Abstract
Background: Vitamin D is a potent immunomodulator, but its impact on morbidity and mortality among infants remains unclear.
Objective: The objective of the study was to prospectively assess the association of vitamin D status with mortality, morbidity, and growth during the first 2 y of life.
Methods: A prospective cohort of 253 HIV-infected and 948 HIV-exposed Tanzanian infants enrolled in a randomized trial of multivitamins (not including vitamin D) was studied. Serum 25-hydroxyvitamin D [25(OH)D] concentrations were measured at 5–7 wk of age and infants were followed at monthly clinic visits until 24 mo. Physicians performed a clinical exam every 3 mo or when an illness was noted.
Results: Serum 25(OH)D concentrations were (means ± SDs) 18.6 ± 10.3 ng/mL and 18.1 ± 9.2 ng/mL for HIV-infected and HIV-exposed infants, respectively. Unexpectedly, serum 25(OH)D concentrations ≥30 ng/mL were significantly associated with higher mortality as compared to the 20–29.9 ng/mL reference for HIV-infected (HR: 2.47; 95% CI: 1.13, 5.44; P = 0.02) and HIV-exposed (HR: 4.00; 95% CI: 1.67, 9.58; P < 0.01) infants after multivariate adjustment. We found no statistically significant association between 25(OH)D concentrations <10 ng/mL and mortality for HIV-infected (HR: 1.43; 95% CI: 0.74, 2.78; P = 0.29) and HIV-exposed (HR: 1.56; 95% CI: 0.60, 4.03; P = 0.36) infants. Among HIV-exposed infants, 25(OH)D concentrations ≥30 ng/mL were significantly associated with clinical [incidence ratio rate (IRR): 1.34; 95% CI: 1.06,1.70; P = 0.02] and confirmed (IRR: 1.71; 95% CI: 1.71; 1.15, 2.54; P < 0.01) malaria diagnoses, whereas concentrations of <10 ng/mL were associated with oral candidiasis (IRR: 1.47; 95% CI: 1.00–2.15; P = 0.046) and wasting (HR: 1.71; 95% CI: 1.20, 2.43; P < 0.01).
Conclusion: The observational design of this study does not allow for causal interpretation; however, the results indicate a strong need for additional studies of vitamin D among HIV-infected and -exposed children, particularly in malaria-endemic settings. The parent trial was registered at clinicaltrials.gov as NCT00197730.
Keywords: HIV, vitamin D, infant, malaria, child nutrition sciences, cohort studies, micronutrients
Introduction
HIV-infected infants have long been recognized to have high rates of morbidity and mortality; however, research has also indicated HIV-exposed (uninfected) infants may also be at higher risk of poor health outcomes than HIV-unexposed peers (1). As a result, interventions for infants born to HIV-infected mothers may target a highly vulnerable population and substantially aid in the achievement of Millennium Development Goals in countries with a high HIV burden.
Vitamin D is a potent immunomodulator of adaptive and innate immune responses, but whether this translates into reduced child infectious disease morbidity and mortality remains unclear (2, 3). Observational studies have found that low vitamin D concentrations during infancy and early childhood are associated with increased risk of incident lower and upper respiratory tract infections; however, randomized trials of vitamin D supplementation have produced mixed results (4–9). A recent randomized trial among Afghani infants found that vitamin D supplements had no impact on the incidence of first episode of pneumonia, but increased the risk of repeat pneumonia episodes (7). Another randomized trial of infant vitamin D supplementation conducted among low-birth-weight Indian infants determined no effect on death or hospitalization, but noted nonsignificant increases in length, weight, and arm circumference among vitamin D–supplemented infants (7, 8). A recent trial in Bangladesh also supports a beneficial effect on child growth, as maternal vitamin D supplementation during the third trimester significantly improved linear growth of infants, but it is unclear if these benefits were produced due to effects during the antenatal or postnatal period (9).
Vitamin D studies in the context of HIV primarily have been observational and conducted among adults, with only a few studies having examined associations for children of HIV-infected mothers (10–13). A prospective cohort study conducted among HIV-infected Tanzanian women found that low vitamin D concentration during the second trimester of pregnancy was significantly associated with an increased risk of infant cough, stunting, wasting, and death (12, 13). An additional prospective cohort study of HIV-infected children in North America determined vitamin D–related genetic variants were associated with HIV disease progression, but vitamin D concentrations were not directly measured (14). To date no longitudinal studies have assessed the relation between vitamin D status during infancy and mortality, morbidity, and growth for HIV-infected and HIV-exposed infants. Accordingly, we present a prospective cohort study among Tanzanian infants born to HIV-infected women.
Methods
Trial design and procedures.
The parent trial for this prospective cohort study was a randomized, double-blind, placebo-controlled trial of infant multivitamin supplementation conducted in Dar es Salaam, Tanzania, from February 2004 to June 2007, and was registered at clinicaltrials.gov as NCT00197730 (15). Pregnant women aged >18 y who presented for prenatal care at or before week 32 of gestation at 8 antenatal clinics in Dar es Salaam were offered HIV screening with pre- and postcounseling. Maternal HIV-1 status was determined by using 2 sequential ELISAs: Murex HIV antigen/antibody (Abbott Murex) followed by the Enzygnost anti–HIV-1/2 Plus (Dade Behring). Discordant results were resolved by using a Western blot test (Bio-Rad Laboratories).
Infants born to HIV-infected mothers were then eligible for enrollment if they were a singleton birth, were 5–7 wk of age at the time of the enrollment visit, and the mother intended to reside in Dar es Salaam for at least 2 y. Infants were excluded if they were of multiple gestation or had a serious congenital anomaly. Enrolled infants were randomly assigned to receive either daily multivitamin or placebo supplements from age 6 wk to the end of the follow-up. The multivitamin contained vitamin C, vitamin E, thiamin, riboflavin, niacin, vitamin B-6, folate, and vitamin B-12. All mothers received single RDA oral multivitamins that did not contain vitamin D during the pregnancy and postpartum periods as part of the HIV treatment standard of care in Tanzania (16).
Infants were tested for HIV infection at 6 wk of age using the Amplicor HIV-1 DNA PCR assay, version 1.5 (Roche Molecular Systems), and at 18 mo of age using 2 sequential HIV ELISAs (Murex HIV antigen/antibody followed by the Enzygnost anti–HIV-1/2 Plus), and discordant results were resolved by using a Western blot assay (Bio-Rad Laboratories). At the start of the trial in 2004, the standard of care for prevention of mother-to-child transmission of HIV in Tanzania was a single dose of nevirapine to the mother at the onset of labor and a single dose of nevirapine to the infant within 72 h of birth. Beginning in July 2005, the availability of antiretrovirals increased, and children were eligible for treatment if they were aged <18 mo with a CD4 percentage of <20% or at pediatric WHO HIV disease stage III, or if they were aged ≥18 mo with a CD4 percentage of <15% or at pediatric WHO HIV disease stage III. Adults were eligible if they had WHO stage IV HIV disease and a CD4 cell count of <200 cells/μL or WHO stage III HIV disease and a CD4 cell count of <350 cells/μL.
Study population and serum 25-hydroxyvitamin D quantification.
A total of 2387 infants were enrolled in the parent trial and the presented prospective cohort selected a subset of these infants. We sampled all infants who were HIV-infected at the baseline 5–7-wk visit and took a random sample of HIV-exposed infants who remained HIV-uninfected for the duration of the trial. Infants who became HIV-infected after baseline were excluded from this study.
We used HPLC tandem MS at Children’s Hospital Boston using an API-5000 (AB Sciex) to quantify serum 25-hydroxyvitamin D [25(OH)D]11 concentrations from blood samples collected at the baseline infant visit (17). Day-to-day CV ranged from 5.6% to 8.5%.
Baseline covariate assessment.
At the baseline visit study nurses conducted a structured interview to collect information on demographic characteristics and breastfeeding methods. Exclusive breastfeeding was defined as feeding a child with breast milk only without any additional foods. A household asset score was calculated as the sum of asset items that included a sofa, television, radio, refrigerator, and fan. Blood samples were obtained from mothers and infants to measure complete blood counts, including hemoglobin concentration (AcT5 Diff AL hematology analyzer) and absolute CD4 T-cell count (FACSCalibur system). Baseline maternal anemia was defined as a hemoglobin concentration of <11 g/dL and child anemia was defined as a concentration of <10 g/dL (18). Nurses collected infant height and weight measurements by using a digital infant balance (Tanita) and a rigid length board with a movable foot piece.
Outcome assessment and definitions.
Mothers and infants were followed at monthly clinic visits for 24 mo after random assignment. Participants who missed a clinic visit were followed at home where relatives or neighbors were asked about the vital status of the infant. During follow-up visits, standardized assessments of child morbidity were made from symptom report diaries completed by the mothers. Study physicians also performed clinical exams every 3 mo and/or when acute complaints of illness were noted by nurses at monthly visits. Mothers were encouraged also to present to the study clinic for a sick visit including a physician exam when the child was unwell. Standardized diagnostic criteria were used to make physician diagnoses. Diarrhea was defined as ≥3 loose or watery stools within a 24-h period. Clinical malaria was symptomatically diagnosed with or without laboratory testing for parasitemia, following the WHO’s Integrated Management of Childhood Illness guidelines, in order to minimize malaria-related deaths (19). Confirmed malaria was defined as a malaria episode with clinical suspicion that was confirmed by observation of any parasitemia in a Giemsa-stained blood smear.
Infant height and weight measurements were also collected at monthly follow-up visits. Length-for-age z score (LAZ), weight-for-length z score (WLZ), and weight-for-age z score (WAZ) were calculated by using WHO child growth standards (20). Stunting, wasting, and underweight were defined as a LAZ, WLZ, and WAZ of 2 or more SDs below the WHO population median, respectively. Complete blood counts for infants, including hemoglobin concentration, were assessed every 6 mo during follow-up.
Statistical methods.
All analyses were stratified by HIV-infection status of the infant because of the potential for effect modification. We did not evaluate the association between 25(OH)D and the incidence of morbidity, anthropometric, and hemoglobin outcomes for HIV-infected infants because of small sample size and the likelihood of competing risks due to high mortality.
We placed baseline infant serum 25(OH)D concentrations into 4 categories: <10 ng/mL, 10–19.9 ng/mL, 20–29.9 ng/mL, and ≥30 ng/mL, based on commonly used clinical cutoffs (21, 22). Kaplan-Meir curves are presented to show the crude cumulative incidence of death overtime by 25(OH)D category. Briefly, we used proportional hazard models to analyze nonrepeatable binomial outcomes, mixed models for continuous outcomes, and generalized estimating equations for repeatable binomial outcomes.
The association between 25(OH)D status and binomial mortality and anthropometric outcomes was investigated using proportional hazard models. Interaction terms of 25(OH)D category with time were used to assess change in the magnitude of associations overtime. The potential nonlinear relation between serum 25(OH)D and mortality and anthropometric outcomes was also examined nonparametrically with restricted cubic splines (23, 24). Tests for nonlinearity used the likelihood ratio test, comparing the model with only the linear term to the model with the linear and the cubic spline terms.
Continuous mean LAZ, WLZ, and WAZ curves by age were constructed by using mixed-effects models employing restricted cubic splines with knots at 3 mo intervals after random assignment and automatic knot selection, using P < 0.05 to determine a parsimonious model (23, 24). Unadjusted models included 25(OH)D category, linear and spline terms for time, and interaction terms of 25(OH)D category with time and its splines as explanatory variables, whereas adjusted models additionally included baseline covariates. Differences in trajectory were tested by using the robust score test.
Poisson regression was used to analyze the incidence rate of physician diagnoses of morbidities, which are binomial repeated events that can occur during both routine and additional sick visits. Generalized estimating equations with an exchangeable working covariance and the log link were used to produce population-averaged RR estimates for maternal report of morbidities from the symptom diaries (25).
Potential confounders were selected a priori and include baseline socioeconomic, maternal health and nutrition, and child nutrition factors associated with infant vitamin D status and adverse child health outcomes. Maternal factors included age, education, marital status, number of prior pregnancies, household assets, food expenditure per person, underweight, anemia, CD4 T-cell count, and use of antiretrovirals during pregnancy. Baseline child confounders included sex, exclusive breastfeeding, anemia, stunting, wasting, season of 25(OH)D measurement, and randomized treatment regimen. Child receipt of antiretrovirals was also adjusted for in mortality analyses for HIV-infected infants. Potential effect modifiers were also selected a priori based on biologic plausibility and include infant sex, exclusive breastfeeding, maternal use of antiretrovirals during pregnancy, maternal wasting, and randomized treatment regimen. Child antiretrovirals were also investigated for HIV-infected children. Missing data for covariates were retained in the analysis using the missing indicator method for variables missing >1% of the observations (26). All P values were 2-sided and P < 0.05 was considered to be statistically significant. Statistical analyses were performed by using SAS version 9.2 (SAS Institute).
Ethics.
Written informed consent was obtained from all mothers in the parent trial. Institutional approval was granted by the Harvard School of Public Health Human Subjects Committee, the Muhimbili University of Health and Allied Sciences Committee of Research and Publications, the Tanzanian National Institute of Medical Research, and the Tanzanian Food and Drugs Authority.
Results
Baseline characteristics.
In this prospective cohort study, all 264 infants who were HIV-infected at the baseline 5–7-wk visit in the parent trial and a random sample of 950 HIV-exposed infants (among the total cohort of 2123) were selected for inclusion. Of these selected participants, serum for quantification of 25(OH)D was available for 253 (95.8%) HIV-infected and 948 (99.8%) HIV-exposed infants. Baseline maternal and infant characteristics of the cohort are presented in Table 1. Serum 25(OH)D concentrations at the baseline study visit were (means ± SDs) 18.6 ± 10.3 ng/mL for HIV-infected and 18.1 ± 9.2 ng/mL for HIV-exposed infants.
TABLE 1.
Baseline characteristics of HIV-infected and HIV-exposed, uninfected infants in Tanzania1
| HIV-infected (n = 253) | HIV-exposed, uninfected (n = 948) | |
| Maternal characteristics | ||
| Maternal age, y | 29.0 ± 4.8 | 27.7 ± 5.0 |
| Education | ||
| None | 10 (4.0) | 69 (7.3) |
| 1–7 y | 198 (79.2) | 684 (72.7) |
| ≥8 y | 42 (16.8) | 188 (20.0) |
| Married/living with partner | 209 (82.6) | 821 (86.6) |
| Prior Pregnancies | ||
| None | 88 (34.8) | 233 (24.8) |
| 1–3 | 100 (39.5) | 638 (67.8) |
| ≥4 | 65 (25.7) | 70 (7.4) |
| Household asset score | ||
| 0–1 | 51 (20.5) | 333 (35.1) |
| 2–3 | 174 (69.9) | 373 (39.4) |
| ≥4 | 24 (9.6) | 242 (25.5) |
| Daily food expenditure per person <500 Tanzanian shillings | 138 (54.5) | 462 (48.7) |
| Underweight (BMI <18.5 kg/m2) | 20 (7.9) | 42 (4.4) |
| Hemoglobin, g/dL | 11.4 ± 1.5 | 11.6 ± 1.3 |
| CD4 T-cell count | ||
| <200 cells/μL | 38 (17.6) | 63 (7.4) |
| 200–349 cells/μL | 61 (28.2) | 138 (16.3) |
| ≥350 cells/μL | 117 (54.2) | 646 (76.3) |
| Administered antiretrovirals during pregnancy | 53 (21.0) | 138 (14.6) |
| Child characteristics | ||
| Male | 127 (50.2) | 507 (53.5) |
| Low birth weight (<2500 g) | 34 (13.4) | 51 (5.4) |
| Exclusive breastfeeding | 201 (79.5) | 866 (91.4) |
| LAZ | −0.71 ± 1.48 | −0.26 ± 1.37 |
| WLZ | −0.50 ± 1.33 | −0.22 ± 1.27 |
| WAZ | −0.99 ± 1.27 | −0.43 ± 1.06 |
| Hemoglobin, g/dL | 10.2 ± 1.5 | 10.6 ± 1.8 |
| Administered antiretrovirals | 76 (30.0) | N/A |
| Randomly assigned to multivitamin | 121 (47.8) | 472 (49.8) |
| Season of 25(OH)D measurement | ||
| Long rain (December–March) | 54 (21.3) | 216 (22.8) |
| Harvest (April–May) | 39 (15.4) | 118 (12.5) |
| Postharvest (June–August) | 113 (44.7) | 403 (42.5) |
| Short rain (September–November) | 47 (18.6) | 211 (22.2) |
| 25(OH)D, ng/mL | 18.6 ± 10.3 | 18.1 ± 9.2 |
Values are means ± SDs or n (%). LAZ, length-for-age z score; N/A, not applicable; WAZ, weight-for-age z score; WLZ, weight-for-length z score; 25(OH)D, 25-hydroxyvitamin D.
Mortality.
The median follow-up time for HIV-infected infants was 15.2 mo (IQR: 5.2–23.7) and the median follow-up time for HIV-exposed infants was 20.9 mo (IQR: 17.0–23.9). During follow-up, 107 HIV-infected infant deaths (44.0%) and 53 HIV-exposed infant deaths (5.6%) were recorded. The most common causes of death were lower respiratory infections, septicemia, malaria, and diarrheal diseases. Supplemental Figures 1 and 2 show the Kaplan-Meir curves for cumulative incidence of death among HIV-infected and HIV-exposed infants by vitamin D category, respectively. Univariate and multivariate associations between vitamin D category and mortality stratified by infant HIV-infection status are presented in Table 2.
TABLE 2.
Association between 25(OH)D concentration and mortality in HIV-infected and HIV-exposed, uninfected infants1
| 25(OH)D <10 ng/mL |
25(OH)D 10–19.9 ng/mL |
25(OH)D ≥30 ng/mL |
|||||
| Value | P | Value | P | 25(OH)D 20–29.9 ng/mL (reference) | Value | P | |
| HIV-infected (n = 253) | |||||||
| Mortality, % (deaths/infants) | 52.5 (31/59) | 47.1 (40/85) | 28.2 (22/78) | 45.2 (14/31) | |||
| Crude HR (95% CI) | 2.24 (1.29, 3.87) | <0.01 | 1.90 (1.13, 3.21) | 0.02 | 1.0 | 1.73 (0.89, 3.39) | 0.11 |
| Adjusted HR2 (95% CI) | 1.43 (0.74, 2.78) | 0.29 | 1.37 (0.77, 2.44) | 0.28 | 1.0 | 2.47 (1.13, 5.44) | 0.02 |
| HIV-exposed (n = 948) | |||||||
| Mortality, % (deaths/infants) | 4.2 (9/213) | 6.1 (22/362) | 3.7 (10/272) | 11.9 (12/101) | |||
| Crude HR (95% CI) | 1.15 (0.47, 2.84) | 0.76 | 1.68 (0.80, 3.55) | 0.17 | 1.0 | 3.33 (1.44, 7.72) | <0.01 |
| Adjusted HR2 (95% CI) | 1.56 (0.60, 4.03) | 0.36 | 1.90 (0.89, 4.08) | 0.09 | 1.0 | 4.00 (1.67, 9.58) | <0.01 |
25(OH)D, 25-hydroxyvitamin D.
Adjusted for baseline maternal factors, including age (<25 y, 25–30 y, ≥30 y), education (<8 y), marital status, number of prior pregnancies (0, 1–2, ≥3), household assets (0–1, 2–3, ≥4), food expenditure per person (<500 Tanzanian shillings), underweight (BMI <18.5 kg/m2), anemia (<11 g/dL), CD4 T-cell count (<200, 200–350, ≥350 cells/μL), and use of antiretrovirals during pregnancy, and baseline child factors, including sex, exclusive breastfeeding, stunting, wasting, low birth weight (<2500 g), anemia (<10 g/dL), season of 25(OH)D measurement [long rain (December–March), harvest (April–May), postharvest (June–August), and short rain (September–November)], and randomized treatment regimen. HIV-infected analysis was also adjusted for child receipt of antiretrovirals.
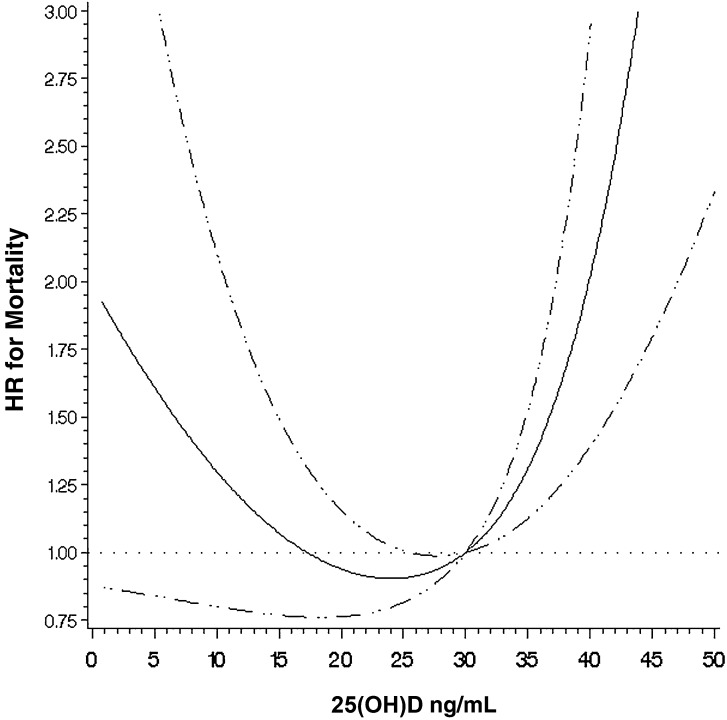
In crude analyses for HIV-infected infants, 25(OH)D concentrations of <10 ng/mL (HR: 2.24; 95% CI: 1.29, 3.87; P < 0.01) and 10–19.9 ng/mL(HR: 1.90; 95% CI: 1.13, 3.21; P = 0.02) were significantly associated with increased risk of mortality compared with the reference 25(OH)D concentration of 20–29.9 ng/mL; however, after multivariate adjustment, there was no statistically significant association. In multivariate analysis, 25(OH)D concentrations of ≥30 ng/mL were significantly associated with increased mortality (HR: 2.47; 95% CI: 1.13, 5.44; P < 0.01). In a sensitivity analysis, 25(OH)D concentrations of 30–40 ng/mL appeared to have increased risk compared with those of 20–29.9 ng/mL, but results were not statistically significant (HR: 2.17; 95% CI: 0.92, 5.13; P = 0.08). A continuous analysis of 25(OH)D concentrations among HIV-infected infants with restricted cubic splines determined a significant, nonlinear U-shaped relation with the lowest risk of mortality occurring at ∼25 ng/mL (P value for nonlinear relation <0.01) (Figure 1).
FIGURE 1.
Adjusted nonlinear relation between 25(OH)D concentrations and mortality, with 25 ng/mL as the reference for HIV-infected children (n = 253). The solid line shows the estimated HR for 25(OH)D concentrations relative to the reference concentration of 25 ng/mL, with the horizontal dotted line designating an HR of 1.0. The 95% CIs for the HR are represented by the dashed lines. The P value for the nonlinear relation is <0.01. 25(OH)D, 25-hydroxyvitamin D.
Among HIV-exposed infants, there was no significant association between 25(OH)D concentrations of <10 ng/mL and 10–19.9 ng/mL and mortality in crude and adjusted analysis but point estimates indicated possible increased risk. 25(OH)D concentrations of ≥30 ng/mL were significantly associated with increased mortality in adjusted analyses (HR: 4.00; 95% CI: 1.67, 9.58; P < 0.01). In a sensitivity analysis, 25(OH)D concentrations of 30–40 ng/mL were significantly associated with increased risk compared with those of 20–29.9 ng/mL (HR: 4.15; 95% CI:1.65, 10.43; P < 0.01). There was no indication of effect modification, and the magnitude of the associations did not appear to change over time.
Morbidity.
The association between 25(OH)D concentrations and physician diagnosis of morbidities among HIV-exposed infants is presented in Table 3. After multivariate adjustment, 25(OH)D concentrations of ≥30 ng/mL were significantly associated with increased clinical [incidence rate ratio (IRR): 1.34; 95% CI: 1.06, 1.70; P = 0.02] and confirmed malaria diagnoses (IRR: 1.71; 95% CI: 1.15, 2.54; P < 0.01), whereas 25(OH)D concentrations of <10 ng/mL were associated with increased incidence of oral candidiasis (IRR: 1.47; 95% CI: 1.00, 2.15; P = 0.046). Supplemental Table 1 presents an analysis of 25(OH)D with incidence of maternal reported morbidities in which no statistically significant relations were found.
TABLE 3.
Association between 25(OH)D concentration and physician diagnosis of selected morbidities and incident anthropometric and anemia outcomes among HIV-exposed, uninfected infants1
| 25(OH)D <10 ng/mL |
25(OH)D 10–19.9 ng/mL |
25(OH)D ≥30 ng/mL |
|||||
| Value | P | Value | P | 25(OH)D 20-29.9 ng/mL (reference) | Value | P | |
| Diarrhea | |||||||
| Mean diagnoses/y | 2.92 | 2.98 | 2.91 | 2.81 | |||
| Crude IRR (95% CI) | 1.26 (0.99, 1.60) | 0.06 | 1.03 (0.83, 1.29) | 0.76 | 1.0 | 1.04 (0.75, 1.44) | 0.81 |
| Adjusted IRR2 (95% CI) | 1.04 (0.81, 1.33) | 0.75 | 0.95 (0.76, 1.19) | 0.68 | 1.0 | 1.10 (0.80, 1.52) | 0.56 |
| Acute lower respiratory infection | |||||||
| Mean diagnoses/y | 0.66 | 0.61 | 0.72 | 0.73 | |||
| Crude IRR (95% CI) | 0.92 (0.71, 1.19) | 0.53 | 0.85 (0.68, 1.07) | 0.18 | 1.0 | 1.01 (0.73, 1.39) | 0.95 |
| Adjusted IRR2 (95% CI) | 0.95 (0.73, 1.24) | 0.71 | 0.90 (0.71, 1.13) | 0.36 | 1.0 | 1.08 (0.77, 1.50) | 0.66 |
| Clinical malaria | |||||||
| Mean diagnoses/y | 1.88 | 1.96 | 1.90 | 2.60 | |||
| Crude IRR (95% CI) | 1.00 (0.81, 1.23) | 0.98 | 1.04 (0.87, 1.25) | 0.63 | 1.0 | 1.37 (1.09, 1.73) | <0.01 |
| Adjusted IRR2 (95% CI) | 1.12 (0.90, 1.38) | 0.30 | 1.11 (0.93, 1.32) | 0.27 | 1.0 | 1.34 (1.06, 1.70) | 0.02 |
| Confirmed malaria | |||||||
| Mean diagnoses/y | 0.24 | 0.28 | 0.28 | 0.43 | |||
| Crude IRR (95% CI) | 0.87 (0.60, 1.26) | 0.45 | 1.00 (0.73, 1.37) | 0.99 | 1.0 | 1.55 (1.05, 2.29) | 0.03 |
| Adjusted IRR2 (95% CI) | 0.96 (0.66, 1.40) | 0.83 | 1.10 (0.80, 1.49) | 0.56 | 1.0 | 1.71 (1.15, 2.54) | <0.01 |
| Oral candidiasis | |||||||
| Mean diagnoses/y | 0.20 | 0.16 | 0.13 | 0.06 | |||
| Crude IRR (95% CI) | 1.55 (1.08, 2.24) | 0.02 | 1.25 (0.89, 1.76) | 0.20 | 1.0 | 0.48 (0.24, 0.98) | 0.04 |
| Adjusted IRR2 (95% CI) | 1.47 (1.00, 2.15) | 0.046 | 1.28 (0.90, 1.81) | 0.17 | 1.0 | 0.61 (0.31, 1.22) | 0.16 |
| Stunting (LAZ <−2) | |||||||
| % (number of events/infants) | 29.0 (56/193) | 26.1 (87/334) | 28.2 (70/248) | 35.7 (35/98) | |||
| Crude HR (95% CI) | 1.10 (0.77, 1.56) | 0.61 | 0.98 (0.71, 1.34) | 0.89 | 1.0 | 1.43 (0.95, 2.14) | 0.09 |
| Adjusted HR2 (95% CI) | 1.22 (0.84, 1.77) | 0.31 | 0.99 (0.71, 1.37) | 0.94 | 1.0 | 1.19 (0.78, 1.82) | 0.42 |
| Wasting (WLZ <−2) | |||||||
| % (number of events/infants) | 37.6 (74/197) | 29.5 (96/325) | 26.9 (65/242) | 33.3 (33/99) | |||
| Crude HR (95% CI) | 1.51 (1.08, 2.11) | 0.02 | 1.14 (0.83, 1.56) | 0.43 | 1.0 | 1.29 (0.85, 1.96) | 0.23 |
| Adjusted HR2 (95% CI) | 1.71 (1.20, 2.43) | <0.01 | 1.19 (0.86, 1.64) | 0.30 | 1.0 | 1.12 (0.72, 1.73) | 0.62 |
| Underweight (WAZ <−2) | |||||||
| % (number of events/infants) | 29.0 (55/190) | 25.5 (85/333) | 27.2 (68/250) | 25.0 (24/96) | |||
| Crude HR (95% CI) | 1.06 (0.74, 1.52) | 0.74 | 0.95 (0.69, 1.30) | 0.74 | 1.0 | 0.93 (0.58, 1.48) | 0.75 |
| Adjusted HR2 (95% CI) | 1.20 (0.82, 1.74) | 0.35 | 1.00 (0.72, 1.39) | 0.99 | 1.0 | 0.79 (0.49, 1.28) | 0.34 |
| Severe anemia (<8.5 g/dL) | |||||||
| % (number of events/infants) | 24.9 (48/193) | 20.4 (66/324) | 20.1 (48/239) | 25.9 (22/85) | |||
| Crude HR (95% CI) | 1.28 (0.86, 1.90) | 0.23 | 1.01 (0.69, 1.46) | 0.97 | 1.0 | 1.35 (0.81, 2.23) | 0.25 |
| Adjusted HR2 (95% CI) | 1.44 (0.92, 2.26) | 0.12 | 1.08 (0.72, 1.62) | 0.71 | 1.0 | 1.23 (0.69, 2.17) | 0.49 |
n = 948. IRR, incidence rate ratio; LAZ, length-for-age z score; WAZ, weight-for-age z score; WLZ, weight-for-length z-score; 25(OH)D, 25-hydroxyvitamin D.
Adjusted for baseline maternal factors, including age, education, marital status, number of prior pregnancies, household assets, food expenditure per person, underweight, anemia, CD4 T-cell count, and use of antiretrovirals during pregnancy, and baseline child factors, including sex, exclusive breastfeeding, stunting, wasting, low birth weight, anemia, season of 25(OH)D measurement, and randomized treatment regimen. Baseline child anemia (<10 g/dL) adjusted for in anthropometric analysis and stunting and wasting adjusted for in anemia analysis.
Growth and anemia.
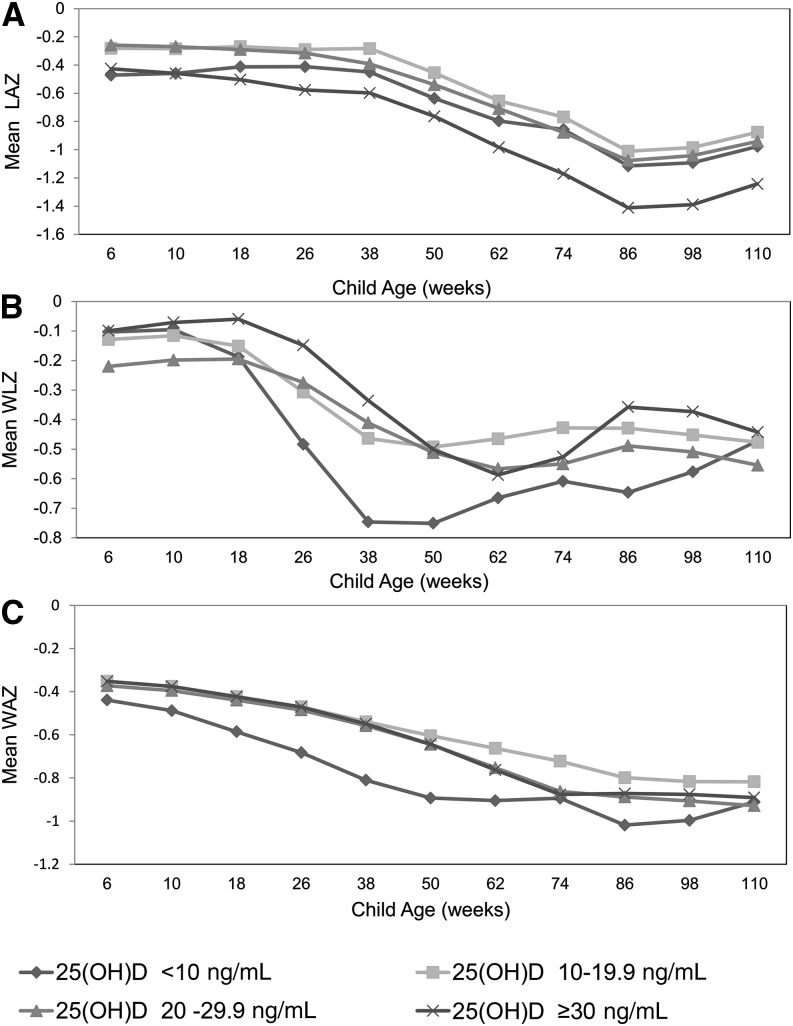
Mean LAZ, WLZ, and WAZ curves over time for HIV-exposed infants are presented in Figure 2 stratified by 25(OH)D category. In multivariate analysis, the trajectory of WLZ significantly differed for infants with 25(OH)D concentrations of <10 ng/mL compared with those with concentrations of 20–29.9 ng/mL (P < 0.01). Infants with 25(OH)D concentrations of <10 ng/mL experienced a rapid decrease in WLZ from 10–38 wk of age, but then experienced catch-up, and WLZ was comparable to other groups by 110 wk of age. HIV-exposed infants with 25(OH)D concentrations of <10 ng/mL also had a significant increase in the hazard of incident wasting in multivariate analyses (P < 0.01) (Table 3). There was no significant difference in LAZ and WAZ trajectory or hazard of stunting, underweight, or anemia by 25(OH)D category (Figure 2 and Table 3).
FIGURE 2.
Mean LAZ (A), WLZ (B), and WAZ (C) by age in weeks among HIV-exposed uninfected infants (n = 948) stratified by 25(OH)D concentration group. LAZ, length-for-age z score; WAZ, weight-for-age z score; WLZ, weight-for-length z score; 25(OH)D, 25-hydroxyvitamin D.
Discussion
In this prospective cohort study of Tanzanian infants born to HIV-infected mothers, the relation between 25(OH)D concentrations at 5–7 wk of age and mortality appeared to be U-shaped for both HIV-infected and HIV-exposed infants. Among HIV-exposed infants, 25(OH)D concentrations of <10 ng/mL were associated with increased risk of oral candidiasis and wasting, whereas 25(OH)D concentrations of ≥30 ng/mL were associated with increased risk of malaria diagnosis. There was no indication of effect modification for any of these relations by any factor, including duration of exclusive breastfeeding.
Our finding that low concentrations of vitamin D may increase the risk of mortality based on categorical point estimates and the shape of the spline analysis has also been noted in observational studies of HIV-infected adult populations (10, 11). Nevertheless, the association between low vitamin D and mortality did not reach statistical significance in either analysis. Infants with low concentrations of vitamin D may have impaired innate immune responses resulting in increased incidence and severity of opportunistic infections. The presence of vitamin D increases expression of antimicrobial peptides, including cathelicidin and β-defensin 2, and is also required for functioning of an IFN-γ–mediated pathway which leads to autophagy, phagosomal maturation, and antimicrobial activity of macrophages (27–29). Vitamin D also may directly inhibit replication of HIV-1 in macrophages (30). In support of this mechanism, our study found that 25(OH)D concentrations of <10 ng/mL were significantly associated with a rapid decrease in WLZ and incident wasting, which may be indicative of increased risk or severity of infectious morbidities.
We also found that 25(OH)D concentrations of ≥30 ng/mL were significantly associated with mortality for both HIV-infected and HIV-exposed infants which may seem unexpected; however, previous studies have suggested vitamin D may be harmful for some populations at high concentrations. A recent randomized trial conducted among infants in Afghanistan found that vitamin D supplements significantly increased the incidence of repeat episodes of pneumonia and also gave some indication that supplementation may lead to an increased risk of severe first pneumonia episodes and very severe respiratory disease (7). Observational studies among HIV-affected populations in Tanzania have also suggested that high vitamin D concentrations may be unfavorable, because there was some indication that 25(OH)D concentrations of >70 ng/mL in HIV-infected pregnant women during the second trimester increased the risk of child mortality. There was also a suggestion that concentrations of >40 ng/mL may increase mortality for HIV-infected adult men and women (10, 12). Studies in HIV-uninfected older adult and elderly populations also found that high concentrations of vitamin D may be linked to increased mortality (31, 32).
As a potential mechanism for the mortality association, 25(OH)D concentrations of ≥30 ng/mL were associated with increased risk of clinical and confirmed malaria. This finding is in contrast to a previous study conducted in an HIV-infected Tanzanian adult cohort, which found no association between vitamin D and incidence of malaria; however, age differences in antimalarial immunity may have led to effect modification (33). Balanced T-helper (Th) 1 and Th2 cell responses are essential to clear malarial parasites while not inducing serious host pathology (34). Vitamin D promotes anti-inflammatory Th2 cell responses that may have reduced clearance of the malaria parasite for HIV-exposed infants with high vitamin D concentrations (3, 34). In addition, vitamin D increases the production of IL-10 and Foxp3 regulatory T-cells, both of which have been associated with increased malaria parasitemia (35–37). Nevertheless, the observational nature of this study leaves the possibility of unmeasured and residual confounding for both the adverse mortality and malaria associations with 25(OH)D concentrations of ≥30 ng/mL. Specifically, infants who are exposed to the outdoors and sun during infancy may also be at high risk of exposure to mosquitos, malaria, and other pathogens that produce malaria-like symptoms. There also may be other unmeasured behaviors or characteristics that are associated with high infant vitamin D concentrations that lead to the observed relation with mortality and malaria diagnosis.
The results of this study suggest that there may be a U-shaped relation between vitamin D and morbidity and mortality among HIV-infected and HIV-exposed Tanzanian infants. We strongly put forth that these associations cannot be considered causal because of the observational design of the study and the consequent risk of confounding and other forms of bias. Nevertheless, this study supports the need for further vitamin D research among diverse infant populations, including those living in equatorial settings, because low vitamin D concentrations are highly prevalent.
Supplementary Material
Acknowledgments
CD, SA, R Kupka, KPM, R Kisenge, and WWF conducted the parent trial (NCT00197730). CRS, CD, and WWF designed the vitamin D study. CRS conducted the statistical analysis and drafted the manuscript. All authors read and approved the final manuscript.
Footnotes
Abbreviations used: IRR, incidence rate ratio; LAZ, length-for-age z score; Th, T-helper; WAZ, weight-for-age z score; WLZ, weight-for-length z score; 25(OH)D, 25-hydroxyvitamin D.
References
- 1.Filteau S. The HIV-exposed, uninfected African child. Trop Med Int Health 2009;14:276–87. [DOI] [PubMed] [Google Scholar]
- 2.Villamor E. A potential role for vitamin D on HIV infection? Nutr Rev 2006;64:226–33. [DOI] [PubMed] [Google Scholar]
- 3.Kamen DL, Tangpricha V. Vitamin D and molecular actions on the immune system: modulation of innate and autoimmunity. J Mol Med 2010;88:441–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Walker VP, Modlin RL. The vitamin D connection to pediatric infections and immune function. Pediatr Res 2009;65:106R–13R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Muhe L, Lulseged S, Mason KE, Simoes EA. Case-control study of the role of nutritional rickets in the risk of developing pneumonia in Ethiopian children. Lancet 1997;349:1801–4. [DOI] [PubMed] [Google Scholar]
- 6.Science M, Maguire JL, Russell ML, Smieja M, Walter SD, Loeb M. Low serum 25-hydroxyvitamin D level and risk of upper respiratory tract infection in children and adolescents. Clin Infect Dis 2013;57:392–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Manaseki-Holland S, Maroof Z, Bruce J, Mughal MZ, Masher MI, Bhutta ZA, Walraven G, Chandramohan D. Effect on the incidence of pneumonia of vitamin D supplementation by quarterly bolus dose to infants in Kabul: a randomised controlled superiority trial. Lancet 2012;379:1419–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kumar GT, Sachdev HS, Chellani H, Rehman AM, Singh V, Arora H, Filteau S. Effect of weekly vitamin D supplements on mortality, morbidity, and growth of low birthweight term infants in India up to age 6 months: randomised controlled trial. BMJ 2011;342:d2975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Roth DE, Perumal N, Al Mahmud A, Baqui AH. Maternal vitamin D3 supplementation during the third trimester of pregnancy: effects on infant growth in a longitudinal follow-up study in Bangladesh. J Pediatr 2013;163:1605–11. [DOI] [PubMed] [Google Scholar]
- 10.Sudfeld CR, Wang M, Aboud S, Giovannucci EL, Mugusi FM, Fawzi WW. Vitamin D and HIV progression among Tanzanian adults initiating antiretroviral therapy. PLoS ONE 2012;7:e40036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Viard JP, Souberbielle JC, Kirk O, Reekie J, Knysz B, Losso M, Gatell J, Pedersen C, Bogner JR, Lundgren JD, et al. EuroSIDA Study Group. Vitamin D and clinical disease progression in HIV infection: results from the EuroSIDA study. AIDS 2011;25:1305–15. [DOI] [PubMed] [Google Scholar]
- 12.Mehta S, Hunter DJ, Mugusi FM, Spiegelman D, Manji KP, Giovannucci EL, Hertzmark E, Msamanga GI, Fawzi WW. Perinatal outcomes, including mother-to-child transmission of HIV, and child mortality and their association with maternal vitamin D status in Tanzania. J Infect Dis 2009;200:1022–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Finkelstein JL, Mehta S, Duggan C, Manji KP, Mugusi FM, Aboud S, Spiegelman D, Msamanga GI, Fawzi WW. Maternal vitamin D status and child morbidity, anemia, and growth in human immunodeficiency virus-exposed children in Tanzania. Pediatr Infect Dis J 2012;31:171–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Moodley A, Qin M, Singh KK, Spector SA. Vitamin D-related host genetic variants alter HIV disease progression in children. Pediatr Infect Dis J 2013;32:1230–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Duggan C, Manji KP, Kupka R, Bosch RJ, Aboud S, Kisenge R, Okuma J, Fawzi WW. Multiple micronutrient supplementation in Tanzanian infants born to HIV-infected mothers: a randomized, double-blind, placebo-controlled clinical trial. Am J Clin Nutr 2012;96:1437–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fawzi WW, Msamanga GI, Spiegelman D, Urassa EJ, McGrath N, Mwakagile D, Antelman G, Mbise R, Herrera G, Kapiga S, et al. Randomised trial of effects of vitamin supplements on pregnancy outcomes and T cell counts in HIV-1-infected women in Tanzania. Lancet 1998;351:1477–82. [DOI] [PubMed] [Google Scholar]
- 17.Lensmeyer GL, Wiebe DA, Binkley N, Drezner MK. HPLC method for 25-hydroxyvitamin D measurement: comparison with contemporary assays. Clin Chem 2006;52:1120–6. [DOI] [PubMed] [Google Scholar]
- 18.Nathan DG, Orkin SH. Nathan and Oski’s hematology of infancy and childhood. 7th ed. Philadelphia: Saunders/Elsevier; 2009.
- 19.Erdman LK, Kain KC. Molecular diagnostic and surveillance tools for global malaria control. Travel Med Infect Dis 2008;6:82–99. [DOI] [PubMed] [Google Scholar]
- 20.WHO Multicentre Growth Reference Study Group. WHO child growth standards: length/height-for-age, weight-for-age, weight-for-length, weight-for-height and body mass index-for-age: methods and development. WHO, Geneva; 2006.
- 21.Heaney RP. Functional indices of vitamin D status and ramifications of vitamin D deficiency. Am J Clin Nutr 2004; 80(6, Suppl)1706S–9S. [DOI] [PubMed] [Google Scholar]
- 22.Institute of Medicine. Dietary reference intakes for calcium and vitamin D. Washington: The National Academies Press; 2011. [PubMed]
- 23.Durrleman S, Simon R. Flexible regression models with cubic splines. Stat Med 1989;8:551–61. [DOI] [PubMed] [Google Scholar]
- 24.Govindarajulu US, Spiegelman D, Thurston SW, Ganguli B, Eisen E. Comparing smoothing techniques in Cox models for exposure-response relationships. Stat Med 2007;26:3735–52. [DOI] [PubMed] [Google Scholar]
- 25.Diggle PJ, Heagerty P, Liang KY, Zeger SL. Analysis of longitudinal data. 2nd ed. Oxford: Oxford University Press; 2002.
- 26.Miettinen O. Theoretical Epidemiology. New York: John Wiley & Sons; 1985.
- 27.Liu PT, Stenger S, Li H, Wenzel L, Tan BH, Krutzik SR, Ochoa MT, Schauber J, Wu K, Meinken C, et al. Toll-like receptor triggering of a vitamin D-mediated human antimicrobial response. Science 2006;311:1770–3. [DOI] [PubMed] [Google Scholar]
- 28.Yuk JM, Shin DM, Lee HM, Yang CS, Jin HS, Kim KK, Lee ZW, Lee SH, Kim JM, Jo EK. Vitamin D3 induces autophagy in human monocytes/macrophages via cathelicidin. Cell Host Microbe 2009;6:231–43. [DOI] [PubMed] [Google Scholar]
- 29. Fabri M, Stenger S, Shin DM, Yuk JM, Liu PT, Realegeno S, Lee HM, Krutzik SR, Schenk M, Sieling PA, et al. Vitamin D is required for IFN-{gamma}-mediated antimicrobial activity of human macrophages. Sci Transl Med 2011; 3:104ra102. [DOI] [PMC free article] [PubMed]
- 30.Campbell GR, Spector SA. Hormonally active vitamin D3 (1alpha,25-dihydroxycholecalciferol) triggers autophagy in human macrophages that inhibits HIV-1 infection. J Biol Chem 2011;286:18890–902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dror Y, Giveon SM, Hoshen M, Feldhamer I, Balicer RD, Feldman BS. Vitamin D levels for preventing acute coronary syndrome and mortality: evidence of a nonlinear association. J Clin Endocrinol Metab 2013;98:2160–7. [DOI] [PubMed] [Google Scholar]
- 32.Michaëlsson K, Baron JA, Snellman G, Gedeborg R, Byberg L, Sundström J, Berglund L, Arnlöv J, Hellman P, Blomhoff R, et al. Plasma vitamin D and mortality in older men: a community-based prospective cohort study. Am J Clin Nutr 2010;92:841–8. [DOI] [PubMed] [Google Scholar]
- 33.Sudfeld CR, Giovannucci EL, Isanaka S, Aboud S, Mugusi FM, Wang M, Chalamilla G, Fawzi WW. Vitamin D status and incidence of pulmonary tuberculosis, opportunistic infections, and wasting among HIV-infected Tanzanian adults initiating antiretroviral therapy. J Infect Dis 2013;207:378–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Langhorne J, Ndungu FM, Sponaas AM, Marsh K. Immunity to malaria: more questions than answers. Nat Immunol 2008;9:725–32. [DOI] [PubMed] [Google Scholar]
- 35.Niikura M, Inoue S, Kobayashi F. Role of interleukin-10 in malaria: focusing on coinfection with lethal and nonlethal murine malaria parasites. J Biomed Biotechnol 2011; 2011:383962. [DOI] [PMC free article] [PubMed]
- 36.Walther M, Tongren JE, Andrews L, Korbel D, King E, Fletcher H, Andersen RF, Bejon P, Thompson F, Dunachie SJ, et al. Upregulation of TGF-beta, FOXP3, and CD4+CD25+ regulatory T cells correlates with more rapid parasite growth in human malaria infection. Immunity 2005;23:287–96. [DOI] [PubMed] [Google Scholar]
- 37.Hansen DS, Schofield L. Natural regulatory T cells in malaria: host or parasite allies? PLoS Pathog 2010;6:e1000771. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.