Abstract
Alzheimer's disease (AD) is a lethal progressive neurological disorder affecting the memory. Recently, US Food and Drug Administration mitigated the standard for drug approval, allowing symptomatic drugs that only improve cognitive deficits to be allowed to accelerate on to clinical trials. Our study focuses on taurine, an endogenous amino acid found in high concentrations in humans. It has demonstrated neuroprotective properties against many forms of dementia. In this study, we assessed cognitively enhancing property of taurine in transgenic mouse model of AD. We orally administered taurine via drinking water to adult APP/PS1 transgenic mouse model for 6 weeks. Taurine treatment rescued cognitive deficits in APP/PS1 mice up to the age-matching wild-type mice in Y-maze and passive avoidance tests without modifying the behaviours of cognitively normal mice. In the cortex of APP/PS1 mice, taurine slightly decreased insoluble fraction of Aβ. While the exact mechanism of taurine in AD has not yet been ascertained, our results suggest that taurine can aid cognitive impairment and may inhibit Aβ-related damages.
Recovery from dementia is the key clinical benefit to the patients of Alzheimer's disease (AD). This has become evident after consecutive failures in clinical trials for disease-modifying drugs that target neuropathological hallmarks. Accordingly, the US Food and Drug Administration loosened the standard for AD drug approval1. Their new guidance suggests accelerated regulatory pathways for drugs that improve cognitive deficits alone in early stages of AD. Albeit flexible in mechanisms of action, these symptomatic drugs must be assessed in early-stage AD patients with overt dementia and apparent biomarkers, such as amyloid plaques and tau tangles. The next generation acetylcholinesterase inhibitors may well fit into the accelerated pathways. However, the unnecessary stimulation of the normal cholinergic systems in the brains of AD patients and, even, non-demented subjects remains to be solved2.
Taurine, 2-aminoethanesulfonic acid, is the second most abundant endogenous amino acid in the central nervous system (CNS) and plays multiple roles in our body: thermoregulation, stabilization of protein folding, anti-inflammatory effects, antioxidation, osmoregulation, calcium homeostasis and CNS development3,4,5,6,7,8,9 (Figure 1). Due to its nontoxic and curative properties, taurine is frequently found in food, drinks and drugs for treating liver and heart disorders10,11,12,13. Recently, taurine has shown therapeutic effects as a cognitive enhancer in animal models of non-AD neurological disorders. Taurine recovers memory impairments of mice induced by alcohol, pentobarbital, sodium nitrite and cycloheximide without any observable effects on other behaviours including motor coordination, exploratory activity and locomotor activity14. Cognitive deficits of rats from excess manganese exposure are improved, and upregulated acetylcholinesterase activity is partially restored after taurine administration15. The intracerebroventricular administration of taurine protects mice from hypoxia-induced learning impairment16. In addition, intravenously administered taurine significantly improves post-injury functional impairments of traumatic brain injury in rats17. Taurine supplementation has also been found to rescue ageing-dependent loss of visual discrimination in mice18. In streptozotocin-induced sporadic dementia rat models, cognitive impairment and abnormal acetylcholinesterase activity is ameliorated by taurine19. Notably, taurine does not enhance learning and memory in cognitively intact adult rodents20.
Figure 1. Structures of taurine and homotaurine.
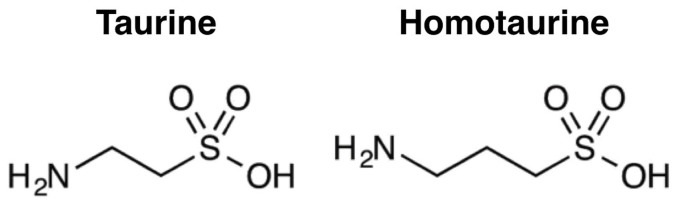
Taurine also has multiple disease-modifying roles to prevent or cease neuropathology of AD. During the development of AD, amyloid-β (Aβ) progressively misfolds into toxic aggregates, which are strongly associated with neuronal loss, synaptic damages and brain atrophy. The electron microscopy study indicates that taurine weakly inhibits Aβ aggregation21. Anti-inflammatory and anti-oxidant properties of taurine also protect neuronal cells and mitochondria from neurotoxicity of Aβ. By activating GABA and glycine receptors, taurine inhibits excitotoxicity caused by Aβ-induced glutamatergic transmission activation22. Taurine is also observed to attenuate Aβ-associated neuronal cell death, mitochondrial permeability transition pore opening, mitochondrial dysfunction and intracellular reactive oxygen species generation by activating Sirtuin 123,24,25,26. Therapeutic effects of taurine remain to be investigated in demented animal models with AD pathology. Considering the reevaluation of anti-amyloidogenic homotaurine for the potential role to ameliorate the cholinergic transmission in early AD, its analog compounds such as taurine are valuable therapeutic candidates for both cognitive enhancement and disease-modification (Figure 1)27.
In this study, we examined both symptomatic and disease-modifying effects of taurine in the demented adult APP/PS1 transgenic AD mouse model. Taurine was orally administered to the mice via drinking water for 6 weeks. The Y-maze and the passive avoidance tasks were then performed in succession to test for improvement of the spatial working memory and the contextual learning abilities, respectively. After sacrificing the animals for their cerebrospinal fluid (CSF) and brains, we measured alterations of Aβ levels in soluble, insoluble and plaque forms by sandwich enzyme-linked immunosorbent assays (ELISA) and Aβ burden assay (ThS staining). In addition, we measured the level of reactive astrocytes by immunohistochemistry (IHC) and western blots.
Results
Animal model and oral administration of taurine
To examine therapeutic efficacy of orally administered taurine as a cognitive enhancer in the early dementing stage of AD, we utilized APPswe/PS1-dE9 transgenic mouse model at the age of 7 months and dissolved taurine in the drinking water for 6-week administration. This mouse model produces elevated amount of human Aβ peptides by expressing mutant human amyloid precursor protein (APP) and presenilin protein 1 (PS1)28. This model is reported to express abnormal learning and memory behaviours with Aβ/tau alterations as early as the age of 6 months29,30. The 7-month old APP/PS1 mice (n = 19, male) and their age-matched wild-type littermates (n = 20, male) were divided into groups depending on presence of taurine in the drinking water. To orally administer 1,000 mg/kg/day of taurine to the mice, amounts of taurine in each water container was calculated based on daily water consumption and weekly check-up on body weights. Oral dosage of 1,000 mg/kg/day to mice was justified based on previously reported taurine in vivo studies and the median lethal dose (over 7,000 mg/kg)14,15,16,17,18,19,20. Throughout the experiment we did not observe any changes in hair loss, water consumption or body weight.
Taurine improves spatial working memory in APP/PS1 mice in the Y-maze task
To assess the spatial working memory of APP/PS1 mice, we performed the Y-maze test at the end of 6-week taurine administration. In the 3-armed Y-shaped maze, a mouse is free to explore, and the sequence of entries is recorded to determine the number of visits to 3 different arms in a row. The analyzed percent alternation reflects the function of visual cortex function of the subjected mouse, and higher percent alternation indicates better spatial memory. In this study, we found that taurine supplementation significantly improved behavioural performance of the APP/PS1 mice on the Y-maze test as compared to the water-only APP/PS1 group (Figure 2A). The spatial working memory of APP/PS1 mice was recovered up to wild-type levels (Figure 2A). We found insignificant changes among the total number of arm entries, dismissing hyperactivity as a possible argument for cognitive improvement (Figure 2A).
Figure 2. Improvement in spatial and hippocampal learning behaviours in taurine-treated transgenic mice.
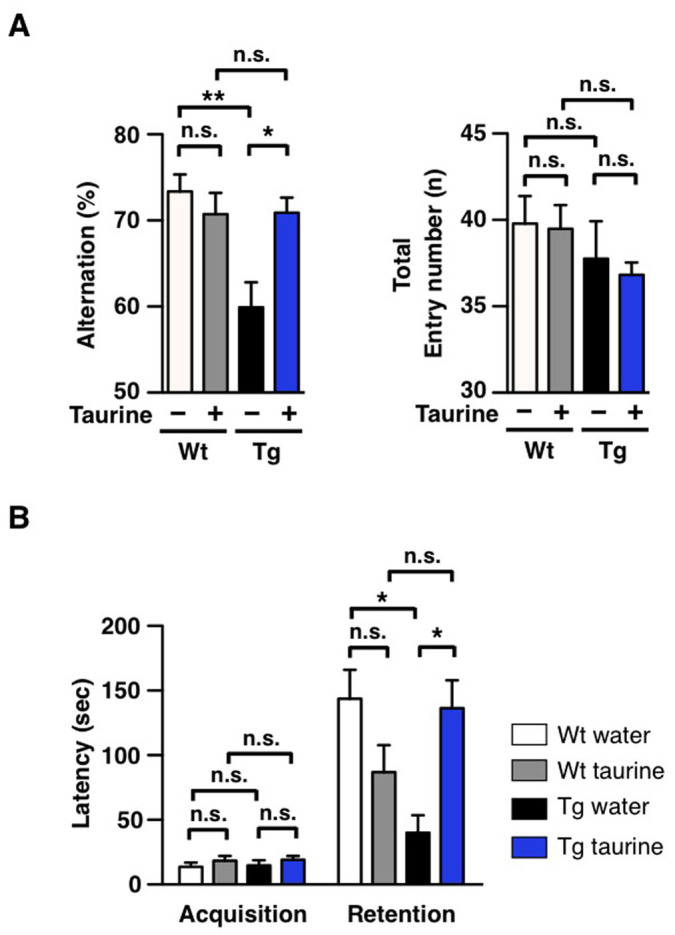
7-month old wild-type (Wt) and age-matched APP/PS1 transgenic (Tg) male mice were orally administered water or taurine (1,000 mg/kg/day) for 6 weeks (n = 8–10 per group). After 6 weeks, behavioural tests were administered to the 8.5-month old mice. (A) Y-maze. Average alternation (%) of each group of mice was calculated. (B) Passive avoidance. Average latency time in seconds for each group of mice was measured. One-way ANOVA followed by Bonferroni's post-hoc comparisons tests were performed in all statistical analyses (*P < 0.05, **P < 0.01, ***P < 0.001, n.s.: no significance).
Taurine improves hippocampal memory in APP/PS1 mice in the passive avoidance task
To evaluate the hippocampal memory of APP/PS1 mice, we performed the passive avoidance test 2 days after the Y-maze test. The passive avoidance test is a fear-motivated test to assess the function of hippocampus and amygdala of the subject. The test requires rodents to resist their affinity for the darker chamber and remain in the lighter chamber of a 2-chamber box. In the acquisition phase, a mouse is placed inside the bright chamber and receives a shock when it traverses to the dark side. After 24 hrs, the mouse is again placed in the bright chamber of the box, and how well it remembers the shock is measured by the latency in avoiding the dark chamber. Higher latency value translates to better retention of memory from the foot-shock given during the learning phase. Consistent with the results obtained from the Y-maze, taurine was observed to significantly enhance behavioural performance of the APP/PS1 mice in the pass avoidance tasks as compared to the non-treated APP/PS1 group (Figure 2B). The hippocampal memory of the taurine-treated APP/PS1 mice was recovered to the level similar to that of wild-type mice (Figure 2B). Similar to the results from the Y-maze test, behavioural alterations of the wild-type by taurine treatment was insignificant (Figure 2B).
Taurine decreases insoluble Aβ42 in the cortex of APP/PS1 mice
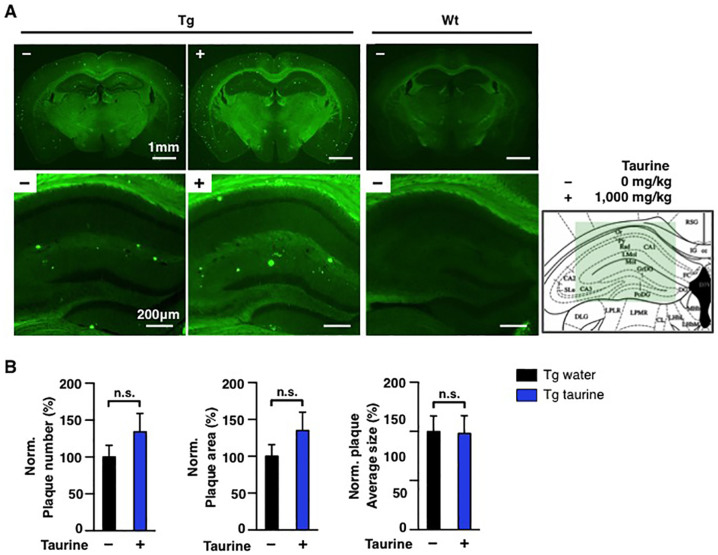
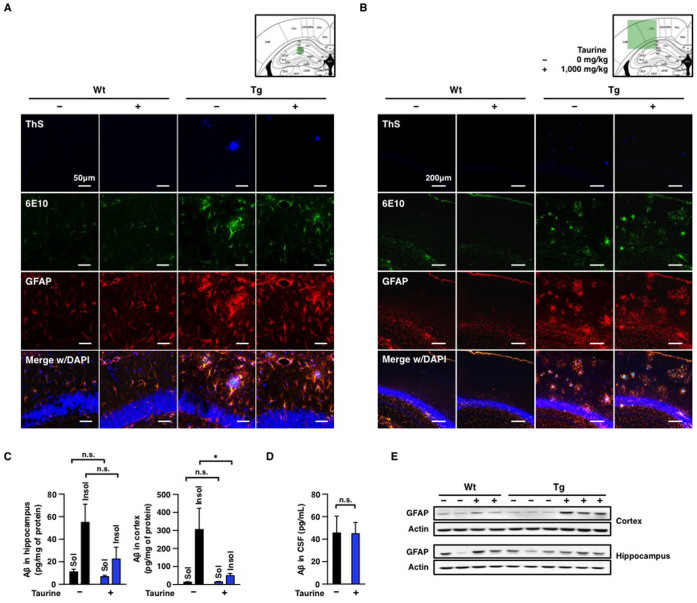
Aβ accumulation in the brain reflects the onset of AD31. As taurine was reported to bind Aβ peptides with weak anti-fibrillogenic effect, we measured alterations of plaque, soluble and insoluble forms of Aβ21,32. To examine the effect of orally administered taurine on the alteration of plaque burden, brains of APP/PS1 mice were sectioned after behavioural tests, and then stained with thioflavin S (ThS). ThS was used to visualize β-sheet-rich Aβ plaques. In comparison to the non-treated APP/PS1 group, no significant difference was found in numbers, area or average size of plaques in taurine-treated APP/PS1 group (Figure 3). Consistent with the results from ThS staining, we did not observe alterations in levels of plaques and amyloid precursor protein by IHC using the monoclonal anti-Aβ antibody, 6E10 (Figure 4A and 4B). Among various isomers of Aβ, Aβ42 is the most amyloidogenic and neurotoxic. In order to determine whether Aβ42 peptides were involved in amelioration of cognitive deficits in APP/PS1 mice, we prepared brain lysates of animals subjected to aforementioned behavioural studies and isolated soluble and insoluble Aβ fractions of both the hippocampus and the cortex for sandwich-ELISA. In the hippocampal region, levels of soluble and insoluble Aβ42 were not altered by taurine administration (Figure 4C). In addition, we did not observe changes in soluble Aβ42 levels in the cortices of APP/PS1 mice by taurine treatment. On the contrary, we found a significant decrease in the level of Aβ42 in the cortical insoluble fraction of the taurine-treated APP/PS1 mice as compared to the non-treated APP/PS1 group (Figure 4C). CSF Aβ42 and tau levels are associated with neuropathological changes in AD brains33. In comparison between 2 transgenic groups, we did not observe differences in CSF Aβ42 (Figure 4D) or tau levels (data not shown). Collectively, these results indicate that 6-week oral administration of taurine (1,000 mg/kg/day) only reduced levels of Aβ42 insoluble fractions of the cortex.
Figure 3. Aβ burden tests in the hippocampi and whole brains in mice.
7-month old wild-type (Wt) or age-matched APP/PS1 transgenic (Tg) male mice were orally administrated water or taurine (1,000 mg/kg/day) for 6 weeks (n = 8–10 per group). (A) ThS-stained Aβ burden in whole brains (scale bar, 1 mm) and hippocampal regions (scale bar, 200 μm) of each group. (B) normalized (%) number, area or average size of Aβ burden to 8.5-month old mice level in whole brains. The mouse brain schematic diagram was adapted from the Mouse Brain Atlas48 (green box: regions of brain images). One-way ANOVA followed by Bonferroni's post-hoc comparisons tests were performed in all statistical analyses (*P < 0.05, **P < 0.01, ***P < 0.001, n.s.: no significance).
Figure 4. Biochemical analyses of GFAP and Aβ in the hippocampi and the cortices.
7-month old wild-type (Wt) and age-matched APP/PS1 transgenic (Tg) male mice were orally administered water or taurine (1,000 mg/kg/day) for 6 weeks (n = 8–10 per group). Immunohistochemical analyses of (A) hippocampal regions and (B) cortical regions of 8.5-month old mice were perfused and sectioned. Aβs in the brain sections were stained by 6E10 antibody and ThS. Aβ plaques with ThS staining (1st row): blue, All Aβs including APP (2nd row): green, GFAP was stained by anti-GFAP (3rd row): red, DAPI: blue (a location indicator). The bottom rows show merged images with DAPI staining. Scale bars, 50 or 200 μm, respectively. The mouse brain schematic diagram was adapted from the Mouse Brain Atlas48 (green box: regions of brain images). (C) Quantifications of Aβ in brain lysates or (D) CSF Aβ analyses by sandwich-ELISA. Aβ-soluble (Sol.) fraction (sucrose-tris lysis buffer) and Aβ-insoluble (Insol.) fraction (guanidine-HCl lysis buffer) of brain lysates were analyzed. β-actin is a loading control. (E) Western blot analyses of brain lysates obtained from hippocampal and cortical regions. One-way ANOVA followed by Bonferroni's post-hoc comparisons tests were performed in all statistical analyses (*P < 0.05, **P < 0.01, ***P < 0.001, n.s.: no significance).
Taurine treatment increases levels of reactive astrocytes in the hippocampus and the cortex
Reactive astrocytes are found in various CNS disease brains to limit inflammation and to protect neurons from tissue degeneration34. In AD, reactive astrocytes cluster around Aβ plaques as a glial response to the neural injury associated with Aβ35. Therefore, we measured glial fibrillary acidic protein (GFAP), a marker for astrocytosis, in the brains of taurine-treated mice by IHC and western blots. In IHC analyses, reactive astrocytes were colocalized with both ThS- and 6E10-stained plaques in transgenic mouse brains (Figure 4A and 4B). To quantify levels of GFAP expression, we performed western blot analyses. Interestingly, we found that oral administration of taurine induced increase of reactive astrocytes in both the wild-type and transgenic mice (Figure 4E). Because taurine treatment selectively enhanced behavioural performance of APP/PS1 groups in Y-maze and passive avoidance tasks without affecting wild-type mice, it is difficult to correlate the increase of astrocytosis with learning and memory in this study.
Discussion
Here we report that taurine in drinking water rescues Alzheimer-like learning and memory deficits of adult APP/PS1 double transgenic mice without modifying the behaviours of cognitively normal mice. Our current study complements a previous study that reported the ability of taurine to improve learning and retention in aged FVB/NJ mouse model compared to their untreated controls36. Unlike APP/PS1 mouse model, which expresses human Aβ and amyloidogenesis, FVB/NJ mouse model induces retinal degeneration. The cognitive impairment induced in their study was through ageing alone and the following consequences, while APP/PS1 mouse model acquired cognitive deficits through increased production and aggregation of human Aβ peptides. In addition to ameliorating deficits associated with ageing and Aβ, taurine proved to be effective with other forms of dementia: hypoxia-induced learning impairment, ischemic stroke-induced learning impairment, chemical-induced sporadic dementia of Alzheimer's type, and alcohol-induced brain impairment14,16,19,37,38. Consistent with our findings, taurine has been reported as ineffective to enhance spatial learning and memory in cognitively normal mice20. Accordingly, unlike acetylcholinesterase inhibitors, taurine seems to be dementia-specific, which may have great clinical impacts as a selective cognitive enhancer.
The results from our study indicate that taurine may play a role in preventing cognitive impairment in AD-like mouse model. However, the exact mechanism is not clear how taurine induces improvement of abnormal behaviours in AD model mice without the significant inhibition of Aβ amyloidogenesis. The sandwich-ELISA results suggest that taurine weakly decreases Aβ levels in the insoluble fraction of brain lysates but rarely alters concentrations of soluble Aβ, including monomers and oligomers. In addition, histochemical analyses reveal that taurine does not affect β-sheet-rich plaques. As the current methods to isolate Aβ in brain lysates into soluble, insoluble and guanidine-soluble fractions do not clearly define the contents, it is difficult to indicate specific alterations of monomers, oligomers, protofibrils and plaques. However, it is considerable that the levels of protofibrils with immature β-structures may be decreased by taurine treatment. Existence of protofibrils often provides confusing results in biochemical analyses measuring levels of high molecular weight Aβ aggregates39. It is also unclear exactly how taurine interacts with Aβ or by what mechanism it decreases the Aβ level. There have been proposals regarding calcium and chloride modulation, but further studies are needed to reveal how taurine decreases Aβ concentration in the brain. One hypothesis on how taurine can affect Aβ levels is the direct interaction between taurine and Aβ peptides in the brain. Previous studies on influences of taurine on amyloidogenesis have been controversial. Taurine in 1 mM slightly prevented Aβ peptide comprising the residues 25–35 from polymerizing into fibrils21, suggesting a small inhibiting effect of taurine on Aβ peptide aggregation. However, in the presence of 20 mM of taurine at pH of 5.5, Aβ40 peptides accelerated in aggregation but not at pH of 7.439. Another hypothesis is that the sulfonic acid group in taurine may bind to Aβ peptides and prevent glycosaminoglycans from binding to Aβ, thereby inhibiting Aβ aggregation32,40. The structural similarity of homotaurine (tramiprosate), a former drug candidate, and taurine (Figure 4) suggests that taurine may also interfere in glycosaminoglycans recruiting Aβ41.
We observed the increased expression of GFAP by taurine, in both wild-type and transgenic mice. Because many investigations reported reduced reactive astrocytes by taurine treatment, additional studies are warranted to determine correlation of taurine supplementation and GFAP alterations. Although such explorations may be beyond the scope of the current study, it is noteworthy that long-term administration of high-dose taurine (200 mg/kg/day, intraperitoneal) was also found to induce over expression of GFAP during improvement of the spatial learning and memory ability in Sprague-Dawley rats15.
Our results suggest that taurine has a potential in treating deleterious effects on cognitive functions of AD. Taurine is already in clinical uses for congestive heart failure and liver disease with no known side-effects. Current prescription limits taurine supplementation to one year, but there is a dearth of adverse evidence for long-term taurine use. Previous studies assert that there are signs of beneficial effects in athletes42 and in sleep-deprived students43. Moreover, the fact that taurine is effective via drinking water is a great convenience for the AD patients. Additional studies are warranted to determine whether these favorable actions of taurine will translate into a therapy that might potentially be useful in the early stage of AD.
Methods
Materials
DMSO, sodium carboxymethyl cellulose, taurine (median lethal dose: greater than 7,000 mg/kg), thioflavin S, glycine and sucrose were purchased from Sigma-Aldrich (St. Louis, Missouri, USA). PBS was obtained from Gibco (Grand Island, New York, USA). 96-well plates (clear, black) were purchased from Corning (New York, New York, USA). Deionized water was generated by Milli-Q plus water system from Milliopore (Bedford, Massachusetts, USA).
Animals
Double APP/PS1 transgenic mice (strain name: B6C3-Tg (APPswe, PSEN1De9) 85Dbo/J) and wild type (B6C3F1) mice were obtained from Jackson Laboratory (Bar Harbor, Maine, USA). Both genes APP and PSEN1 were confirmed before the experiment began via a PCR instrument from Bio-Rad (S1000 Thermal-Cycler) using the standard PCR condition from Jackson Laboratory, the PCR-remix provided by Cosmo-Genetech (G-taq PCR premix kit, CMT-6002), and DNA from mice tails. The mice were 7 months of age at the beginning of the experiment, and they were housed in a room under controlled temperature, with an alternating 12 hrs light-dark cycle and access to food and water ad libitum. The behavioural tests were performed during the light period in a sound-attenuated and air-regulated experiment room. There was at least 30 min of habituation time before the behavioural tests began. All animal experiments were carried out in accordance with the National Institutes of Health guide for the care and use of laboratory animals (NIH Publications No. 8023, revised 1978). The animal studies were approved by the Institutional Animal Care and Use Committee of Korea Institute of Science and Technology.
Repeated oral dose studies
Taurine was orally administered via water at dose level of 0 (control) or 1,000 mg/kg/day for 6 weeks to 7-month old male APP/PS1 and wild-type mice: taurine-treated APP/PS1 (n = 11), non-treated APP/PS1 (n = 8), taurine-treated wild-type (n = 10) and non-treated wild-type (n = 10). We speculated that administering taurine orally via water should be effective since taurine can cross the blood-brain barrier, albeit in small amounts44. Amount of water intake per mouse was calculated by measuring the water consumption every day for each cage. Body weight of each animal was measured on the 0th day and every 7 days afterwards. After the behavioural tests, the brains and CSF were collected under ether anesthesia. 19 Mice were perfused, and the other 20 mice had their cortices and hippocampi extracted.
Y-maze
The Y-maze test was performed after 6 weeks of oral taurine administration. The apparatus was a black, plastic maze with 3 arms (40 L × 10 W × 12 H cm) labeled A, B and C that converged to the middle, forming an equilateral triangle with 4 cm at its longest axis. Mice were placed at the end of one arm and allowed to move freely through the maze for 8 min, and the sequence of arm movements was manually recorded. When all 4 limbs of the mouse were within the pathway, it was considered an entry. An alternation was counted when a mouse successively entered 3 different arms. Spontaneous alternation behaviour was calculated based on the following equation:
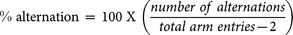 |
Passive avoidance
The passive avoidance test was performed 2 days after the Y-maze test. A 2-compartment shuttle chamber with a light compartment and a darker one containing a shock generator was used. For the acquisition trial, each mouse was first placed in a light chamber. After 10 sec, the door between the light/dark compartments was opened so that the mouse could move freely into the dark chamber. Upon entering the dark chamber, the door closed immediately and an electric foot shock (0.5 mA, 1 sec, once) was delivered through the floor grid. The mouse was then returned to its original cage for 24 hrs. For the retention trial after the 24 hrs, each mouse was placed into the light chamber, and the latency time between placement and entry into the dark chamber was measured (maximum 300 sec). Data was recorded and analyzed using a video camera-based Ethovision System (Nodulus, Netherlands).
Data analyses including recordings of all behavioural responses were transcribed manually into the computer-acceptable format by keeping research colleagues blind.
Brain and CSF sample preparation
After the behavioural tests, mice were anesthetized with 2% avertin (20 μg/g, i.p.). Each group was divided approximately into half for perfusion and the other half for brain lysate. Brain lysates were developed by dissecting the hippocampal and cortical regions of mouse brains and homogenized using a lysis buffer (10 mM Tris-HCl, 5 mM EDTA in 320 mM sucrose, pH 7.4) containing 1× proteinase inhibitors cocktail for 30 min on ice45. The homogenates were centrifuged at 13,500 rpm, 4 °C for 30 min. Concentrations of cortical and hippocampal lysate supernatants were determined by Bradford protein assay. The perfusion began with 0.9% saline followed by ice cold 4% paraformaldehyde (pH 7.4). Excised brains were post-fixed overnight in 4% paraformaldehyde and immersed in 30% sucrose for 48 hrs for cryoprotection. The perfused brain samples were cut at 35 μm using a Cryostat (Microm HM 525, Thermo Scientific, Waltham, MA, USA) and mounted onto glass slides. CSF sampling was performed according to the method described previously46. Anesthetized mice were placed prone, and their cisterna magna were surgically exposed. The exposed meninges were penetrated with laboratory-produced capillary tube that had a tapered tip to obtain CSF. About 3–5 μL of CSF samples were obtained from each mouse.
Western blot
20 μg of brain lysates were analyzed using a 10% SDS-gel electrophoresis. The proteins on the gel were transferred to a PVDF membrane. After the transfer, the membrane was blocked, then antibodies were employed. Immunoreactive bands were visualized using an enhanced chemiluminescence technique (Bio-Rad). The primary antibody information is the following: glial fibrillary acidic protein (GFAP, Millipore AB5541, 1:1,000) and actin as the loading control (Millipore MAB1501R, 1:10,000). The secondary antibody information is the following: anti-mouse (Santa-Cruz, 1:20,000) and anti-rabbit (Santa Cruz, 1:3,000).
ThS staining and immunohistochemistry
Aβ plaque burden in cryo-sectioned brains were visualized using ThS staining. ThS was dissolved in 50% of ethanol at 500 μM and brain sections were stained for 7 min. Then, to remove non-specific binding of ThS dye, the sections were rinsed through 100, 95 and 90% of ethanol for 10 sec each and moved into PBS in succession. The brain sections were also stained for Aβ and GFAP (Millipore AB5541). Aβ was stained by monoclonal antibody (6E10). DAPI was also used for indication of the brain region. The images were taken on a Carl Zeiss LSM700 confocal microscope and a Leica DM2500 fluorescence microscope47. Data analyses including recordings of all behavioural responses were transcribed manually into the computer-acceptable format by keeping research colleagues blind. The mouse brain schematic diagrams in data were adapted from the Mouse Brain Atlas48.
Sandwich-ELISA
Sandwich-ELISA kit was purchased from Invitrogen, and the assays were performed on brain lysates and CSF following the manufacturer's directions and using the antibodies provided in the kit.
Statistics
Graphs were obtained with GraphPad Prism 5, and the statistical analyses were performed with one-way ANOVA followed by Bonferroni's post-hoc comparisons (*P < 0.05, **P < 0.01, ***P < 0.001, n.s.: no significance). The error bars represent the SEMs.
Author Contributions
H.Y.K., H.V.K., J.H.Y., B.R.K. and Y.K. designed the experiments. S.L. synthesized Aβ42. J.W.K., Y.C. and J.W. performed animal preparation and H.V.K. and J.Y.K. performed animal behavioural tasks. H.Y.K. and S.M.C. prepared CSF samples and performed sandwich ELISA. H.Y.K., H.V.K. and B.R.K. performed the brain tissue staining, HPLC and western blots analyses. H.Y.K., H.V.K., J.H.Y. and Y.K. wrote the manuscript.
Acknowledgments
This work was supported by KIST Institutional Programs (Open Research 2E24582 and Flagship 2E25023), KHIDI (H14C04660000), UST Research Internship Program and MIT International Science & Technology Initiatives (MISTI). We appreciate Dr Jun-Seok Lee and Young-Hwa Yoo for the Y-maze analysis program.
References
- Kozauer N. & Katz R. Regulatory innovation and drug development for early-stage Alzheimer's disease. N Engl J Med 368, 1169–1171 10.1056/NEJMp1302513 (2013). [DOI] [PubMed] [Google Scholar]
- Aisen P. S., Cummings J. & Schneider L. S. Symptomatic and nonamyloid/tau based pharmacologic treatment for Alzheimer disease. Cold Spring Harb Perspect Med 2, a006395 10.1101/cshperspect.a006395 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huxtable R. J. Physiological actions of taurine. Physiol Rev 72, 101–163 (1992). [DOI] [PubMed] [Google Scholar]
- Frosini M. et al. A specific taurine recognition site in the rabbit brain is responsible for taurine effects on thermoregulation. Br J Pharmacol 139, 487–494 10.1038/sj.bjp.0705274 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar R. Role of naturally occurring osmolytes in protein folding and stability. Arch Biochem Biophys 491, 1–6 10.1016/j.abb.2009.09.007 (2009). [DOI] [PubMed] [Google Scholar]
- Miao J. et al. Taurine attenuates Streptococcus uberis-induced mastitis in rats by increasing T regulatory cells. Amino Acids 42, 2417–2428 10.1007/s00726-011-1047-3 (2012). [DOI] [PubMed] [Google Scholar]
- Schaffer S. W., Azuma J. & Madura J. D. Mechanisms underlying taurine-mediated alterations in membrane function. Amino Acids 8, 231–246 10.1007/BF00806821 (1995). [DOI] [PubMed] [Google Scholar]
- Schaffer S., Takahashi K. & Azuma J. Role of osmoregulation in the actions of taurine. Amino Acids 19, 527–546 (2000). [DOI] [PubMed] [Google Scholar]
- Su J. H., Anderson A. J., Cummings B. J. & Cotman C. W. Immunohistochemical evidence for apoptosis in Alzheimer's disease. Neuroreport 5, 2529–2533 (1994). [DOI] [PubMed] [Google Scholar]
- Matsuyama Y., Morita T., Higuchi M. & Tsujii T. The effect of taurine administration on patients with acute hepatitis. Prog Clin Biol Res 125, 461–468 (1983). [PubMed] [Google Scholar]
- Azuma J. et al. Therapeutic effect of taurine in congestive heart failure: a double-blind crossover trial. Clin Cardiol 8, 276–282 (1985). [DOI] [PubMed] [Google Scholar]
- Azuma J., Sawamura A. & Awata N. Usefulness of taurine in chronic congestive heart failure and its prospective application. Jpn Circ J 56, 95–99 (1992). [DOI] [PubMed] [Google Scholar]
- Xu Y. J., Arneja A. S., Tappia P. S. & Dhalla N. S. The potential health benefits of taurine in cardiovascular disease. Exp Clin Cardiol 13, 57–65 (2008). [PMC free article] [PubMed] [Google Scholar]
- Vohra B. P. & Hui X. Improvement of impaired memory in mice by taurine. Neural Plast 7, 245–259 10.1155/NP.2000.245 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu C. L. et al. Taurine improves the spatial learning and memory ability impaired by sub-chronic manganese exposure. J Biomed Sci 21, 51 10.1186/1423-0127-21-51 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malcangio M. et al. Effect of ICV taurine on the impairment of learning, convulsions and death caused by hypoxia. Psychopharmacology (Berl) 98, 316–320 (1989). [DOI] [PubMed] [Google Scholar]
- Su Y. et al. Taurine improves functional and histological outcomes and reduces inflammation in traumatic brain injury. Neuroscience 266, 56–65 10.1016/j.neuroscience.2014.02.006 (2014). [DOI] [PubMed] [Google Scholar]
- Suge R. et al. Specific timing of taurine supplementation affects learning ability in mice. Life Sci 81, 1228–1234 10.1016/j.lfs.2007.08.028 (2007). [DOI] [PubMed] [Google Scholar]
- Javed H. et al. Taurine ameliorates neurobehavioral, neurochemical and immunohistochemical changes in sporadic dementia of Alzheimer's type (SDAT) caused by intracerebroventricular streptozotocin in rats. Neurol Sci 34, 2181–2192 10.1007/s10072-013-1444-3 (2013). [DOI] [PubMed] [Google Scholar]
- Ito K., Arko M., Kawaguchi T., Kuwahara M. & Tsubone H. The effect of subacute supplementation of taurine on spatial learning and memory. Exp Anim 58, 175–180 (2009). [DOI] [PubMed] [Google Scholar]
- Santa-Maria I., Hernandez F., Moreno F. J. & Avila J. Taurine, an inducer for tau polymerization and a weak inhibitor for amyloid-beta-peptide aggregation. Neurosci Lett 429, 91–94 10.1016/j.neulet.2007.09.068 (2007). [DOI] [PubMed] [Google Scholar]
- Pan C., Prentice H., Price A. L. & Wu J. Y. Beneficial effect of taurine on hypoxia- and glutamate-induced endoplasmic reticulum stress pathways in primary neuronal culture. Amino Acids 43, 845–855 10.1007/s00726-011-1141-6 (2012). [DOI] [PubMed] [Google Scholar]
- Louzada P. R. et al. Taurine prevents the neurotoxicity of beta-amyloid and glutamate receptor agonists: activation of GABA receptors and possible implications for Alzheimer's disease and other neurological disorders. FASEB J 18, 511–518 10.1096/fj.03-0739com (2004). [DOI] [PubMed] [Google Scholar]
- Wu J. et al. Taurine activates glycine and gamma-aminobutyric acid A receptors in rat substantia gelatinosa neurons. Neuroreport 19, 333–337 10.1097/WNR.0b013e3282f50c90 (2008). [DOI] [PubMed] [Google Scholar]
- Paula-Lima A. C., De Felice F. G., Brito-Moreira J. & Ferreira S. T. Activation of GABA(A) receptors by taurine and muscimol blocks the neurotoxicity of beta-amyloid in rat hippocampal and cortical neurons. Neuropharmacology 49, 1140–1148 10.1016/j.neuropharm.2005.06.015 (2005). [DOI] [PubMed] [Google Scholar]
- Sun Q. et al. Taurine attenuates amyloid beta 1-42-induced mitochondrial dysfunction by activating of SIRT1 in SK-N-SH cells. Biochem Biophys Res Commun 447, 485–489 10.1016/j.bbrc.2014.04.019 (2014). [DOI] [PubMed] [Google Scholar]
- Martorana A., Di Lorenzo F., Manenti G., Semprini R. & Koch G. Homotaurine induces measurable changes of short latency afferent inhibition in a group of mild cognitive impairment individuals. Front Aging Neurosci 6, 254 10.3389/fnagi.2014.00254 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jankowsky J. L. et al. Co-expression of multiple transgenes in mouse CNS: a comparison of strategies. Biomol Eng 17, 157–165 (2001). [DOI] [PubMed] [Google Scholar]
- Filali M., Lalonde R. & Rivest S. Cognitive and non-cognitive behaviors in an APPswe/PS1 bigenic model of Alzheimer's disease. Genes Brain Behav 8, 143–148 10.1111/j.1601-183X.2008.00453.x (2009). [DOI] [PubMed] [Google Scholar]
- Maia L. F. et al. Changes in amyloid-beta and Tau in the cerebrospinal fluid of transgenic mice overexpressing amyloid precursor protein. Sci Transl Med 5, 194re192 10.1126/scitranslmed.3006446 (2013). [DOI] [PubMed] [Google Scholar]
- Rodrigue K. M. et al. beta-Amyloid burden in healthy aging: regional distribution and cognitive consequences. Neurology 78, 387–395 10.1212/WNL.0b013e318245d295 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gervais F. et al. Targeting soluble Abeta peptide with Tramiprosate for the treatment of brain amyloidosis. Neurobiol Aging 28, 537–547 10.1016/j.neurobiolaging.2006.02.015 (2007). [DOI] [PubMed] [Google Scholar]
- Tapiola T. et al. Cerebrospinal fluid {beta}-amyloid 42 and tau proteins as biomarkers of Alzheimer-type pathologic changes in the brain. Arch Neurol 66, 382–389 10.1001/archneurol.2008.596 (2009). [DOI] [PubMed] [Google Scholar]
- Sofroniew M. V. Reactive astrocytes in neural repair and protection. Neuroscientist 11, 400–407 10.1177/1073858405278321 (2005). [DOI] [PubMed] [Google Scholar]
- Pike C. J., Cummings B. J. & Cotman C. W. Early association of reactive astrocytes with senile plaques in Alzheimer's disease. Exp Neurol 132, 172–179 (1995). [DOI] [PubMed] [Google Scholar]
- El Idrissi A. Taurine improves learning and retention in aged mice. Neurosci Lett 436, 19–22 10.1016/j.neulet.2008.02.070 (2008). [DOI] [PubMed] [Google Scholar]
- Sun M., Gu Y., Zhao Y. & Xu C. Protective functions of taurine against experimental stroke through depressing mitochondria-mediated cell death in rats. Amino Acids 40, 1419–1429 10.1007/s00726-010-0751-8 (2011). [DOI] [PubMed] [Google Scholar]
- Sun M., Zhao Y., Gu Y. & Xu C. Anti-inflammatory mechanism of taurine against ischemic stroke is related to down-regulation of PARP and NF-kappaB. Amino Acids 42, 1735–1747 10.1007/s00726-011-0885-3 (2012). [DOI] [PubMed] [Google Scholar]
- Kim H. Y., Kim Y., Han G. & Kim D. J. Regulation of in vitro Abeta1-40 aggregation mediated by small molecules. J Alzheimers Dis 22, 73–85 10.3233/JAD-2010-100183 (2010). [DOI] [PubMed] [Google Scholar]
- Fraser P. E., Nguyen J. T., Chin D. T. & Kirschner D. A. Effects of sulfate ions on Alzheimer beta/A4 peptide assemblies: implications for amyloid fibril-proteoglycan interactions. J Neurochem 59, 1531–1540 (1992). [DOI] [PubMed] [Google Scholar]
- Aisen P. S. et al. Tramiprosate in mild-to-moderate Alzheimer's disease - a randomized, double-blind, placebo-controlled, multi-centre study (the Alphase Study). Arch Med Sci 7, 102–111 10.5114/aoms.2011.20612 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alford C., Cox H. & Wescott R. The effects of red bull energy drink on human performance and mood. Amino Acids 21, 139–150 (2001). [DOI] [PubMed] [Google Scholar]
- Aggarwal R., Mishra A., Crochet P., Sirimanna P. & Darzi A. Effect of caffeine and taurine on simulated laparoscopy performed following sleep deprivation. Br J Surg 98, 1666–1672 10.1002/bjs.7600 (2011). [DOI] [PubMed] [Google Scholar]
- Olive M. F. Interactions between taurine and ethanol in the central nervous system. Amino Acids 23, 345–357 10.1007/s00726-002-0203-1 (2002). [DOI] [PubMed] [Google Scholar]
- Dunah A. W. et al. Sp1 and TAFII130 transcriptional activity disrupted in early Huntington's disease. Science 296, 2238–2243 10.1126/science.1072613 (2002). [DOI] [PubMed] [Google Scholar]
- Liu L. & Duff K. A technique for serial collection of cerebrospinal fluid from the cisterna magna in mouse. J Vis Exp 10.3791/960 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z., Herrmann K. & Pohlenz F. Lateral scanning confocal microscopy for the determination of in-plane displacements of microelectromechanical systems devices. Opt Lett 32, 1743–1745 (2007). [DOI] [PubMed] [Google Scholar]
- Paxinos G. & Franklin B. J. The Mouse Brain in Stereotaxic Coordinates. 2nd edn, (Academic Press, 2001). [Google Scholar]