Key Points
GPIbα–VWF-A1 bond kinetics regulates platelet–VWF interactions and can be altered to correct defects in hemostasis or prevent thrombosis.
Targeting a distinct GPIbα−VWF-A1 binding interface may offer a unique therapeutic approach to reducing platelet-driven thrombosis.
Abstract
Platelet–von Willebrand factor (VWF) interactions must be tightly regulated in order to promote effective hemostasis and prevent occlusive thrombus formation. However, it is unclear what role the inherent properties of the bond formed between the platelet receptor glycoprotein Ibα and the A1 domain of VWF play in these processes. Using VWF-A1 knock-in mice with mutations that enhance (I1309V) or disrupt (R1326H) platelet receptor glycoprotein Ibα binding, we now demonstrate that the kinetic interplay between two distinct contact surfaces influences the site and extent to which platelets bind VWF. Incorporation of R1326H mutation into the major site shortened bond lifetime, yielding defects in hemostasis and thrombosis comparable to VWF-deficient animals. Similarly, disrupting this region of contact with an allosteric inhibitor impaired human platelet accrual in damaged arterioles. In contrast, the I1309V mutation near the minor site prolonged bond lifetime, which was essential for the development of a type 2B–like VWD phenotype. However, combining the R1326H and I1309V mutations normalized both bond kinetics and the hemostatic and thrombotic properties of VWF. These findings broaden our understanding of mechanisms governing platelet–VWF interactions in health and disease, and underscore the importance of combined biophysical and genetic approaches in identifying potential therapeutic avenues for treating bleeding and thrombotic disorders.
Introduction
von Willebrand factor (VWF) is a large multimeric plasma glycoprotein that adheres to sites of vascular injury where it can serve as a homing beacon for platelets.1,2 It is composed of functionally distinct types of domains that are either duplicated or triplicated in the following order from the N-terminus: D′-D3-A1-A2-A3-D4-B1-B2-B3-C1-C2-CK.3,4 However, it is the interaction between the VWF-A1 domain and the platelet receptor glycoprotein Ibα (GPIbα) that initiates primary hemostasis by bringing platelets into close proximity with reactive substrates generated at sites of arterial damage.5-8 The clinical significance of this adhesive event is underscored by the increased bleeding tendencies of individuals who either lack VWF or possess mutations within its A1 domain. In the latter case, this includes mutations that limit (type 2M) or paradoxically enhance binding (type 2B), both of which are associated with von Willebrand disease (VWD).3,4
Against this background, structure analyses of native and mutant complexes have provided insight into the nature of the interactions that support adhesion between this receptor–ligand pair and possible mechanisms by which mutations alter this process. The structure reveals a major and minor binding site for GPIbα on the surface of the A1 domain that is bridged by an area of solvated charge interaction.9-12 The major contact surface involves a region known as the “β-switch,” which emanates from the C-terminal flank of the leucine rich repeats (LRR) of GPIbα (residues 227 to 241), forming a β-hairpin structure that is tightly packed between the β-sheet of the VWF-A1 domain on one side and the concave face of the LRR on the other, constraining its movement. The importance of the β-hairpin structure is suggested by the ability of a cyclic peptide (CTERMALHNLC) designated OS1 to impair ristocetin-induced human platelet–VWF aggregation by stabilizing an alternate conformation of this regulatory loop that is incapable of forming key interactions with the A1 domain.13,14 The minor contact surface involves interactions of the NH2-terminal β-hairpin structure and first LRR of GPIbα with loops α1β2, β3α2, and α3β4 at the bottom face of the A1 domain.10 Type 2M mutations, located in or directly adjacent to the larger contact surface, may disrupt electrostatic interactions or induce structural changes that reduce GPIbα binding. In contrast, type 2B mutations, clustered in close proximity to the minor site, may alter the conformation of a region known as the α1-β2 loop.9-12 Consequently, this is thought to either reinforce electrostatic interactions that augment binding9 or reduce a steric clash that would impede adhesion,12 which may result in the spontaneous formation and clearance of platelet–VWF aggregates from the circulation. Although crystal structures are informative, they cannot take into account the role that other domains or external forces such as shear flow play in regulating platelet–VWF interactions.15 Indeed, several mechanisms have been identified that may prevent inappropriate aggregate formation in the circulation. This includes shielding of VWF-A1 by the adjacent D′D3 domains,16 the inhibitory effects of the β2-glycoprotein I,17 and the ability of shear flow to activate the VWF-A1 so that it can then engage GPIbα.18 Moreover, forces generated by flowing blood have been shown to promote the unfolding and elongation of VWF, so that it exposes multiple A1 domains.19
Another mechanism that has not been fully explored in vivo is the contribution that the physicochemical properties of the bond formed between GPIbα and VWF-A1 play in regulating hemostasis and thrombus formation. Analyses of the kinetic and mechanical properties of this receptor–ligand pair have yielded insight into the biophysical consequences of the predicted allosteric changes associated with type 2M and 2B mutations.20-24 That said, it remains unclear whether specific properties of this receptor–ligand bond are involved in regulating adhesion under complex and dynamic conditions that exists in the mammalian circulation.25-27 In this study, we sought to determine the extent to which the biophysical properties of the GPIbα–VWF-A1 bond may limit platelet–VWF aggregate formation in blood, yet maximize adhesion at sites of vascular injury. By generating transgenic mice harboring modified VWF-A1 domains, we define the relationship between the physicochemical properties of GPIbα–VWF-A1 binding interfaces, their contribution to the lifetime of the interaction, and the importance of this kinetic parameter in regulating hemostasis and thrombosis in a living animal.
Methods
Modeling of murine GPIbα–VWF-A1 complexes
Crystal structures of the human GPIbα–VWF-A1 complex have been described for wild-type (WT) and for the complex formed between the type 2B mutations (R1306Q and I1309V) in VWF-A1 and the platelet-type mutation (M239V) in GPIbα.9-12 Human GPIbα was used as a template for the mouse receptor. Consensus rotamers with minimal steric clashes were chosen, followed by adjustments to create reasonable van der Waals interactions and H-bonding using COOT (http://www.biop.ox.ac.uk/coot/).
A consensus model of the human receptor–ligand pair was used to build its murine counterpart. This included the double-mutant VWF-A1 (I1309V and R1326H) complex that was based on the human VWF-A1 (R1306Q)10 mutant complex using the program SWISS-MODEL (http://swissmodel.expasy.org/). Figures were created using PyMOL.
Mice
The generation of the VWFR1326H mutant mouse has been previously described.28 A similar targeting strategy was used to introduce the double mutation (I1309V and R1326H) into exon 28 of the murine VWF gene or to substitute in the human A1 domain. VWF-deficient mice were purchased from The Jackson Laboratory. VWF mutant animals were on a C57BL/6J background and kept in a pathogen-free facility. Experiments were performed in accordance with the guidelines set forth by the Institutional Animal Care and Use Committee at Columbia University Medical Center.
Analysis of VWF expression and function
Detection and/or measurement of murine VWF A1-A2-A3 domain transcripts, plasma VWF antigen levels, functional factor VIII levels, and multimer pattern were performed as previously described.28-31
Platelet adhesion and aggregation
A parallel-plate flow chamber was used to assess platelet accumulation on surface-immobilized plasma VWF or recombinant VWF-A1 protein (amino acids 1238 to 1471).11,21,28 The ability of ristocetin (Chrono-Log Corporation, Havertown, PA) to aggregate human platelets in plasma was assessed using a Lumi-Aggregometer (model 540 VS; Chrono-Log).32
In vivo thrombus formation
Administration of anesthesia, insertion of vascular catheters, fluorescent labeling of platelets, and surgical preparation of the cremaster muscle have been previously described.28,32 A pulsed nitrogen dye laser was used to induce arteriole injury in the cremaster muscle of anesthetized 8- to 12-week-old animals. Mouse or human platelet-vessel wall interactions were visualized by fluorescence microscopy.28
Tail bleeding assay
Bleeding times were measured in 8-week-old mice after amputating 1 cm of the tail tip and then placing the tail in a physiological saline solution (37°C) as previously described.28
Statistical analyses
Details for the calculation of maximum likelihood estimates (MLEs) for the off-rates (koff) and Bell model parameters (k0off, σ), and additional information on adhesion, intravital, and biophysical studies can be found in the supplemental Methods available on the Blood Web site. Statistical analysis of all other data was determined using unpaired Student t test (GraphPad Prism software, La Jolla, CA). Values were considered significant at P < .05.
Results
Structural and functional changes associated with I1309V and R1326H mutations
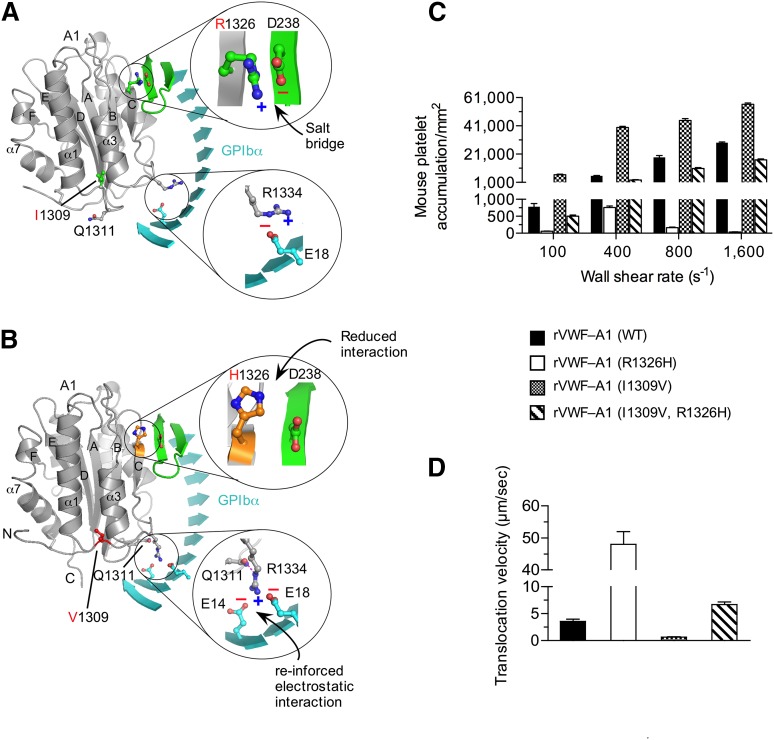
To determine the interplay between known contact surfaces and to establish the role that the biophysical properties of the GPIbα–VWF-A1 bond play in regulating platelet–VWF interactions, we chose mutations within the major or minor contact interfaces of the murine A1 domain that enhance (I1309V) or disrupt (R1326H) binding. The I1309V mutation is associated with type 2B VWD,33 whereas the R1326H substitution impairs interactions with mouse GPIbα but supports binding to the human platelet receptor.28 Interestingly, several mammalian species including humans have a histidine in lieu of arginine at position 1326.34 Thus, murine VWF is the ideal system for studying the effect that this amino acid substitution has on both the biophysical properties of the GPIbα–VWF-A1 bond and platelet–VWF interactions in vivo. Models of WT and mutant murine complexes were built to better understand how these mutations might affect binding (Figure 1A-B). Importantly, mouse and human VWF-A1 domains bear a high degree of structural homology suggesting that the mutations would have similar effects on key contact surfaces.11,28 Although the precise mechanism by which the R1326H substitution reduces function is unknown, we speculate that the loss of the salt bridge with D238 of murine GPIbα to be the most likely possibility. Similarly, crystal structure analyses have yet to identify the exact mechanism by which the I1309V mutation augments adhesion in the human complex10,12; it is located in the α1-β2 loop adjacent to the type 2B mutation R1306Q.9-12,35 The latter mutation has been reported to result in a local conformational change with a repositioning of residues Q1311 and R1334 that reinforce interactions with human GPIbα.9 Figure 1B illustrates the position of residue 1309 in murine VWF-A1 in the context of the complex formed with murine GPIbα. Structural analysis also reveals that the murine system is capable of supporting a similar repositioning of these residues in the presence of the type 2B mutation.
Figure 1.
I1309V mutation rescues the defect in platelet adhesion associated with the R1326H mutation. (A-B) Proposed models for the WT (A) and double-mutant (I1306V and R1326H) (B) murine VWF-A1 domains (gray) in complex with murine GPIbα (cyan). The β-switch hairpin loop of GPIbα is shown in green and key side chains involved in forming the interface are shown in stick. Zoomed images reveal details of the electrostatic interactions that may be altered by the mutations R1326H and I1309V, respectively. (C-D) Accumulation (C) and translocation velocity (WSR of 1,600 s−1) (D) of mouse platelets on surface-immobilized rVWF-A1 proteins. Platelet attachment and their subsequent motion were digitally recorded on an inverted Nikon microscope (Eclipse TE2000) with a plan ×10 or ×20 objective, respectively. The number of platelets attached per unit area and translocation velocities was determined by off-line analysis (Image-Pro Plus, Media Cybernetics, Rockville, MD). Results are representative of 3 separate experiments performed in triplicate (mean ± SD).
To analyze the effect of the mutations on adhesion, we first generated recombinant murine VWF-A1 (rVWF-A1) proteins containing the R1326H and I1309V substitutions and determined their ability to support interactions with mouse platelets in a parallel plate flow system.28 The R1326H mutation impaired platelet accumulation and increased translocation velocity ∼13-fold, as compared with WT rVWF-A1 (Figure 1C-D). In contrast, I1309V mutation greatly augmented platelet accumulation and decreased translocation velocity ∼fivefold, as reported for the human complex.21 Strikingly, rVWF–A1 containing both amino acid substitutions yielded an adhesive phenotype comparable to WT.
I1309V and R1326H mutations differentially alter bond formation and dissociation
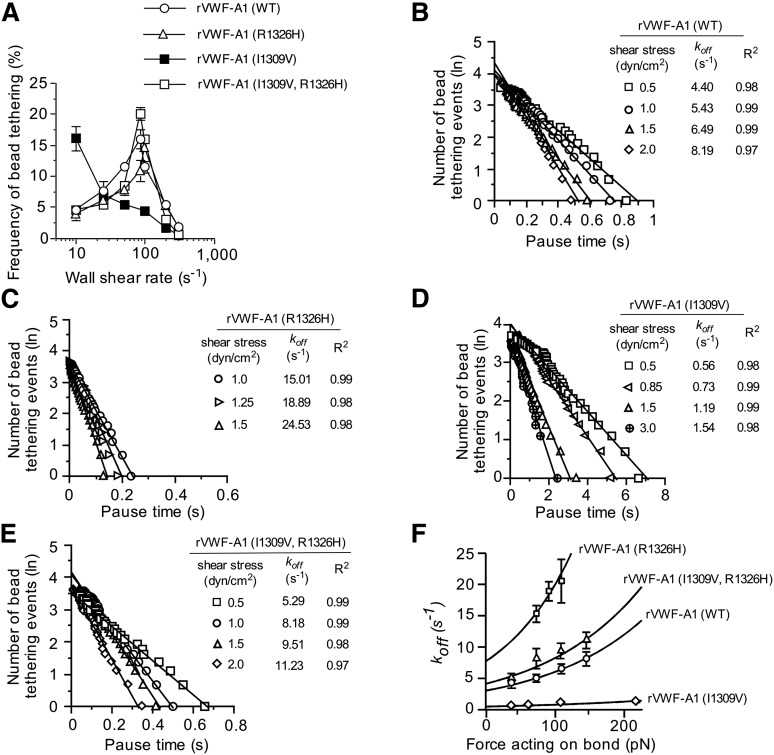
To determine whether the biophysical properties of rVWF-A1 expressing both mutations truly reflect that of the WT complex, we analyzed the formation and dissociation of transient adhesive events known as “tether bonds” formed between free-flowing microspheres coated with low site densities of murine rVWF-A1 and surface-immobilized mouse platelets.21,22 In this system, the formation of a tether bond is detected as an abrupt halt in the forward motion of the microsphere that can occur over a wide range of shear rates only if the coating concentration of rVWF-A1 protein is sufficiently low to prevent multiple bond formation. To this end, we found that a coating concentration of 5 µg/mL (∼35 sites/µm2) was the lowest concentration capable of supporting transient tethering events at wall shear rates (WSRs) ranging from 10 s−1 to 300 s−1 (supplemental Figure 1A-B). The effect of the mutations on bond formation was determined by evaluating the frequency with which microspheres interacted with surface-immobilized mouse platelets at various WSRs. Although the I1309V gain-of-function mutation enhanced the rate of association ∼3.5-fold at the lowest WSR tested (as reported for the human mutant complex),21 this was abolished when combined with the R1326H substitution (Figure 2A). In fact, murine rVWF-A1 possessing the double mutation displayed a rate of association that more closely resembled the WT complex, with a maximal tethering frequency at a WSR of 85 s−1.
Figure 2.
Kinetics of tether bond formation and dissociation. (A) Frequency of transient adhesive interactions between microspheres coated with recombinant VWF-A1 protein and surface-immobilized mouse platelets (WSRs of 10 s−1 to 300 s−1). (B-E) Representative graphs depicting the distribution of pause times for more than 30 individual transiently tethered VWF-A1–coated microspheres at the indicated wall shear stresses. The dissociation rate constant (koff) is the negative slope of the linear regression through the experimental data. (F) Effect of force on the GPIbα–VWF-A1 tether bond as a function of koff. Tethering frequency and estimation of koff values for VWF-A1–coated microspheres transiently interacting with surface-immobilized platelets were determined by recording digital images with a Nikon ×10 plan or 60× DIC objective (oil immersion) at 30 fps or 235 fps, respectively (Speed Vision Technologies, San Diego, CA). Data represent the mean ± SD. pN, piconewtons.
The effects of the mutations on the kinetics of dissociation (koff) were determined by measuring the lifetime of tether bonds and their response to an applied force.21,22 Previously, we have shown the utility of this system for assessing the biophysical properties of the interaction between human GPIbα and rVWF-A1. Moreover, the use of microspheres with a uniform size and shape permits the calculation of the amount of shear force acting on the tether bond. As previously observed for the human complex, the distribution of interaction times indicated that the majority of WT and mutant tether bonds follow first-order kinetics that fit a straight line, the regressed slope of which corresponded to -koff (Figure 2B-E). In addition, the rate of dissociation behaved in accordance with the equation of Bell, which states that koff = k0off exp(σFb/kT), where Fb is the applied force, k0off is the dissociation rate constant in the absence of force, kT is the thermal energy, and σ is the reactive compliance, relates to the sensitivity of bond off-rate to an applied force.36 The larger the values for k0off and σ, the shorter the bond lifetime (1/k0off) and the more prone the receptor–ligand interaction is to force-driven dissociation, respectively. MLE of the kinetics of GPIbα–VWF-A1 interactions and their subsequent fit to the Bell equation were performed.37 Regression analyses of these simulations fit the data at bond forces ranging from 36 pN to 217 pN, and thus permitted the determination of k0off and σ (Figure 2F and Table 1). Although the major effect of the I1309V substitution was to prolong the intrinsic lifetime of the tether bond (sixfold), R1326H shortened its duration (2.5-fold). However, the intrinsic kinetic and mechanical properties of rVWF-A1 bearing both mutations were nearly identical to that of the native complex, suggesting that its biological function may be restored as well.
Table 1.
Intrinsic dissociation rate constants (k0off) and reactive compliance (σ) values for WT and mutant murine GPIbα–VWF-A1 tether bonds
| Mouse rVWF–A1 | k0off (s−1) | σ (nm) | R2 |
|---|---|---|---|
| WT | 3.05 ± 0.55 | 0.028 ± 0.001 | 0.97 |
| R1326H | 7.80 ± 1.15 | 0.039 ± 0.002 | 0.93 |
| I1309V | 0.49 ± 0.04 | 0.020 ± 0.003 | 0.95 |
| I1309V, R1326H | 4.15 ± 0.67 | 0.028 ± 0.001 | 0.94 |
Values were determined by using statistical MLE of Bell parameters.
Hemostatic and thrombotic properties of murine VWF possessing mutant A1 domains
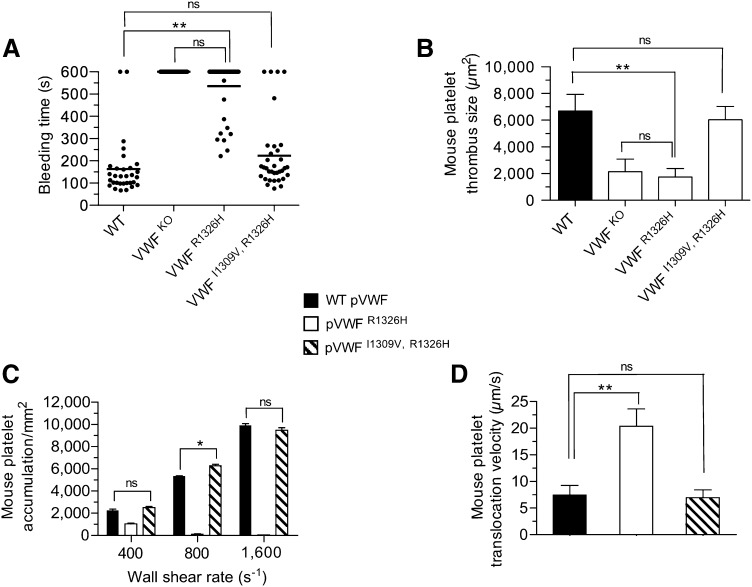
To establish whether our biophysical observations truly have biological relevance, we generated knock-in mice expressing either the R1326H (VWFR1326H) mutation alone28 or in combination with I1309V (VWFI1309V, R1326H) (supplemental Figure 2A-C). VWF gene transcription, multimer pattern, antigen levels, as well as factor VIII function and platelet counts were similar to WT littermates (supplemental Figure 2D-H). Consistent with the reduction in bond lifetime and impaired adhesion, VWFR1326H mice had prolonged tail bleeding times and a diminished capacity to form thrombi in laser-injured arterioles (Figure 3A-B). In fact, they possessed a phenotype similar to VWF-deficient animals. Remarkably, the additional incorporation of the I1309V mutation corrected the observed perturbations in platelet–VWF interactions. Not only did VWF-A1 double-mutant animals have hemostatic properties similar to WT littermates, but also thrombi formed at sites of arterial injury were comparable in size. Direct evidence that plasma VWFI1309V, R1326H has adhesive properties equivalent to the WT plasma protein is demonstrated by its ability to support mouse platelet accumulation and translocation velocities at similar levels (Figure 3C-D). This was not the case for plasma VWFR1326H, which impaired platelet adhesion and yielded a ∼threefold increase in translocation velocity. These data support a close association between the lifetime of the GPIbα–VWF-A1 bond and the ability of these hemostatic elements to support critical biological processes.
Figure 3.
Hemostatic and thrombotic properties of plasma VWFI1309V, R1326H. (A) Tail bleeding times for WT and mutant VWF-A1 mice. Each point represents 1 individual animal; lines show the mean of each group. (B) Thrombus formation in laser-injured arterioles of WT and VWF mutant animals (n = 7 mice per genotype; 1 arteriole per mouse). Platelet-vessel wall interactions were visualized through a ×20 water-immersion objective (Olympus LUMPlanFl, 0.5 NA) using a Zeiss Axiotech Vario microscope equipped with a Yokogawa CSU-22 spinning disk confocal scanner, iXon EM camera, and 561 nm laser line to detect rhodamine-labeled mouse platelets (Revolution XD, Andor Technology, South Windsor, CT). The extent of thrombus formation was assessed for 2 minutes postinjury and the area (µm2) of coverage determined by off-line analysis (Image IQ, Andor Technology and Image-Pro Plus). (C-D) Accumulation (C) and translocation velocities (WSR of 1,600 s−1) (D) of mouse platelets on surface-immobilized WT or mutant plasma VWF at the indicated WSRs (n = 3 separate experiments). Images were obtained using a CCD camera and analyzed using Image-Pro Plus. Data are the mean ± SD. *P < .05 and **P < .0001 relative to control. ns, not significant (P > .05).
Prolongation in bond lifetime yields a type 2B–like VWD phenotype
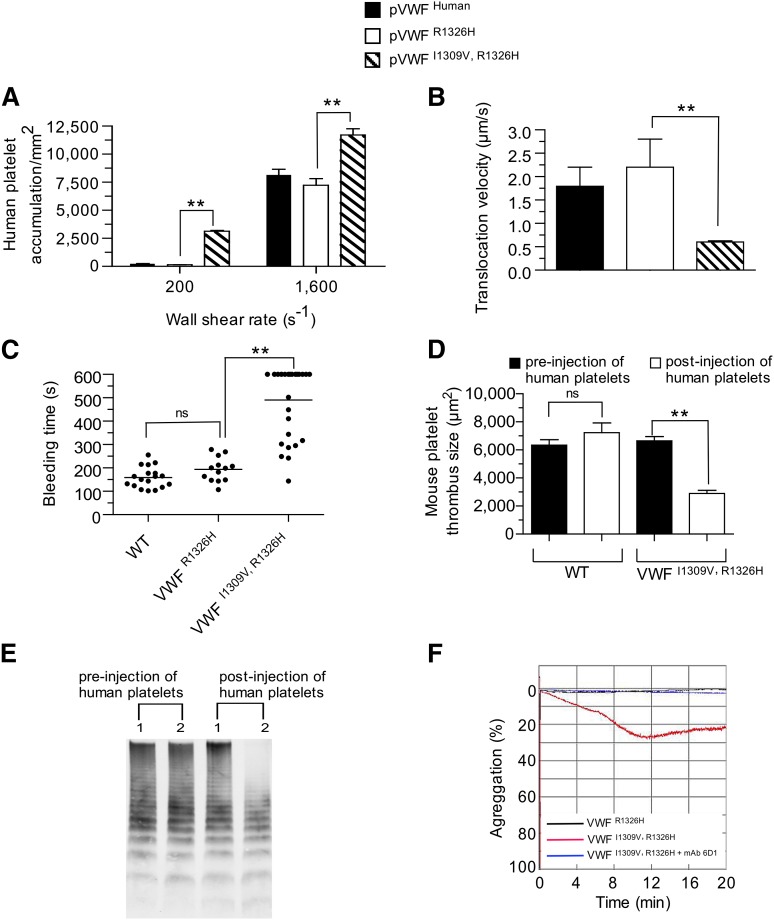
Previously, we have shown that murine VWF possessing the R1326H mutation alone could support human platelet-mediated hemostasis and thrombosis.28 This is thought to result from the abolishment of an unfavorable electrostatic interaction between lysine 238 in human GPIbα and arginine 1326 in WT murine VWF-A1. Therefore, we speculated that the addition of the I1309V mutation would further enhance human platelet interactions and may yield a phenotype reminiscent of type 2B VWD if a prolongation in bond lifetime was essential for this process. Consistent with the known effects of this gain-of-function mutation on the biophysical properties of the human complex,21 surface-immobilized plasma VWFI1309V, R1326H did augment accumulation and reduce the translocation velocities of human platelets in flow (Figure 4A-B). Importantly, it also enhanced the frequency of tether bond formation and prolonged the duration of the interaction between human GPIbα and mouse rVWF-A1 I1309V, R1326H protein (supplemental Figure 3A-B). Evidence that the I1309V mutation disrupts hemostasis and clot formation is demonstrated by the ∼threefold prolongation in tail bleeding time and >50% reduction in murine thrombus size observed in VWFI1309V, R1326H animals 20 minutes postadministration of human platelets (Figure 4C-D). This was associated with the depletion of high molecular weight multimers (HMWM) of VWF from the blood of double-mutant mice (Figure 4E). However, the number of circulating mouse platelets remained essentially unchanged (777 ± 13 × 103/µL pre-administration vs 835 ± 33 × 103/µL postadministration, mean ± standard deviation [SD]; P = .15). Moreover, plasma VWFI1309V, R1326H but not VWFR1326H supported the spontaneous aggregation of human platelets in suspension (Figure 4F).
Figure 4.
Prolongation in bond lifetime favors spontaneous platelet–VWF interactions. (A-B) Accumulation (A) and translocation velocities (WSR of 1,600 s−1) (B) of human platelets on surface-immobilized plasma VWFR1326H, VWFI1309V, R1326H, or human VWF at the indicated WSRs. Data are representative of 3 separate experiments performed in duplicate. (C-D) Tail bleeding times (C) and mouse platelet-mediated thrombus formation (D) for WT and mutant VWF-A1 mice pre- and 20 minutes postadministration of human platelets. Platelet-vessel wall interactions were visualized through a ×20 water-immersion objective using a Zeiss Axiotech Vario microscope equipped with a Yokogawa CSU-22 spinning disk confocal scanner, iXon EM camera, and 561 nm laser line. (E) Representative VWF multimer gel of plasma obtained from mutant mice before and 20 minutes after the administration of human platelets. Numbers 1 and 2 denote mice possessing plasma VWFR1326H or VWFI1309V, R1326H, respectively. (F) Representative tracing of light transmitted aggregometry of human platelets resuspended in plasma obtained from VWFR1326H or VWFI1309V, R1326H mice. MAb 6D1 is a function blocking antibody to human GPIbα. Data are the mean ± SD. **P < .0001 as compared with VWFR1326H mice. ns, not significant (P > .05) by unpaired Student t test.
Human GPIbα–VWF-A1 major binding interface is essential for thrombus formation
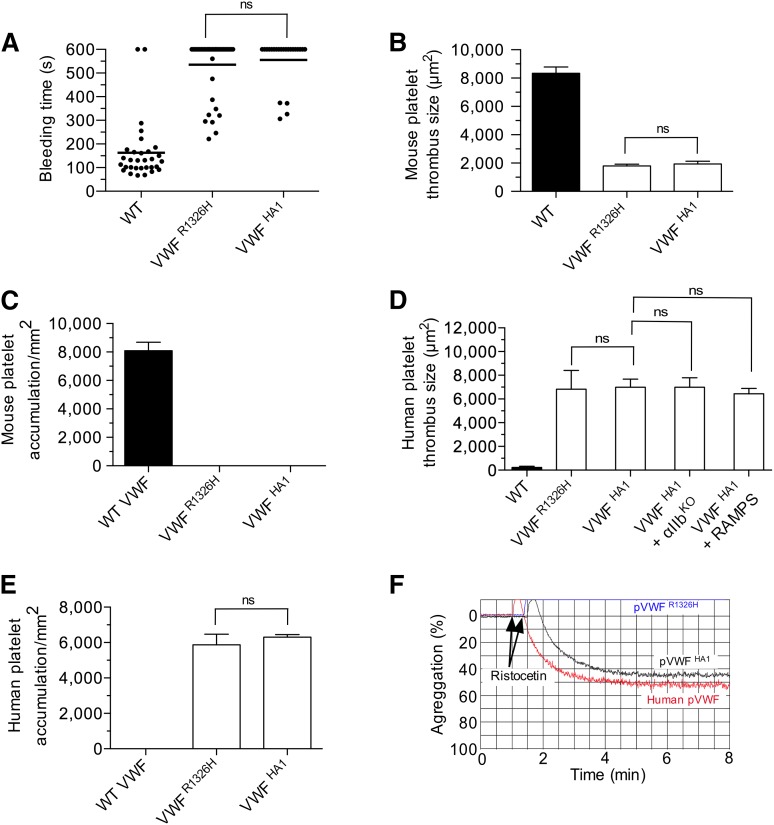
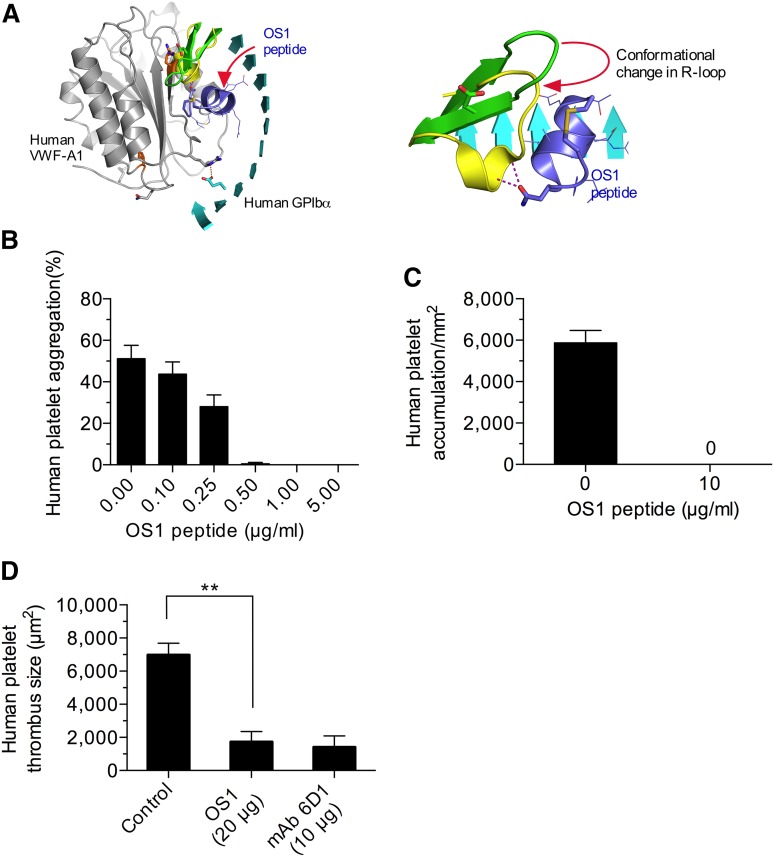
Given the importance of the major contact site in supporting mouse platelet–VWF interactions, we next determined whether this was also the case for the human complex. To enable the study of this interaction in vivo, we generated an animal in which the majority of the murine A1 domain was replaced with its human counterpart (supplemental Figure 4A-B). VWF gene transcription, multimer pattern, antigen levels, and platelet counts in VWFHA1 mice were similar to WT littermate controls (supplemental Figure 4C-F). In addition, plasma VWF from these animals could not support hemostasis and thrombosis due to a lack of effective adhesive interactions with mouse platelets under conditions of high shear flow (Figure 5A-C). This is consistent with the previous observation that human VWF cannot support these processes in mice unless it contains the murine A1 domain.38 In the presence of human platelets, however, VWFHA1 mice formed large and often occlusive thrombi in laser-injured arterioles (Figure 5D). Moreover, murine αIIbβ3, or for that matter murine platelets, were not essential for this process as VWFHA1 animals lacking αΙIβ or injected with rabbit serum that depletes mouse platelets39 were still capable of supporting human platelet-mediated thrombus formation (Figure 5D and supplemental Figure 5). Similarly, human platelets accumulated on surface-immobilized plasma from VWFHA1 or VWFR1326H mice but not from WT littermates in flow (Figure 5E). However, only plasma VWFHA1 could promote ristocetin-induced platelet agglutination (Figure 5F), which could be inhibited by pretreating human platelets with the OS1 peptide (Figure 6A-B). This impaired response may result from lack of aspartic acid at residue 1472 in murine VWF-A1, which is known to be involved in ristocetin binding to the human A1 domain.40 The importance of the major contact surface in supporting effective interactions between human GPIbα and VWF-A1 was also suggested by the ability of the cyclic peptide to reduce human platelet accumulation on surface-immobilized plasma VWFHA1 and at sites of arterial injury (Figure 6C-D).
Figure 5.
Hemostatic and thrombotic properties of plasma VWFHA1. (A) Tail bleeding times for WT, VWFR1326H, and VWFHA1 mice. Each point represents 1 individual animal; lines show the mean of each group. (B) Thrombus formation in laser-injured arterioles of WT and VWF mutant mice (n = 7 mice per genotype; 1 arteriole per animal). Fluorescent images were obtained using a 20× water-immersion objective, a Yokogawa CSU-22 spinning disk confocal scanner, and a 561 nm laser line to detect rhodamine-labeled mouse platelets. The extent of thrombus formation was assessed for 2 minutes postinjury and its area determined by off-line analysis (Image IQ and Image-Pro Plus). (C) Accumulation of mouse platelets on surface-immobilized WT or mutant plasma VWF at a WSR of 1,600 s−1 (n = 3). Images were obtained using a CCD camera and analyzed using Image-Pro Plus. (D) Human platelet-mediated thrombus formation in laser injured arterioles of WT or VWF mutant animals (n = 7 mice per genotype; 1 arteriole per animal). A 488 nm laser line was used to detect BCECF-labeled human platelets. Rabbit anti-mouse platelet serum (RAMPS) was used to deplete mouse platelets. (E) Accumulation of human platelets on surface-immobilized WT or mutant plasma VWF at a WSR of 1,600 s−1 (n = 3). (F) Representative plot depicting the extent of ristocetin-induced aggregation of lyophilized human platelets in plasma from human volunteers or VWF mutant animals (n = 2 experiments performed in duplicate). Data are the mean ± SD. ns, not significant (P > .05).
Figure 6.
Disrupting the major contact interface limits human platelet–VWF interactions. (A) Ribbon representation of the human GPIbα–VWF-A1–OS1 ternary complex (left). The change in R-loop conformation imposed upon OS1 cyclic peptide (purple) binding to GPIbα (right). (B) Ristocetin-induced aggregation of human platelets in plasma VWFHA1 in the presence and absence of OS1. (C) Accumulation of human platelets on surface-immobilized plasma VWFHA1 (WSR of 1,600 s−1) in the absence or presence of OS1 (n = 3). Images were obtained using a CCD camera and analyzed using Image-Pro Plus. (D) Human platelet-mediated thrombus formation in laser injured arterioles of VWFHA1 mice who received an infusion of OS1 peptide or the human GPIbα function blocking antibody, mAb 6D1 (n = 7 mice per treatment condition; 1 arteriole per mouse). Fluorescent images were obtained using a ×20 water-immersion objective, a Yokogawa CSU-22 spinning disk confocal scanner, and a 488 nm laser line. The extent of thrombus formation was assessed for 2 minutes postinjury and its area determined by off-line analysis (Image IQ and Image-Pro Plus). Data are the mean ± SD. **P < .0001. ns, not significant (P > .05).
Discussion
The co-existence of platelets and VWF in blood mandates mechanisms that prevent spontaneous aggregate formation yet still support primary hemostasis. In the present study, we demonstrate how the combined use of biophysical analysis and animals with genetically modified VWF-A1 domains provide unparalleled insight into the contribution that the kinetic properties of two distinct GPIbα contact surfaces play in regulating platelet–VWF interactions. We have found that the composite lifetime of this bidentate interaction must be maintained within a specific range to support effective hemostasis and prevent the loss of circulating high molecular weight VWF multimers. Moreover, evidence is presented that targeting a distinct binding interface between human GPIbα and VWF-A1 may have therapeutic utility in reducing arterial thrombosis.
Over the past decade, detailed analyses of the kinetic and mechanical properties of small regions of contact between proteins have yielded important insight into the relationships between force-lifetime-and-chemistry of receptor-ligand bonds involved in cell adhesion.41-43 This is also true for the human GPIbα–VWF-A1 complex where type 2B mutations such as R1306Q, I1309V, and R1450E have been reported to enhance the formation and increase the longevity of this interaction.21,44,45 However, in order to determine whether a prolongation in bond lifetime is a prerequisite for the ability of the I1309V mutation to disrupt hemostasis in a mammalian system, first required evidence that the biophysical properties of murine GPIbα–VWF-A1 bond and its response to an applied force were comparable to the human complex. Indeed, values for the intrinsic off-rate (k0off) and the mechanical stability (σ) of native and mutant tether bonds were similar between the species, including the gain-of-function mutation I1309V, as previously reported by our group.21,22 Surprisingly, the observed alterations in adhesion associated with the I1309V mutation did not persist when combined with R1326H. In fact, the biophysical properties of the double-mutant bond more closely resembled that of the native complex. These observations led us to predict that murine plasma VWFI1309V, R1326H should possess hemostatic and thrombotic properties similar to the native plasma protein if bond lifetime plays an important role in these processes. This was borne out by the observation that double knock-in mice had tail bleeding times and formed arterial thrombi of similar size as observed for WT littermate controls. This suggests that the I1309V mutation can only yield a type 2B phenotype if it can prolong the lifetime of the interactions beyond that of the native complex. The ability of plasma murine VWFR1326H to support interactions with human platelets comparable to human VWF, permitted us to test this hypothesis. Indeed, rVWF-A1 expressing the I1309V, R1326H double mutation was able to support interactions with human GPIbα at reduced shear rates and for a greater length of time. Consequently, the administration of human platelets to VWFI1309V, R1326H mice impaired, rather than supported, hemostasis and arterial thrombus formation.
Mechanistically, we propose that the enhancement in cellular on-rate and lifetime of the interaction would favor the formation of multiple GPIbα–VWF-A1 bonds as platelets and VWF randomly collide in flowing blood, thus stabilizing interactions between these hemostatic elements. Moreover, as the highest molecular weight multimers have more A1 domains exposed on the outer surface, it is reasonable to hypothesize that this would favor interactions with platelets in the context of a type 2B mutation; thus, preferentially targeting HMWM for clearance from the circulation. This indeed appears to be the case, as not only did human platelets spontaneously interact with plasma VWFI1309V,R1326H in solution, they were also capable of depleting VWF multimers possessing the greatest hemostatic capacity from the circulation of these animals. However, this was not associated with a reduction in murine platelet counts, demonstrating that the impairment in hemostasis was due to an absence of HMWM of VWF.
It is important to note that shear flow has a profound effect on the duration of the interaction between GPIbα–VWF-A1. For instance, shear flow can initially stabilize binding by prolonging the interaction (termed a “catch bond”) and then shorten its duration (termed a “slip bond”) as it is progressively increased.12,24 This is reflected in our system as the requirement for a specific level of WSR (∼85 s−1) to promote maximal interactions between WT VWF-A1–coated beads and surface adherent platelets, which is mitigated upon the inclusion of the type 2B mutation. Future studies to evaluate the effect of the R1326H and I1309V mutations on the “slip-catch” and “catch-slip” transitions will be required to better understand how this contributes to the regulation of hemostasis and thrombosis in our animal models.
The ability to prolong the lifetime of the GPIbα–VWF-A1 bond is not unique to type 2B mutations as it has also been reported for snake venoms. This is best exemplified by the ability of botrocetin, a component of the Bothrops jararaca venom, to promote platelet–VWF aggregation in plasma, as well as contribute to the loss of VWF multimers from the circulation.46 We have shown that botrocetin increases bond lifetime twofold for both human and mouse complexes without affecting the rate of association.11 This relied on the ability of the venom protein to form a biological clasp that prolonged their embrace. These results further support our current observations that the lifetime of the GPIbα–VWF-A1 bond plays an important role in regulating platelet–VWF interactions.
It is noteworthy that the R1326H substitution had such a profound effect on hemostasis and thrombosis in mice. There has been much speculation on whether one or both of the identified binding surfaces between GPIbα and VWF-A1 could be targets for the prevention or treatment of thrombosis.47 The current study helps clarify this issue by demonstrating that a cyclic peptide (OS1) proven to alter the conformation of GPIbα so that is incapable of forming several key interactions with the major binding interface of VWF-A1, significantly reduced human platelet-mediated arterial thrombosis. The therapeutic implication of blocking this interaction has been born out in previous studies in which a recombinant human GPIbα chimeric protein and an anti–VWF-A1 aptamer were shown to either prevent thrombosis in a canine model of coronary injury or promote an increase in platelet count in a thrombotic thrombocytopenic purpura patient, respectively.48,49 Thus, the development of agents that can alter interactions between this receptor–ligand pair may offer a powerful approach to reducing disease processes related to alterations in platelet–VWF interactions.
In conclusion, we propose an additional mechanism that contributes to the regulation of platelet–VWF interactions in vivo: the inherent physicochemical properties of the bond formed between GPIbα and VWF-A1. Our results also provide insight into the pathophysiology of a type 2B mutation and the importance of bond kinetics on influencing the site and degree to which platelets adhere to VWF.
Acknowledgments
The authors thank Aychyn C. Huang and Jeffrey S. Jhang for performing factor VIII function analysis, Gray Shaw for supplying the OS1 peptide, and Ian Laurenzi for performing the analysis of tether bond formation. The authors are grateful to Barry Coller and Michael Rosen for critical review of the manuscript.
This study was supported by grants from the National Institutes of Health National Heart, Lung, and Blood Institute (HL103989 and HL097971).
Footnotes
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Authorship
Contribution: T.G.D. conceived the study, supervised the project, conducted experiments, and wrote the manuscript; J.C. generated VWF mutant mice and conducted experiments; H.Z., A.D., and X.L.Z. performed experiments; and J.E. generated atomic models of the murine GPIbα–VWF complex.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Thomas G. Diacovo, Columbia University, 1130 St. Nicholas Ave, Room 924, New York, NY 10032; e-mail: td2142@cumc.columbia.edu.
References
- 1.Ruggeri ZM, Orje JN, Habermann R, Federici AB, Reininger AJ. Activation-independent platelet adhesion and aggregation under elevated shear stress. Blood. 2006;108(6):1903–1910. doi: 10.1182/blood-2006-04-011551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sakariassen KS, Bolhuis PA, Sixma JJ. Human blood platelet adhesion to artery subendothelium is mediated by factor VIII-Von Willebrand factor bound to the subendothelium. Nature. 1979;279(5714):636–638. doi: 10.1038/279636a0. [DOI] [PubMed] [Google Scholar]
- 3.Nichols WC, Ginsburg D. von Willebrand disease. Medicine (Baltimore) 1997;76(1):1–20. doi: 10.1097/00005792-199701000-00001. [DOI] [PubMed] [Google Scholar]
- 4.Sadler JE. New concepts in von Willebrand disease. Annu Rev Med. 2005;56:173–191. doi: 10.1146/annurev.med.56.082103.104713. [DOI] [PubMed] [Google Scholar]
- 5.Cruz MA, Diacovo TG, Emsley J, Liddington R, Handin RI. Mapping the glycoprotein Ib-binding site in the von willebrand factor A1 domain. J Biol Chem. 2000;275(25):19098–19105. doi: 10.1074/jbc.M002292200. [DOI] [PubMed] [Google Scholar]
- 6.Piétu G, Meulien P, Cherel G, et al. Production in escherichia coli of a biologically active subfragment of von Willebrand factor corresponding to the platelet glycoprotein Ib, collagen and heparin binding domains. Biochem Biophys Res Commun. 1989;164(3):1339–1347. doi: 10.1016/0006-291x(89)91816-0. [DOI] [PubMed] [Google Scholar]
- 7.Savage B, Saldívar E, Ruggeri ZM. Initiation of platelet adhesion by arrest onto fibrinogen or translocation on von Willebrand factor. Cell. 1996;84(2):289–297. doi: 10.1016/s0092-8674(00)80983-6. [DOI] [PubMed] [Google Scholar]
- 8.Sugimoto M, Ricca G, Hrinda ME, et al. Functional modulation of the isolated glycoprotein Ib binding domain of von Willebrand factor expressed in Escherichia coli. Biochemistry. 1991;30(21):5202–5209. doi: 10.1021/bi00235a013. [DOI] [PubMed] [Google Scholar]
- 9.Dumas JJ, Kumar R, McDonagh T, et al. Crystal structure of the wild-type von Willebrand factor A1-glycoprotein Ibalpha complex reveals conformation differences with a complex bearing von Willebrand disease mutations. J Biol Chem. 2004;279(22):23327–23334. doi: 10.1074/jbc.M401659200. [DOI] [PubMed] [Google Scholar]
- 10.Huizinga EG, Tsuji S, Romijn RA, et al. Structures of glycoprotein Ibalpha and its complex with von Willebrand factor A1 domain. Science. 2002;297(5584):1176–1179. doi: 10.1126/science.107355. [DOI] [PubMed] [Google Scholar]
- 11.Fukuda K, Doggett T, Laurenzi IJ, Liddington RC, Diacovo TG. The snake venom protein botrocetin acts as a biological brace to promote dysfunctional platelet aggregation. Nat Struct Mol Biol. 2005;12(2):152–159. doi: 10.1038/nsmb892. [DOI] [PubMed] [Google Scholar]
- 12.Blenner MA, Dong X, Springer TA. Structural basis of regulation of von Willebrand factor binding to glycoprotein Ib. J Biol Chem. 2014;289(9):5565–5579. doi: 10.1074/jbc.M113.511220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Benard SA, Smith TM, Cunningham K, et al. Identification of peptide antagonists to glycoprotein Ibalpha that selectively inhibit von Willebrand factor dependent platelet aggregation. Biochemistry. 2008;47(16):4674–4682. doi: 10.1021/bi702428q. [DOI] [PubMed] [Google Scholar]
- 14.McEwan PA, Andrews RK, Emsley J. Glycoprotein Ibalpha inhibitor complex structure reveals a combined steric and allosteric mechanism of von Willebrand factor antagonism. Blood. 2009;114(23):4883–4885. doi: 10.1182/blood-2009-05-224170. [DOI] [PubMed] [Google Scholar]
- 15.Lenting PJ, Pegon JN, Groot E, de Groot PG. Regulation of von Willebrand factor-platelet interactions. Thromb Haemost. 2010;104(3):449–455. doi: 10.1160/TH09-11-0777. [DOI] [PubMed] [Google Scholar]
- 16.Ulrichts H, Udvardy M, Lenting PJ, et al. Shielding of the A1 domain by the D’D3 domains of von Willebrand factor modulates its interaction with platelet glycoprotein Ib-IX-V. J Biol Chem. 2006;281(8):4699–4707. doi: 10.1074/jbc.M513314200. [DOI] [PubMed] [Google Scholar]
- 17.Hulstein JJ, Lenting PJ, de Laat B, Derksen RH, Fijnheer R, de Groot PG. beta2-Glycoprotein I inhibits von Willebrand factor dependent platelet adhesion and aggregation. Blood. 2007;110(5):1483–1491. doi: 10.1182/blood-2006-10-053199. [DOI] [PubMed] [Google Scholar]
- 18.Dong JF, Berndt MC, Schade A, McIntire LV, Andrews RK, López JA. Ristocetin-dependent, but not botrocetin-dependent, binding of von Willebrand factor to the platelet glycoprotein Ib-IX-V complex correlates with shear-dependent interactions. Blood. 2001;97(1):162–168. doi: 10.1182/blood.v97.1.162. [DOI] [PubMed] [Google Scholar]
- 19.Schneider SW, Nuschele S, Wixforth A, et al. Shear-induced unfolding triggers adhesion of von Willebrand factor fibers. Proc Natl Acad Sci USA. 2007;104(19):7899–7903. doi: 10.1073/pnas.0608422104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Arya M, Kolomeisky AB, Romo GM, Cruz MA, López JA, Anvari B. Dynamic force spectroscopy of glycoprotein Ib-IX and von Willebrand factor. Biophys J. 2005;88(6):4391–4401. doi: 10.1529/biophysj.104.046318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Doggett TA, Girdhar G, Lawshé A, et al. Selectin-like kinetics and biomechanics promote rapid platelet adhesion in flow: the GPIb(α)-vWF tether bond. Biophys J. 2002;83(1):194–205. doi: 10.1016/S0006-3495(02)75161-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Doggett TA, Girdhar G, Lawshe A, et al. Alterations in the intrinsic properties of the GPIbalpha-VWF tether bond define the kinetics of the platelet-type von Willebrand disease mutation, Gly233Val. Blood. 2003;102(1):152–160. doi: 10.1182/blood-2003-01-0072. [DOI] [PubMed] [Google Scholar]
- 23.Kumar RA, Dong JF, Thaggard JA, Cruz MA, López JA, McIntire LV. Kinetics of GPIbalpha-vWF-A1 tether bond under flow: effect of GPIbalpha mutations on the association and dissociation rates. Biophys J. 2003;85(6):4099–4109. doi: 10.1016/S0006-3495(03)74822-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ju L, Dong JF, Cruz MA, Zhu C. The N-terminal flanking region of the A1 domain regulates the force-dependent binding of von Willebrand factor to platelet glycoprotein Ibα. J Biol Chem. 2013;288(45):32289–32301. doi: 10.1074/jbc.M113.504001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dufrêne YF, Evans E, Engel A, Helenius J, Gaub HE, Müller DJ. Five challenges to bringing single-molecule force spectroscopy into living cells. Nat Methods. 2011;8(2):123–127. doi: 10.1038/nmeth0211-123. [DOI] [PubMed] [Google Scholar]
- 26.Neuman KC, Nagy A. Single-molecule force spectroscopy: optical tweezers, magnetic tweezers and atomic force microscopy. Nat Methods. 2008;5(6):491–505. doi: 10.1038/nmeth.1218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Weisel JW, Shuman H, Litvinov RI. Protein-protein unbinding induced by force: single-molecule studies. Curr Opin Struct Biol. 2003;13(2):227–235. doi: 10.1016/s0959-440x(03)00039-3. [DOI] [PubMed] [Google Scholar]
- 28.Chen J, Tan K, Zhou H, et al. Modifying murine von Willebrand factor A1 domain for in vivo assessment of human platelet therapies. Nat Biotechnol. 2008;26(1):114–119. doi: 10.1038/nbt1373. [DOI] [PubMed] [Google Scholar]
- 29.Cao W, Krishnaswamy S, Camire RM, Lenting PJ, Zheng XL. Factor VIII accelerates proteolytic cleavage of von Willebrand factor by ADAMTS13. Proc Natl Acad Sci USA. 2008;105(21):7416–7421. doi: 10.1073/pnas.0801735105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Laje P, Shang D, Cao W, et al. Correction of murine ADAMTS13 deficiency by hematopoietic progenitor cell-mediated gene therapy. Blood. 2009;113(10):2172–2180. doi: 10.1182/blood-2008-08-173021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Niiya M, Endo M, Shang D, et al. Correction of ADAMTS13 deficiency by in utero gene transfer of lentiviral vector encoding ADAMTS13 genes. Mol Ther. 2009;17(1):34–41. doi: 10.1038/mt.2008.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Magallon J, Chen J, Rabbani L, et al. Humanized mouse model of thrombosis is predictive of the clinical efficacy of antiplatelet agents. Circulation. 2011;123(3):319–326. doi: 10.1161/CIRCULATIONAHA.110.951970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Federici AB, Mannucci PM, Stabile F, et al. A type 2b von Willebrand disease mutation (Ile546—>Val) associated with an unusual phenotype. Thromb Haemost. 1997;78(3):1132–1137. [PubMed] [Google Scholar]
- 34.Jenkins PV, Pasi KJ, Perkins SJ. Molecular modeling of ligand and mutation sites of the type A domains of human von Willebrand factor and their relevance to von Willebrand’s disease. Blood. 1998;91(6):2032–2044. [PubMed] [Google Scholar]
- 35.Fukuda K, Doggett TA, Bankston LA, Cruz MA, Diacovo TG, Liddington RC. Structural basis of von Willebrand factor activation by the snake toxin botrocetin. Structure. 2002;10(7):943–950. doi: 10.1016/s0969-2126(02)00787-6. [DOI] [PubMed] [Google Scholar]
- 36.Bell GI. Models for the specific adhesion of cells to cells. Science. 1978;200(4342):618–627. doi: 10.1126/science.347575. [DOI] [PubMed] [Google Scholar]
- 37.Uz B, Arslan E, Laurenzi IJ. Maximum likelihood estimation of the kinetics of receptor-mediated adhesion. J Theor Biol. 2010;262(3):478–487. doi: 10.1016/j.jtbi.2009.10.015. [DOI] [PubMed] [Google Scholar]
- 38.Navarrete AM, Casari C, Legendre P, et al. A murine model to characterize the antithrombotic effect of molecules targeting human von Willebrand factor. Blood. 2012;120(13):2723–2732. doi: 10.1182/blood-2012-03-420042. [DOI] [PubMed] [Google Scholar]
- 39.Radley JM, Hodgson GS, Levin J. Platelet production after administration of antiplatelet serum and 5-fluorouracil. Blood. 1980;55(1):164–166. [PubMed] [Google Scholar]
- 40.Flood VH, Gill JC, Morateck PA, et al. Common VWF exon 28 polymorphisms in African Americans affecting the VWF activity assay by ristocetin cofactor. Blood. 2010;116(2):280–286. doi: 10.1182/blood-2009-10-249102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chen W, Evans EA, McEver RP, Zhu C. Monitoring receptor-ligand interactions between surfaces by thermal fluctuations. Biophys J. 2008;94(2):694–701. doi: 10.1529/biophysj.107.117895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Evans EA, Calderwood DA. Forces and bond dynamics in cell adhesion. Science. 2007;316(5828):1148–1153. doi: 10.1126/science.1137592. [DOI] [PubMed] [Google Scholar]
- 43.Thomas WE. Mechanochemistry of receptor-ligand bonds. Curr Opin Struct Biol. 2009;19(1):50–55. doi: 10.1016/j.sbi.2008.12.006. [DOI] [PubMed] [Google Scholar]
- 44.Yago T, Lou J, Wu T, et al. Platelet glycoprotein Ibalpha forms catch bonds with human WT vWF but not with type 2B von Willebrand disease vWF. J Clin Invest. 2008;118(9):3195–3207. doi: 10.1172/JCI35754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Coburn LA, Damaraju VS, Dozic S, Eskin SG, Cruz MA, McIntire LV. GPIbα-vWF rolling under shear stress shows differences between type 2B and 2M von Willebrand disease. Biophys J. 2011;100(2):304–312. doi: 10.1016/j.bpj.2010.11.084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sanders WE, Read MS, Reddick RL, Garris JB, Brinkhous KM. Thrombotic thrombocytopenia with von Willebrand factor deficiency induced by botrocetin. An animal model. Lab Invest. 1988;59(4):443–452. [PubMed] [Google Scholar]
- 47.Sadler JE. Biomedicine. Contact—how platelets touch von Willebrand factor. Science. 2002;297(5584):1128–1129. doi: 10.1126/science.1075452. [DOI] [PubMed] [Google Scholar]
- 48.Wadanoli M, Sako D, Shaw GD, et al. The von Willebrand factor antagonist (GPG-290) prevents coronary thrombosis without prolongation of bleeding time. Thromb Haemost. 2007;98(2):397–405. [PubMed] [Google Scholar]
- 49.Knöbl P, Jilma B, Gilbert JC, Hutabarat RM, Wagner PG, Jilma-Stohlawetz P. Anti-von Willebrand factor aptamer ARC1779 for refractory thrombotic thrombocytopenic purpura. Transfusion. 2009;49(10):2181–2185. doi: 10.1111/j.1537-2995.2009.02232.x. [DOI] [PubMed] [Google Scholar]