Abstract
Using manometric and enzymic techniques, H2 and CO2 evolution in darkness and light has been studied in the green alga Chlamydomonas reinhardtii F-60. F-60 is a mutant strain characterized by an incomplete photosynthetic carbon reduction cycle but an intact electron transport chain.
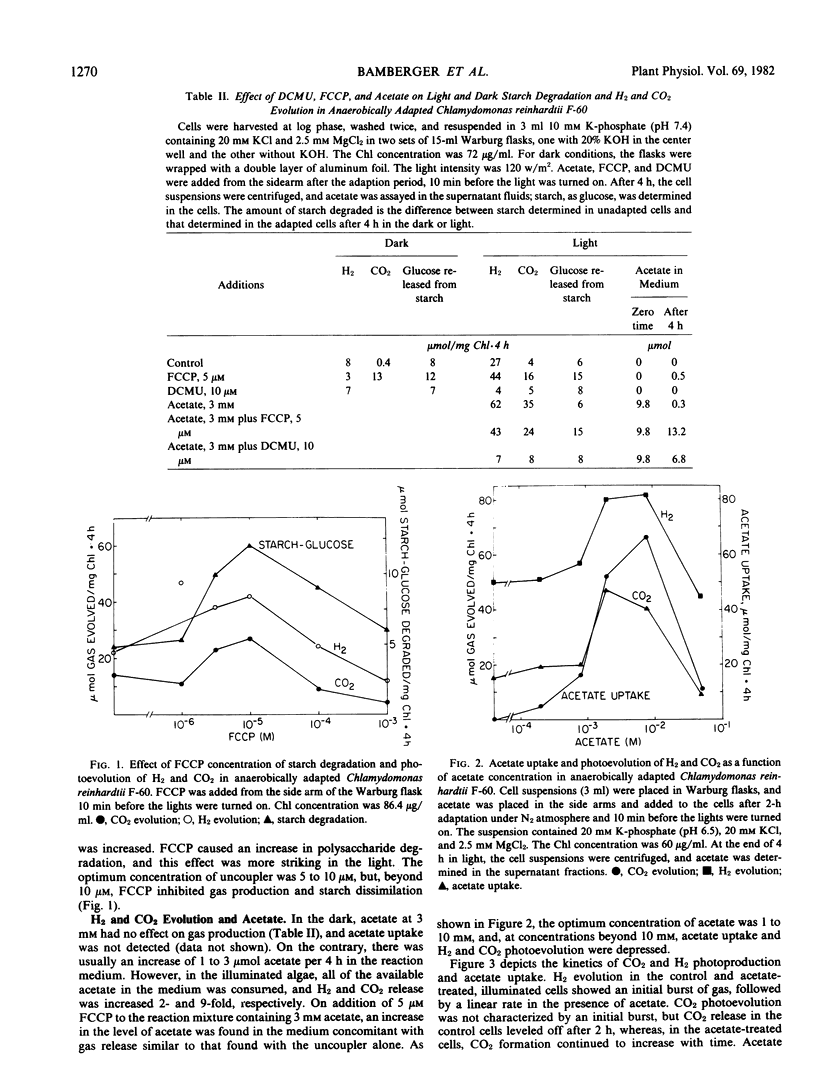
In the dark, starch was broken down, and H2 and CO2 was released. The uncoupler, carbonyl cyanide m-fluorophenylhydrazone with an optimum concentration of 5 to 10 micromolar, increased the rate of CO2 release and starch breakdown but depressed H2 formation. It was suggested that carbonyl cyanide m-fluorophenylhydrazone increased the rate of starch breakdown by making the chloroplast membrane permeable to H+, removing a rate-limiting step, and leading to an altered fermentative pattern.
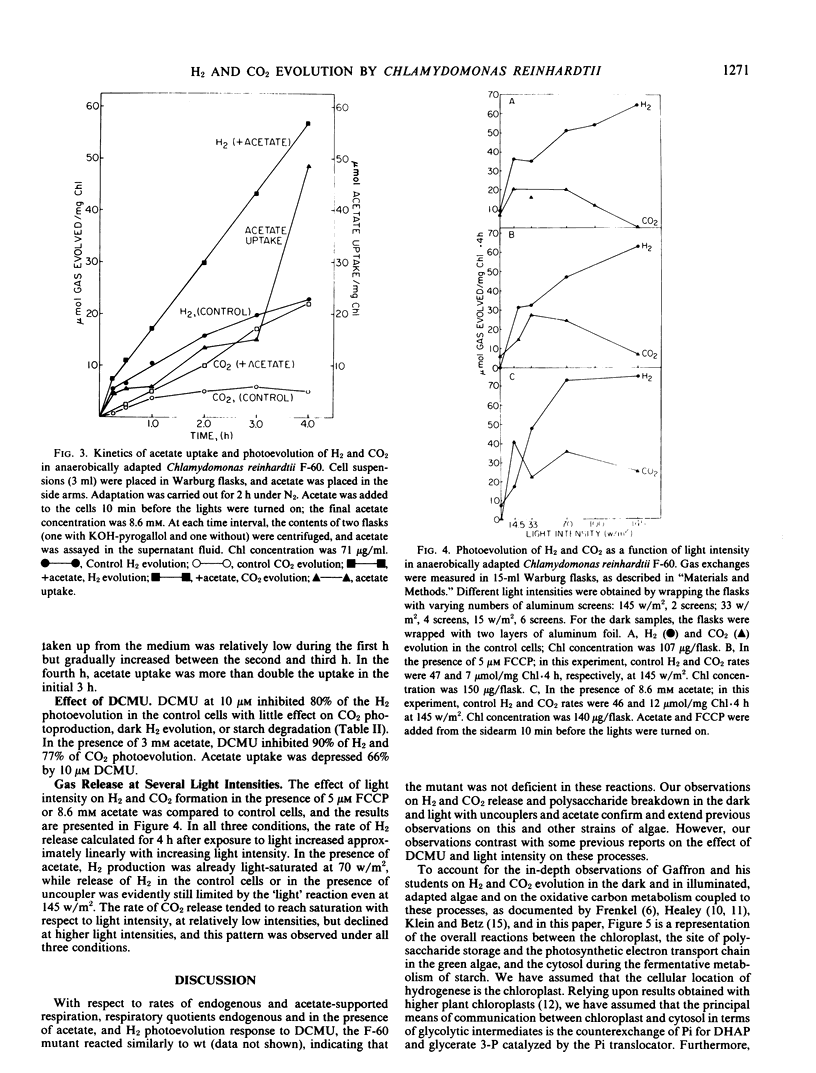
Photoevolution of H2 and CO2, but not starch breakdown, was stimulated by acetate. Maximum stimulation occurred at concentrations from 1 to 10 millimolar. Carbonyl cyanide m-fluorophenylhydrazone stimulated starch breakdown and CO2 and H2 release in the light, but not to the extent of acetate. Inasmuch as the uptake and subsequent metabolism of acetate required ATP, it was suggested that acetate, like carbonyl cyanide m-fluorophenylhydrazone, stimulated H2 photoproduction by removing ATP which limited the sequence of reactions. The contribution of photosystem II to the photoproduction of H2, as judged from the effect of 10 micromolar 3-(3,4-dichlorophenyl)-1, 1-dimethylurea, was at least 80%.
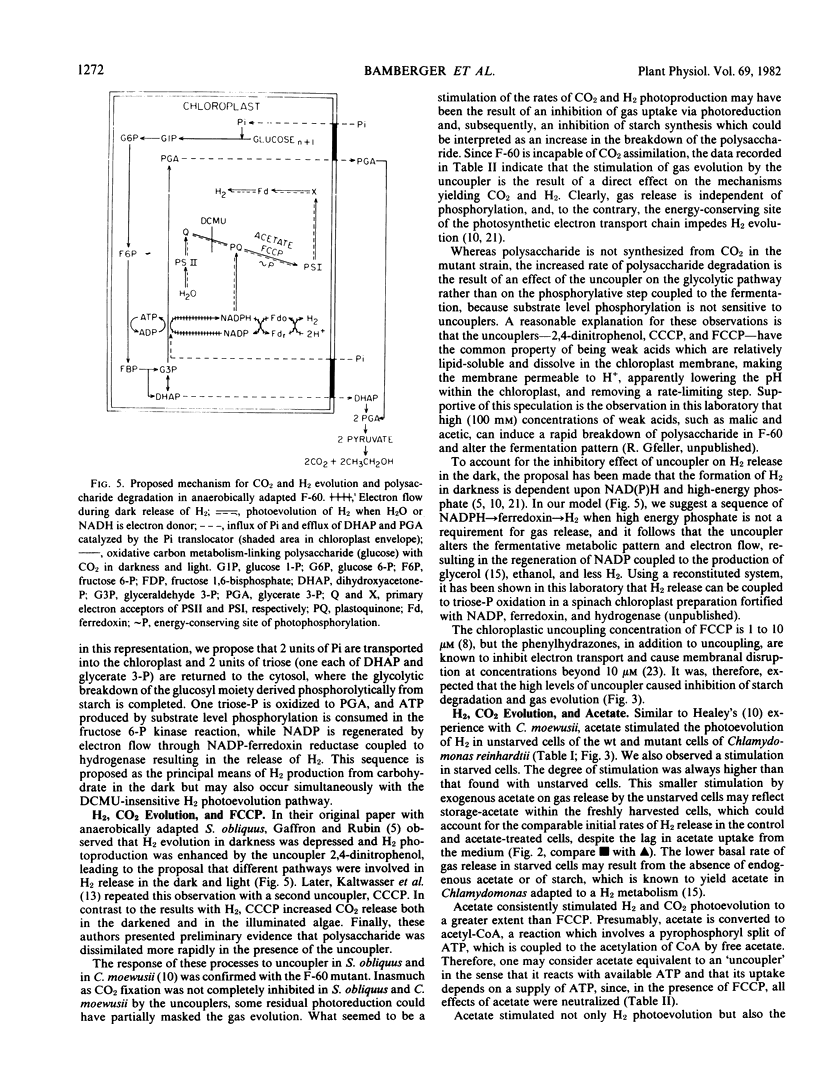
CO2 photoevolution increased linearly with time, but H2 photoevolution occurred in two phases: a rapid initial phase followed by a second slower phase. The rate of H2 release increased hyperbolically with light intensity, but the rate of CO2 production tended to level off and decrease with increasing light intensity, up to 145 watts per square meter. It was proposed that a changing CO2 and H2 ratio is the result of interaction between the carbon and hydrogen metabolism and the photosynthetic electron transport chain.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ben-Amotz A., Gibbs M. H2 metabolism in photosynthetic organisms. II. Light-dependent H2 evolution by preparations from Chlamydomonas, Scenedesmus and spinach. Biochem Biophys Res Commun. 1975 May 5;64(1):355–359. doi: 10.1016/0006-291x(75)90261-2. [DOI] [PubMed] [Google Scholar]
- FRENKEL A. W. Hydrogen evolution by the flagellate green alga, Chlamydomonas moewusii. Arch Biochem Biophys. 1952 Jul;38:219–230. doi: 10.1016/0003-9861(52)90026-x. [DOI] [PubMed] [Google Scholar]
- Gorman D. S., Levine R. P. Cytochrome f and plastocyanin: their sequence in the photosynthetic electron transport chain of Chlamydomonas reinhardi. Proc Natl Acad Sci U S A. 1965 Dec;54(6):1665–1669. doi: 10.1073/pnas.54.6.1665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Healey F. P. The Mechanism of Hydrogen Evolution by Chlamydomonas moewusii. Plant Physiol. 1970 Feb;45(2):153–159. doi: 10.1104/pp.45.2.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein U., Betz A. Fermentative Metabolism of Hydrogen-evolving Chlamydomonas moewusii. Plant Physiol. 1978 Jun;61(6):953–956. doi: 10.1104/pp.61.6.953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moll B., Levine R. P. Characterization of a Photosynthetic Mutant Strain of Chlamydomonas reinhardi Deficient in Phosphoribulokinase Activity. Plant Physiol. 1970 Oct;46(4):576–580. doi: 10.1104/pp.46.4.576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pow T., Krasna A. I. Photoproduction of hydrogen from water in hydrogenase-containing algae. Arch Biochem Biophys. 1979 May;194(2):413–421. doi: 10.1016/0003-9861(79)90635-0. [DOI] [PubMed] [Google Scholar]
- Russell G. K., Gibbs M. Regulation of Photosynthetic Capacity in Chlamydomonas mundana. Plant Physiol. 1966 May;41(5):885–890. doi: 10.1104/pp.41.5.885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SYRETT P. J., WONG H. A. THE FERMENTATION OF GLUCOSE BY CHLORELLA VULGARIS. Biochem J. 1963 Nov;89:308–315. doi: 10.1042/bj0890308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinbach E. C., Garbus J. Mechanism of action of reagents that uncouple oxidative phosphorylation. Nature. 1969 Mar 15;221(5185):1016–1018. doi: 10.1038/2211016a0. [DOI] [PubMed] [Google Scholar]
- de la Roche A. I. Increase in linolenic Acid is not a prerequisite for development of freezing tolerance in wheat. Plant Physiol. 1979 Jan;63(1):5–8. doi: 10.1104/pp.63.1.5. [DOI] [PMC free article] [PubMed] [Google Scholar]


