Abstract
Objective: To explore the clinical and pathological characteristics of small-cell carcinoma (SmCC) of the prostate and applicable treatment methods. Methods: We reported three cases of SmCC of the prostate diagnosed from 1999 to 2011 at the Chinese PLA General Hospital. We also reviewed clinical and pathological data of 26 cases in China reported over the same period. Results: Serum prostate-specific antigen (PSA) levels were normal in 20 cases (76.9%) and elevated in six (23.1%). There was local invasion in 12 cases (46.2%) at the time of diagnosis; lymphatic vessel invasion and distant metastases were detected in eight (30.8%) and nine cases (34.6%) respectively. At the end of follow-up, 16 cases (61.5%) had died, eight (30.8%) survived, and two (7.7%) were missing. The median survival time was 8 months, and the 1-year survival rate was 23.2%. Statistical analysis showed that survival time was significantly correlated with chemotherapy treatment (p<0.05). However, serum PSA levels, surgical approach, pathological type, local invasion, lymphatic vessel invasion, and distant metastasis had no significant relationship with survival (p>0.05). Conclusions: SmCC of the prostate is a rare neoplasm typified by high malignancy, rapid progress, and poor prognosis. Pathological analysis is an important tool for confirming a diagnosis. Pure SmCC is usually not associated with an increase in serum PSA. Surgery, mixed with acinar adenocarcinoma components, and clinical staging do not correlate with prognosis; and chemotherapy was the only prognostic factor. For patients, diagnosed by preoperative biopsy, administering chemotherapy as the first-line treatment may improve outcomes.
Keywords: prostate, small cell carcinoma, clinical characteristics, pathological characteristics, prognosis, immunohistochemistry.
Introduction
Small-cell carcinoma (SmCC) is a poorly differentiated tumor that can occur in many organs and tissues, although it is rare outside the lung. Approximately 10% of cases occur in the prostate. SmCC of the prostate is an uncommon type of prostate cancer, accounting for only 1% of cases 1. The first case of SmCC of the prostate was reported by Wenk et al. 2 in 1977. To date, about 160 cases have been reported in the English-language literature, and 26 cases in the Chinese literature 3-21.
Due to its rare occurrence, most studies have only provided case reports, and few have carried out mediation sample analysis. Thus clinical and pathological characteristics and the biological behavior of SmCC of the prostate remain uncertain.
Here, we present three cases of SmCC of the prostate treated at the China PLA General Hospital (PLAGH) from 1999 to 2011. We also reviewed similar cases in the Chinese literature over the same period, focusing on clinical and pathological characteristics. Considerations of biological behaviors as well as the treatment options are also analyzed.
Materials and Methods
Clinical and pathological information of the cases from PLAGH
In total, 1,002 cases of prostate cancer were diagnosed pathologically at the Chinese PLA General Hospital from 1999 to 2011, and seven of them were suspected of SmCC. All pathologic sections from these seven cases were reviewed by three pathologists. Immunohistochemical staining for prostate-specific antigen (PSA), CD56, synaptophysin (Syn), chromogranin A (CgA), and cytokeratin (CK) was performed. Four cases were excluded because of the negative staining for CD56, Syn, and CgA, which were suspected to be poorly differentiated acinar adenocarcinomas with Gleason scores 9-10. The remaining three cases (0.3%) were confirmed as SmCC. Detailed clinical and pathological information is presented below.
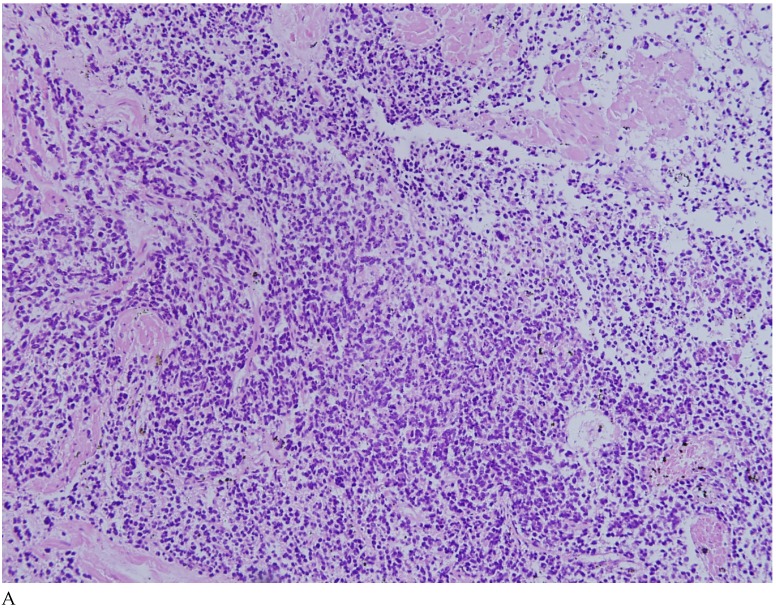
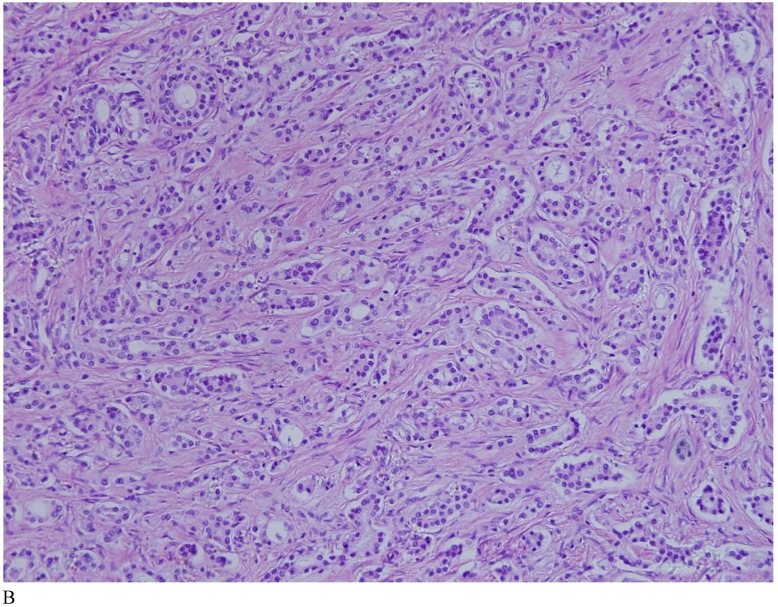
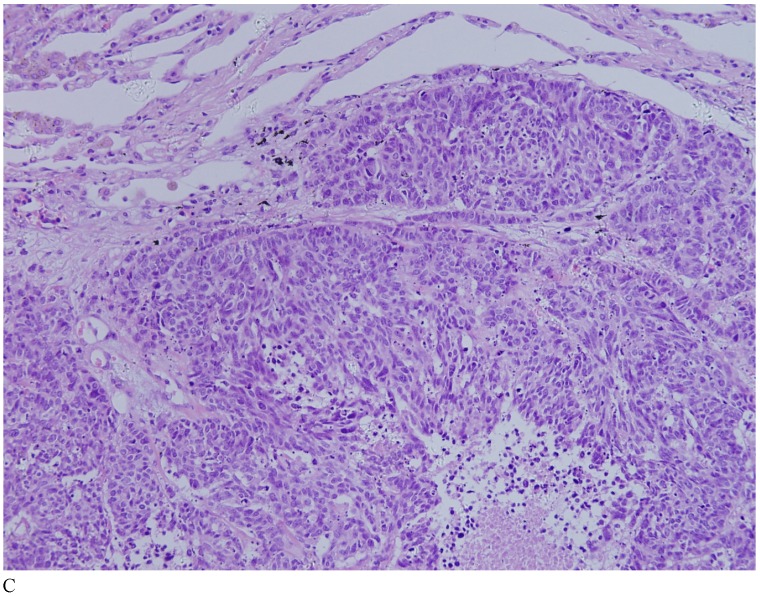
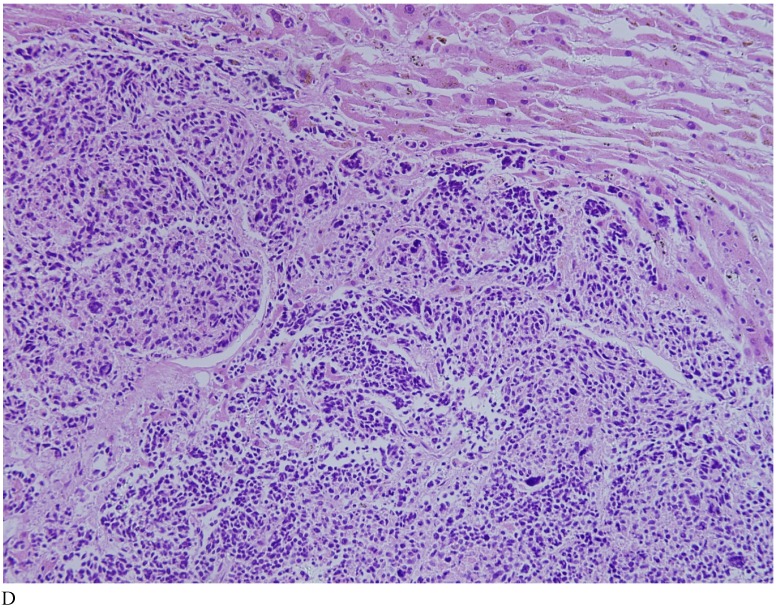
Case 1. A 79-year-old patient with a history of slowly increasing serum PSA levels for 2 years presented to the hospital with a 3-day history of fever. The patient's serum PSA level was 18.12ng/mL (normal <4ng/mL). Digital rectal examination (DRE) indicated grade III prostate enlargement. Magnetic resonance imaging (MRI) showed prostate cancer combined with right seminal vesicle invasion, pelvic lymph node, multi-bone, and liver metastases. An ultrasound-guided prostate biopsy was performed. Pathological examination suggested acinar adenocarcinoma. Gleason scores were 3+4=7. The patient was treated only with endocrine therapy because of his poor general condition. The patient died of respiratory failure caused by multiple metastases of the prostate cancer to both lungs 2 months later. Autopsy pathology revealed mixed SmCC of the prostate, combined with bilateral lung, liver, bilateral adrenal gland, pancreas, bone, and lymph node metastases. The prostate cancer was classified as SmCC with a mixture of acinar adenocarcinoma (Fig. 1A). All metastatic cancers were determined as pure SmCC without component of the acinar adenocarcinoma (Fig. 1B, 1C, 1D).
Figure 1.
Case 1. Mixed SmCC. (A) Components of SmCC. (B) Components of acinar adenocarcinoma. Gleason scores were 3+3=6 combined with bilateral lung (C) and liver (D) metastases.
Case 2. A 65-year-old patient was referred to our hospital with symptoms of dysuria and frequent urination. The serum PSA level was 20.67ng/mL (normal range: 0-4 ng/mL). DRE indicated the prostate was homogeneously enlarged and the central sulcus disappeared. MRI showed a mass in the prostate, combined with pelvic lymph node metastasis. He underwent an ultrasound-guided prostate biopsy. Combined SmCC and acinar adenocarcinoma (Gleason scores, 4+3=7) were confirmed pathologically. The patient received endocrine therapy. The 4-month follow-up showed tumor-bearing survival, and the general condition of the patient was poor.
Case 3. A 76-year-old patient was admitted to the hospital for frequent urination and difficulty urinating. Serum PSA level measured on admission was normal. DRE suggested grade III prostate enlargement. An ultrasound scan showed a visible heterogeneous low echo pattern in the prostate region, with involvement of the trigone of the bladder. An ultrasound-guided prostate biopsy was performed and SmCC was confirmed pathologically. The patient refused any treatment following the biopsy confirmation of pure SmCC. The 8-month follow-up indicated tumor-bearing survival.
Retrieval method of the related literature
All literature reports relating to SmCC of the prostate were accessed from the China Hospital Knowledge Database (CHKD), the Chinese Medical Journal Database (CMJD), the Chinese Medical Citation Index (CMCI), and the Wanfang Database (WFD) using the keywords “small cell carcinoma”, “prostate”, and “neuroendocrine.” The search located 29 cases in 19 reports 3-21. The contents of these reports were then reviewed. Two cases were found to be duplicated and were eliminated 19-20. Another case was also eliminated due to incomplete clinical and pathological data 21. In total, 26 cases were eligible 3-18. Data on age, clinical symptoms and signs, serum PSA levels, DRE findings, ultrasound scans and MRI, surgical approach, postoperative therapy, follow-up time, and survival were collected.
Statistical methods
Statistical analyses were performed using SPSS 13.0 software. Overall survival was analyzed using the Kaplan-Meier method. The correlation between each clinical and pathological variable and survival time was determined using Fisher's exact test. The relationship between pathological type and serum PSA levels was evaluated using the chi-squared test. Multivariate analysis was performed for prognostic factors using a Cox risk model. Two-tailed tests were used for all processes and p values <0.05 were considered statistically significant.
Results
Clinical information of the patients reported in literatures
The 26 cases were aged from 21 to 82 years (average 59 years). Because the median age was 61 years, patients were divided into two groups: <59 years old and ≥60 years old. There were 15 cases in the ≥60 group (57.7%). Most cases (18, 69.2%) suffered from difficulty urinating, and 20 cases (76.9%) showed normal serum PSA levels. DRE indicated grade III prostate enlargement and hardening. Space-occupying lesions were detected by imaging examinations. Among them, 11 cases (42.3%) underwent prostate biopsies; eight (30.8%) underwent transurethral resection of the prostate (TURP) and seven (26.9%) underwent radical prostatectomy. For postoperative treatment, 13 cases (50.0%) received chemotherapy, two (7.7%) received radiotherapy, and 13 (50.0%) had a history of endocrine therapy (the clinicopathologic features were summarized in table 1).
Table 1.
Clinicopathologic features of 26 cases of prostatic small cell carcinomas from Chinese literature.
| No. | Age | PSA level (ng/mL) |
Diagnostic procedure |
Treatment | Pathologic type | Time of follow-up (months) | Survival status |
|---|---|---|---|---|---|---|---|
| 1 | 76 | 0. 81 | TURP | Non | pure | 6 | death |
| 2 | 32 | 0.45 | radical | Chemotherapy | pure | 10 | death |
| 3 | 61 | 2.4 | biopsy | Non | pure | 1 | lost |
| 4 | 43 | 8.8 | biopsy | Chemotherapy | pure | 1 | Survival with tumor |
| 5 | 64 | <4 | TURP | Endocrine therapy | mixed | 5 | death |
| 6 | 57 | 25.78 | biopsy | Non | mixed | 7 | death |
| 7 | 76 | <4 | TURP | Endocrine therapy | mixed | 8 | death |
| 8 | 56 | <4 | radical | Endocrine therapy | pure | 17 | Survival with tumor |
| 9 | 32 | <4 | biopsy | Chemotherapy | pure | 3 | lost |
| 10 | 65 | <4 | biopsy | Chemo+Radiotherapy | pure | 11 | death |
| 11 | 21 | 0.69 | biopsy | Endocrine therapy | pure | 3 | death |
| 12 | 82 | 2.26 | TURP | Endocrine therapy | pure | 3 | death |
| 13 | 61 | 0.32 | radical | Chemotherapy | pure | 3 | Survival with tumor |
| 14 | 55 | <4 | radical | Endocrine therapy | pure | 3 | death |
| 15 | 34 | <4 | biopsy | Chemotherapy | pure | 13 | death |
| 16 | 62 | <4 | radical | Non | pure | 5 | Survival with tumor |
| 17 | 65 | 1.92 | biopsy | Chemo+Endocrine+Radiotherapy | pure | 11 | death |
| 18 | 55 | <4 | radical | Endocrine therapy | pure | 3 | death |
| 19 | 34 | <4 | biopsy | Chemotherapy | pure | 12 | Survival with tumor |
| 20 | 50 | 0.31 | radical | Non | pure | 1 | death |
| 21 | 82 | 2.61 | TURP | Chemo+Endocrine therapy | pure | 17 | Survival with tumor |
| 22 | 81 | 39.26 | biopsy | Non | pure | 5 | death |
| 23 | 77 | 25.02 | biopsy | Chemo+Endocrine therapy | pure | 2 | Survival with tumor |
| 24 | 79 | 18.12 | biopsy | Endocrine therapy | mixed | 2 | death |
| 25 | 76 | <4 | biopsy | Non | pure | 8 | death |
| 26 | 65 | 20.67 | biopsy | Endocrine therapy | mixed | 4 | Survival with tumor |
Pathological characteristics
Two of our three cases of SmCC were mixed with acinar adenocarcinoma, and one was pure SmCC. Of the 26 cases in the literature reports, 21 cases were pure SmCC. Under the microscope SmCC tissues were flaky and in a nest-like distribution. Coagulative necrosis was also observed. The cells were small, like oat grains, with one end pointed and the other rounded. The cells contained only a small amount of cytoplasm, with the nuclei bared and darkly colored, and the nucleolus obscured; mitotic figures were frequently seen. For most cases, positive immunohistochemical staining for neurone-specific enolase (NSE), CD56, Syn, and CgA were detected, and immunohistochemical staining was positive for CK, producing a dot-like pattern. Local invasion, lymphatic vessel involvment, and distant metastases were found in 12 (46.2%), eight (30.8%), and nine cases (34.6%) respectively.
Correlation between serum PSA levels and pathological type
Among the 21 patients with pure SmCC, serum PSA levels in 18 cases (85.7%) were normal and only elevated in three (14.3%) cases. For the five mixed cases, three (60.0%) had an elevation, and the other two (40.0%) had serum PSA levels in the normal range. However, the results of the chi-square tests revealed no significant differences (p=0.062).
Overall survival analysis
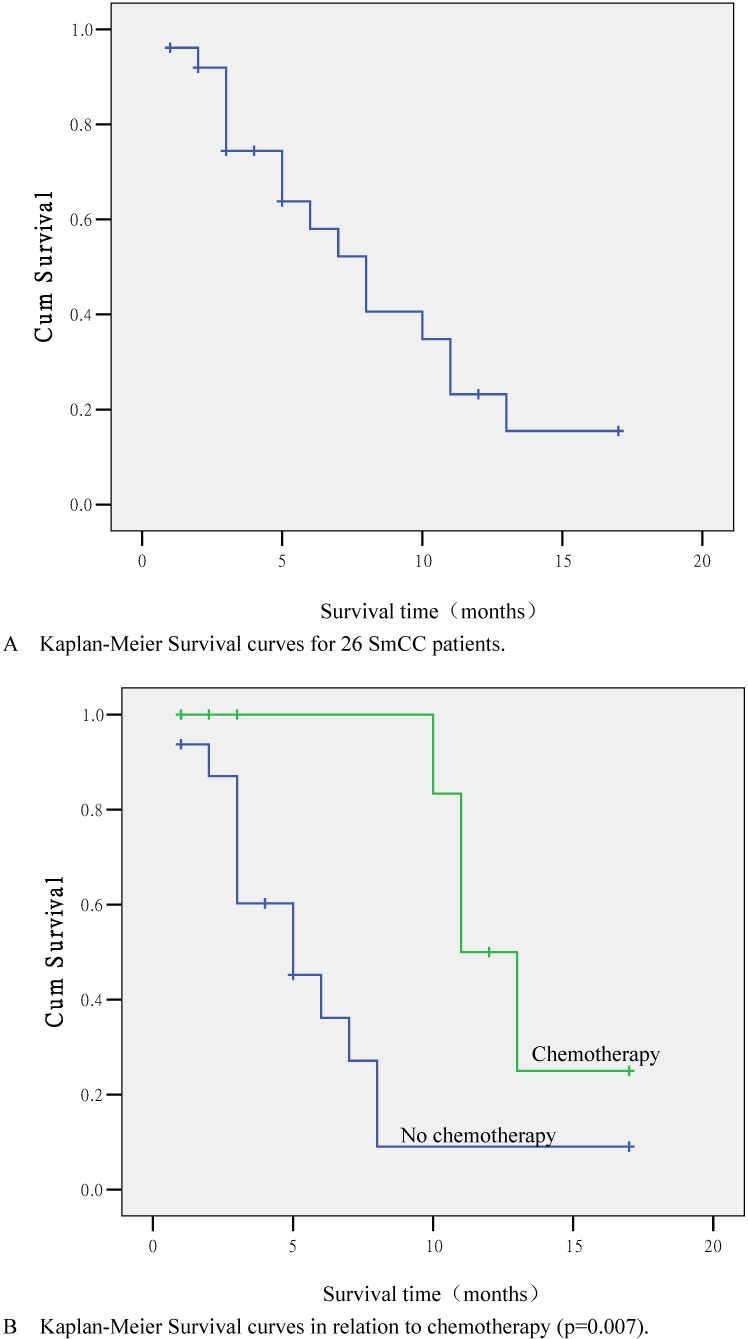
Follow-up was performed in 24 out of 26 cases (92.3%). The longest follow-up was 17 months. There were 16 (61.5%) deaths by the end of follow-up; eight cases lived with tumors. The median survival time was 8 months, and the 1-year survival rate was 23.2% (Fig. 2A).
Figure 2.
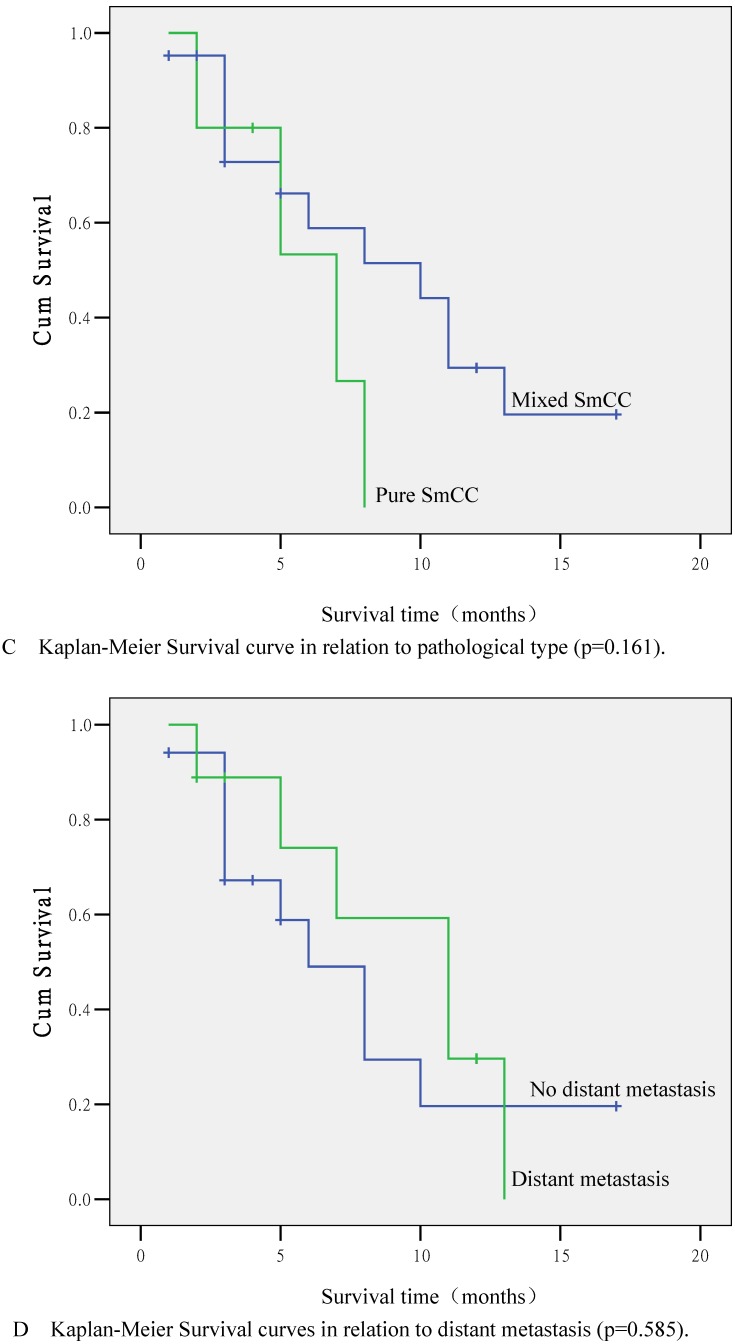
Kaplan-Meier Survival curves. (A) Survival curve for 26 SmCC patients. (B) Survival curve in relation to chemotherapy (p=0.007). (C) Survival curve in relation to pathological type (p=0.161). (D) Survival curve in relation to distant metastasis (p=0.585).
Prognostic factor analysis
According to statistical analyses, the survival time was closely related to chemotherapy administration. The 1-year survival rates for patients treated or not treated with chemotherapy were 50.0% and 9.0% respectively, and the difference was significant (p=0.007; Fig. 2B). Other factors, including age (p=0.863), clinical symptoms, serum PSA levels (p=0.163), surgical approach (p=0.967), pathological type (p=0.161; Fig. 2C), endocrine therapy (p=0.730), local invasion (p=0.477), lymphatic vessel invasion (p=0.695), and distant metastases (p=0.585; Fig. 2C) were not significantly correlated with survival time (p>0.05). Multivariate survival analysis using Cox's risk model indicated that chemotherapy was the only prognostic factor (p=0.005).
Discussion
SmCC of the prostate is a poorly differentiated tumor with a very low incidence (less than 1% of all prostate cancers) 1, 22. We reviewed pathological specimens of all 1,002 confirmed cases of prostate cancer from 1999 to 2011 in the Chinese PLA General Hospital; only three cases (0.3%) of SmCC were confirmed.
SmCC of the prostate is most common in elderly people. The average age is 69 at the time of diagnosis according to one report 23. However, the average age of the 26 Chinese cases in the present study was 59, lower than the reported age. Whether this is associated with ethnicity remains to be determined. The common clinical symptoms for the 26 cases were dysuria and urinary tract irritation. Occasionally, there was hematuria, which might be due to the invasion into the bladder or the urinary tract 14. DRE and imaging examinations are generally non-specific and can barely differentiate SmCC from acinar adenocarcinoma 1.
Over 95% of the cases of acinar adenocarcinoma exhibit an elevated serum PSA level. However, in SmCC, the serum PSA remains generally within the normal range. This is the case even for widespread metastasis 16. Of the 26 cases in the present study, only six (23.1%) showed high serum PSA levels, and three of them were mixed with conventional acinar adenocarcinoma. Thus serum PSA level is not an appropriate marker for SmCC of the prostate.
The confirmation of SmCC of the prostate relies mainly on pathological findings. Microscopically, SmCC cells are consistently round or short-spindle-shaped, arranged in a flaky or nest-like pattern. The cells only contain a small amount of cytoplasm, with the nuclei bared and darkly colored. The nucleolus is obscured and mitotic figures are frequently observed. Necrosis is also common. Immunohistochemical staining of SmCC cells for NSE, Syn, CgA, and CD56 is usually carried out for diagnosis 24. Combined use of these four markers often results in a definitive diagnosis. Moreover, PSA, PAP, and P504S expression are rare in SmCC 8 and a CK-positive staining pattern in SmCC is characteristically dot-like. This can be used to differentiate SmCC from the poorly differentiated acinar adenocarcinoma.
SmCC of the prostate is commonly combined with acinar adenocarcinoma according to the literature. Only about 35% of reported cases are pure SmCC 1, 13. Two of the three cases at our hospital were mixed SmCC, consistent with findings in the literature. Among the other 26 cases reported in China, 21 cases were pure SmCC, accounting for 87.0%, which is higher than the percentage reported in foreign literature. The reasons may lie in: (1) they were mainly collected from cases report; (2) eleven out of 26 cases only underwent prostate biopsies. Tissues within a limited scope were sampled which could not rule out a mixture with acinar adenocarcinoma. The survival time was short for both pure and mixed SmCC, however, for the mixed SmCC cases, the recurrence and metastasis were related to the components of SmCC. Thus pathologists should carefully observe the specimens to identify and report any component of SmCC.
Currently, there are no established guidelines for the treatment of SmCC of the prostate due to the small number of cases 1. In some reports, it has been proposed that surgery is the first choice for early and confined lesions 25. However, most SmCC cases have already been in advanced stages when diagnosed. It is difficult to achieve the best outcome with surgery for such advanced cases. SmCC is a hormone-independent tumor, and endocrine therapy is not effective for SmCC 6. Seven of 26 cases (26.9%) underwent radical prostatectomy, eight (30.8%) underwent transurethral resection of the prostate, 11 (42.3%) underwent prostate biopsies, and 13 cases (50.0%) received endocrine therapy. Statistical analyses showed that the performance of surgical resection and the administration of endocrine therapy were not related to survival time. Some believe that local radiotherapy might improve patients' quality of life. However, due to the small number of cases, we could not assess the efficacy of radiotherapy.
It is believed that SmCC of the prostate is more sensitive to chemotherapy, which should be considered as the first-line treatment. Amato et al. 26 used chemotherapy in 21 SmCC patients, and efficacy was demonstrated in 13 cases (62%). As yet, no standard chemotherapy regime has been formally acknowledged for SmCC of the prostate. Chemotherapy regimens for SmCC of the lung are typically used to treat SmCC of the prostate. It has been reported that the combined use of cisplatin and etoposide had an efficacy of 61% in SmCC of the prostate 27. The efficiency and remission rate of this regimen are lower in SmCC of the prostate, compared to those for SmCC of the lung, with a median survival time of only 7 months and a 5-year survival rate below 1% 28. In the present study, only 13 out of 26 cases (50.0%) were treated with chemotherapy. Statistical analysis demonstrated that chemotherapy was the only independent prognostic factor. The 1-year survival rates were 50.0% and 9.0% between patients treated with or without chemotherapy, respectively. The difference was statistically significant.
SmCC of the prostate is a high-grade malignant tumor, characterized by rapid growth, short survival time, and poor prognosis 12. Most cases have already progressed to the advanced stage at the time of diagnosis and are already combined with distant metastases 29, 30 to the liver, lung, bone, and central nervous system. In our study, combined local invasion, lymph node involvement, and distant metastasis were detected in 46.2%, 30.8%, and 34.6% of the cases, respectively, at the time of diagnosis. The average survival time was only 8 months and the 1-year survival rate was only 23.2%. Some reports had stated that only the components of SmCC could lead to metastasis in mixed cases. Case one treated in our hospital was finally conducted autopsy, and confirmed a diagnosis of mixed SmCC, but all the components in the metastatic tumor were pure SmCC, which implies that SmCC is more likely to cause early metastasis than acinar adenocarcinoma.
In conclusion, SmCC of the prostate is a rare, high-malignancy neuroendocrine tumor, and is not associated with elevated serum PSA levels. SmCC can be distinguished from poorly differentiated acinar adenocarcinoma based on histomorphological characteristics and immunophenotype. SmCC of the prostate is more sensitive to chemotherapy, which is recommended as the first-line treatment for the cases confirmed by biopsy. Moreover, the prognosis of mixed SmCC of the prostate depends on the components of SmCC. For cases with any component of SmCC in the prostate cancer specimens, the treatment and prognosis prediction should be regarded as the same as those for pure SmCC.
Acknowledgments
We thank Professor Li Zhiwen at the Epidemiological Research Branch, Institute of Reproductive and Child Health, Peking University for carrying out the statistical analysis.
References
- 1.Antonio C, Evangelia P, Nafsika S. et al. Pure Small Cell Carcinoma of the Prostate: A Case Report and Literature Review. Case Rep Oncol. 2011;4:88–95. doi: 10.1159/000324717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wenk RE, Bhagavan BS, Levy R, Miller D, Weisburger W. Ectopic ACTH, prostatic oat cell carcinoma, and marked hypernatremia. Cancer. 1977;40:773–778. doi: 10.1002/1097-0142(197708)40:2<773::aid-cncr2820400226>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 3.Shu RY, Dong P, Zhang QW. Prostatic small cell carcinoma: a case and clinic-pathologic features. Anhui Medical and Pharmaceutical Journal. 2008;12(9):852–853. [Google Scholar]
- 4.Luo YH, Zhao ZM, Li H. et al. Small Cell Car cinoma of t he Prost at e: Repor t of 3 Cases. Journal of Kunming Medical University. 2008;2B(29):57–60. [Google Scholar]
- 5.Huang HH, Chen ZJ. Prostatic mixed small cell carcinoma: 3 cases report. Chinese Community Doctors. 2009;20(11):213. [Google Scholar]
- 6.Zeng Q, Zhu XY, Jiang XZ. A case of prostatic neuroendocrine carcinoma. J Clin Urol. 2004;10(19):626. [Google Scholar]
- 7.Cheng JW, Meng QG, Tang Y. Diagnosis and Treatment of Neuroendocrine Cancers of the Prostate: A Case Report. J Oncol. 2009;8:780–781. [Google Scholar]
- 8.Xiao L, Lu XY, Pan SH. A case of prostatic small cell carcinoma. J Clin Exp Pathol. 2007;23(6):737–739. [Google Scholar]
- 9.Zhang GX, Xu KX, Wang YB. Case report of small cell carcinoma of prostate and literature review. Chin J Androl. 2008;22:31–33. [Google Scholar]
- 10.Guo SL, Zhang CY, Sun KZ. Prostatic small cell carcinoma: 2 cases report [J] China Foreign Medical Treatment. 2009;8:167. [Google Scholar]
- 11.Xie H, Xu YM, Wu DL. Small cell carcinoma of the prostate (report of 3 cases and review of the literature) Chin J Androl. 2009;11:27–29. [Google Scholar]
- 12.Qian WQ, Sun ZQ, Xu J. et al. Small cell carcinoma of prostate: a case report and review of the literature. Geriatr Health Care. 2007;3:150–154. [Google Scholar]
- 13.Wu DL, Jin SB, Xu YZ. et al. Prostatic small cell carcinoma: 2 cases report. Chin J Urol. 2000;4:226. [Google Scholar]
- 14.Li JS, Tang LK, Huang WL. et al. Small cell carcinoma of the prostate (report of 2 cases and review of the literature) Chin J Urol. 2006;8:559–562. [Google Scholar]
- 15.Wei ZF, Xu H, Wang H. et al. Clinicopatho logical Character ization of Prostatic Sma ll Cell Carcinoma (A Case Report and Review of the Literature) National J Androl. 2009;15:829–832. [PubMed] [Google Scholar]
- 16.Liu C, Li J, Jia ZL. et al. Small cell carcinoma of the prostate and mistreatment analysis: 1 case report. Chin J Mis diagn. 2008;29:7275–7276. [Google Scholar]
- 17.Chunhai HU, Hengcheng ZHU, Xiuheng LIU. et al. Small cell carcinoma of the prostate recurred from prostatic adenocarcinoma after the androgen depletion treatment (report of one case and review of the literature) J Clin Urol. 2010;25:930–933. [Google Scholar]
- 18.Fan JH, Wang L, Nan XY. et al. The diagnosis and treatment of small cell carcinoma of the prostate and a review of the literature. Chin J Urol. 2011;32:591–594. [Google Scholar]
- 19.Liu C, Ruan JX, Li J. et al. A case of prostatic small cell carcinoma. Medical Journal of National Defending Forces in North China. 2009;1:91–92. [Google Scholar]
- 20.Sun ZQ, Qian WQ, Xu J. et al. Prostatic small cell carcinoma: a case report. Chin J Urol. 2006;12:820. [Google Scholar]
- 21.Xie LP, Qin J, Zeng XY. et al. Age and Pathological Features of 481 Prostate Cancer Patients. National J Androl. 2005;6:428–430. [PubMed] [Google Scholar]
- 22.Eble JN, Sauter G, Epstein JI, WHO classification of tumours of the urinary system and male genital organs. Lyon: IARC Press; 2004. [Google Scholar]
- 23.Wang W, E pstein JI. Small cell carcinoma of the prostate. A morphologic and immunohistochemical study of 95 cases. Am J Surg Pathol. 2008;32:65–71. doi: 10.1097/PAS.0b013e318058a96b. [DOI] [PubMed] [Google Scholar]
- 24.Sun Y, Niu JY, Huang JT. Neuroendocrine differentiation in prostate cancer. Am J Transl Res. 2009;1:148–162. [PMC free article] [PubMed] [Google Scholar]
- 25.Stein ME, Bernstein Z, Abacioglu U. et al. Small cell (neuroendocrine) carcinoma of the prostate: etiology, diagnosis, prognosis, and therapeutic implications a retrospective study of 30 patients from the rare cancer network. Am J Med Sci. 2008;336:478–488. doi: 10.1097/MAJ.0b013e3181731e58. [DOI] [PubMed] [Google Scholar]
- 26.Amato RJ, Logothetis CJ, Hallinan R. et al. Chemotherapy for small cell carcinoma of prostatic origin. J Urol. 1992;147:935–937. doi: 10.1016/s0022-5347(17)37427-x. [DOI] [PubMed] [Google Scholar]
- 27.Papandreou CN, Daliani DD, Thall PF. et al. Results of a phase II study with doxorubicin, etoposide, and cisplatin in patients with fully characterized small cell carcinoma of the prostate. J Clin Oncol. 2002;20:3072–3080. doi: 10.1200/JCO.2002.12.065. [DOI] [PubMed] [Google Scholar]
- 28.Rubenstein JH, Katin MJ, Mangano MM. et al. Small cell anaplastic carcinoma of the prostate: seven new cases, review of the literature, and discussion of a therapeutic strategy. Am J Clin Oncol. 1997;20:376–338. doi: 10.1097/00000421-199708000-00011. [DOI] [PubMed] [Google Scholar]
- 29.Venkatesh PK, Motwani B, Sherman N. et al. Metastatic pure small cell carcinoma of prostate. Am J Med Sci. 2004;328:286–289. doi: 10.1097/00000441-200411000-00009. [DOI] [PubMed] [Google Scholar]
- 30.Mackey JR, Au HJ, Hugh J. et al. Genitourinary small cell carcinoma: determination of clinical and therapeutic factors associated with survival. J Urol. 1998;159:1624–1629. doi: 10.1097/00005392-199805000-00058. [DOI] [PubMed] [Google Scholar]