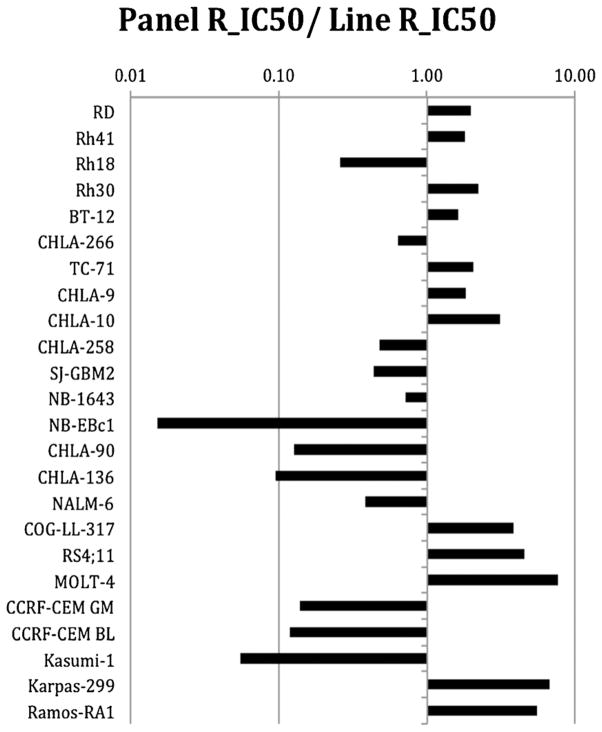
Eribulin demonstrated potent cytotoxic activity, with T/C% (treated/control %) values approaching 0% for many of the cell lines at the highest concentration tested, and with a median Relative In/Out% (Relative I/O%) value of −89% (Table I). Relative I/O% values represent the percentage difference between the Ymin value and the estimated starting cell number and either the control cell number (for agents with Ymin > starting cell number) or 0 (for agents with Ymin < estimated starting cell number). Relative I/O% values range between 100% (no treatment effect) and −100% (complete cytotoxic effect), with a Relative I/O% value of 0 being observed for a completely effective cytostatic agent. The cell line panels showed distinctive median Relative I/O% values, with the four rhabdomyosarcoma cell lines having a median value of only −38% (P = 0.027 for comparison to non-rhabdomyosarcoma cell lines) and with the ALL cell lines have a median value of −99% (P = 0.005 for comparison to non-ALL cell lines). The median relative IC50 (rIC50) value for the PPTP cell lines was 0.27 nM, with a range from <0.1 to 14.8 nM. The rIC50 for the neuroblastoma cell lines was higher than that of the other PPTP cell lines (2.07 nM vs. 0.14 nM, respectively, P = 0.02). The most sensitive cell lines, each with rIC50 values < 0.1 nM, were all of lymphoid origin (three ALL, one ALCL, and one NHL; Fig. 1).
TABLE I.
In Vitro Activity of Eribulin Against PPTP Cell Lines
| Cell line | Histotype | rIC50 (nM) | Panel rIC50/Line rIC50 | Ymin % (observed) | Relative in/out% |
|---|---|---|---|---|---|
| RD | Rhabdomyosarcoma | 0.14 | 1.92 | 4.49 | −18 |
| Rh41 | Rhabdomyosarcoma | 0.14 | 2.00 | 6.63 | −70 |
| Rh18 | Rhabdomyosarcoma | 0.83 | 0.33 | 20.92 | −53 |
| Rh30 | Rhabdomyosarcoma | 0.11 | 2.47 | 12.75 | −23 |
| BT-12 | Rhabdoid | 0.15 | 1.83 | 2.12 | −74 |
| CHLA-266 | Rhabdoid | 0.38 | 0.71 | 5.54 | −79 |
| TC-71 | Ewing sarcoma | 0.11 | 2.45 | 0.01 | −99 |
| CHLA-9 | Ewing sarcoma | 0.16 | 1.68 | 1.82 | −49 |
| CHLA-10 | Ewing sarcoma | 0.11 | 2.58 | 4.54 | −28 |
| CHLA-258 | Ewing sarcoma | 0.50 | 0.54 | 6.29 | −84 |
| SJ-GBM2 | Glioblastoma | 0.51 | 0.53 | 0.46 | −95 |
| NB-1643 | Neuroblastoma | 0.47 | 0.57 | 0.53 | −97 |
| NB-EBc1 | Neuroblastoma | 14.80 | 0.02 | 1.76 | −92 |
| CHLA-90 | Neuroblastoma | 1.77 | 0.15 | 9.26 | −67 |
| CHLA-136 | Neuroblastoma | 2.37 | 0.11 | 9.96 | −65 |
| NALM-6 | ALL | 0.59 | 0.46 | 0.03 | −99 |
| COG-LL-317 | ALL | <0.10 | >2.74 | 0.03 | −99 |
| RS4;11 | ALL | <0.10 | >2.74 | 0.22 | −99 |
| MOLT-4 | ALL | <0.10 | >2.74 | 0.05 | −99 |
| CCRF-CEM (1) | ALL | 1.62 | 0.17 | 0.01 | −100 |
| CCRF-CEM (2) | ALL | 1.88 | 0.14 | 0.02 | −100 |
| Kasumi-1 | AML | 4.11 | 0.07 | 3.92 | −86 |
| Karpas-299 | ALCL | <0.10 | >2.74 | 0.03 | −100 |
| Ramos-RA1 | NHL | <0.10 | >2.74 | 0.00 | −100 |
| Median | 0.27 | 1.20 | 1.79 | −89 | |
| Minimum | 0.01 | 0.02 | 0.00 | −100 | |
| Maximum | 14.80 | >2.74 | 20.92 | −18 |
Fig. 1.
Eribulin in vitro activity: The median rIC50 ratio graph shows the relative rIC50 values for the cell lines of the PPTP panel. Each bar represents the ratio of the panel rIC50 to the rIC50 value of the indicated cell line. Bars to the right represent cell lines with higher sensitivity, while bars to the left indicate cell lines with lesser sensitivity.
In Vivo Testing
Eribulin was tested against the PPTP solid tumor xenografts using a dose of 1 mg/kg administered by the IP route Q4Dx3 with the treatment repeated at Day 21. For the ALL panel (using NOD-SCID mice), the maximum tolerated dose was 1.5 mg/kg, and this dose was used for efficacy testing on the same schedule as used for the solid tumor xenografts. The total planned treatment and observation period was 6 weeks. Eribulin was generally well tolerated, with only a 1.5% toxicity rate in the treated groups (6 of 409), not much higher than that observed for control animals (1 of 401). All 43 tested xenograft models were considered evaluable for efficacy. A complete summary of results is provided in Supplementary Table I, including total numbers of mice, number of mice that died (or were otherwise excluded), numbers of mice with events and average times to event, tumor growth delay, as well as numbers of responses and T/C values.
Eribulin induced significant differences in EFS distribution compared to control in 29 of 35 (83%) of the evaluable solid tumor xenografts and in 8 of 8 (100%) of the evaluable ALL xenografts (Table II). For those xenografts with a significant difference in EFS distribution between treated and control groups, the EFS T/C activity measure additionally requires an EFS T/C value of >2.0 for intermediate activity and indicates a substantial agent effect in slowing tumor growth. High activity further requires a reduction in final tumor volume compared to the starting tumor volume. Twenty-four of 30 (80%) solid tumor models evaluable for the EFS T/C activity metric demonstrated EFS T/C >2.0, with seven lines showing intermediate activity and 17 showing high activity. High activity was observed across most histotypes, including three of four evaluable Ewing sarcoma xenografts, six of seven rhabdomyosarcoma xenografts, two of four glioblastoma xenografts, and three of five evaluable osteosarcoma xenografts. For the ALL panel, six of eight (75%) xenografts met criteria for high EFS T/C activity and two met criteria for intermediate activity.
TABLE II.
Summary of In Vivo Activity of Eribulin
| Line | Tumor type | Time to event | P-value | EFS T/C | Median final RTV (CD45%) | Tumor volume T/Ca | T/C volume activity | EFS activityb | Response activityc |
|---|---|---|---|---|---|---|---|---|---|
| BT-29 | Rhabdoid | >EP | 0.012 | >1.6 | >4 | 0.65 | Low | NE | Int |
| KT-16 | Rhabdoid | 12.1 | 0.865 | 1.1 | >4 | 0.76 | Low | Low | Low |
| KT-10 | Wilms | >EP | <0.001 | >3.7 | 0.0 | 0.01 | High | High | High |
| KT-13 | Wilms | >EP | <0.001 | >3.3 | 1.1 | 0.15 | High | Int | High |
| SK-NEP-1 | Ewing | >EP | <0.001 | >4.3 | 0.0 | 0.00 | High | High | High |
| EW5 | Ewing | 41.3 | <0.001 | 3.3 | >4 | 0.13 | High | Int | Int |
| EW8 | Ewing | >EP | <0.001 | >3.1 | 0.0 | 0.00 | High | High | High |
| TC-71 | Ewing | >EP | <0.001 | >1.5 | 2.3 | 0.19 | Int | NE | High |
| CHLA258 | Ewing | >EP | <0.001 | >4.2 | 0.0 | 0.01 | High | High | High |
| Rh10 | ALV Rhabdomyosarcoma | >EP | <0.001 | >3.8 | 0.0 | 0.08 | High | High | High |
| Rh28 | ALV Rhabdomyosarcoma | >EP | <0.001 | >2.4 | 0.0 | 0.29 | Int | High | High |
| Rh30 | ALV Rhabdomyosarcoma | >EP | <0.001 | >3.8 | 0.0 | 0.06 | High | High | High |
| Rh30R | ALV Rhabdomyosarcoma | >EP | <0.001 | >4.3 | 0.0 | 0.11 | High | High | High |
| Rh41 | ALV Rhabdomyosarcoma | >EP | <0.001 | >3.0 | 0.0 | 0.07 | High | High | High |
| Rh18 | EMB Rhabdomyosarcoma | >EP | 0.001 | >3.2 | 1.8 | 0.76 | Low | Int | Int |
| Rh36 | EMB Rhabdomyosarcoma | >EP | <0.001 | >6.3 | 0.0 | 0.10 | High | High | High |
| BT-28 | Medulloblastoma | >EP | <0.001 | >3.3 | 0.7 | 0.44 | Int | High | Int |
| BT-50 | Medulloblastoma | >EP | 0.444 | >1.1 | >4 | 0.78 | Low | NE | Low |
| BT-41 | Ependymoma | >EP | 1.000 | — | 1.3 | 0.42 | Int | NE | Int |
| GBM2 | Glioblastoma | >EP | 0.012 | >3.5 | 0.6 | 1.00 | Low | High | High |
| BT-39 | Glioblastoma | >EP | 0.045 | >3.6 | 0.5 | 1.00 | Low | High | Int |
| D645 | Glioblastoma | >EP | <0.001 | >7.1 | 3.8 | 0.07 | High | Int | High |
| D456 | Glioblastoma | 19.8 | <0.001 | 3.4 | >4 | 1.00 | Low | Int | Int |
| NB-SD | Neuroblastoma | 17.5 | <0.001 | 2.4 | >4 | 0.53 | Low | Int | Int |
| NB-1771 | Neuroblastoma | 14.8 | <0.001 | 2.0 | >4 | 0.56 | Low | Low | Int |
| NB-1691 | Neuroblastoma | 7.9 | 0.953 | 1.1 | >4 | 0.80 | Low | Low | Low |
| NB-EBc1 | Neuroblastoma | 4.5 | 0.207 | 1.1 | >4 | 0.82 | Low | Low | Low |
| CHLA-79 | Neuroblastoma | 13.0 | 0.360 | 1.9 | >4 | 0.71 | Low | Low | Int |
| NB-1643 | Neuroblastoma | >EP | <0.001 | >4.7 | 0.6 | 0.01 | High | High | High |
| OS-1 | Osteosarcoma | >EP | <0.001 | >1.5 | 0.4 | 0.64 | Low | NE | High |
| OS-2 | Osteosarcoma | >EP | <0.001 | >2.5 | 0.8 | 0.21 | Int | High | Int |
| OS-17 | Osteosarcoma | >EP | <0.001 | >2.3 | 0.5 | 0.25 | Int | High | High |
| OS-9 | Osteosarcoma | 26.3 | <0.001 | 1.4 | >4 | 0.61 | Low | Low | Low |
| OS-33 | Osteosarcoma | >EP | <0.001 | >2.7 | 0.2 | 0.24 | Int | High | High |
| OS-31 | Osteosarcoma | >EP | <0.001 | >2.6 | 1.8 | 0.27 | Int | Int | Int |
| ALL-2 | ALL B-precursor | >EP | <0.001 | >2.8 | 0.0 | — | High | High | |
| ALL-4 | ALL B-precursor | >EP | <0.001 | >4.3 | 0.0 | — | High | High | |
| ALL-7 | ALL B-precursor | >EP | 0.009 | >4.4 | 3.0 | — | Int | High | |
| ALL-8 | ALL T-cell | >EP | <0.001 | >7.9 | 0.0 | — | High | High | |
| ALL-17 | ALL B-precursor | >EP | 0.001 | >5.3 | 0.0 | — | High | High | |
| ALL-19 | ALL B-precursor | >EP | <0.001 | >8.4 | 0.0 | — | High | High | |
| ALL-31 | Tertiary, T-cell ALL | >EP | <0.001 | >3.7 | 12.9 | — | Int | High | |
| MLL-7 | Tertiary, Infant, Precursor B-ALL | >EP | <0.001 | >4.7 | 0.0 | — | High | High |
Tumor Volume T/C value Relative tumor volumes (RTV) for control (C) and treatment (T) mice were calculated at Day 21 or when all mice in the control and treated groups still had measurable tumor volumes (if <21 days). The T/C value is the mean RTV for the treatment group divided by the mean RTV for the control group. High activity = T/C ≤0.15; Intermediate activity = T/C ≤0.45 but >0.15; and Low activity = T/C >0.45;
Objective response measures are described in detail in the Supplemental Response Definitions Section. PD1 = progressive disease with EFS T/C ≤1.5, and PD2 = progressive disease with EFS T/C >1.5;
EFS T/C values = the ratio of the median time to event of the treatment group and the median time to event of the respective control group. High activity requires: (a) an EFS T/C >2; (b) a significant difference in EFS distributions, and (c) a net reduction in median tumor volume for animals in the treated group at the end of treatment as compared to at treatment initiation. Intermediate activity = criteria (a) and (b) above, but not having a net reduction in median tumor volume for treated animals at the end of the study. Low activity = EFS T/C <2. P values in bold are significantly different from control time to event.
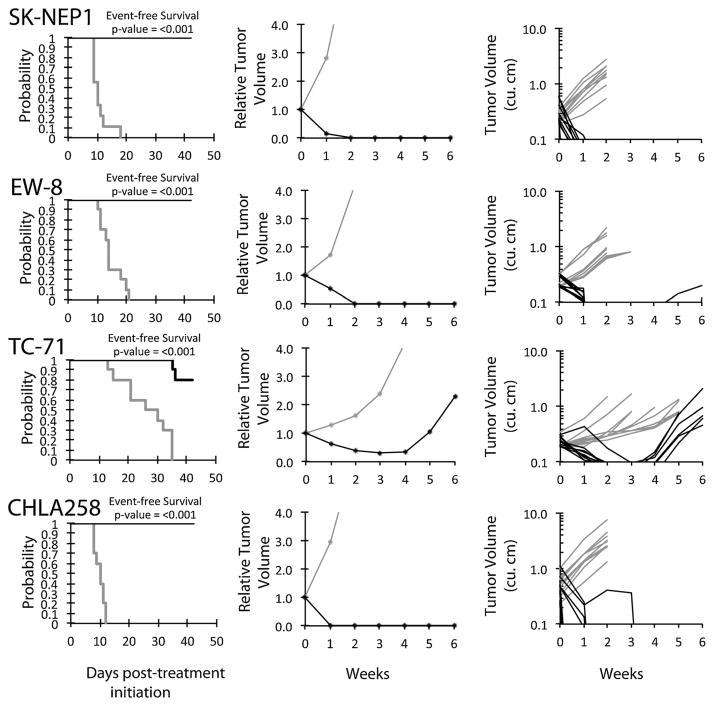
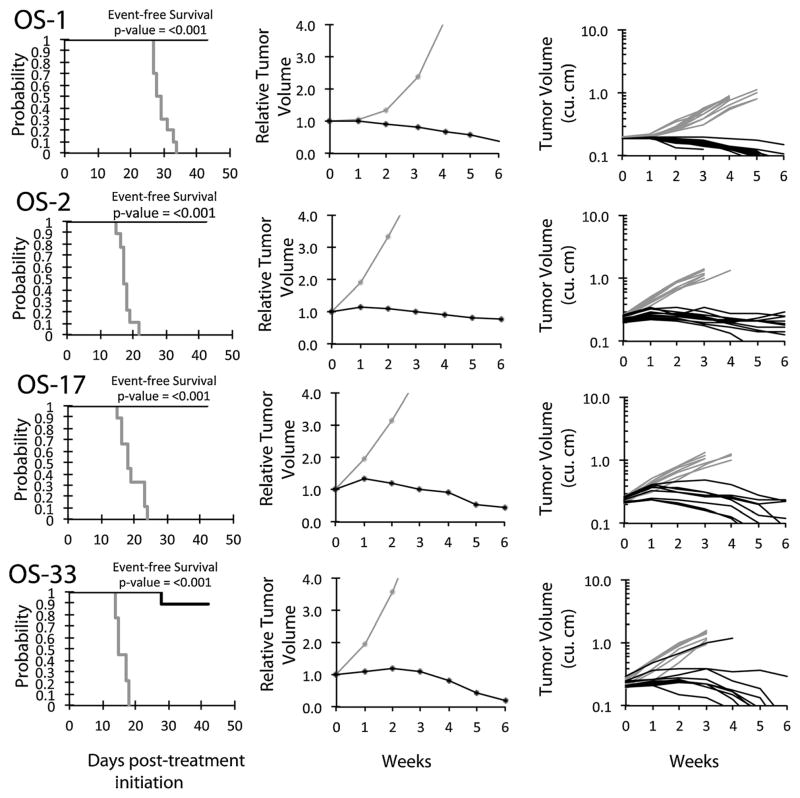
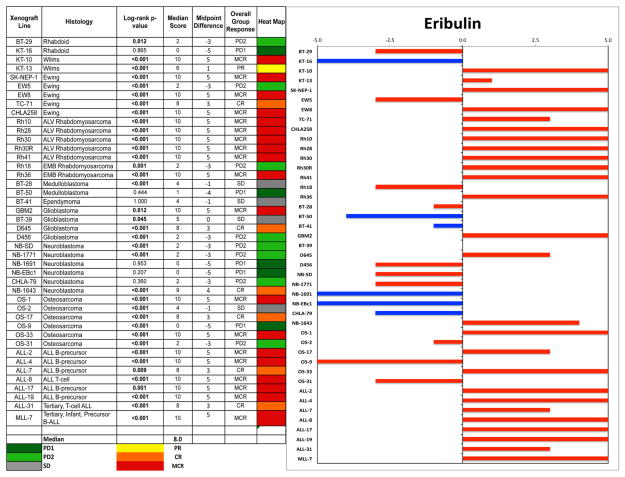
An objective response was observed in 18 of 35 (51%) solid tumor xenografts. Complete responses (CR) or maintained CR (MCR) were observed in one of two Wilms tumor, four of five Ewing sarcoma (Fig. 2), six of seven rhabdomyosarcoma, two of four glioblastoma, and three of six osteosarcoma xenografts, Figure 3. For the ALL panel, all eight xenografts achieved CR or MCR. The in vivo testing results for the objective response measure of activity are presented in Figure 4 in a “heat-map” format as well as a “COMPARE”-like format, based on the scoring criteria described in the Supplemental Response Definitions section. The latter analysis demonstrates relative tumor sensitivities around the midpoint score of 5 (stable disease).
Fig. 2.
Eribulin in vivo objective response activity for Ewing sarcoma models. Ewing sarcomas (SK-NEP1, EW-8, TC-71, and CHLA258): Kaplan–Meier curves for EFS (left), median relative tumor volume graphs (center), and individual tumor volume graphs (right) are shown for selected lines. Controls (gray lines); treated (black lines), statistical significance (P-values) of the difference between treated and control groups are included.
Fig. 3.
Eribulin in vivo objective response activity for osteosarcoma models. Osteosarcomas (OS-1, OS-2, OS-17, and OS-33): Kaplan–Meier curves for EFS (left), median relative tumor volume graphs (center), and individual tumor volume graphs (right) are shown for selected lines. Controls (gray lines); treated (black lines), statistical significance (P-values) of the difference between treated and control groups are included.
Fig. 4.
Left: The colored heat map depicts group response scores. A high level of activity is indicated by a score of 6 or more, intermediate activity by a score of ≥2 but <6, and low activity by a score of <2. Right: representation of tumor sensitivity based on the difference of individual tumor lines from the midpoint response (stable disease). Bars to the right of the median represent lines that are more sensitive, and to the left are tumor models that are less sensitive. Red bars indicate lines with a significant difference in EFS distribution between treatment and control groups, while blue bars indicate lines for which the EFS distributions were not significantly different.
Eribulin Pharmacokinetics
Eribulin drug levels at the 1 mg/kg dose used for solid tumor efficacy testing were evaluated so that the eribulin systemic exposure in mice could be compared to that of eribulin in humans at clinically tolerated doses. The area under the plasma drug concentration–time curve (AUC) for eribulin at the 1 mg/kg dose is 658 ng hour/ml (summarized in Supplementary Tables II and III and Supplementary Fig. 1). Previous murine pharmacokinetic results using a different mouse strain noted an AUC of 1,132 ng hour/ml for an intravenous dose of 2 mg/kg, which for a 1 mg/kg dose would produce an AUC of approximately 566 ng hour/ml [21]. Hence, the PPTP results in SCID mice are similar to those previously reported for eriubulin. The systemic exposure in humans at the 1.4 mg/m2 dose is approximately 790 ng hour/ml [11,22–25]. The number of doses of eribulin administered per treatment course for preclinical testing is three (i.e., Q4Dx3 repeated at Day 21), while in the clinic two doses are administered per course (Day 1 and 8 of each 21-day course). The AUC per course of treatment in the preclinical setting (1,974 ng hour/ml) is approximately 25% higher than the AUC per course of treatment in the clinical setting (1,580 ng hr/ml). Thus, the 1.0 mg/kg/dose used by the PPTP provides systemic exposures for eribulin that are reasonably comparable to those achieved in humans treated at the recommended Phase 2 dose of 1.4 mg/m2 (summarized in Supplementary Table IV).
DISCUSSION
Eribulin demonstrated a remarkably high level of activity against the PPTP solid tumor and ALL in vivo models. The obvious comparison for the eribulin in vivo results are to those previously obtained for vincristine. Supplementary Table V shows this comparison while Supplementary Figure 2 provides a comparison of the objective response heat map for eribulin and vincristine. There are a number of similarities between the activity pattern for the two agents, with both showing activity for Wilms tumor, rhabdomyosarcoma, glioblastoma, and osteosarcoma. The most noticeable difference between the two agents is the high level of activity observed for eribulin for the Ewing sarcoma xenografts (4 of 5 with CR or MCR) compared to vincristine (0 of 5 with CR or MCR). Additionally, activity for eribulin for rhabdomyosarcoma appears to be greater than that observed for vincristine (6 of 7 with MCR for eribulin vs. 2 MCR/1 PR out of 7 for vincristine).
Identifying potential roles for eribulin for childhood cancers must be approached with a clear appreciation for how vincristine is currently utilized. Vincristine is an important component of standard therapy for Wilms tumor and rhabdomyosarcoma and for each of these diagnoses, patients with favorable prognosis receive only vincristine and actinomycin-D. Data from the 1960s and 1970s support single agent activity for each agent against these diagnoses [26–28]. However, there are limited single agent response data for vincristine for other solid tumors, with most of the data coming from reports from the 1960s. For example, for Ewing sarcoma, two patients with this tumor had objective regressions to vincristine [29,30], and another report described one of seven patients with a partial response [31].
Current standard therapy in North America for newly diagnosed children with rhabdomyosarcoma is vincristine, actinomycin-D, and cyclophosphamide (VAC), with 30 doses of vincristine prescribed over an approximately 10-month treatment period for children with “intermediate risk” rhabdomyosarcoma. Therapy for favorable histology Wilms tumor is similar in its use of vincristine, with 15 doses given during a 25-week treatment course for standard risk patients. Vincristine is also used in upfront regimens for patients with Ewing sarcoma, with the current COG standard therapy for newly diagnosed non-metastatic Ewing sarcoma prescribing 18 doses of vincristine over an approximately 9-month treatment course. Current treatment regimens for children with neuroblastoma use little or no vincristine, an approach that is supported by the PPTP results for neuroblastoma xenografts and by the limited single agent clinical activity observed for vincristine against this disease [27]. Vincristine is not utilized in current protocols for children with intermediate risk neuroblastoma, and for high risk neuroblastoma vincristine is used in only two of six courses of induction therapy and is not employed as part of the stem cell transplant preparative regimen.
For osteosarcoma, vincristine is not used in standard upfront therapy regimens and in commonly employed retrieval regimens. Vincristine was highly active against one osteosarcoma xenograft, while eribulin showed high activity against three osteosarcoma xenografts. While vincristine has been used in the past to treat osteosarcoma [32], it is a drug not normally considered active in this disease. There are, however, only limited single agent data for vincristine for patients with osteosarcoma, most of which is from patients treated in the 1960s [27,31]. Vincristine was a component of a multi-agent regimen that was found to be no better than a two-drug regimen (cisplatin and doxorubicin) in a Phase 3 trial for children and adolescents with non-metastatic, operable osteosarcoma [33]. A small randomized study comparing high-dose methotrexate and vincristine administered every 3 weeks as adjuvant therapy for osteosarcoma provided no evidence for benefit for this combination [34]. A randomized trial of two methotrexate regimens, each administered as adjuvant therapy with doxorubicin and vincristine, showed approximately 40% 4-year DFS, suggesting that neither regimen was particularly effective compared to cisplatin-containing regimens [35]. Better results were obtained in single institution studies evaluating high-dose methotrexate, vincristine, and doxorubicin, with EFS rates in the 60% range. A randomized trial showing the benefit of adjuvant chemotherapy included vincristine, although the presumed primary active agents in the regimen were high-dose methotrexate and doxorubicin [36]. A two drug regimen of vincristine and cyclophosphamide evaluated as adjuvant therapy for osteosarcoma in a small clinical trial in the 1960s at St. Jude Children’s Research Hospital (Study OST 68) appeared to be ineffective [37].
Other tubulin targeted agents have been evaluated against childhood cancers. For example, vinorelbine, a semi-synthetic vinca-alkaloid with a modified catharanthine ring, has been studied in children with recurrent solid tumors. In a Phase 2 trial by the Children’s Oncology Group (COG), 4 of 11 patients with rhabdomyosarcoma showed objective responses, as did 1 of 2 patients with medulloblastoma and 1 of 4 patients with astrocytoma [38]. There were no responses among eight children with neuroblastoma and nine patients with non-rhabdomyosarcoma soft tissue sarcoma (NRSTS). Vinorelbine was also evaluated in an Italian pediatric solid tumor Phase 2 study in which 28 patients were assessable for response [39]. Objective responses were observed in 6 of 12 patients with rhabdomyosarcoma (five of six of the alveolar subtype), in one of five patients with osteosarcomas, and in one of seven patients with Ewing sarcomas.
ABT-751, which is an orally bioavailable sulfonamide that binds to the colchicine-binding site on β-tubulin to inhibit microtubule polymerization, has been recently studied in pediatric preclinical models as well as in children with solid tumors, and thus it provides a comparator to the activity observed for eribulin. ABT-751 induced regression in 4 of 25 preclinical models (16%) including models of neuroblastoma (2 of 4) that are refractory to vincristine and paclitaxel [40]. Among six rhabdomyosarcoma xenografts, one achieved CR (IRS-56), while each of four osteosarcoma xenografts showed PD1 responses and two Ewing sarcoma xenografts showed PD2 responses. Comparing the ABT-751 results to those obtained with eribulin, one of the two responding neuroblastoma xenografts (NB-1643) also achieved an objective response to eribulin, while the other (NB-EBc1) was not responsive to eribulin. Eribulin showed substantially greater activity than ABT-751 against osteosarcoma and rhabdomyosarcoma xenografts. ABT-751 clinical experience includes the absence of objective responses among 45 children with neuroblastoma treated with the agent in Phase 1 and pilot clinical trials, although the median EFS was longer for children with neuroblastoma compared to those with other solid tumors [41].
A common reason for over-prediction of clinical activity by preclinical in vivo models is that mice may tolerate higher drug levels of the test compound compared to humans. However, incorporating modeling of mouse and human pharmacokinetic data with xenograft efficacy data enhance the predictive capability of in vivo testing [42]. Both prior murine pharmacokinetic data for eribulin as well as the pharmacokinetic data presented herein suggest that the systemic exposure in mice per treatment course at the 1 mg/kg dose is similar to that observed in humans at the 1.4 mg/m2 recommended Phase 2 dose [11,21]. Further testing will include a dose–response evaluation against several responsive tumors which, when combined with pharmacokinetic results, will allow a more refined comparison of the systemic exposures associated with high level activity in the preclinical setting to those achievable in humans.
Combination testing is another potentially contributory approach. In developing combinations for clinical testing, it will be important to consider that eribulin, unlike vincristine, shows myelosuppression as its primary dose-limiting toxicity. For osteosarcoma, combinations with cisplatin and ifosfamide may be relevant in the clinical setting, as cisplatin is one of the most active agents in newly diagnosed patients and ifosfamide in relapsed patients. Combinations with a topoisomerase I inhibitor would have general applicability, given the activity of these agents for several childhood solid tumors and given evidence for supra-additive effects for topoisomerase I inhibitors with vincristine. The combination with irinotecan may be especially relevant as primary toxicities are non-overlapping (diarrhea for irinotecan and myelosuppression for eribulin).
In conclusion, eribulin showed potent in vitro activity against the PPTP cell lines and showed high level in vivo activity across a range of pediatric preclinical models. To some extent, the results are similar to those previously observed for vincristine, with activity noted for such vincristine-responsive tumors as rhabdomyosarcoma, Wilms tumor, and ALL. The in vivo activity for eribulin against Ewing sarcoma xenografts exceeded that previously observed for vincristine. These results support evaluation of eribulin in children with relapsed/refractory cancers, and if robust single agent activity is observed then proceeding with further development for the cancers identified as clinically responsive.
Supplementary Material
Acknowledgments
Grant sponsor: National Cancer Institute; Grant numbers: NO1-CM-42216; CA21765; CA108786
This work was supported by NO1-CM-42216, CA21765, and CA108786 from the National Cancer Institute and used eribulin supplied the Esai Company, Woodcliff Lake, NJ. In addition to the authors this article represents work contributed by the following: Sherry Ansher, Ingrid Boehm, Joshua Courtright, Kathryn Evans, Edward Favours, Henry S. Friedman, Danuta Gasinski, Nicholas Pettit, Melissa Sammons, Joe Zeidner, Jianrong Wu, Ellen Zhang, and Jian Zhang. Children’s Cancer Institute Australia for Medical Research is affiliated with the University of New South Wales and the Sydney Children’s Hospitals Network.
Footnotes
Conflict of interest: Nothing to declare.
Additional Supporting Information may be found in the online version of this article.
References
- 1.Bai RL, Paull KD, Herald CL, et al. Halichondrin B and homohalichondrin B, marine natural products binding in the vinca domain of tubulin. Discovery of tubulin-based mechanism of action by analysis of differential cytotoxicity data. J Biol Chem. 1991;266:15882–15889. [PubMed] [Google Scholar]
- 2.Towle MJ, Salvato KA, Budrow J, et al. In vitro and in vivo anticancer activities of synthetic macrocyclic ketone analogues of halichondrin B. Cancer Res. 2001;61:1013–1021. [PubMed] [Google Scholar]
- 3.Jordan MA, Wilson L. Microtubules as a target for anticancer drugs. Nat Rev Cancer. 2004;4:253–265. doi: 10.1038/nrc1317. [DOI] [PubMed] [Google Scholar]
- 4.Smith JA, Wilson L, Azarenko O, et al. Eribulin binds at microtubule ends to a single site on tubulin to suppress dynamic instability. Biochemistry. 2010;49:1331–1337. doi: 10.1021/bi901810u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jordan MA, Kamath K, Manna T, et al. The primary antimitotic mechanism of action of the synthetic halichondrin E7389 is suppression of microtubule growth. Mol Cancer Ther. 2005;4:1086–1095. doi: 10.1158/1535-7163.MCT-04-0345. [DOI] [PubMed] [Google Scholar]
- 6.Towle MJ, Salvato KA, Wels BF, et al. Eribulin induces irreversible mitotic blockade: Implications of cell-based pharmacodynamics for in vivo efficacy under intermittent dosing conditions. Cancer Res. 2011;71:496–505. doi: 10.1158/0008-5472.CAN-10-1874. [DOI] [PubMed] [Google Scholar]
- 7.Jain S, Vahdat LT. Eribulin mesylate. Clin Cancer Res. 2011;17:6615–6622. doi: 10.1158/1078-0432.CCR-11-1807. [DOI] [PubMed] [Google Scholar]
- 8.Rubin E, Rosen L, Rajeev V, et al. Phase I study of E7389 administed by 1 hour infusion every 21 days. J Clin Oncol. 2005;23:2054. [Google Scholar]
- 9.Tan AR, Rubin EH, Walton DC, et al. Phase I study of eribulin mesylate administered once every 21 days in patients with advanced solid tumors. Clin Cancer Res. 2009;15:4213–4219. doi: 10.1158/1078-0432.CCR-09-0360. [DOI] [PubMed] [Google Scholar]
- 10.Synold T, Tsao-Wei DD, Quinn DI, et al. Phase I and pharmacokinetic (PK) study of eribulin (E7389) in patients with renal dysfunction and advanced urothelial cancer: A California Cancer Consortium Trial. J Clin Oncol. 2010;28:2527. [Google Scholar]
- 11.Goel S, Mita AC, Mita M, et al. A phase I study of eribulin mesylate (E7389), a mechanistically novel inhibitor of microtubule dynamics, in patients with advanced solid malignancies. Clin Cancer Res. 2009;15:4207–4212. doi: 10.1158/1078-0432.CCR-08-2429. [DOI] [PubMed] [Google Scholar]
- 12.Synold T, Morgan RJ, Newman EW, et al. A Phase I pharmacokinetic and target validation study of the novel anti-tubulin agent E7389: A California Cancer Consortium trial. J Clin Oncol. 2005;23:3036. [Google Scholar]
- 13.Vahdat LT, Pruitt B, Fabian CJ, et al. Phase II study of eribulin mesylate, a halichondrin B analog, in patients with metastatic breast cancer previously treated with an anthracycline and a taxane. J Clin Oncol. 2009;27:2954–2961. doi: 10.1200/JCO.2008.17.7618. [DOI] [PubMed] [Google Scholar]
- 14.Cortes J, O’Shaughnessy J, Loesch D, et al. Eribulin monotherapy versus treatment of physician’s choice in patients with metastatic breast cancer (EMBRACE): A phase 3 open-label randomised study. Lancet. 2011;377:914–923. doi: 10.1016/S0140-6736(11)60070-6. [DOI] [PubMed] [Google Scholar]
- 15.Preston JN, Trivedi MV. Eribulin: A novel cytotoxic chemotherapy agent. Ann Pharmacother. 2012;46:802–811. doi: 10.1345/aph.1Q636. [DOI] [PubMed] [Google Scholar]
- 16.Schoffski P, Ray-Coquard IL, Cioffi A, et al. Activity of eribulin mesylate in patients with soft-tissue sarcoma: A phase 2 study in four independent histological subtypes. Lancet Oncol. 2011;12:1045–1052. doi: 10.1016/S1470-2045(11)70230-3. [DOI] [PubMed] [Google Scholar]
- 17.Frgala T, Kalous O, Proffitt RT, et al. A fluorescence microplate cytotoxicity assay with a 4-log dynamic range that identifies synergistic drug combinations. Mol Cancer Ther. 2007;6:886–897. doi: 10.1158/1535-7163.MCT-04-0331. [DOI] [PubMed] [Google Scholar]
- 18.Kang MH, Smith MA, Morton CL, et al. National Cancer Institute Pediatric Preclinical Testing Program: Model description for in vitro cytotoxicity testing. Pediatr Blood Cancer. 2011;56:239–249. doi: 10.1002/pbc.22801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Houghton PJ, Morton CL, Tucker C, et al. The pediatric preclinical testing program: Description of models and early testing results. Pediatr Blood Cancer. 2007;49:928–940. doi: 10.1002/pbc.21078. [DOI] [PubMed] [Google Scholar]
- 20.Liem NL, Papa RA, Milross CG, et al. Characterization of childhood acute lymphoblastic leukemia xenograft models for the preclinical evaluation of new therapies. Blood. 2004;103:3905–3914. doi: 10.1182/blood-2003-08-2911. [DOI] [PubMed] [Google Scholar]
- 21.Taur JS, Desjardins CS, Schuck EL, et al. Interactions between the chemotherapeutic agent eribulin mesylate (E7389) and P-glycoprotein in CF-1 abcb1a-deficient mice and Caco-2 cells. Xenobiotica. 2011;41:320–326. doi: 10.3109/00498254.2010.542256. [DOI] [PubMed] [Google Scholar]
- 22.Devriese LA, Mergui-Roelvink M, Wanders J, et al. Eribulin mesylate pharmacokinetics in patients with solid tumors receiving repeated oral ketoconazole. Invest New Drugs. 2012;31:381–389. doi: 10.1007/s10637-012-9829-3. [DOI] [PubMed] [Google Scholar]
- 23.Devriese LA, Witteveen PO, Wanders J, et al. Pharmacokinetics of eribulin mesylate in patients with solid tumors receiving repeated oral rifampicin. Br J Clin Pharmacol. 2012;75:507–515. doi: 10.1111/j.1365-2125.2012.04381.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Devriese LA, Witteveen PO, Marchetti S, et al. Pharmacokinetics of eribulin mesylate in patients with solid tumors and hepatic impairment. Cancer Chemother Pharmacol. 2012;70:823–832. doi: 10.1007/s00280-012-1976-x. [DOI] [PubMed] [Google Scholar]
- 25.Mukohara T, Nagai S, Mukai H, et al. Eribulin mesylate in patients with refractory cancers: A Phase I study. Invest New Drugs. 2012;30:1926–1933. doi: 10.1007/s10637-011-9741-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sullivan MP, Sutow WW, Cangir A, et al. Vincristine sulfate in management of Wilms’ tumor. Replacement of preoperative irradiation by chemotherapy. JAMA. 1967;202:381–384. [PubMed] [Google Scholar]
- 27.Sutow WW. Vincristine (NSC-67574) therapy for malignant solid tumors in children (except Wilms’ tumor) Cancer Chemother Rep. 1968;52:485–487. [PubMed] [Google Scholar]
- 28.Sutow WW, Berry DH, Haddy TB, et al. Vincristine sulfate therapy in children with metastatic soft tissue sarcoma. Pediatrics. 1966;38:465–472. [PubMed] [Google Scholar]
- 29.James DH, Jr, George P. Vincristine in children with malignant solid tumors. J Pediatr. 1964;64:534–541. doi: 10.1016/s0022-3476(64)80343-7. [DOI] [PubMed] [Google Scholar]
- 30.Selawry OS, Hananian J. Vincristine treatment of cancer in children. JAMA. 1963;183:741–746. doi: 10.1001/jama.1963.03700090061010. [DOI] [PubMed] [Google Scholar]
- 31.Sutow WW, Vietti TJ, Fernbach DJ, et al. Evaluation of chemotherapy in children with metastatic Ewing’s sarcoma and osteogenic sarcoma. Cancer Chemother Rep. 1971;55:67–78. [PubMed] [Google Scholar]
- 32.Rosen G, Nirenberg A, Caparros B, et al. Osteogenic sarcoma: Eight-percent, three-year, disease-free survival with combination chemotherapy (T-7) Natl Cancer Inst Monogr. 1981;56:213–220. [PubMed] [Google Scholar]
- 33.Souhami RL, Craft AW, Van der Eijken JW, et al. Randomised trial of two regimens of chemotherapy in operable osteosarcoma: A study of the European Osteosarcoma Intergroup. Lancet. 1997;350:911–917. doi: 10.1016/S0140-6736(97)02307-6. [DOI] [PubMed] [Google Scholar]
- 34.Edmonson JH, Green SJ, Ivins JC, et al. A controlled pilot study of high-dose methotrexate as postsurgical adjuvant treatment for primary osteosarcoma. J Clin Oncol. 1984;2:152–156. doi: 10.1200/JCO.1984.2.3.152. [DOI] [PubMed] [Google Scholar]
- 35.Krailo M, Ertel I, Makley J, et al. A randomized study comparing high-dose methotrexate with moderate-dose methotrexate as components of adjuvant chemotherapy in childhood nonmetastatic osteosarcoma: A report from the Childrens Cancer Study Group. Med Pediatr Oncol. 1987;15:69–77. doi: 10.1002/mpo.2950150205. [DOI] [PubMed] [Google Scholar]
- 36.Eilber F, Giuliano A, Eckardt J, et al. Adjuvant chemotherapy for osteosarcoma: A randomized prospective trial. J Clin Oncol. 1987;5:21–26. doi: 10.1200/JCO.1987.5.1.21. [DOI] [PubMed] [Google Scholar]
- 37.Pratt CB, Champion JE, Fleming ID, et al. Adjuvant chemotherapy for osteosarcoma of the extremity. Long-term results of two consecutive prospective protocol studies. Cancer. 1990;65:439–445. doi: 10.1002/1097-0142(19900201)65:3<439::aid-cncr2820650311>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- 38.Kuttesch JF, Jr, Krailo MD, Madden T, et al. Phase II evaluation of intravenous vinorelbine (Navelbine) in recurrent or refractory pediatric malignancies: A Children’s Oncology Group study. Pediatr Blood Cancer. 2009;53:590–593. doi: 10.1002/pbc.22133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Casanova M, Ferrari A, Spreafico F, et al. Vinorelbine in previously treated advanced childhood sarcomas: Evidence of activity in rhabdomyosarcoma. Cancer. 2002;94:3263–3268. doi: 10.1002/cncr.10600. [DOI] [PubMed] [Google Scholar]
- 40.Morton CL, Favours EG, Mercer KS, et al. Evaluation of ABT-751 against childhood cancer models in vivo. Invest New Drugs. 2007;25:285–295. doi: 10.1007/s10637-007-9042-y. [DOI] [PubMed] [Google Scholar]
- 41.Meany HJ, Sackett DL, Maris JM, et al. Clinical outcome in children with recurrent neuroblastoma treated with ABT-751 and effect of ABT-751 on proliferation of neuroblastoma cell lines and on tubulin polymerization in vitro. Pediatr Blood Cancer. 2010;54:47–54. doi: 10.1002/pbc.22267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wong H, Choo EF, Alicke B, et al. Antitumor activity of targeted and cytotoxic agents in murine subcutaneous tumor models correlates with clinical response. Clin Cancer Res. 2012;18:3846–3855. doi: 10.1158/1078-0432.CCR-12-0738. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.