Key Points
Posttransplantation cyclophosphamide is effective as sole GVHD prophylaxis for myeloablative HLA-matched–related or –unrelated BMT.
Despite low chronic GVHD with PTCy, relapse and survival are comparable with outcomes reported using other GVHD prophylactic approaches.
Abstract
High-dose, posttransplantation cyclophosphamide (PTCy) reduces severe graft-versus-host disease (GVHD) after allogeneic blood or marrow transplantation (alloBMT), but the impact of PTCy on long-term, disease-specific outcomes is unclear. We conducted a retrospective study of 209 consecutive adult patients transplanted for acute myeloid leukemia (AML, n = 138), myelodysplastic syndrome (n = 28), or acute lymphoblastic leukemia (ALL, n = 43) using PTCy as sole GVHD prophylaxis after myeloablative conditioning and HLA-matched–related or –unrelated T-cell–replete allografting. At alloBMT, 30% of patients were not in morphologic complete remission. The cumulative incidences of grades II to IV and III to IV acute GVHD at 100 days and chronic GVHD at 2 years were 45%, 11%, and 13%, respectively. Forty-three percent of patients did not require immunosuppression for any reason beyond PTCy. At 3 years, relapse cumulative incidence was 36%, disease-free survival was 46%, survival free of disease and chronic GVHD was 39%, and overall survival was 58%. Lack of remission at alloBMT, adverse cytogenetics, and low allograft nucleated cell dose were associated with inferior survival for AML patients. Minimal residual disease but not t(9;22) was associated with inferior outcomes for ALL patients. The ability to limit posttransplantation immunosuppression makes PTCy a promising transplantation platform for the integration of postgrafting strategies to prevent relapse.
Introduction
Historically, graft-versus-host disease (GVHD), particularly chronic GVHD (cGVHD), has been associated with a reduced risk of relapse after allogeneic blood or marrow transplantation (alloBMT).1-3 However, cGVHD is also the leading cause of late nonrelapse mortality (NRM) posttransplantation, contributing to the substantially elevated mortality risk in alloBMT survivors that continues for at least 15 years after transplantation.4 In this context, the development of effective pharmacologic strategies for preventing cGVHD has proved to be challenging, and an estimated 41% to 60% of patients treated with calcineurin inhibitor (CNI) -based GVHD prophylaxis after HLA-matched–related or –unrelated allografting develop cGVHD.5-7 T-cell depletion (TCD), whether in vivo or in vitro, is effective in reducing cGVHD, but it can be associated with higher rates of infection, posttransplantation lymphoproliferative disorder, NRM, and disease relapse.8,9
Initially developed as an approach to facilitate HLA-haploidentical alloBMT, high-dose, posttransplantation cyclophosphamide (PTCy) was found to be effective in preventing both acute GVHD (aGVHD) and cGVHD.10-12 Subsequently, the efficacy of PTCy as sole GVHD prophylaxis after myeloablative conditioning (MAC) and HLA-matched–related or –unrelated alloBMT was shown, resulting in cumulative incidences of grade III to IV aGVHD and cGVHD of ∼10% each.13
Despite the clinical efficacy of PTCy in preventing severe GVHD after T-cell–replete allografting, the potential detrimental effect of PTCy on relapse has been of concern. PTCy is known to preferentially eliminate alloreactive T cells and preserve regulatory T cells,14,15 both of which could theoretically diminish a robust graft-versus-tumor response. Moreover, relatively high rates of relapse in the first 2 studies of the PTCy approach have been observed.11,13 Undoubtedly, these relapse rates are attributable, in part, to the enrollment of patients with very high-risk hematologic malignancies (54% with active disease in the first MAC study13); lower rates of relapse have been seen in a recent multi-institutional study in which only 27% of patients had active disease at the time of alloBMT.16 Interpretation of these studies is further complicated by marked patient heterogeneity, particularly in the disease types enrolled.
There are currently no published data on disease-specific outcomes using PTCy as sole GVHD prophylaxis after MAC and HLA-matched allografting, an approach which has been in use at our institution for a decade. Herein, we report outcomes of all adult patients with acute myeloid leukemia (AML), myelodysplastic syndrome (MDS), or acute lymphoblastic leukemia (ALL) who were transplanted with this approach at our institution from 2004 to 2011.
Methods
Study design
We conducted a retrospective, institutional review board–approved study of patients from Johns Hopkins Hospital who underwent alloBMT with the following transplantation platform: MAC, HLA-matched–related or –unrelated alloBMT, and PTCy as sole GVHD prophylaxis. All 209 consecutive adult (age ≥18 years) patients with AML, MDS, or ALL treated at Johns Hopkins Hospital with this approach from its inception in June 2004 through December 2011 were included in this study. One hundred twenty-three patients were treated on institutional review board–approved protocols.13,16 Because of either lack of insurance with clinical trial coverage or enrollment after clinical trial completion,13 86 patients were treated identically off-study as standard of care after they provided written, informed consent in accordance with the Declaration of Helsinki. The primary objectives of this study were to report extended follow-up and disease-specific outcomes for this patient cohort.
Transplantation platform
For the 172 patients receiving busulfan (Bu)/cyclophosphamide (Cy) MAC (82 previously reported13), Bu was given every 6 hours for 4 consecutive days followed by Cy 50 mg/kg per day for 2 consecutive days. Bu dosing was started at either 0.8 mg/kg per dose intravenously or 1 mg/kg per dose orally and was adjusted for the fifth and subsequent doses on the basis of measured pharmacokinetics to achieve a targeted area under the concentration curve of 800 to 1400 μmol × min/L.13 For the 36 patients receiving Bu/fludarabine MAC on protocol,16 Bu was administered once per day for 4 days starting at 130 mg/m2 intravenously with Bu dosing adjusted to a targeted daily systemic exposure of 4600 μMol-min with an acceptable range of 3600 to 5600 μMol-min; fludarabine was given at 40 mg/m2 per day intravenously immediately before Bu on all 4 days.16 For 1 ALL patient, MAC entailed cyclophosphamide 50 mg/kg per day intravenously for 2 consecutive days followed by total body irradiation of 1200 cGy total dose (six 200-cGy fractions administered twice per day for 3 consecutive days).
Allografts were 10/10 HLA-matched (HLA-A, -B, -C, -DRB1, and -DQB1) and were derived from a sibling, a first-degree relative, or an unrelated donor. T-cell–replete bone marrow at a targeted collection of 4 × 108 nucleated cells per kilogram of the recipient’s ideal body weight was the allograft source in all patients except for one patient who received unrelated peripheral blood stem cells because of donor-related issues in collecting bone marrow. All 209 patients received PTCy at 50 mg/kg per day on days +3 and +4 as the only GVHD prophylaxis. Mesna was administered on days of cyclophosphamide treatment in 4 divided doses at a total daily dose of 80% of the cyclophosphamide dose. Growth factor support was not allowed except in cases of delayed engraftment or secondary graft failure at the discretion of the treating physician. Supportive care was provided per institutional standard practice as previously described.13
Definitions and endpoints
Primary graft failure was defined as failure to achieve a sustained absolute neutrophil count of ≥500 cells per microliter in the absence of persistent or relapsed disease. Secondary graft failure was defined as a decline in the neutrophil count to <500 cells per microliter and loss of donor chimerism after initial engraftment in the absence of disease relapse. GVHD was diagnosed by standard criteria.17-19 aGVHD was treated per our institutional standard practice, which entailed observation or corticosteroids alone for cutaneous grade II aGVHD or corticosteroids plus tacrolimus for grade II aGVHD involving other organs or for grade III to IV aGVHD. Eighteen patients received first-line therapy with a pharmacologic agent other than tacrolimus as treatment on BMT Clinical Trials Network (CTN) studies 0302 or 0802.20,21 Secondary systemic immunosuppressants used for GVHD treatment were defined as any systemically absorbed pharmacologic agent or phototherapy (ultraviolet or extracorporeal photopheresis).
Pretransplantation disease status evaluations were performed prior to the start of conditioning and included at a minimum a bone marrow biopsy and bone marrow aspirate for flow cytometry and cytogenetics. Morphologic complete remission (CR) was determined by standard criteria22-24 and was defined as <5% blasts on bone marrow biopsy and absence of active extramedullary disease. Patients not in morphologic CR were considered to have active disease. Untreated MDS patients or MDS patients without response to pretransplantation therapy were considered to have active disease regardless of blast count. The presence of minimal residual disease (MRD) in patients in morphologic CR was assessed retrospectively and was defined as any disease detectable by flow cytometry, cytogenetics, fluorescence in situ hybridization, and/or polymerase chain reaction.22,23 Pretransplantation remission status was assessed by investigators blinded to patient outcomes. Posttransplantation disease status was assessed by bone marrow biopsy at approximately day +60. However, patients with circulating blasts, delayed engraftment, or other clinical concern for relapse had their disease status evaluated prior to day +60. Relapse was defined as any detectable disease posttransplantation, even at a flow cytometric, cytogenetic, or molecular level.
Statistical analysis
For disease-free survival (DFS), an event was defined as relapse or death from any cause. For cGVHD-free DFS (cGVHD-DFS), events included cGVHD (any severity grade), relapse, or death from any cause. DFS, overall survival (OS), and cGVHD-DFS times were defined from transplantation date to the date of event occurrence or censored at last follow-up for patients without an observed event. Kaplan-Meier estimates25 of DFS, OS, and cGVHD-DFS were compared between groups via log-rank statistics and the Cox proportional hazards model. Cumulative incidences were estimated or compared between groups by using Fine and Gray’s method.26,27 NRM was a competing risk for relapse and vice versa. Competing risks for GVHD were defined as graft failure, relapse, donor lymphocyte infusion (DLI), or death from any cause. Multivariate analyses were conducted for all patients and for AML patients by including variables derived from stepwise procedures based on P values ≤.10. Significance in multivariate analyses or in direct comparisons between groups was based on P values ≤ .05. Statistical analysis was performed by using R 3.0.2 (R Foundation for Statistical Computing, Vienna, Austria). Follow-up and the database were locked on January 7, 2014.
Results
Patient, donor, and allograft characteristics
Patient, donor, and allograft characteristics are summarized in Table 1. The median patient age was 50 years (range, 18 to 66 years). HLA-matched–unrelated allografts were used in 93 patients (44%). The median hematopoietic cell transplantation comorbidity index (HCT-CI)28 score was 2 (range, 0 to 12), with 43% of patients having a score ≥3. Thirty percent of patients had active disease at the time of transplantation, including 27% of AML patients. Other high-risk AML disease characteristics included adverse cytogenetics by refined Medical Research Council (MRC) criteria29 in 22% of tested patients (31% unfavorable by the Southwest Oncology Group criteria30), Flt3 internal tandem duplication (Flt3/ITD) positivity in 27% of AML patients (40% of tested patients because only 67% of patients had this molecular test performed), and AML secondary from antecedent hematologic disorder or treatment-related in 38% (supplemental Table 1 available at the Blood Web site). Fifty-seven percent of MDS patients had poor-risk cytogenetics by the International Prognostic Scoring System.31 Forty-four percent of ALL patients had disease positive for the Philadelphia chromosome t(9;22). The median follow-up of survivors was 4.0 years (range, 0.8 to 8.0 years).
Table 1.
Patient, donor, and allograft characteristics
| Characteristic | AML patients (n = 138) | MDS patients (n = 28) | ALL patients (n = 43) | Entire cohort (n = 209) | ||||
|---|---|---|---|---|---|---|---|---|
| No. | % | No. | % | No. | % | No. | % | |
| Age at BMT, years | ||||||||
| Median | 51 | 55.5 | 47 | 50 | ||||
| Range | 20-66 | 28-63 | 18-61 | 18-66 | ||||
| Female patients | 72 | 52 | 18 | 64 | 24 | 56 | 114 | 55 |
| HCT-CI | ||||||||
| Median | 2 | 3 | 1 | 2 | ||||
| Range | 0-12 | 0-8 | 0-5 | 0-12 | ||||
| HCT-CI | ||||||||
| 0 | 25 | 18 | 3 | 11 | 16 | 37 | 44 | 21 |
| 1-2 | 50 | 36 | 9 | 32 | 16 | 37 | 75 | 36 |
| ≥3 | 63 | 46 | 16 | 57 | 11 | 26 | 90 | 43 |
| Pretransplant remission status | ||||||||
| CR without MRD | 76 | 55 | 1 | 4 | 31 | 72 | 108 | 52 |
| CR with MRD | 25 | 18 | 1 | 4 | 12 | 28 | 38 | 18 |
| Active disease | 37 | 27 | 26 | 93 | 0 | 0 | 63 | 30 |
| Conditioning | ||||||||
| Bu/Cy | 120 | 87 | 23 | 82 | 29 | 67 | 172 | 82 |
| Bu/Flu | 18 | 13 | 5 | 18 | 13 | 30 | 36 | 17 |
| Cy/TBI | 0 | 0 | 0 | 0 | 1 | 2 | 1 | 0.5 |
| Graft source | ||||||||
| Bone marrow | 137 | 99.3 | 28 | 100 | 43 | 100 | 208 | 99.5 |
| Peripheral blood | 1 | 0.7 | 0 | 0 | 0 | 0 | 1 | 0.5 |
| Donor | ||||||||
| Matched-related | 78 | 57 | 14 | 50 | 24 | 56 | 116 | 56 |
| Matched-unrelated | 60 | 43 | 14 | 50 | 19 | 44 | 93 | 44 |
| Donor age, years | ||||||||
| Median | 44 | 48.5 | 41 | 43 | ||||
| Range | 17-75 | 21-66 | 21-62 | 17-75 | ||||
| Female donors | 66 | 48 | 14 | 50 | 16 | 37 | 96 | 46 |
| Female-into-male allografting | 26 | 19 | 4 | 14 | 4 | 9 | 34 | 16 |
| CMV serostatus | ||||||||
| Patient positive | 79 | 57 | 12 | 43 | 19 | 44 | 110 | 53 |
| Donor positive | 53 | 38 | 7 | 25 | 15 | 35 | 75 | 36 |
| Graft cell counts* | ||||||||
| Nucleated cells × 108 | ||||||||
| Median | 4.04 | 4.29 | 3.90 | 4.06 | ||||
| Range | 0.99-8.07 | 0.95-8.82 | 0.88-7.02 | 0.88-8.82 | ||||
| CD34+ cells × 106 | ||||||||
| Median | 3.35 | 3.34 | 3.78 | 3.45 | ||||
| Range | 0.97-8.36 | 1.52-9.87 | 1.63-8.17 | 0.97-9.87 | ||||
| CD3+ cells × 107 | ||||||||
| Median | 3.70 | 3.92 | 3.73 | 3.73 | ||||
| Range | 0.60-8.28 | 1.95-9.32 | 2.37-9.59 | 0.60-9.59 | ||||
| CD4+ cells × 107 | ||||||||
| Median | 2.08 | 2.06 | 1.77 | 2.03 | ||||
| Range | 0.37-9.90 | 0.95-5.00 | 1.10-5.25 | 0.37-9.90 | ||||
| CD8+ cells × 107 | ||||||||
| Median | 1.58 | 1.45 | 1.62 | 1.58 | ||||
| Range | 0.48-22.4 | 0.70-3.60 | 0.97-4.01 | 0.48-22.4 | ||||
BMT, blood or marrow transplantation; Flu, fludarabine, TBI, total body irradiation.
Cell counts shown are for the 208 patients treated with bone marrow allografts. Nucleated, CD34+, and CD3+ cell counts were available for all patients. The CD4+ and CD8+ cell counts shown reflect available data from 139 patients (67%).
Graft failure
Primary graft failure occurred in 5 patients (2.4%), of whom 2 received HLA-matched–related and 3 received HLA-matched–unrelated allografts. All 5 received a second alloBMT with 4 engrafting (2 long-term survivors) and the fifth dying of infection during aplasia after the second alloBMT. Secondary graft failure occurred in 4 patients (2.0%), 1 of whom received an HLA-matched–related allograft and 3 of whom received HLA-matched–unrelated allografts. One patient died of infection during aplasia, and the other 3 engrafted after a second alloBMT (2 long-term survivors).
GVHD
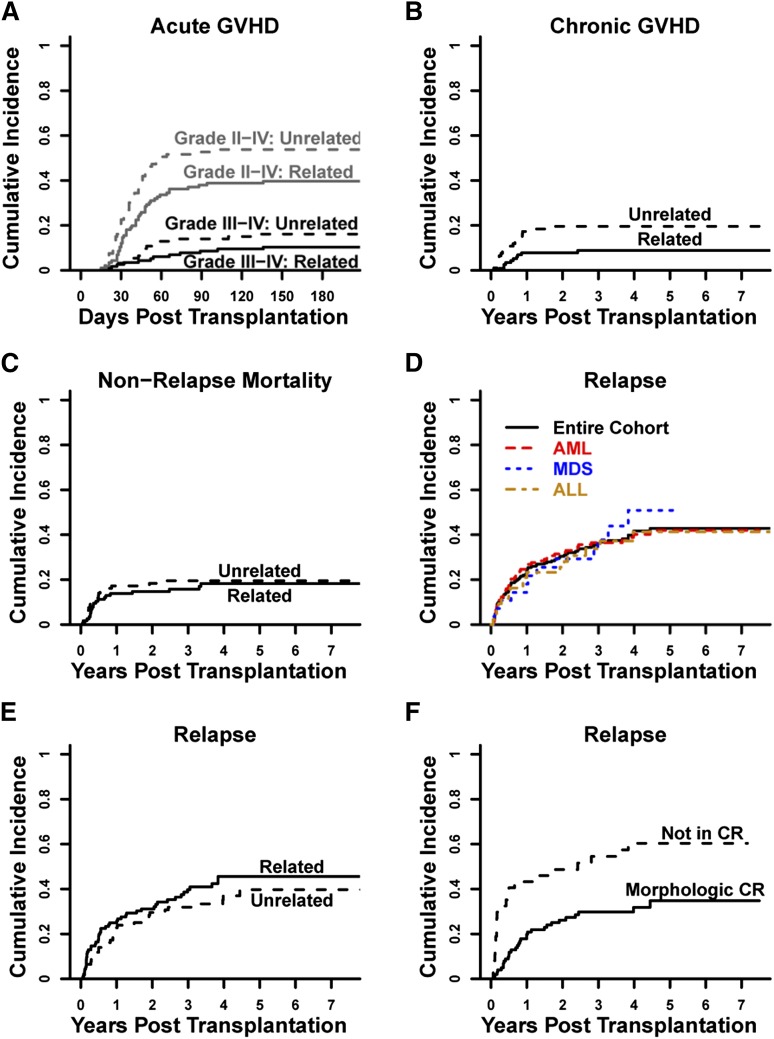
On competing-risk analysis, the estimated cumulative incidence of grade II to IV aGVHD at 100 days was 45% (95% confidence interval [CI], 39% to 52%) with a higher rate seen after HLA-matched–unrelated (54%; 95% CI, 44% to 64%) vs HLA-matched–related (39%; 95% CI, 30% to 48%) allografting (subdistribution hazard ratio [SDHR], 1.58; 95% CI, 1.07 to 2.35; P = .023) (Figure 1A). The estimated cumulative incidence of grade III to IV aGVHD at 100 days was 11% (95% CI, 7% to 15%) with no significant difference seen between patients receiving either HLA-matched–unrelated (14%; 95% CI, 7% to 21%) or HLA-matched–related (9%; 95% CI, 3% to 14%) alloBMT (SDHR, 1.73; 95% CI, 0.82 to 3.64; P = .15) (Figure 1A). The estimated cumulative incidence of cGVHD at 2 years was 13% (95% CI, 8% to 18%) and was significantly higher in patients receiving HLA-matched–unrelated (20%; 95% CI, 11% to 28%) vs HLA-matched–related (8%; 95% CI, 3% to 13%) alloBMT (SDHR, 2.42; 95% CI, 1.13 to 5.22; P = .024) (Figure 1B). The single patient who received peripheral blood stem cells as the allograft source had neither aGVHD nor cGVHD. For treatment of aGVHD and/or cGVHD at any time, 99 patients (47%) received corticosteroids, 78 (37%) received any secondary systemic immunosuppressant, and 65 (31%) received a CNI. CNI use for any reason, including GVHD prophylaxis after second alloBMT or treatment of GVHD after DLI, occurred in 82 patients (39%). Ninety patients (43%) did not require immunosuppression for any reason after PTCy.
Figure 1.
GVHD, NRM, and relapse. Cumulative incidences are shown for (A) aGVHD, (B) cGVHD, (C) NRM, (D) relapse for the entire cohort by disease type, (E) relapse for the entire cohort by donor type, and (F) relapse for AML patients by their pretransplantation remission status.
NRM
The estimated cumulative incidences of NRM for the entire cohort at 100 days, 1 year, and 3 years were 7% (95% CI, 4% to 11%), 15% (95% CI, 10% to 20%), and 17% (95% CI, 12% to 23%), respectively. There were no differences in NRM rates between patients receiving HLA-matched–unrelated vs HLA-matched–related allografts (SDHR, 1.15; 95% CI, 0.61 to 2.17; P = .66) (Figure 1C). Causes of death for the 38 cases of NRM are shown in supplemental Table 2 and include aGVHD in 6 patients (2.9% of all patients), cGVHD in 4 patients (1.9% of all patients, all from complications of bronchiolitis obliterans syndrome), infection in 4 patients (1.9% of all patients), and veno-occlusive disease in 2 cases (1.0% of all patients). Despite no patients dying of cytomegalovirus (CMV) infection, in multivariate analysis, patients serologically positive for CMV had a higher cumulative incidence of NRM compared with patients serologically negative for CMV even when age-adjusted (Table 2).
Table 2.
Multivariate analyses
| Variables | NRM | Relapse | DFS | OS | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| HR | 95% CI | P | HR | 95% CI | P | HR | 95% CI | P | HR | 95% CI | P | |
| Entire cohort | ||||||||||||
| Age at alloBMT | 1.04 | 1.01-1.07 | .016 | 1.02 | 1.00-1.04 | .0292 | 1.02 | 1.00-1.04 | .0167 | |||
| HCT-CI 1-2 vs 0 | 1.39 | 0.75-2.6 | .2975 | |||||||||
| HCT-CI ≥3 vs 0 | 2.1 | 1.17-3.77 | .0127 | |||||||||
| Bu/Flu vs Bu/Cy | 0.61 | 0.35-1.08 | .091 | |||||||||
| Active disease vs morphologic CR | 2.31 | 1.46-3.64 | .0003 | 1.55 | 1.05-2.29 | .0279 | ||||||
| Patient CMV serology positive vs negative | 2.74 | 1.33-5.66 | .0063 | 1.66 | 1.15-2.40 | .0073 | 1.44 | 0.96-2.16 | .081 | |||
| Donor CMV serology positive vs negative | 1.63 | 1.05-2.53 | .03 | |||||||||
| TNC dose | 0.79 | 0.67-0.93 | .005 | 0.82 | 0.71-0.95 | .0066 | 0.79 | 0.68-0.92 | .0018 | |||
| AML patients | ||||||||||||
| Age at alloBMT | 1.04 | 1-1.07 | .032 | 1.01 | 0.99-1.03 | .2568 | 1.01 | 0.99-1.04 | .2352 | |||
| HCT-CI 1-2 vs 0 | 1.52 | 0.71-3.25 | .2831 | 1.51 | 0.69-3.32 | .303 | ||||||
| HCT-CI ≥3 vs 0 | 1.9 | 0.96-3.78 | .0662 | 1.82 | 0.89-3.71 | .0986 | ||||||
| Bu/Flu vs Bu/Cy | 0.48 | 0.21-1.11 | .0858 | 0.39 | 0.14-1.08 | .0699 | ||||||
| Active disease vs morphologic CR | 2.51 | 1.35-4.66 | .0036 | 2.26 | 1.39-3.67 | .001 | 1.87 | 1.12-3.12 | .0159 | |||
| Patient CMV serology positive vs negative | 4.13 | 1.58-10.8 | .0038 | 1.91 | 1.16-3.13 | .0105 | 1.61 | 0.96-2.71 | .0696 | |||
| TNC dose | 0.75 | 0.59-0.95 | .018 | 0.77 | 0.64-0.94 | .0095 | 0.74 | 0.6-0.91 | .0035 | |||
| Cytogenetics* adverse vs favorable/intermediate | 2.7 | 1.46-4.98 | .0015 | 2.2 | 1.31-3.7 | .0028 | 2.16 | 1.27-3.68 | .0044 | |||
By refined MRC criteria as per Grimwade et al.29
Relapse
The estimated cumulative incidence of relapse at 3 years for the entire cohort was 36% (95% CI, 29% to 43%) (Figure 1D). Relapse rates after HLA-matched–unrelated allografting were not significantly different than rates after HLA-matched–related allografting (SDHR, 0.8; 95% CI, 0.51 to 1.24; P = .32) (Figure 1E). The estimated cumulative incidence of relapse at 3 years for AML patients was 36% (95% CI, 28% to 45%), 30% (95% CI, 21% to 39%) for AML patients in morphologic CR, and 55% (95% CI, 38% to 71%) for AML patients with active disease (Figure 1F). The estimated cumulative incidences of relapse at 3 years for MDS and ALL patients were 38% (95% CI, 19% to 57%) and 34% (95% CI, 19% to 49%), respectively. In multivariate analysis for the entire cohort, active disease at alloBMT, donor serologic positivity for CMV, and lower allograft total nucleated cell (TNC) dose (treated as a continuous variable) were associated with higher cumulative incidence of relapse (Table 2). In multivariate analysis of AML patients, active disease, lower TNC dose, and adverse cytogenetics were associated with higher cumulative incidence of relapse (Table 2).
Treatment of relapse included DLI in 28 patients (35% of relapses) at a median 1.3 years (range, 0.2 to 3.9 years) posttransplantation or a reduced-intensity conditioning HLA-haploidentical alloBMT11 in 3 patients (4% of relapses) at a range of 0.7 to 2.4 years posttransplantation. Of the 19 AML patients receiving DLI, 6 had long-term CRs (follow-up: median, 4.2 years; range, 2.2 to 5.2 years), 3 had transient CRs (remissions of 0.6, 2.8, and 3.0 years in length), 2 patients died in CR of infectious complications, 2 died early post-DLI with unclear response, and 6 patients had no response. Four MDS patients received DLI with 1 maintained CR of 4.9 years, 2 transient CRs of 1.0 and 1.3 years, and 1 without response. Five ALL patients received DLI with 2 transient CRs (0.6 and 3.1 years), 1 death early post-DLI with unclear response, and 2 with no response. Second alloBMTs using HLA-haploidentical donors were associated with 2 sustained CRs (1.3 and 2.5 years) in ALL patients and 1 transient CR (1.3 years) in an AML patient.
Survival outcomes for the entire cohort
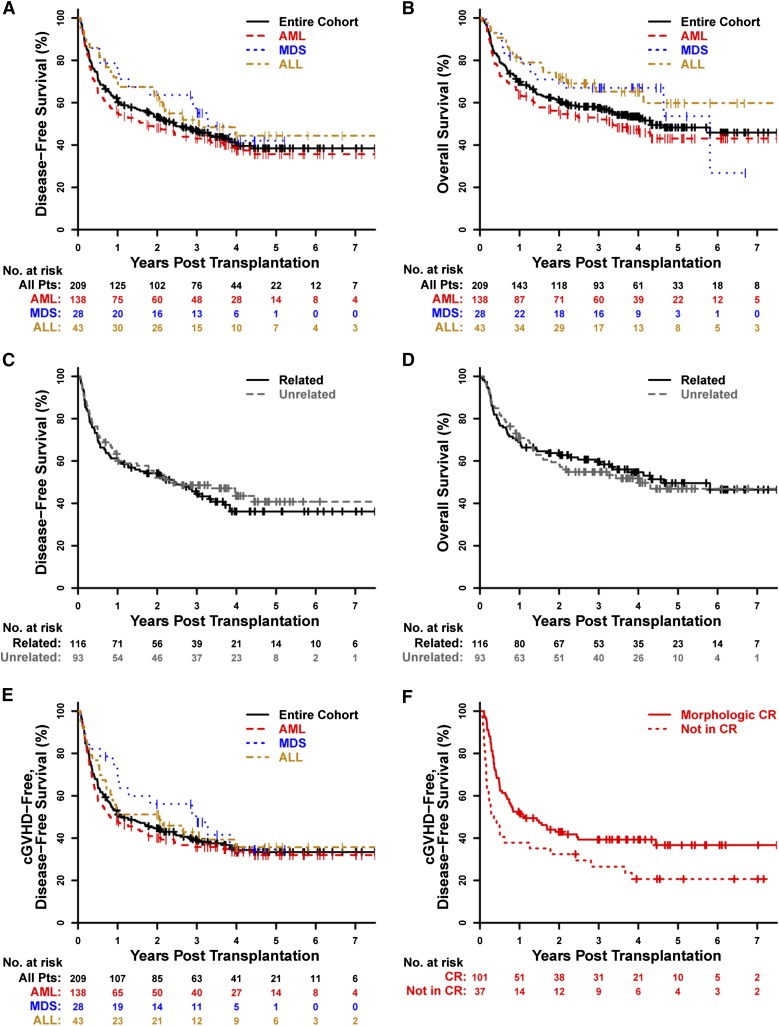
In the entire cohort, the 3-year probabilities of DFS and OS were 46% (95% CI, 40% to 54%) and 58% (95% CI, 51% to 65%), respectively (Figure 2A-B). Three-year DFS and OS outcomes were similar between patients receiving HLA-matched–unrelated (DFS: 49%; 95% CI, 39% to 60%; OS: 55%; 95% CI, 45% to 66%) or HLA-matched–related (DFS: 44%; 95% CI, 36% to 55%; OS: 60%; 95% CI, 51% to 69%) allografts (Figure 2C-D). In multivariate analysis, older age (treated as a continuous variable), active disease at alloBMT, patient serologic positivity for CMV, and lower allograft nucleated cell dose were each associated with inferior DFS (Table 2). Older age, HCT-CI ≥3, and lower TNC dose were also associated with inferior OS in multivariate analysis (Table 2). cGVHD-DFS at 3 years for the entire cohort was 39% (95% CI, 33% to 46%) (Figure 2E).
Figure 2.
Survival outcomes. Kaplan-Meier estimates of DFS and OS are shown for the entire cohort by (A-B) disease type and (C-D) donor type. Kaplan-Meier estimates of the composite outcome of cGVHD-DFS are shown for the entire cohort (E) by disease type and (F) for AML patients by remission status at the time of allogeneic transplantation. Pts, patients.
Survival outcomes for AML patients
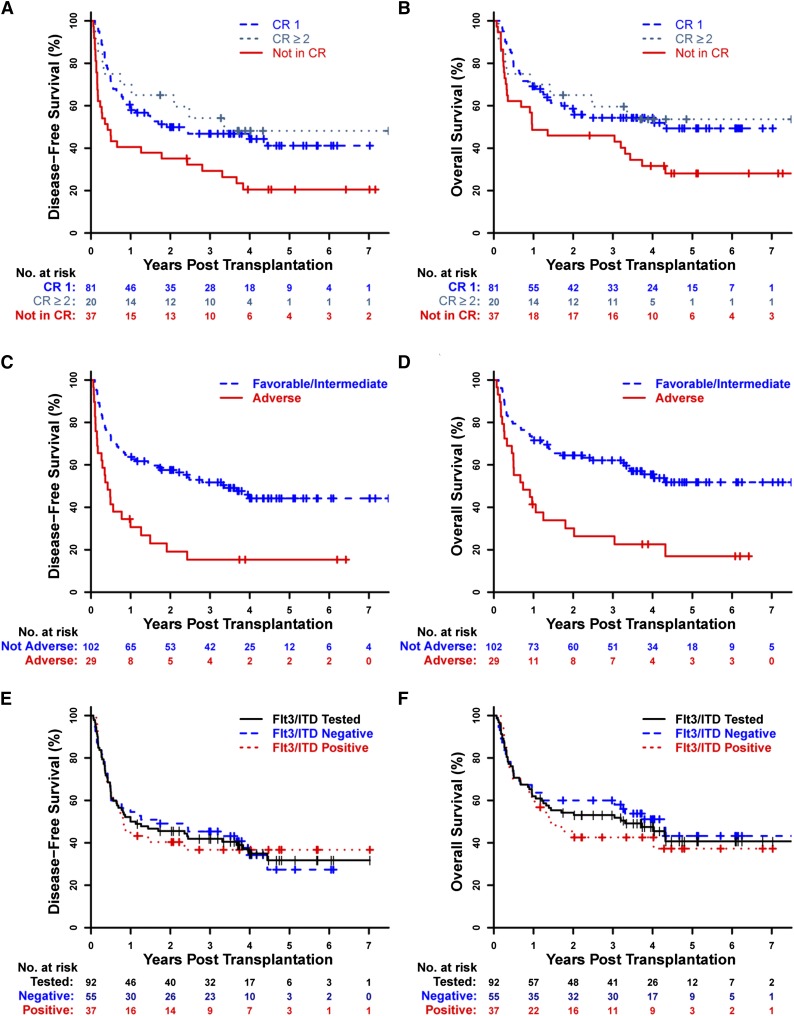
In AML patients, the 3-year probabilities of DFS, OS, and cGVHD-DFS were 43% (95% CI, 35% to 52%), 53% (95% CI, 45% to 62%), and 36% (95% CI, 28% to 45%), respectively (Figure 2A-B,E). AML patients in morphologic CR had significantly higher DFS (48% vs 29% at 3 years), OS (55% vs 50% at 3 years), and cGVHD-DFS (39% vs 27% at 3 years) than AML patients with active disease (DFS: hazard ratio [HR], 2.05; 95% CI, 1.31 to 3.23; P = .0018; OS: HR, 1.78; 95% CI, 1.1 to 2.87; P = .019; cGVHD-DFS: HR, 1.77; 95% CI, 1.14 to 2.75; P = .012) (Figures 2F and 3A-B), but outcomes were similar regardless of whether patients were in first or subsequent CR (Figure 3A-B).
Figure 3.
Survival outcomes for AML patients. Kaplan-Meier estimates of DFS and OS are shown for AML patients (n = 138) by (A-B) their pretransplantation remission status, (C-D) cytogenetics by the refined MRC criteria in those tested (n = 131), and (E-F) Flt3/ITD status in those tested (n = 92).
Cytogenetics were significantly associated with outcomes in AML patients. AML patients with adverse cytogenetics by the refined MRC classification had markedly lower DFS and OS than the combined group of AML patients with favorable- or intermediate-risk cytogenetics (Figure 3C-D). This combined favorable/intermediate-risk group was used because there were few (n = 9) AML patients with favorable-risk cytogenetics by the refined MRC criteria, and their outcomes were similar to those of patients with intermediate-risk cytogenetics (supplemental Figure 1A-B). Of note, all AML patients in our cohort with monosomal karyotype32 were already included in the adverse group on the basis of refined MRC criteria. Lower DFS and OS were also seen for unfavorable or adverse cytogenetic risk groups when using the Southwest Oncology Group30 or Armand et al33 cytogenetic criteria (supplemental Figure 1C-F).
Higher HCT-CI scores were also associated with inferior survival outcomes in AML patients (supplemental Figure 2A-B). There was no impact on survival outcomes of AML being either de novo (n = 86) or secondary (n = 52). Among those tested for Flt3/ITD, DFS and OS were similar for AML patients with (n = 37) or without (n = 55) Flt3/ITD (Figure 3E-F). Similarly, there was no apparent effect of Flt3/ITD status on DFS or OS when examining only Flt3/ITD-tested patients with a normal karyotype (n = 48). In AML patients in morphologic CR at initiation of alloBMT, detectable MRD did not appear to have an impact on DFS or OS outcomes (supplemental Figure 2C-D). In multivariate analysis for AML patients, active disease at alloBMT, adverse cytogenetics, and lower TNC dose were associated with inferior DFS and OS; patient serologic positivity for CMV was also associated with inferior DFS (Table 2).
Survival outcomes for ALL and MDS patients
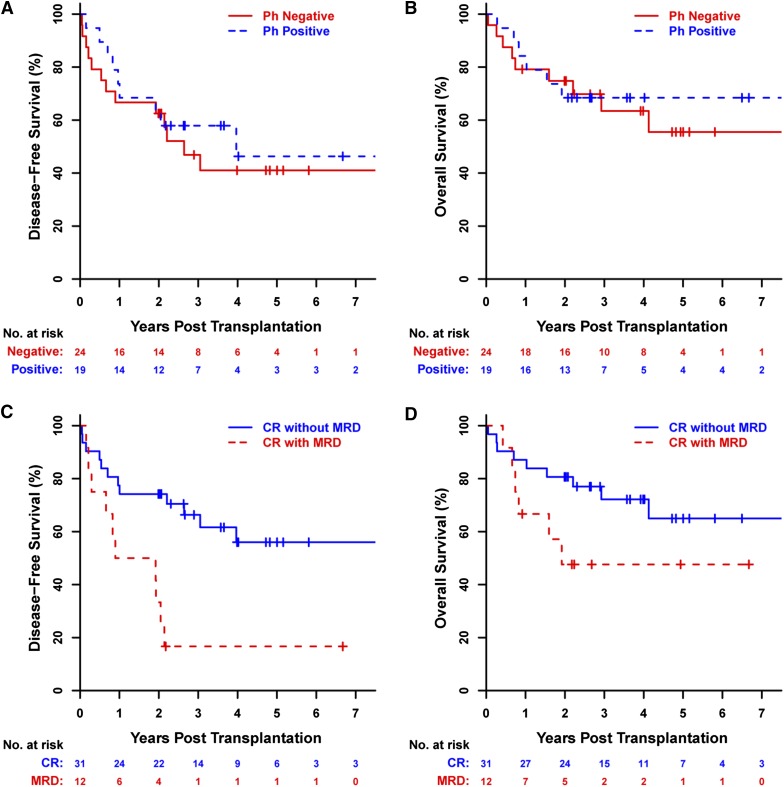
In ALL patients, the 3-year probabilities of DFS and OS were 52% (95% CI, 39% to 70%) and 65% (95% CI, 52% to 82%), respectively (Figure 2A-B). There was no apparent impact of the Philadelphia chromosome on DFS or OS outcomes of ALL patients (Philadelphia chromosome negative as reference; DFS: HR, 0.8; 95% CI, 0.34 to 1.86; P = .60; OS: HR, 0.84; 95% CI, 0.3 to 2.37; P = .74) (Figure 4A-B). However, MRD in ALL patients was associated with significantly lower DFS (HR, 3.51; 95% CI, 1.47 to 8.43; P = .0048) with a trend toward lower OS as well (HR, 2.24; 95% CI, 0.79 to 6.38; P = .13) (Figure 4C-D).
Figure 4.
Survival outcomes for ALL patients. Kaplan-Meier estimates of DFS and OS for ALL patients by (A-B) the presence of the Philadelphia chromosome t(9;22) (Ph) and (C-D) the presence of MRD at their pretransplantation assessment.
DFS and OS at 3 years for MDS patients were 55% (95% CI, 39% to 78%) and 67% (95% CI, 51% to 87%), respectively (Figure 2A-B). Although the comparative power was limited by low numbers (n = 28), there was no significant effect of the International Prognostic Scoring System cytogenetic risk groups detected on DFS or OS outcomes for MDS patients (supplemental Figure 3).
Discussion
Data from this retrospective analysis of longer-term outcomes of AML, MDS, and ALL patients treated at our institution with PTCy as sole GVHD prophylaxis after MAC and HLA-matched allografting support that this approach is associated with a low incidence of cGVHD. The cumulative incidence of cGVHD using PTCy is lower than rates reported using standard CNI-based GVHD prophylaxis with or without antithymocyte globulin5-7 or even using TCD34,35 for either HLA-matched–related or –unrelated alloBMT. Yet, the mechanisms underlying cGVHD prevention by PTCy remain to be fully defined. Since grade II to IV aGVHD rates are similar to those seen with CNI-based GVHD prophylaxis,5,6 elimination of alloreactive T cells cannot fully explain cGVHD prevention after PTCy.14 Preservation of regulatory T cells by PTCy may complement reductions in alloreactive T cells,15 thus allowing tolerance induction to occur. Another possibility is that aGVHD and cGVHD are mediated by T-cell subsets that are differentially affected by PTCy. The role of non–T-cell immune subsets and thymic clonal selection36 in the prevention of cGVHD by PTCy are actively being studied.
In multivariate analysis, 4 factors (pre-alloBMT remission status, cytogenetics, allograft TNC dose, and patient CMV seropositivity) were found to be associated with inferior DFS and/or OS for AML patients treated with our transplantation platform. Although outcomes of patients with active disease or adverse cytogenetics are discouraging, they are similar to those seen using other transplantation approaches.33,37 The strong protective influence of high TNC dose in the allograft on survival outcomes has been consistently found in previous reports,38-42 wherein the improvement in survival has been differentially attributed to positive impacts on NRM,38,39 relapse (as in our patients), or both.40,41 The lack of an impact of allograft TNC dose on NRM in our patients may be reflective of the high median TNC dose given, with only 17% of patients receiving a TNC dose <3.0 × 108 cells per kilogram. The mechanism of reduced relapse by higher TNC dose remains unknown but could reflect natural killer cell antitumor immunity since allograft CD34+, CD3+, CD4+, or CD8+ cell doses did not have an impact on relapse or survival outcomes in our patients. The association of patient CMV seropositivity with worse survival outcomes has been previously reported.43-45 However, the mechanism underlying this interaction in our patients is unclear because none died of invasive CMV disease. Potentially, stimulation by CMV antigens may be skewing the immune response in a negative manner, even in the absence of CMV disease.
A few of our results differed from those previously reported with the use of other transplantation platforms. Donor CMV seropositivity was associated in our patient cohort with an increased risk of relapse. Because this result is contrary to the results of several prior studies46-49 and because there was no association between donor CMV seropositivity and relapse within the AML cohort and no effect of donor CMV seropositivity on survival outcomes, this relationship may represent a type I statistical error. The apparent lack of an effect of MRD on outcomes for our AML patients directly contrasts with a recent report suggesting that any detectable disease in AML patients portends a worse prognosis.50 Beyond a smaller sample size, our failure to show an impact of MRD in AML patients likely reflects several factors. First, flow cytometric assessment for MRD by the Seattle group50 has been performed prospectively since 2006 (before the clinical import of MRD in adult AML had been recognized) and thus is more comprehensive than that used at our institution. By contrast, the practices used at our institution have greater sensitivity for detecting MRD in ALL, which showed a dramatic effect on outcomes in our patients. Second, many of our patients were treated at a time when the available molecular tests were limited. Finally, the specific tests performed for restaging of disease status pre-alloBMT were not always consistent, even for patients who had a molecular marker that could be followed. Thus, some patients who truly had MRD may not have been recognized as such. The reported impact of Flt3/ITD positivity on outcomes after alloBMT has been inconsistent between various studies,51 but no detectable influence of Flt3/ITD positivity (n = 37) was seen in our study among the two-thirds of AML patients (n = 92) who were tested. Whether differences between our results and those of other studies reflect true differential effects of the PTCy transplantation platform, low statistical power, or confounding factors would require assessment in larger independent cohorts of patients.
Although comparing outcomes among studies is problematic, relapse, DFS, and OS outcomes for our patients appear similar to those seen in contemporaneous studies using CNIs or TCD (Table 3),6,7,35,52,53 despite the low incidence of cGVHD in our patients. There has been increasing interest in defining new clinical endpoints that fully integrate the potential for optimal long-term patient outcomes that may be missed by simple DFS. Given that cGVHD is a leading cause of late posttransplant morbidity and mortality,4 cGVHD-DFS has been proposed as a new endpoint. This endpoint will be used in upcoming BMT CTN studies, including being used as the primary endpoint in BMT CTN 1301, which will directly compare PTCy vs TCD vs CNI-based pharmacologic prophylaxis for the treatment of patients with acute leukemia or MDS undergoing MAC and HLA-matched allografting. Because our patients have a low incidence of cGVHD and thus NRM beyond 1 year, their DFS and cGVHD-DFS rates were similar. The absence of prophylactic immunosuppression beyond day +4 after HLA-matched alloBMT is an additional advantage of PTCy GVHD prophylaxis. In fact, almost half our patients did not receive further immunosuppression after PTCy. The relatively low incidences of cGVHD and NRM and the ability to limit immunosuppression after alloBMT make PTCy a promising transplantation platform for the integration of pharmacologic and immunologic postgrafting strategies to prevent or treat relapse.
Table 3.
Comparison with other contemporaneous studies
| Reference | Approach | Study time period | No. of patients | MAC (%) | Related or unrelated allografts | % AML, MDS, or ALL patients | Median follow-up, y | GVHD CI (%) | NRM CI (%) | Relapse CI (%) | DFS (%) | OS (%) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Acute grade II-IV at 100 d | Acute grade III-IV, at 100 d | Chronic at 2 y | At 1 y | At 3 y | At 2 y (AML in CR only) | At 3 y (AML in CR only) | At 3 y | At 2 y | At 3 y (AML in CR only) | At 3 y | At 2 y | At 3 y | ||||||||
| This study | PTCy | 2004-2011 | 209 | 100 | Both | 100 | 4.0 | 45 | 11 | 13 | 15 | 17 | 26 | 30 | 36 | 53 | 48 | 46 | 61 | 58 |
| 6* | CNI/ MTX | 2004-2009 | 278 | 80 | Unrelated | 87 | 3.0 | 46 | 14 | 41 | 23 | 27 | NR | NR | 30 | 44 | NR | 43 | 49 | 46 |
| 7† | ATG-F/ CsA/MTX | 2003-2007 | 103 | 100 | Unrelated | 94 | 3.0 | 33 | 12 | 30 | NR | 19 | NR | NR | 33 | NR | NR | NR | NR | 55 |
| 7† | CsA/MTX | 2003-2007 | 98 | 100 | Unrelated | 86 | 3.0 | 51 | 25 | 60 | NR | 34 | NR | NR | 28 | NR | NR | NR | NR | 43 |
| 35 | TCD‡ | 2005-2008 | 44 | 100 | Related | 100 AML CR1/2 | 2.8 | 23 | 4.5 | 19 | NR | 21 (2 y) | 24 | NR | NR | 55 | NR | NR | 59 | NR |
| 35 | CNI-based§ | 2003-2006 | 84 | 100 | Related | 100 AML CR1/2 | 4.0 | 39 | 9.5 | 50 | NR | 19 (2 y) | 27 | NR | NR | 54 | NR | NR | 65 | NR |
| 52 | TCD|| | 2001-2010 | 115 | 100 | Both | 100 AML CR1 | 2.7 | 5 | 1 | 13 | 18 | 24 | NR | 18 | NR | NR | 58 | NR | NR | 57 |
| 52 | Tacro/ mini-MTX¶ | 2001-2010 | 181 | 100 | Both | 100 AML CR1 | 2.4 | 18 | 3 | 53 | 13 | 16 | NR | 25 | NR | NR | 60 | NR | NR | 66 |
ATG, antithymocyte globulin; ATG-F, antithymocyte globulin-Fresenius; CI, cumulative incidence; CsA, cyclosporine-A; MTX, methotrexate; NR, not reported; tacro, tacrolimus.
Data are shown for patients receiving bone marrow allografts. Data were obtained from the original publication and personal communication with the study statistician, Brent Logan (Medical College of Wisconsin). Ninety percent of patients received a CNI and MTX; for the other 10%, treatment was not specified.
Results for early posttransplantation outcomes are taken from Finke et al.53
TCD was performed both ex vivo (Miltenyi CliniMACS CD34 selection) and in vivo (rabbit ATG on day −4).
Included patients received tacrolimus and MTX (n = 39); tacrolimus and mycophenolate mofetil (n = 4); tacrolimus and corticosteroids (n = 1); tacrolimus, MTX, and mycophenolate mofetil (n = 1); or tacrolimus alone (n = 3).
TCD was performed ex vivo, but 45% of patients also received ATG.
Forty-three percent of patients also received ATG, and 8% also received pentostatin.
Acknowledgments
The authors thank Janice Davis Sproul, M. Victor Lemas, and the Johns Hopkins Cell Therapy Laboratory for providing graft composition and donor lymphocyte infusion data on the patients studied. The authors also thank Brent Logan and Claudio Anasetti for kindly providing specific estimates of outcomes for the bone marrow allografting group from their randomized study. The authors thank all patients and research/clinical staff who made this work possible.
This work was supported by a P01 grant from the National Cancer Institute of the National Institutes of Health (CA 015396). C.G.K. was supported by a Leukemia & Lymphoma Society Special Fellow in Clinical Research award.
Footnotes
The online version of this article contains a data supplement.
There is an Inside Blood Commentary on this article in this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Authorship
Contribution: C.G.K. designed the study, planned analyses, collected data, provided clinical care of patients, and wrote the manuscript; H.-L.T. planned and performed statistical analyses and prepared figures; J.B.-M. provided clinical care of patients, particularly the scoring and treatment of patients with graft-versus-host disease, and revised the manuscript; B.D.S., I.G., J.A.K., and Y.L.K. helped define endpoints, assessed the pretransplantation remission status of patients, provided clinical care of patients, and revised the manuscript; D.E.G., W.M., I.B., C.A.H., L.J.S., J.D.P., K.W.P., A.E.D., M.M.S., M.A.M., R.A.B., M.J.L., and R.F.A. provided clinical care of patients; G.L.R. planned and supervised statistical analyses; E.J.F. and R.J.J. provided clinical care of patients, contributed to the analytical plan, and revised the manuscript; and L.L. designed the study, planned analyses, provided clinical care of patients, and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Leo Luznik, Cancer Research Building I, Room 2M88, 1650 Orleans St, Baltimore, MD 21287; e-mail: luznile@jhmi.edu.
References
- 1.Weiden PL, Sullivan KM, Flournoy N, Storb R, Thomas ED. Antileukemic effect of chronic graft-versus-host disease: contribution to improved survival after allogeneic marrow transplantation. N Engl J Med. 1981;304(25):1529–1533. doi: 10.1056/NEJM198106183042507. [DOI] [PubMed] [Google Scholar]
- 2.Horowitz MM, Gale RP, Sondel PM, et al. Graft-versus-leukemia reactions after bone marrow transplantation. Blood. 1990;75(3):555–562. [PubMed] [Google Scholar]
- 3.Weisdorf D, Zhang MJ, Arora M, Horowitz MM, Rizzo JD, Eapen M. Graft-versus-host disease induced graft-versus-leukemia effect: greater impact on relapse and disease-free survival after reduced intensity conditioning. Biol Blood Marrow Transplant. 2012;18(11):1727–1733. doi: 10.1016/j.bbmt.2012.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bhatia S, Francisco L, Carter A, et al. Late mortality after allogeneic hematopoietic cell transplantation and functional status of long-term survivors: report from the Bone Marrow Transplant Survivor Study. Blood. 2007;110(10):3784–3792. doi: 10.1182/blood-2007-03-082933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ratanatharathorn V, Nash RA, Przepiorka D, et al. Phase III study comparing methotrexate and tacrolimus (prograf, FK506) with methotrexate and cyclosporine for graft-versus-host disease prophylaxis after HLA-identical sibling bone marrow transplantation. Blood. 1998;92(7):2303–2314. [PubMed] [Google Scholar]
- 6.Anasetti C, Logan BR, Lee SJ, et al. Blood and Marrow Transplant Clinical Trials Network. Peripheral-blood stem cells versus bone marrow from unrelated donors. N Engl J Med. 2012;367(16):1487–1496. doi: 10.1056/NEJMoa1203517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Socié G, Schmoor C, Bethge WA, et al. ATG-Fresenius Trial Group. Chronic graft-versus-host disease: long-term results from a randomized trial on graft-versus-host disease prophylaxis with or without anti-T-cell globulin ATG-Fresenius. Blood. 2011;117(23):6375–6382. doi: 10.1182/blood-2011-01-329821. [DOI] [PubMed] [Google Scholar]
- 8.Wagner JE, Thompson JS, Carter SL, Kernan NA Unrelated Donor Marrow Transplantation Trial. Effect of graft-versus-host disease prophylaxis on 3-year disease-free survival in recipients of unrelated donor bone marrow (T-cell Depletion Trial): a multi-centre, randomised phase II-III trial. Lancet. 2005;366(9487):733–741. doi: 10.1016/S0140-6736(05)66996-6. [DOI] [PubMed] [Google Scholar]
- 9.Soiffer RJ, Lerademacher J, Ho V, et al. Impact of immune modulation with anti-T-cell antibodies on the outcome of reduced-intensity allogeneic hematopoietic stem cell transplantation for hematologic malignancies. Blood. 2011;117(25):6963–6970. doi: 10.1182/blood-2011-01-332007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Luznik L, Fuchs EJ. High-dose, post-transplantation cyclophosphamide to promote graft-host tolerance after allogeneic hematopoietic stem cell transplantation. Immunol Res. 2010;47(1-3):65–77. doi: 10.1007/s12026-009-8139-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Luznik L, O’Donnell PV, Symons HJ, et al. HLA-haploidentical bone marrow transplantation for hematologic malignancies using nonmyeloablative conditioning and high-dose, posttransplantation cyclophosphamide. Biol Blood Marrow Transplant. 2008;14(6):641–650. doi: 10.1016/j.bbmt.2008.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Munchel A, Kesserwan C, Symons HJ, et al. Nonmyeloablative, HLA-haploidentical bone marrow transplantation with high dose, post-transplantation cyclophosphamide. Pediatr Rep. 2011;3(Suppl 2):e15. doi: 10.4081/pr.2011.s2.e15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Luznik L, Bolaños-Meade J, Zahurak M, et al. High-dose cyclophosphamide as single-agent, short-course prophylaxis of graft-versus-host disease. Blood. 2010;115(16):3224–3230. doi: 10.1182/blood-2009-11-251595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Luznik L, O’Donnell PV, Fuchs EJ. Post-transplantation cyclophosphamide for tolerance induction in HLA-haploidentical bone marrow transplantation. Semin Oncol. 2012;39(6):683–693. doi: 10.1053/j.seminoncol.2012.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kanakry CG, Ganguly S, Zahurak M, et al. Aldehyde dehydrogenase expression drives human regulatory T cell resistance to posttransplantation cyclophosphamide. Sci Transl Med. 2013;5(211):211ra157. [DOI] [PMC free article] [PubMed]
- 16.Kanakry CG, O’Donnell PV, Furlong T, et al. Multi-institutional study of post-transplantation cyclophosphamide as single-agent graft-versus-host disease prophylaxis after allogeneic bone marrow transplantation using myeloablative busulfan and fludarabine conditioning. J Clin Oncol. 2014;32(31):3497–3505. doi: 10.1200/JCO.2013.54.0625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Przepiorka D, Weisdorf D, Martin P, et al. 1994 Consensus Conference on Acute GVHD Grading. Bone Marrow Transplant. 1995;15(6):825–828. [PubMed] [Google Scholar]
- 18.Shulman HM, Sullivan KM, Weiden PL, et al. Chronic graft-versus-host syndrome in man. A long-term clinicopathologic study of 20 Seattle patients. Am J Med. 1980;69(2):204–217. doi: 10.1016/0002-9343(80)90380-0. [DOI] [PubMed] [Google Scholar]
- 19.Filipovich AH, Weisdorf D, Pavletic S, et al. National Institutes of Health consensus development project on criteria for clinical trials in chronic graft-versus-host disease: I. Diagnosis and staging working group report. Biol Blood Marrow Transplant. 2005;11(12):945–956. doi: 10.1016/j.bbmt.2005.09.004. [DOI] [PubMed] [Google Scholar]
- 20.Alousi AM, Weisdorf DJ, Logan BR, et al. Blood and Marrow Transplant Clinical Trials Network. Etanercept, mycophenolate, denileukin, or pentostatin plus corticosteroids for acute graft-versus-host disease: a randomized phase 2 trial from the Blood and Marrow Transplant Clinical Trials Network. Blood. 2009;114(3):511–517. doi: 10.1182/blood-2009-03-212290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bolaños-Meade J, Logan BR, Alousi AM, et al. Phase III clinical trial steroids/mycophenolate mofetil vs steroids/placebo as therapy for acute graft-versus-host disease: BMT CTN 0802. Blood. 2014 Aug 28. [Epub ahead of print]
- 22.Cheson BD, Bennett JM, Kopecky KJ, et al. International Working Group for Diagnosis, Standardization of Response Criteria, Treatment Outcomes, and Reporting Standards for Therapeutic Trials in Acute Myeloid Leukemia. Revised recommendations of the International Working Group for Diagnosis, Standardization of Response Criteria, Treatment Outcomes, and Reporting Standards for Therapeutic Trials in Acute Myeloid Leukemia. J Clin Oncol. 2003;21(24):4642–4649. doi: 10.1200/JCO.2003.04.036. [DOI] [PubMed] [Google Scholar]
- 23.Brüggemann M, Schrauder A, Raff T, et al. European Working Group for Adult Acute Lymphoblastic Leukemia (EWALL); International Berlin-Frankfurt-Münster Study Group (I-BFM-SG) Standardized MRD quantification in European ALL trials: proceedings of the Second International Symposium on MRD assessment in Kiel, Germany, 18-20 September 2008. Leukemia. 2010;24(3):521–535. doi: 10.1038/leu.2009.268. [DOI] [PubMed] [Google Scholar]
- 24.Cheson BD, Greenberg PL, Bennett JM, et al. Clinical application and proposal for modification of the International Working Group (IWG) response criteria in myelodysplasia. Blood. 2006;108(2):419–425. doi: 10.1182/blood-2005-10-4149. [DOI] [PubMed] [Google Scholar]
- 25.Kaplan EL, Meier P. Nonparametric-Estimation from Incomplete Observations. J Am Stat Assoc. 1958;53:457–481. [Google Scholar]
- 26.Fine JP, Gray RJ. A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc. 1999;94:496–509. [Google Scholar]
- 27.Gray RJ. A Class of K-Sample Tests for Comparing the Cumulative Incidence of a Competing Risk. Ann Stat. 1988;16:1141–1154. [Google Scholar]
- 28.Sorror ML, Maris MB, Storb R, et al. Hematopoietic cell transplantation (HCT)-specific comorbidity index: a new tool for risk assessment before allogeneic HCT. Blood. 2005;106(8):2912–2919. doi: 10.1182/blood-2005-05-2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Grimwade D, Hills RK, Moorman AV, et al. National Cancer Research Institute Adult Leukaemia Working Group. Refinement of cytogenetic classification in acute myeloid leukemia: determination of prognostic significance of rare recurring chromosomal abnormalities among 5876 younger adult patients treated in the United Kingdom Medical Research Council trials. Blood. 2010;116(3):354–365. doi: 10.1182/blood-2009-11-254441. [DOI] [PubMed] [Google Scholar]
- 30.Slovak ML, Kopecky KJ, Cassileth PA, et al. Karyotypic analysis predicts outcome of preremission and postremission therapy in adult acute myeloid leukemia: a Southwest Oncology Group/Eastern Cooperative Oncology Group Study. Blood. 2000;96(13):4075–4083. [PubMed] [Google Scholar]
- 31.Greenberg P, Cox C, LeBeau MM, et al. International scoring system for evaluating prognosis in myelodysplastic syndromes. Blood. 1997;89(6):2079–2088. [PubMed] [Google Scholar]
- 32.Breems DA, Van Putten WL, De Greef GE, et al. Monosomal karyotype in acute myeloid leukemia: a better indicator of poor prognosis than a complex karyotype. J Clin Oncol. 2008;26(29):4791–4797. doi: 10.1200/JCO.2008.16.0259. [DOI] [PubMed] [Google Scholar]
- 33.Armand P, Kim HT, Zhang MJ, et al. Classifying cytogenetics in patients with acute myelogenous leukemia in complete remission undergoing allogeneic transplantation: a Center for International Blood and Marrow Transplant Research study. Biol Blood Marrow Transplant. 2012;18(2):280–288. doi: 10.1016/j.bbmt.2011.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Devine SM, Carter S, Soiffer RJ, et al. Low risk of chronic graft-versus-host disease and relapse associated with T cell-depleted peripheral blood stem cell transplantation for acute myelogenous leukemia in first remission: results of the blood and marrow transplant clinical trials network protocol 0303. Biol Blood Marrow Transplant. 2011;17(9):1343–1351. doi: 10.1016/j.bbmt.2011.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pasquini MC, Devine S, Mendizabal A, et al. Comparative outcomes of donor graft CD34+ selection and immune suppressive therapy as graft-versus-host disease prophylaxis for patients with acute myeloid leukemia in complete remission undergoing HLA-matched sibling allogeneic hematopoietic cell transplantation. J Clin Oncol. 2012;30(26):3194–3201. doi: 10.1200/JCO.2012.41.7071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Eto M, Mayumi H, Tomita Y, Yoshikai Y, Nishimura Y, Nomoto K. Sequential mechanisms of cyclophosphamide-induced skin allograft tolerance including the intrathymic clonal deletion followed by late breakdown of the clonal deletion. J Immunol. 1990;145(5):1303–1310. [PubMed] [Google Scholar]
- 37.Armand P, Kim HT, DeAngelo DJ, et al. Impact of cytogenetics on outcome of de novo and therapy-related AML and MDS after allogeneic transplantation. Biol Blood Marrow Transplant. 2007;13(6):655–664. doi: 10.1016/j.bbmt.2007.01.079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Barker JN, Scaradavou A, Stevens CE. Combined effect of total nucleated cell dose and HLA match on transplantation outcome in 1061 cord blood recipients with hematologic malignancies. Blood. 2010;115(9):1843–1849. doi: 10.1182/blood-2009-07-231068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dominietto A, Lamparelli T, Raiola AM, et al. Transplant-related mortality and long-term graft function are significantly influenced by cell dose in patients undergoing allogeneic marrow transplantation. Blood. 2002;100(12):3930–3934. doi: 10.1182/blood-2002-01-0339. [DOI] [PubMed] [Google Scholar]
- 40.Rocha V, Labopin M, Gluckman E, et al. Acute Leukemia Working Party of the European Blood and Marrow Transplant Registry. Relevance of bone marrow cell dose on allogeneic transplantation outcomes for patients with acute myeloid leukemia in first complete remission: results of a European survey. J Clin Oncol. 2002;20(21):4324–4330. doi: 10.1200/JCO.2002.11.058. [DOI] [PubMed] [Google Scholar]
- 41.Barrett AJ, Ringdén O, Zhang MJ, et al. Effect of nucleated marrow cell dose on relapse and survival in identical twin bone marrow transplants for leukemia. Blood. 2000;95(11):3323–3327. [PubMed] [Google Scholar]
- 42.Peffault de Latour R, Purtill D, Ruggeri A, et al. Influence of nucleated cell dose on overall survival of unrelated cord blood transplantation for patients with severe acquired aplastic anemia: a study by eurocord and the aplastic anemia working party of the European group for blood and marrow transplantation. Biol Blood Marrow Transplant. 2011;17(1):78–85. doi: 10.1016/j.bbmt.2010.06.011. [DOI] [PubMed] [Google Scholar]
- 43.Castro-Malaspina H, Harris RE, Gajewski J, et al. Unrelated donor marrow transplantation for myelodysplastic syndromes: outcome analysis in 510 transplants facilitated by the National Marrow Donor Program. Blood. 2002;99(6):1943–1951. doi: 10.1182/blood.v99.6.1943. [DOI] [PubMed] [Google Scholar]
- 44.Lin YF, Lairson DR, Chan W, et al. Children with acute leukemia: a comparison of outcomes from allogeneic blood stem cell and bone marrow transplantation. Pediatr Blood Cancer. 2011;56(1):143–151. doi: 10.1002/pbc.22677. [DOI] [PubMed] [Google Scholar]
- 45.Boeckh M, Nichols WG. The impact of cytomegalovirus serostatus of donor and recipient before hematopoietic stem cell transplantation in the era of antiviral prophylaxis and preemptive therapy. Blood. 2004;103(6):2003–2008. doi: 10.1182/blood-2003-10-3616. [DOI] [PubMed] [Google Scholar]
- 46.Jacobsen N, Keiding N, Ryder L, et al. Graft-versus-leukaemia activity associated with cytomegalovirus antibody positive bone marrow donors in acute myeloid leukaemia. Lancet. 1987;1(8530):456–457. doi: 10.1016/s0140-6736(87)90166-8. [DOI] [PubMed] [Google Scholar]
- 47.Nachbaur D, Clausen J, Kircher B. Donor cytomegalovirus seropositivity and the risk of leukemic relapse after reduced-intensity transplants. Eur J Haematol. 2006;76(5):414–419. doi: 10.1111/j.1600-0609.2005.00625.x. [DOI] [PubMed] [Google Scholar]
- 48.Ljungman P, Brand R, Einsele H, Frassoni F, Niederwieser D, Cordonnier C. Donor CMV serologic status and outcome of CMV-seropositive recipients after unrelated donor stem cell transplantation: an EBMT megafile analysis. Blood. 2003;102(13):4255–4260. doi: 10.1182/blood-2002-10-3263. [DOI] [PubMed] [Google Scholar]
- 49.Schmidt-Hieber M, Labopin M, Beelen D, et al. CMV serostatus still has an important prognostic impact in de novo acute leukemia patients after allogeneic stem cell transplantation: a report from the Acute Leukemia Working Party of EBMT. Blood. 2013;122(19):3359–3364. doi: 10.1182/blood-2013-05-499830. [DOI] [PubMed] [Google Scholar]
- 50.Walter RB, Buckley SA, Pagel JM, et al. Significance of minimal residual disease before myeloablative allogeneic hematopoietic cell transplantation for AML in first and second complete remission. Blood. 2013;122(10):1813–1821. doi: 10.1182/blood-2013-06-506725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hu B, Vikas P, Mohty M, Savani BN. Allogeneic stem cell transplantation and targeted therapy for FLT3/ITD+ acute myeloid leukemia: an update. Expert Rev Hematol. 2014;7(2):301–315. doi: 10.1586/17474086.2014.857596. [DOI] [PubMed] [Google Scholar]
- 52.Bayraktar UD, de Lima M, Saliba RM, et al. Ex vivo T cell-depleted versus unmodified allografts in patients with acute myeloid leukemia in first complete remission. Biol Blood Marrow Transplant. 2013;19(6):898–903. doi: 10.1016/j.bbmt.2013.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Finke J, Bethge WA, Schmoor C, et al. Standard graft-versus-host disease prophylaxis with or without anti-T-cell globulin in haematopoietic cell transplantation from matched unrelated donors: a randomised, open-label, multicentre phase 3 trial. Lancet Oncol. 2009;10:855–864. doi: 10.1016/S1470-2045(09)70225-6. [DOI] [PubMed] [Google Scholar]