Abstract
6-Benzylaminopurine, 6-(Δ2-isopentenylamino)purine, 6-furfurylaminopurine, rotenone, and antimycin A inhibited oxidation of NADH by mitochondrial sonicates or submitochondrial particles (but not by intact mitochondria) from pea (Pisum sativum L., cult. Alaska) stems and mung bean (Vigna radiata L. Wilczak) hypocotyls. The above purine cytokinins can interfere with electron transport from NADH to the cytochrome system in the inner mitochondrial membrane. Adenine did not inhibit oxidation by sonicated mitochondria, and zeatin was almost ineffective. Zeatin scarcely inhibited state 3 malate respiration by intact mitochondria, but the O-formyl and O-n-propionyl esters of zeatin and the O-acetyl ester of 2-chlorozeatin were more active. Perhaps zeatin is ineffective because it does not get into the inner membranes of the isolated mitochondria, whereas the esters and other cytokinins mentioned above do. N-4-(2-chloropyridyl)-N′-Phenylurea, which has cytokinin-like effects on plant growth and development, inhibited NADH oxidation by sonicated mitochondria. It also inhibited malate, succinate, and NADH oxidation by intact mitochondria; in contrast, the latter two oxidations were not decreased by purine cytokinins.
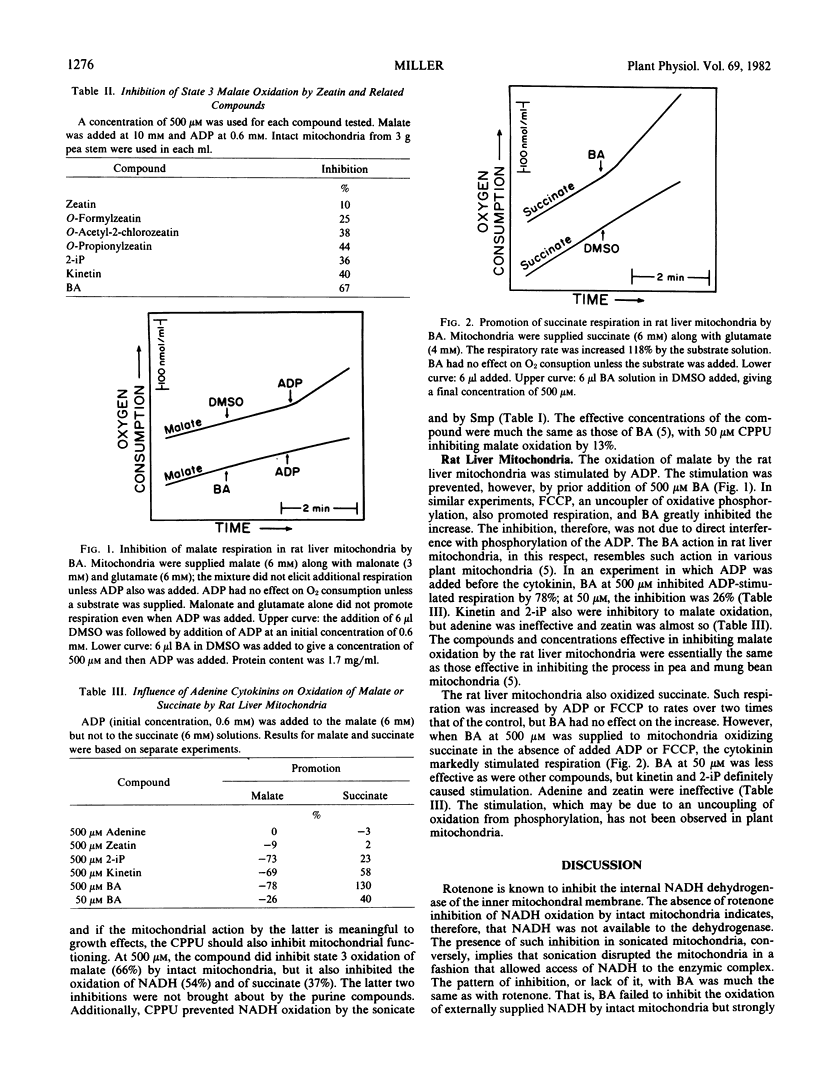
The benzyl, isopentenyl, and furfuryl aminopurines inhibited malate (but not succinate) oxidation by rat liver mitochondria, if ADP was present. In the absence of ADP, these cytokinins promoted succinate (but not malate) oxidation. Zeatin and adenine had slight, if any, effect in either situation.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Halevy A. H., Dilley D. R., Wittwer S. H. Senescence inhibition and respiration induced by growth retardants and N-benzyladenine. Plant Physiol. 1966 Sep;41(7):1085–1089. doi: 10.1104/pp.41.7.1085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Miller C. O. Cytokinin inhibition of respiration in mitochondria from six plant species. Proc Natl Acad Sci U S A. 1980 Aug;77(8):4731–4735. doi: 10.1073/pnas.77.8.4731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore T. S., Miller C. O. Effects of cytokinins on the respiration of soybean callus tissue. Plant Physiol. 1972 Nov;50(5):594–598. doi: 10.1104/pp.50.5.594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satler S. O., Thimann K. V. The influence of aliphatic alcohols on leaf senescence. Plant Physiol. 1980 Sep;66(3):395–399. doi: 10.1104/pp.66.3.395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tetley R. M., Thimann K. V. The Metabolism of Oat Leaves during Senescence: I. Respiration, Carbohydrate Metabolism, and the Action of Cytokinins. Plant Physiol. 1974 Sep;54(3):294–303. doi: 10.1104/pp.54.3.294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thore A., Baltscheffsky H. Inhibitory effects of lower aliphatic alcohols on electron transport phosphorylation systems. I. Straight-chain, primary alcohols. Acta Chem Scand. 1965;19(7):1591–1599. doi: 10.3891/acta.chem.scand.19-1591. [DOI] [PubMed] [Google Scholar]


