Abstract
Ultraviolet photodetectors have been fabricated from ZnO quantum dots/carbon nanodots hybrid films, and the introduction of carbon nanodots improves the performance of the photodetectors greatly. The photodetectors can be used to detect very weak ultraviolet signals (as low as 12 nW/cm2). The detectivity and noise equivalent power of the photodetector can reach 3.1 × 1017 cmHz1/2/W and 7.8 × 10−20 W, respectively, both of which are the best values ever reported for ZnO-based photodetectors. The mechanism for the high sensitivity of the photodetectors has been attributed to the enhanced carrier-separation at the ZnO/C interface.
Ultraviolet (UV) photodetectors have a variety of potential applications in both civilian and military areas including but not limited to flame sensing, UV radiation calibration and monitoring, astronomical studies, optical communication, missile launch detection, and so on1,2,3,4,5. Quantum dots (QDs) have some unique characters such as convenient integration, tunable bandgap, controllable transport and trap state properties6,7, which makes them an ideal candidate for photodetection8,9,10,11,12. Photodetectors fabricated from QDs have been studied extensively in recent years, and CdSe13, PbS14, HgTe15, PbSe16, ZnO17, etc., have been employed as the active layer of QD photodetectors. Amongst these materials, ZnO and its related materials have been considered as a promising candidate for UV photodetection due to their wide direct bandgap, high electron saturation velocity, high irradiation resistance, etc17,18,19. Additionally, one of the most noteworthy properties of ZnO lies in its rich and easy-to-get nanostructures20,21,22. ZnO QDs have been synthesized in various methods21,22, and UV photodetectors have been fabricated from these QDs17,22. Nevertheless, ZnO QDs usually have large surface-to-volume ratio and many grain boundaries, and the boundaries will hinder the mobility of electrons drastically thus deteriorate the performance of the photodetectors23,24,25. Therefore it is of great importance and significance if some method can be employed to enhance the performance of ZnO QD based photodetectors.
Carbon based nanostructures like graphene, nanotube, fullerene, etc has been employed to enhance the performance of photodetectors or photovoltaic devices26,27,28. Amongst these nanostructures, carbon nanodots have attracted much attention in recent years for their unique properties including highly luminescent characters, good photo-bleaching resistance, and mild synthesis method, etc29,30,31,32. Hence various potential applications have been expected from carbon nanodots, such as light-emitting devices, photocatalysis, bioimaging, and so on29,30,31,32,33,34. Besides, it has been reported that carbon nanodots can serve as an electron transportation layer35,36. It is speculated that if carbon nanodots are employed to facilitate the carrier transportation and separation of electrons and holes in ZnO QDs, the performance of ZnO QD photodetectors may be improved significantly. However, none such report can be found up to date.
In this work, carbon nanodots have been mixed together with ZnO QDs to form hybrid films, and UV photodetectors have been fabricated from the hybrid films. It is found that the responsivity of the photodetector can be increased greatly with the introduction of carbon nanodots. The detectivity and noise equivalent power (NEP) of the photodetector can reach 3.1 × 1017 cmHz1/2/W and 7.8 × 10−20 W, respectively, both of which correspond to the best values ever reported for ZnO-based photodetectors. The mechanism for the ultrahigh sensitivity has been attributed to the increase carrier separation at the ZnO/C interface.
Results
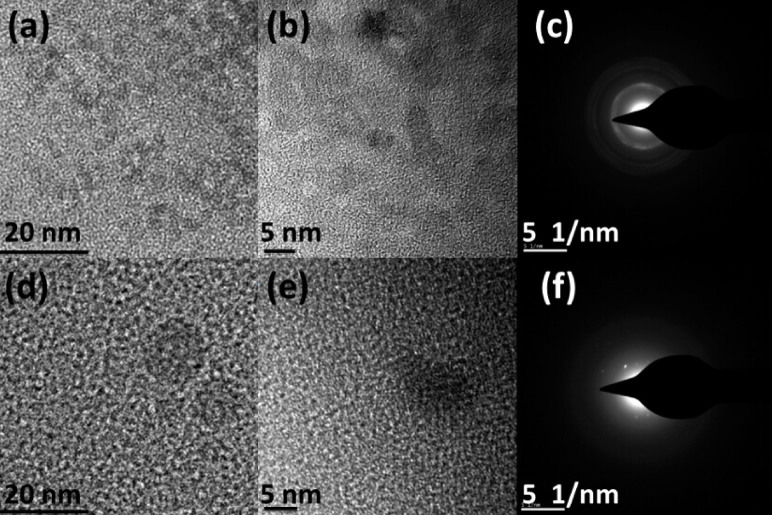
The morphology of the ZnO QDs and carbon nanodots are characterized by transmission electron microscopy (TEM), and the TEM images of the as-prepared ZnO QDs and carbon nanodots are shown in Fig. 1. Figures 1(a) and 1(d) show clearly that both the ZnO QDs and carbon nanodots have nearly spherical shape, and the size of the ZnO QDs is around 5 nm, while that of the carbon nanodots is about 20 nm. Figures 1(b) and 1(e) show the high-resolution TEM images of the ZnO QDs and carbon nanodots, respectively. Figures 1(c) and 1(f) indicate the corresponding selected area electron diffraction patterns of the two kinds of dots. The patterns exhibit lattice spacing and concentric rings, which indicates the crystalline nature of the QDs and nanodots.
Figure 1.
Low-magnification TEM image of the ZnO QDs (a) and carbon nanodots (d); High resolution TEM images of the ZnO QDs (b) and carbon nanodots (e); Selected-area electron diffraction patterns of the ZnO QDs (c) and carbon nanodots (f).
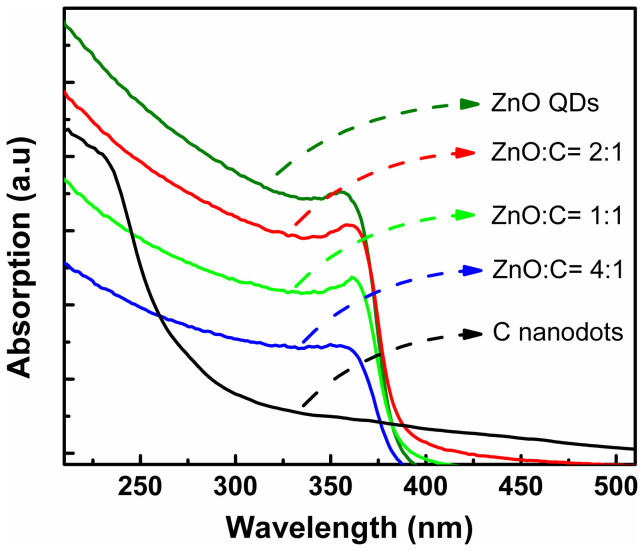
The typical absorption spectra of the ZnO QDs and carbon nanodots films with different volume ratios are shown in Fig. 2. Note that the spectra have been adjusted vertically for clarity. One can see from the spectra that the absorption curve of the hybrid films share the same lineshape with that of the ZnO QDs irrespective of the volume ratio. A strong absorption in UV region can be observed from the figure, while that in the visible region is negligible, which is favorable for high-performance UV photodetectors fabricated from the blending solution37.
Figure 2. Absorption spectra of the ZnO QDs, carbon nanodots, and the blending solutions.
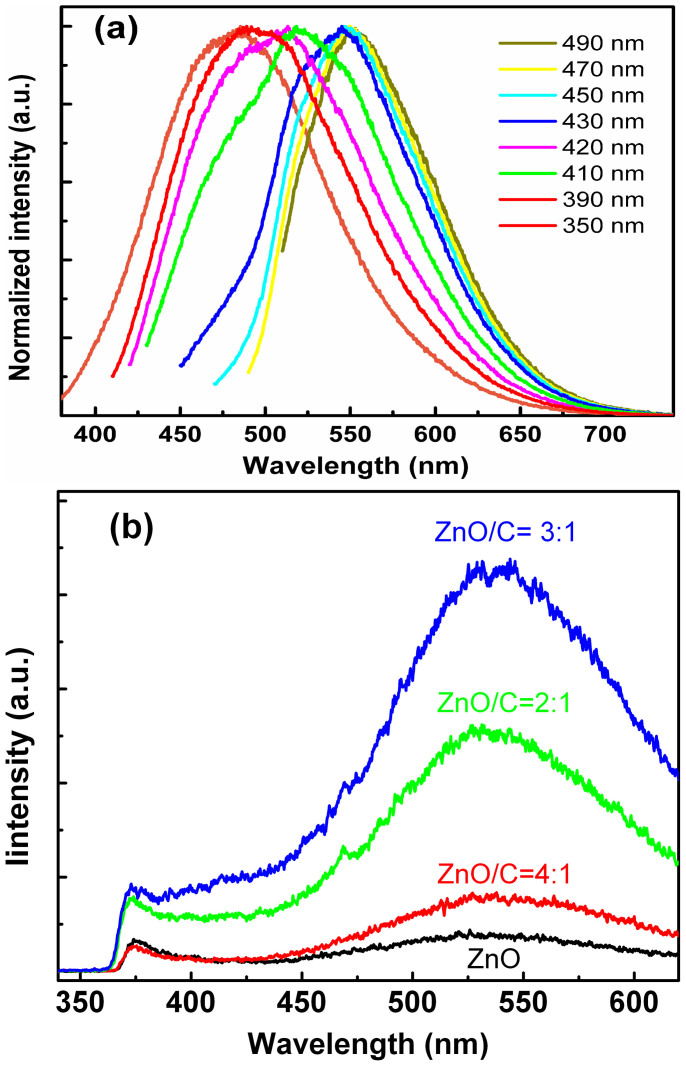
The photoluminescence (PL) spectra of the carbon nanodot solution are shown in Fig. 3. One can see from Fig. 3(a) that obvious emission can be detected from the carbon nanodots and the emission peak of the nanodots redshifts from around 480 nm to 550 nm when the excitation wavelength changes from 350 nm to 490 nm. Note that the above excitation dependent emission is a typical characteristic of carbon nanodots29,30,31. The PL spectra of the ZnO QD solution mixed with carbon nanodots with different volume ratio are shown in Fig. 3(b). All the spectra show a dominant broad emission at around 550 nm, which has been frequently observed in ZnO QDs, and can be attributed to the deep-level related emission of ZnO38,39. Another weak emission at around 375 nm is also visible from the figure, which can be attributed to the near band-edge emission of ZnO39. The PL spectra of the ZnO and carbon nanodots mixed solution share the same lineshape with that of ZnO QDs, but the emission intensity varies, which reveals that effective interaction between ZnO QDs and carbon nanodots occurs in the hybrid system.
Figure 3.
(a) Normalized PL spectra of the carbon nanodots excited by different wavelength illumination source; (b) PL spectra of ZnO/C blending solution with different volume ratios.
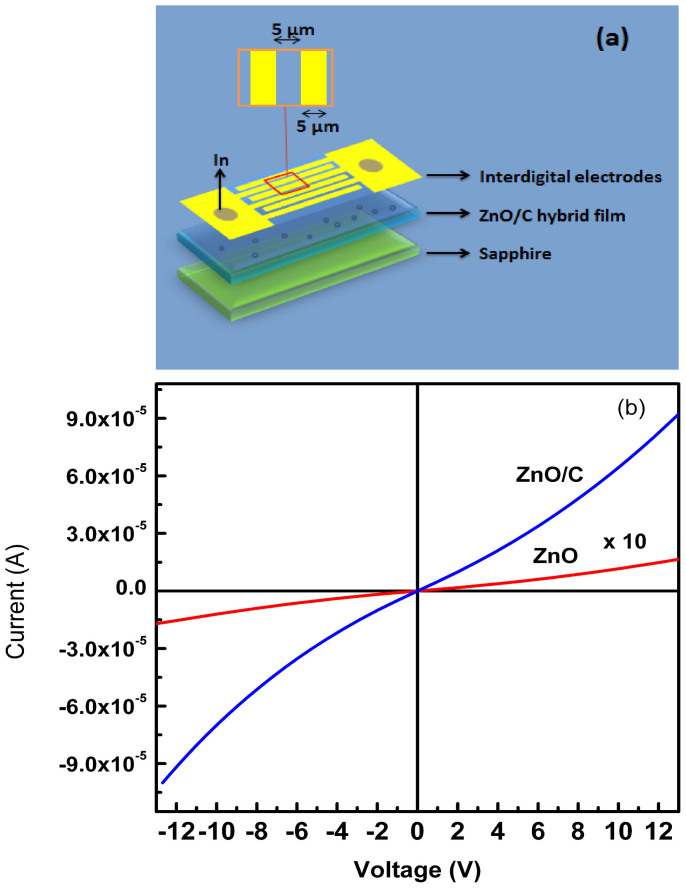
To fabricate the photodetectors, the mixed solution with different ZnO/C ratio was spin coated onto sapphire substrate, and then annealed in air ambient at 400°C for one hour, then at 600°C for another hour. In this way, ZnO QD/carbon nanodot hybrid films have been prepared, and the UV photodetectors have been fabricated from the hybrid films. The schematic diagram of the hybrid photodetector device structure fabricated from the ZnO QDs/carbon nanodots hybrid films is shown in Fig. 4(a). The electrode is composed by 12 pairs of interdigital contact, the length and width of the interdigital contacts are 500 μm and 5 μm, respectively, and the spacing between the adjacent interdigital contacts are 5 μm. Therefore the active area of the devices is around 5.75 × 10−4 cm2 (23 × 5 μm × 500 μm). Figure 4(b) shows the typical current-voltage (I–V) curves of the photodetector fabricated from the ZnO/C hybrid films with a ratio of 2:1 and bare ZnO QD film. One can see from the curves that in the investigated bias range, the current increases almost linearly with the bias voltage. Another noteworthy phenomenon lies in that the current of the hybrid photodetector is much larger than that of the bare ZnO QD photodetector, revealing that the introduction of carbon nanodots helps to decrease the resistivity of the ZnO QD films.
Figure 4.
Schematic illustration (a) and I–V curves (b) of the photodetector fabricated from the ZnO/C hybrid films and bare ZnO QDs, note that the current of the device fabricated from bare ZnO QDs has been magnified by 10 times for clarity sake.
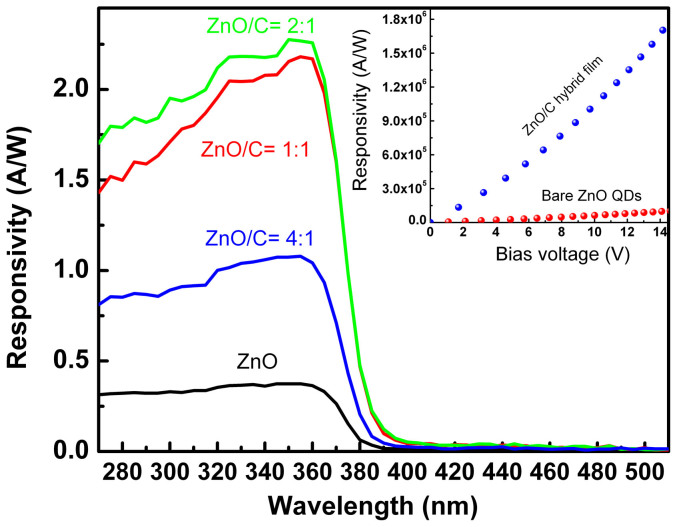
We note that the photodetectors show obvious response even when the illumination source is as weak as 12 nW/cm2. The response spectrum of the photodetectors fabricated from the hybrid films with different ratios at a bias voltage of 50 mV is shown in Fig. 5, and that of the photodetector fabricated from bare ZnO QDs is also plotted for comparison. One can see that the response spectra of the photodetector fabricated from the hybrid films are very similar in shape with that of the device fabricated from bare ZnO QDs, and all the spectra show a maximum response at around 370 nm. A noteworthy phenomenon in the spectra lies in the fact that the responsivity of the photodetectors fabricated from the hybrid films is much higher than that from bare ZnO QDs. Note that the response curves of the devices recorded one year later are almost identical to the ones recorded before as the devices are kept in a tinfoil sealed desiccator, indicating the good reliability of the photodetectors. The device fabricated from ZnO/C ratio of 2: 1 shows the largest responsivity, thus this device has been selected as a representative in the following investigations. The inset of Fig. 5 shows the dependence of the peak responsivity of the photodetectors fabricated from ZnO/C hybrid films and bare ZnO QDs on the bias applied. The responsivity increases monotonically with the bias in both cases, and the responsivity of the photodetector fabricated from ZnO QD/carbon nanodots hybrid films can reach 1.7 × 106 A/W, while that of the photodetector fabricated from bare ZnO QDs is 9.7 × 104 A/W reaches 14 V. That is, the responsivity of the device fabricated from the hybrid films is over two orders higher than that of the device fabricated from bare ZnO QDs.
Figure 5.
(a) Response spectra of the photodetectors fabricated from the ZnO QD/carbon nanodots hybrid film with different ratios under a bias of 50 mV; (b) Dependence of the responsivity of the photodetectors fabricated from the ZnO/C hybrid films and bare ZnO QDs on the bias voltage.
Detectivity (D*) and noise equivalent power (NEP) are also two key figure-of-merit parameters that determine the performance of a photodetector, which usually correspond to the weakest signals that can be detected by the photodetector. D* ad NEP can be expressed by the following formulas40:
 |
 |
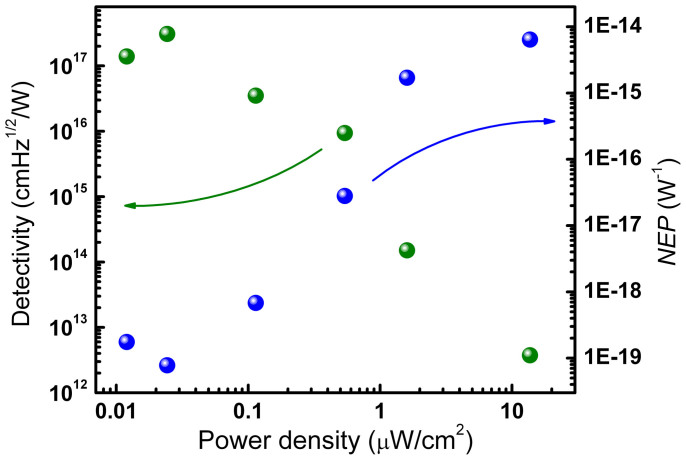
Where Rλ is the responsivity of the devices; R0 is the dark impedance; A is the detector area (5.75 × 10−4 cm2); B is the bandwidth. According to Eqs. 1 and 2, the detectivity and NEP of the photodetectors fabricated from the bare ZnO QDs and ZnO/C hybrid films have been measured. We note that the detectivity and NEP of the photodetector depends greatly on the power density of the illumination source, as shown in Fig. 6. One can see that the detectivity increases significantly with decreasing the illumination power density. The above fact reveals that the photodetectors in our case favors for weak signal detection, which is eagerly wanted in many practical application fields. When the power density of the illumination lamp is reduced to 20 nW/cm2, the detectivity of the photodetector fabricated from bare ZnO QDs can reach 7.8 × 1016 cmHz1/2/W, and the NEP can reach 3.1 × 10−19 W when the bias voltage is 14 V. While the corresponding values for the photodetector fabricated from the ZnO QD/carbon nanodots hybrid films are 3.1 × 1017 cmHz1/2/W and 7.8 × 10−20 W, respectively. Table I summarizes the recent reported relatively high detectivity and NEP values of ZnO-based UV photodetectors, and the corresponding values obtained in this work are also included. It is clear from the table that both the detectivity and NEP of the photodetectors fabricated from hybrid films correspond the best values ever reported for ZnO-based UV photodetectors41,42,43,44,45. Also note that the detectivity of the UV photodetectors fabricated from the hybrid films are significantly higher than that of conventional commercial available 4H-SiC UV photodetectors (~1013–1015 cmHz1/2/W)46,47.
Figure 6. The dependence of the detectivity and NEP of the photodetectors fabricated from the hybrid film on the illumination power density.
Table 1. Comparison of the D* and NEP of the photodetectors fabricated from the ZnO QDs/carbon nanodot hybrid films with the reported values.
| ZnO structure | D* (cmHz1/2/W) | NEP (W) | Voltage (V) | Reference |
|---|---|---|---|---|
| Thin film | 1.19 × 1010 | 2.65 × 10−12 | 5 | 41 |
| Nanowall | 3.38 × 109 | 1.87 × 10−10 | 2 | 42 |
| Nanorod | 1.43 × 1015 | 2.27 × 10−14 | 5 | 43 |
| Nanowire | 2.13 × 109 | 5.88 × 10−13 | 1.5 | 44 |
| Nanoparticle | 3.43 × 1015 | NA | 9 | 45 |
| ZnO/C Hybrid film | 3.1 × 1017 | 7.8 × 10−20 | 14 | This work |
Discussion
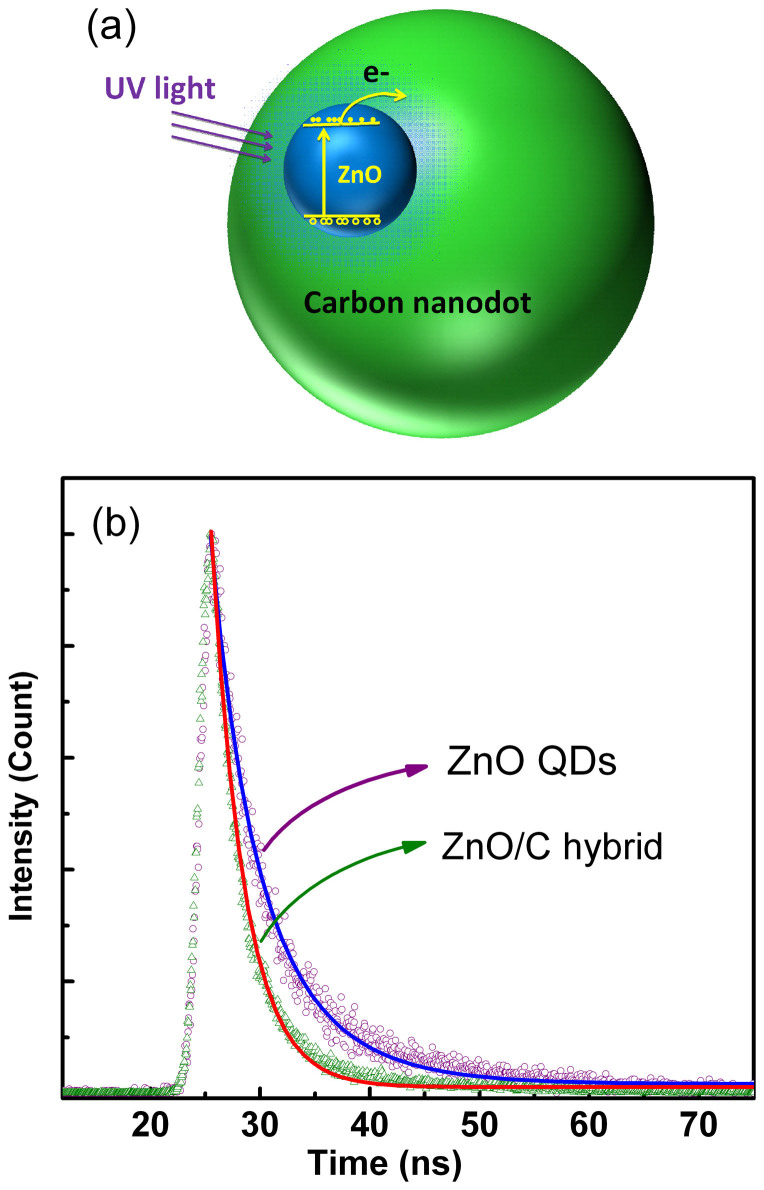
The above results indicate that the incorporation of carbon nanodots has improved the responsivity of the photodetectors greatly. In order to understand the mechanisms of the improved performance, a model has been proposed, as shown in Fig. 7(a). Since the absorption of the hybrid films is dominated by ZnO QDs, under the illumination of UV light, electrons and holes will be generated mainly in the ZnO QDs, and some of the generated electrons can enter into the carbon nanodots. Considering that carbon can serve as electron transportation media32,44, the electrons that entered into the carbon nanodots can be collected by the electrodes soon. To confirm this mechanism, the transient spectra of the emission at 375 nm for the hybrid ZnO/C system and bare ZnO QDs are illustrated in Fig. 7(b), in which the scattered symbols are experimental data, while the solid lines are fitting results to the experimental data using the following two-order exponential decay formula:
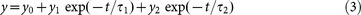 |
Figure 7.
(a) Schematic illustration of the carrier generation and separation in the ZnO QDs/carbon nanodots hybrid structure; (b) Transient spectra of the emission at around 375 nm for bared ZnO QDs and ZnO/C hybrid films.
Where y is the emission intensity, y0, y1, y2 are constant, t is time. One can see that in both cases, the experimental data can be well fitted, and the best fitting yields the lifetime of τ1 = 2.97 ns, and τ2 = 2.97 ns for the ZnO/C hybrid films, while τ1 = 6.28 ns, and τ2 = 3.16 ns for the bare ZnO QDs. Note that τ1 in both cases may come from the exciton recombination inside the ZnO QDs, while τ2 correspond to the exciton captured by the surface defects of the QDs48,49. It can be seen that the carrier lifetime for the ZnO/C hybrid films is distinctively shorter than that for the bare ZnO QDs, which consolidates that some of the carries have been transferred from the ZnO QDs to the carbon nanodots in the ZnO/C hybrid films, as illustrated in Fig. 7(a). The effective electron transportation from the ZnO QDs to the carbon nanodots will facilitate the carrier separation, thus helps to enhance the performance of the photodetector.
In summary, UV photodetectors have been fabricated from ZnO QDs/carbon nanodots hybrid films, and the detectivity and noise equivalent power of the photodetectors can reach 3.1 × 1017 cmHz1/2/W and 7.8 × 10−20 W, respectively, both of which corresponds to the best value ever reported for ZnO-based photodetectors. The mechanism for the high performance has been attributed to the increased carrier-separation at the ZnO QD/carbon nanodots interface. The results reported in this paper may provide a route to UV photodetectors with ultrahigh sensitivity that is eagerly wanted for weak signal detection.
Methods
Materials and synthesis
The ZnO QDs in this study were synthesized via a sol-gel method which involves with Zinc acetate dihydrate, 2-methoxyethanol and monoethanolamine (MEA)50. The synthesis process is illustrated as follows: Firstly, a three-neck flask loaded with 8.3 g zinc acetate dihydrate and a magnet rotor was installed with oil-bath and condenser pipe of which the straight part connecting with flask was sealed with nitrogen and the recycle part connecting with conduit was filled with water flow. After that 48 ml 2-methoxyethanol was added into the three-neck flask. The magnet rotor was kept rotary all the way. When the oil temperature was raised up to 70°C, 2.3 ml MEA was injected into the flask. After 24 hours stirring by rotary rotor, transparent and colourless ZnO QD solution was moved into an Erlenmeyer flask. The synthesis of carbon nanodots was carried out according to the method developed in Ref. 32, in which octadecylene (ODE), 1-hexadecylamine (HDA) and anhydrous citric acid were engaged in the synthesis. Firstly, 15 ml ODE acting as inert solvent and 1.5 g HAD acting as surface passivation agent were loaded in a three-neck flask, and then heated to boiling under argon flow. Secondly, 1.0 g anhydrous citric acid acting as carbon precursor was injected into the flask quickly. After purified 5 times by acetone, jelly-like carbon nanodots were obtained, and the product was then dissolved into toluene.
Device fabrication and characterization
To prepare ZnO/carbon nanodot hybrid films, the ZnO QDs and carbon nanodots solution were mixed in a series of volume ratio (1:0, 4:1, 2:1, 1:1), and the mixed solution was spun onto c-plane sapphire and then the samples were annealed at 400°C for one hour, and then at 600°C for another hour. In this way, ZnO QD and carbon nanodot hybrid films have been prepared. To fabricate UV photodetectors from the hybrid films, a thin Au layer was deposited onto the hybrid films in a vacuum evaporation method acting as contact, and interdigital electrodes were configured via a photolithography and wet etching process. For comparison, ZnO layers without the carbon nanodots have also been prepared, and photodetectors have been fabricated from the ZnO layers. The morphology of the QDs was characterized using a Philips TF-F20 transmission electron microscope operating at 200 kV. The absorption spectra of the ZnO QDs and carbon nanodots were studied in a Shimadzu UV-3101 PC scanning spectrophotometer. The photoluminescence spectra of the carbon nanodots and ZnO QDs are measured in a Shimadzu F4500 spectrometer with a Xe lamp as the excitation source. Photoresponse properties of the hybrid films were measured in a SPEX scanning monochromator employing a 150 W Xe lamp as the illumination source. The current- voltage measurement of the photodetector was measured in a Lakeshore 7707 Hall system.
Author Contributions
D.Y.G. conducted the measurements of the ZnO QDs and carbon nanodots, and also the response properties of the photodetectors, C.X.S. conceive the idea of the paper, and analyze the experimental data. S.N.Q. took part in the discussion and formation of the idea of this paper, D.Z.S. is the principle investigator of the project.
Acknowledgments
This work is financially supported by the National Basic Research Program of China (2011CB302006), the National Science Foundation for Distinguished Young Scholars of China (61425021), the Natural Science Foundation of China (11134009, 11374296, 61376054, 61475153 and 61177040), and the Science and Technology Developing Project of Jilin Province (20111801).
References
- Konstantatos G. et al. Ultrasensitive solution-cast quantum dot photodetectors. Nature 442, 180–183 (2006). [DOI] [PubMed] [Google Scholar]
- Jin Z. W., Gao L., Zhou Q. & Wang J. Z. High-performance flexible ultraviolet photoconductors based on solution-processed ultrathin ZnO/Au nanoparticle composite films. Sci. Rep. 4, 4268 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monroy E., Omnes F. & Calle F. Wide-bandgap semiconductor ultraviolet photodetectors. Semicond. Sci. Technol. 18, R33 (2003). [Google Scholar]
- Peng L., Hu L. & Fang X. Low-dimensional nanostructure ultraviolet photodetectors. Adv. Mater. 25, 5321–5328 (2013). [DOI] [PubMed] [Google Scholar]
- Sang L. W. et al. Arbitrary Multicolor Photodetection by Hetero-integrated Semiconductor Nanostructures. Sci. Rep. 3, 2638 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J. et al. All solution-processed stable white quantum dot light-emitting diodes with hybrid ZnO@TiO2 as blue emitters. Sci. Rep. 4, 2045–2322 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kramer J. & Sargent E. H. Colloidal quantum dot photovoltaics: a path forward. ACS Nano, 5, 8506–8514 (2011). [DOI] [PubMed] [Google Scholar]
- Coe-Sullivan S. Optoelectronics: Quantum dot developments. Nature Photon. 3, 315–316 (2009). [Google Scholar]
- Konstantatos G. & Sargent E. H. Nanostructured materials for photon detection. Nature Nanotechnol. 5, 391–400 (2010). [DOI] [PubMed] [Google Scholar]
- Sargent E. Photodetectors: A sensitive pair. Nature Nanotechnol. 7, 349–350 (2012). [DOI] [PubMed] [Google Scholar]
- Cheng S. H. et al. All Carbon-Based Photodetectors: An eminent integration of graphite quantum dots and two dimensional graphene. Sci. Rep. 4, 5734 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kardynał B. E., Shields A. J. & Beattie N. S. Low-noise photon counting with a radio-frequency quantum-dot field-effect transistor. Appl. Phys. Lett. 84, 419–421 (2004). [Google Scholar]
- Clifford J. P., Konstantatos G. & Johnston K. W. Fast, sensitive and spectrally tunable colloidal-quantum-dot photodetectors. Nature Nanotechnol. 4, 40–44 (2009). [DOI] [PubMed] [Google Scholar]
- Sun Z. H. et al. Infrared photodetectors based on CVD-Grown graphene and PbS quantum dots with ultrahigh responsivity. Adv. Mater. 24, 5878–5883 (2012). [DOI] [PubMed] [Google Scholar]
- Keuleyan S., Lhuillier E., Brajuskovic V. & Guyot-Sionnest P. Mid-infrared HgTe colloidal quantum dot photodetectors. Nature Photon. 5, 489–493 (2011). [Google Scholar]
- McDonald S. A. Solution-processed PbS quantum dot infrared photodetectors and photovoltaics. Nature Mater. 4, 138–142 (2005). [DOI] [PubMed] [Google Scholar]
- Jin Y., Wang J. & Sun B. Solution-processed ultraviolet photodetectors based on colloidal ZnO nanoparticles. Nano Lett. 8, 1649–1653 (2008). [DOI] [PubMed] [Google Scholar]
- Gimenez A. J., Yanez-Limon, J. M. & Seminario, J. M. ZnO− Paper Based Photoconductive UV Sensor. J. Phys. Chem. C 15, 282–287 (2010). [Google Scholar]
- Liu J. S. et al. High responsivity ultraviolet photodetector realized via a carrier-trapping process. Appl. Phys. Lett. 97, 251102 (2010). [Google Scholar]
- Liu X. Y. et al. Pure ultraviolet emission from ZnO nanowire-based p-n heterostructures. Opt. Lett. 39, 422–425 (2014). [DOI] [PubMed] [Google Scholar]
- Zhou W. Y. et al. ZnO nanorods: morphology control, optical properties, and nanodevice applications. Sci China-Phys Mech Astron. 56, 2243 (2013). [Google Scholar]
- Gogurla N. et al. Multifunctional Au-ZnO Plasmonic Nanostructures for Enhanced UV Photodetector and Room Temperature NO Sensing Devices. Sci. Rep. 4, 6483 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asok A., Gandhi M. N. & Kulkarni A. R. Enhanced visible photoluminescence in ZnO quantum dots by promotion of oxygen vacancy formation. Nanoscale 4, 4943–4946 (2012). [DOI] [PubMed] [Google Scholar]
- Sze S. M. & Ng K. K. PHYSICS OF SEMICONDUCTOR DEVICES (John Wiley & Sons, New York, 2006), 3rd Ed. [Google Scholar]
- Son D. I., Kwon B. W. & Park D. H. Emissive ZnO-graphene quantum dots for white-light-emitting diodes. Nature Nanotechnol. 7, 465–471 (2012). [DOI] [PubMed] [Google Scholar]
- Konstantatos G. et al. Hybrid graphene–quantum dot phototransistors with ultrahigh gain. Nature Nanotechnol. 7, 363–368 (2012). [DOI] [PubMed] [Google Scholar]
- Tvrdy K., Frantsuzovc P. A. & Kamata P. V. Photo induced electron transfer from semiconductor quantum dots to metal oxide nanoparticles. Proc. Natl. Acad. Sci. 108, 29–34 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lightcap I. V. & Kamat P. V. Fortification of CdSe Quantum Dots with Graphene Oxide. Excited State Interactions and Light Energy Conversion. J. Am. Chem. Soc. 134, 7109–7116 (2012). [DOI] [PubMed] [Google Scholar]
- Qu S. N., Wang X. Y., Lu Q. P., Liu X. Y. & Wang L. J. A biocompatible fluorescent ink based on water-soluble luminescent carbon nanodots. Angew. Chem. Int. Ed. 51, 12215–12218 (2012). [DOI] [PubMed] [Google Scholar]
- Wei W. L. Non-enzymatic-browning-reaction: a versatile route for production of nitrogen-doped carbon dots with tunable multicolor luminescent display. Sci. Rep. 4, 3564 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qu S. N., Chen H., Zheng X. M., Cao J. S. & Liu X. Y. Ratiometric fluorescent nanosensor based on water soluble carbon nanodots with multiple sensing capacities. Nanoscale 5, 5514–5518 (2013). [DOI] [PubMed] [Google Scholar]
- Wang F. et al. One-step synthesis of highly luminescent carbon dots in noncoordinating solvents. Chem. Mater. 22, 4528–4530 (2010). [Google Scholar]
- Baker S. N. & Baker G. A. Luminescent Carbon Nanodots: Emergent Nanolights. Angew. Chem. Int. Ed. 49, 6726–6744 (2010). [DOI] [PubMed] [Google Scholar]
- Yu H. et al. ZnO/carbon quantum dots nanocomposites: one-step fabrication and superior photocatalytic ability for toxic gas degradation under visible light at room temperature. New J. Chem. 36, 1031–1035 (2012). [Google Scholar]
- Qu S. N., Chen H., Zheng X. M., Cao J. S. & Liu X. Y. Ratiometric fluorescent nanosensor based on water soluble carbon nanodots with multiple sensing capacities. Nanoscale 5, 5514–5518 (2013). [DOI] [PubMed] [Google Scholar]
- Zhang H. et al. Carbon quantum dots/Ag3PO4 complex photocatalysts with enhanced photocatalytic activity and stability under visible light. J. Mater. Chem. 22, 10501–10506 (2012). [Google Scholar]
- Yu J. et al. Enhanced responsivity of photodetectors realized via impact ionization. Sensors. 12, 1280–1287 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang X. S. et al. Synthesis of ZnO Nanoparticles with Tunable Emission Colors and Their Cell Labeling Applications. Chem. Mater. 22, 3383–3388 (2010). [Google Scholar]
- Dutta M., Jana S. & Basak D. Quenching of Photoluminescence in ZnO QDs Decorating Multiwalled Carbon Nanotubes, ChemPhysChem. 11, 1774–1779 (2010). [DOI] [PubMed]
- Zhu H. et al. Metal-oxide-semiconductor-structured MgZnO ultraviolet photodetector with high internal gain. J. Phys. Chem. C 114, 7169–7172 (2010). [Google Scholar]
- Lee H. Y., Hsu Y. T. & Lee C. T. ZnO-based resonant cavity enhanced metal–semiconductor–metal ultraviolet photodetectors. Solid State Sci. 79, 223–226 (2013). [Google Scholar]
- Chen T. P. et al. Noise Properties of ZnO Nanowalls Deposited Using Rapid Thermal Evaporation Technology. IEEE Photon. Technol. Lett. 25, 213–216 (2013). [Google Scholar]
- Chen T. P. et al. IEEE. High Detectivity Mechanism of ZnO-Based Nanorod Ultraviolet Photodetectors. IEEE Photon. Technol. Lett. 25, 348–351 (2013). [Google Scholar]
- Yu H. et al. ZnO nanowire based visible-transparent ultraviolet detectors on polymer substrates. J. Appl. Phys. 111, 102806 (2012). [Google Scholar]
- Guo F. et al. A nanocomposite ultraviolet photodetector based on interfacial trap-controlled charge injection. Nature Nanotechnol. 7, 798–802 (2012). [DOI] [PubMed] [Google Scholar]
- Bai X., Liu H. D., McIntosh D. C. & Campbell J. C. High-detectivity and high-single-photon-detection-efficiency 4H-SiC avalanche photodiodes. IEEE J. Quantum Electron. 45, 300–303. (2009). [Google Scholar]
- Zhu H., Chen X., Cai J. & Wu Z. 4H–SiC ultraviolet avalanche photodetectors with low breakdown voltage and high gain. Solid State Electron. 53, 7–10 (2009). [Google Scholar]
- Son D. I., Yang H. Y., Kim T. W. & Park W. II. Photoresponse mechanisms of ultraviolet photodetectors based on colloidal ZnO quantum dot-graphene nanocomposites. Appl. Phys. Lett. 102, 021105 (2013). [Google Scholar]
- Li F. S. et al. Flexible photovoltaic cells fabricated utilizing ZnO quantum dot/carbon nanotube heterojunctions. Nanotechnology 20, 155202 (2009). [DOI] [PubMed] [Google Scholar]
- Zhu M. W., Gong J. & Sun C. Investigation of correlation between the microstructure and electrical properties of sol-gel derived ZnO based thin films. J. Appl. Phys. 104, 07311 (2008). [Google Scholar]