Abstract
Background: Converging evidence now indicates that aerobic fitness and adiposity are key correlates of childhood cognitive function and brain health. However, the evidence relating dietary intake to executive function/cognitive control remains limited.
Objective: The current study assessed cross-sectional associations between performance on an attentional inhibition task and dietary fatty acids (FAs), fiber, and overall diet quality among children aged 7–9 y (n = 65).
Methods: Attentional inhibition was assessed by using a modified flanker task. Three-day food records were used to conduct nutrient-level analyses and to calculate diet quality (Healthy Eating Index–2005) scores.
Results: Bivariate correlations revealed that socioeconomic status and sex were not related to task performance or diet measures. However, age, intelligence quotient (IQ), pubertal staging, maximal oxygen uptake (V̇O2max), and percentage of fat mass (%fat mass) correlated with task accuracy. Hierarchical regression models were used to determine the relation between diet variables and task accuracy and reaction time across both congruent and incongruent trials of the flanker task. After adjustment of confounding variables (age, IQ, pubertal staging, V̇O2max, and %fat mass), congruent accuracy was positively associated with insoluble fiber (β = 0.26, P = 0.03) and total dietary fiber (β = 0.23, P = 0.05). Incongruent response accuracy was positively associated with insoluble fiber (β = 0.35, P < 0.01), pectins (β = 0.25, P = 0.04), and total dietary fiber (β = 0.32, P < 0.01). Higher diet quality was related to lower accuracy interference (β = −0.26, P = 0.03), whereas higher total FA intake was related to greater accuracy interference (β = 0.24, P = 0.04). No statistically significant associations were observed between diet variables and reaction time measures.
Conclusion: These results demonstrate that children’s diet quality, specifically dietary fiber, is an important correlate of performance on a cognitive task requiring variable amounts of cognitive control.
Keywords: dietary fiber, diet quality, cognition, pediatrics, adiposity, aerobic fitness
Introduction
The human brain continues to undergo extensive changes in structure and functional connectivity throughout childhood and adolescence (1). Thus, it is important to understand what influence childhood behavioral factors—such as diet—can have on cognitive function and brain health during development. Diets consumed by children in the United States fail to meet federally recommended dietary guidelines (2). Excess FA and lower dietary fiber intake have been associated with childhood obesity (3–5), which continues to affect 1 in 5 American children (6). Of further concern is that SFAs and a lower quality diet may be associated with poorer cognitive function and cognitive decline in adulthood (7, 8). However, evidence explicating the role of diet in childhood cognitive function remains limited.
Diet is thought to influence cognitive function by a variety of mechanisms that include, but are not limited to, providing essential nutrients for brain development (9), amelioration of neuroinflammation (10, 11), and provision of energy (12). However, links between nutrients and specific cognitive domains are not well characterized. Cognitive control (also known as “executive control” or “executive function”) refers to “the ability to orchestrate thought and action in accord with internal goals” (13). Cognitive control, which consists of inhibition (resisting distractions or habits to maintain focus), working memory (mentally holding and manipulating information), and cognitive flexibility (multitasking), is thought to be vital to success in school, vocation, and life (14). Although the ability for cognitive control involves widespread neural circuitry, it is well established that the prefrontal cortex is critical for the successful top-down control of these cognitive processes (13). Our laboratory has previously shown that cognitive control—assessed by using both behavioral and neuroimaging measures—is associated with key aspects of physiologic health, including aerobic fitness and adiposity, among prepubertal children (15, 16). The extent to which habitual diet plays a role in cognitive control, however, remains to be discovered.
Studies in rodents and children link poor diet quality to cognitive impairment on hippocampus-dependent memory tasks (17–19). Rodents fed diets higher in SFAs and refined sugars exhibit decrements on memory tasks as well as neurotrophic factors important for neuronal plasticity and learning (17, 18). Among children, dietary lipids were shown to be differentially associated with memory function (19), whereas overall diet quality was found to be positively correlated with academic performance among fifth-grade students (20). However, comparatively less is known about the relation between dietary fiber and cognitive control in childhood. Fiber intake is associated with several beneficial health effects, including reduced risk of obesity, cardiovascular disease, type 2 diabetes, and colon cancer (21, 22). Despite these known benefits, 90% of American children fail to meet their recommended fiber intake (23). This is concerning because the benefits of dietary fiber extend beyond chronic disease prevention and may contribute to cognitive function and brain health as well. Soluble fibers (e.g., pectins, gums, and mucilages) provide substrates for bacterial fermentation, resulting in end products such as SCFAs. One of these SCFAs, butyrate, was shown to increase transcripts for brain-derived neurotrophic factor —a molecule important for neuronal integrity—in the frontal cortex (24). Furthermore, a diet containing 10% pectins ameliorates pre-depression–associated sickness behaviors and decreases markers of neuroinflammation in rodents (10). Insoluble fibers (e.g., lignin, celluloses, and hemicelluloses) are thought to enhance insulin sensitivity (25), which may have implications for cognitive health as well. Therefore, dietary fiber may play a crucial role in not just physical but cognitive health as well. However, to our knowledge, no previous study has delineated the associations between total dietary fiber or specific dietary fibers and cognitive control processes.
The aim of this study was to determine whether dietary fiber intake is associated with performance on an attentional inhibition task, which is one aspect of cognitive control requiring prefrontal cortex function. Given that individuals do not typically consume single nutrients but rather a combinations of foods (26), a comprehensive approach of assessing both diet quality (on the basis of types of foods consumed) and nutrient measures (e.g., dietary fiber and FAs) was used. We hypothesized that, after adjustment for demographic characteristics, fitness, and adiposity, diet quality and fiber intake would be positively associated with cognitive control among prepubertal children.
Methods
Participants.
Prepubertal children aged between 7 and 9 y (n = 65) were recruited from a larger randomized controlled trial [FITKids (Fitness Improves Thinking in Kids)] investigating the effects of a 9-mo afterschool physical activity program on cognitive function and brain health among prepubertal children. All data used in the study reported herein were collected at baseline and thus before the afterschool physical activity program. Participants were recruited from the East-Central Illinois region by using mailings, flyers, and informational meetings targeted at parents of children in the third and fourth grades. Children were screened for neurological disorders, physical disabilities, psychoactive medication status, and normal or corrected-to-normal vision. Data were also collected on 1) intelligence quotient (IQ)5, by using the Kaufman Brief Intelligence Test (27) or the Woodcock-Johnson Tests of Cognitive Abilities (28); 2) socioeconomic status (SES) as estimated on the basis of participation in a school meal-assistance program, maternal and paternal educational levels, and how many parents work full time; and 3) pubertal status by using the modified Tanner staging system (29) The participants provided written assent and legal guardians provided written informed consent. All procedures followed were in accord with the ethical standards and the regulations of the University of Illinois Institutional Review Board.
Diet assessment.
Diet was assessed by using the mean of 3-d food records (2 week days and 1 weekend day). The record was completed by the child with assistance from the parent. Both child and parent received instructions on how to correctly fill out the 3-d food record. In addition, the record contained written instructions for recording food intake (including how to describe food preparation methods, added fats, brand names, and ingredients of mixed dishes and recipes). Trained staff entered food records into the Nutrition Data Systems-Research (Nutrition Coordinating Center) software. Nutrient-level analyses were conducted by using the intake properties file to determine mean macronutrient intake. In addition, subcomponents of the macronutrients were studied, including SFAs, omega-3 (n–3) FAs, and fiber (insoluble, soluble, pectins). Nutrient intake was normalized to mean intake per 1000 kcal within participants before subsequent analyses. Healthy Eating Index (HEI)–2005 scores were derived according to a method described previously (30).
Anthropometric measurements.
Height and weight were measured by using a stadiometer (model 240; Seca) and a Tanita WB-300 Plus digital scale, respectively. Participants were not asked to fast before the anthropometric assessment. The mean of 3 measurements of height and weight were used for the analyses. The 2000 CDC growth charts were used to determine each child’s BMI-for-age percentile (31). Adiposity was assessed by DXA by using a Hologic QDR 4500A bone densitometer (software version 13.4.2). The percentage of whole-body fat mass (%fat mass) was expressed by using the standard software measure of fat mass adjusted for total mass.
Cardiorespiratory fitness assessment.
Maximal oxygen uptake (V̇O2max) was measured by using a modified Balke treadmill protocol (32). Oxygen consumption was measured by using a computerized indirect calorimetry system (ParvoMedics True Max 2400) with the mean for V̇O2 and respiratory exchange ratio assessed every 20 s. V̇O2max was based on maximal effort as evidenced by 1) a peak heart rate ≥185 beats/min (32) and a heart rate plateau (33), 2) a respiratory exchange ratio >1.0 (34), 3) a score on the children’s OMNI ratings of perceived exertion scale >8 (35), and/or 4) a plateau in oxygen consumption corresponding to an increase of <2 mL · kg−1 · min−1 despite an increase in workload (32).
Flanker task.
A modified Eriksen flanker task was used to assess attentional inhibition, a subcomponent of cognitive control (36). The flanker task required attentional inhibition because participants were asked to respond to a centrally presented target stimulus amid an array of 4 flanking stimuli. In the modified version of the task used in the current study, both the target and flanking stimuli were left- or right-oriented fish. The task consisted of congruent trials in which flanking fish faced in the same direction as the target fish and incongruent trials, in which flanking fish faced in the opposite direction of the target fish (37). Successful performance on the incongruent trials, relative to congruent trials, requires cognitive control to suppress the interference presented by the flanking stimuli. Congruent and incongruent trials were equi-probable and random. Participants completed 150 trials (75 trials × 2 blocks). Stimulus duration was 200 ms, with a 1700-ms intertrial interval. In addition to the standard variables of accuracy and reaction time, interference scores for response accuracy (congruent − incongruent) and reaction time (incongruent − congruent) were calculated to index ability to maintain task performance between the 2 trial types. Therefore, higher interference scores reflect poorer maintenance of cognitive control.
Statistical analyses.
An a priori power calculation was performed to determine the appropriate sample size (38). Assuming a medium effect size (f2 = 0.15), an α set at 0.05, and 80% power, the necessary sample size required was 55 participants. We examined whether diet quality and nutrient intake were associated with flanker response accuracy and reaction time using multiple hierarchical linear regression analyses, controlling for confounding variables. Confounding variables were determined by first conducting bivariate correlations (Pearson’s r) between the participant characteristics (age, sex, SES, pubertal staging, V̇O2max, %fat mass), IQ, and flanker task performance. Variables found to be significantly correlated with task performance were then entered in step 1 of the regression models as control variables. HEI scores and macronutrients of interest (dietary fiber and FAs) were then added to step 2 of the regression model. The significance of the change in the R2 value between the 2 steps was used to judge the independent contribution of diet quality and diet measures for explaining variance in task performance, beyond that of the participant demographic, fitness, and adiposity variables. This analysis was performed separately for congruent and incongruent trials. The α level was set at 0.05 and statistics were performed by using SPSS 19 (IBM).
Results
Participant characteristics (demographic and weight status) and flanker task performance are presented in Table 1. Mean reaction times were determined after averaging correct trials. Most (58%) of the participants were of healthy weight, whereas 25% were obese. Participants with low SES constituted 26% of the sample, and 90% were between Tanner stages 1 and 2. There were no differences in flanker performance across sex groups. Table 2 summarizes the means of the diet intake variables among participants. Participants reported meeting ∼51% of their energy needs from carbohydrates, 14% from proteins, and 35% from fat.
TABLE 1.
Demographic characteristics, weight status, and flanker task performance among prepubertal children1
| Value | |
| Age, y | 8.6 ± 0.1 |
| IQ | 112.2 ± 1.7 |
| V̇O2max, mL · kg−1 · min−1 | 40.9 ± 0.8 |
| BMI, kg/m2 | 18.5 ± 0.5 |
| BMI-for-age percentile2 | 65.4 ± 3.7 |
| Weight status,2 % | |
| Underweight | 3 |
| Normal weight | 58 |
| Overweight | 14 |
| Obese | 25 |
| Socioeconomic status, % | |
| Low | 26 |
| Middle | 35 |
| High | 39 |
| Pubertal staging, % | |
| Stage 1 | 91 |
| Stage 2 | 9 |
| Congruent reaction time, ms | 517.7 ± 14.4 |
| Congruent response accuracy, % | 78.9 ± 1.5 |
| Incongruent reaction time, ms | 551.7 ± 126.3 |
| Incongruent response accuracy, % | 71.0 ± 1.4 |
| Reaction time interference, % | 34.0 ± 4.8 |
| Response accuracy interference, ms | 7.8 ± 0.8 |
Values are means ± SEs unless otherwise indicated; n = 65. IQ, intelligence quotient; V̇O2max, maximal oxygen uptake.
Determined by the 2000 CDC growth charts (31).
TABLE 2.
Mean daily dietary intakes among prepubertal children (n = 65)1
| Value | |
| Energy, kcal/d | 1750 ± 46.1 |
| Carbohydrate, g/d | 224 ± 6.3 |
| Protein, g/d | 65.0 ± 2.1 |
| Fat, g/d | 68.2 ± 2.4 |
| SFAs, g/d | 24.1 ± 0.9 |
| Cholesterol, mg/d | 210 ± 14.7 |
| n–3 FAs, g/d | 1.4 ± 0.1 |
| DHA, mg/d | 47.7 ± 16.5 |
| Total dietary fiber, g/d | 14.2 ± 0.5 |
| Insoluble fiber, g/d | 10.0 ± 0.4 |
| Soluble fiber, g/d | 4.1 ± 0.2 |
| Pectins g/d | 1.6 ± 0.1 |
| Healthy Eating Index–2005 | 66.4 ± 1.0 |
Values are means ± SEs.
According to bivariate correlations between task performance and participant characteristics, congruent response accuracy was positively correlated with age (r = 0.32, P < 0.01) and IQ (r = 0.26, P = 0.02). Incongruent response accuracy was positively correlated with IQ (r = 0.26, P = 0.02) and V̇O2max (r = 0.24, P = 0.03) and negatively correlated with %fat mass (r = −0.22, P = 0.04). Response accuracy interference was positively correlated with age (r = 0.30, P < 0.01) and pubertal stage (r = 0.26, P = 0.02). The correlation between response accuracy interference and %fat mass was marginally above the threshold for significance (r = 0.18, P = 0.08). No significant correlations were observed between reaction time measures and IQ, V̇O2max, and %fat mass. Furthermore, SES and sex were not significantly correlated with task performance measures.
There were no significant correlations between IQ and diet variables. Table 3 summarizes bivariate correlations between task performance and diet variables. Congruent response accuracy was positively correlated with total dietary fiber (r = 0.23, P = 0.04) and insoluble (r = 0.24, P = 0.03) but not soluble (r = 0.10, P = 0.21) fiber. Similarly, incongruent response accuracy was positively related to total (r = 0.33, P < 0.01) and insoluble (r = 0.35, P < 0.01) fiber but not soluble fiber (r = 0.14, P = 0.14). However, intake of pectins was also positively (r = 0.24, P = 0.03) related to incongruent response accuracy. Alternatively, incongruent response accuracy was negatively correlated with dietary cholesterol (r = −0.21, P = 0.05). Accuracy interference was positively correlated with total fat (r = 0.23, P = 0.03) and negatively correlated with pectins (r = −0.21, P = 0.03). HEI-2005 scores were positively related to incongruent accuracy (r = 0.24, P = 0.03) and negatively related to response accuracy interference (r = −0.27, P = 0.01). Bivariate correlations between task performance and diet variables revealed no significant associations for reaction time measures.
TABLE 3.
Bivariate correlations between flanker task performance and diet among prepubertal children1
| Congruent RT | Congruent RA | Incongruent RT | Incongruent RA | RT interference | RA interference | |
| Carbohydrates | 0.03 | 0.07 | 0.03 | 0.16 | 0.02 | −0.16 |
| Proteins | −0.09 | −0.19 | −0.04 | −0.13 | 0.14 | −0.10 |
| Fats | 0.03 | 0.02 | 0.01 | −0.11 | −0.08 | 0.23* |
| SFAs | 0.06 | 0.05 | 0.03 | −0.02 | −0.11 | 0.13 |
| n–3 FAs | 0.04 | 0.01 | 0.07 | 0.02 | 0.10 | −0.02 |
| DHA | −0.17 | −0.13 | −0.10 | −0.08 | 0.18 | −0.10 |
| Cholesterol | −0.06 | −0.17 | 0.00 | −0.21* | 0.19 | 0.05 |
| Total dietary fiber | 0.11 | 0.23* | 0.09 | 0.33** | −0.05 | −0.18 |
| Insoluble fiber | 0.14 | 0.24* | 0.13 | 0.35** | 0.00 | −0.20 |
| Soluble fiber | −0.01 | 0.10 | −0.06 | 0.14 | −0.17 | −0.06 |
| Pectins | 0.09 | 0.12 | 0.08 | 0.24* | −0.03 | −0.21* |
| Healthy Eating Index–2005 | 0.16 | 0.08 | 0.15 | 0.24* | 0.01 | −0.27* |
All diet variables were energy adjusted before analysis. *P < 0.05, **P < 0.01 (one-tailed). RA, response accuracy; RT, reaction time.
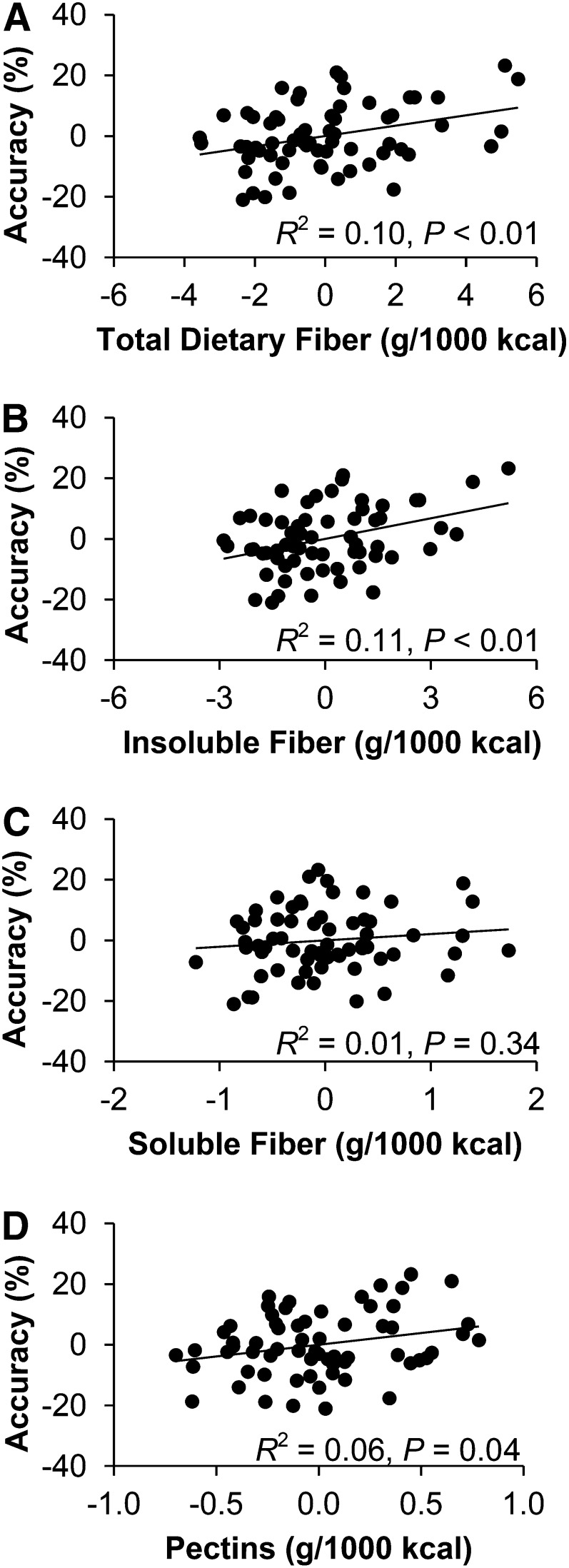
The stepwise hierarchical regression models predicting task accuracy are summarized in Table 4. After the inclusion of step 1 variables (age, IQ, V̇O2max, %fat mass), the addition of total dietary fiber at step 2 significantly increased the model R2 and was positively associated with both congruent (ΔR2 = 0.05, β = 0.23, P = 0.05) and incongruent (ΔR2 = 0.10, β = 0.32, P < 0.01) accuracy. Similarly, the addition of insoluble fiber at step 2 significantly improved the model and was positively associated with flanker accuracy on both the congruent (ΔR2 = 0.06, β = 0.26, P = 0.03) and incongruent (ΔR2 = 0.11, β = 0.35, P < 0.01) trials. Although addition of total soluble fiber intake did not significantly improve the models for task performance, the inclusion of pectins at step 2 indicated a selectively positive relation with incongruent response accuracy (ΔR2 = 0.06, β = 0.25, P = 0.04). Figure 1 illustrates the associations between dietary fiber subcomponents and incongruent response accuracy. Finally, total FAs were positively associated with response accuracy interference (ΔR2 = 0.06, β = 0.24, P = 0.04), whereas HEI scores were negatively associated with response accuracy interference (ΔR2 = 0.08, β = −0.26, P = 0.03).
TABLE 4.
Summary of regression analyses for variables predicting flanker response accuracy and interference among prepubertal children1
| Congruent response accuracy |
Incongruent response accuracy |
Response accuracy interference |
|||||||
| Step and variable | β | ΔR2 | Model P | β | ΔR2 | Model P | β | ΔR2 | Model P |
| Step 1 | — | 0.22 | 0.01 | — | 0.17 | 0.04 | — | 0.15 | 0.07 |
| Age | 0.36* | — | — | 0.22 | — | — | 0.26* | — | — |
| IQ | 0.29* | — | — | 0.29* | — | — | 0.02 | — | — |
| Pubertal stage | 0.05 | — | — | −0.06 | — | — | 0.19 | — | — |
| V̇O2max | 0.10 | — | — | 0.19 | — | — | −0.15 | — | — |
| %Fat mass | −0.07 | — | — | −0.08 | — | — | 0.02 | — | — |
| Step 2 | |||||||||
| Total fiber | 0.23* | 0.05* | <0.01 | 0.32* | 0.10* | <0.01 | −0.15 | 0.02 | 0.08 |
| Insoluble fiber | 0.26* | 0.06* | <0.01 | 0.35* | 0.11* | <0.01 | −0.15 | 0.02 | 0.07 |
| Pectins | 0.15 | 0.02 | 0.01 | 0.25* | 0.06* | 0.02 | −0.17 | 0.03 | 0.07 |
| Total fat | −0.04 | <0.01 | 0.02 | −0.18 | 0.03 | 0.04 | 0.24* | 0.06* | 0.03 |
| HEI-2005 | 0.08 | <0.01 | 0.02 | 0.23 | 0.05 | 0.02 | −0.26* | 0.06* | 0.02 |
*P < 0.05. HEI, Healthy Eating Index; IQ, intelligence quotient; V̇O2max, maximal oxygen uptake.
FIGURE 1 .
Partial regression plots (A–D) illustrating the associations between dietary fiber variables and flanker incongruent response accuracy among prepubertal children after adjustment for age, pubertal stage, intelligence quotient, V̇O2max, and percentage of fat mass. V̇O2max, maximal oxygen uptake.
Discussion
Previous studies have shown that differences in cognitive control can be observed as a function of aerobic fitness level and adiposity among prepubertal children (15, 16). However, data relating dietary components to childhood cognitive control are limited. The current study provided novel evidence relating diet intake to cognitive control among prepubertal children. The major findings were that total dietary fiber was positively associated with accuracy on an attentional inhibition task. Specifically, higher daily intake of total and insoluble fibers was related to accuracy in both the congruent and incongruent trials, whereas pectin intake was selectively associated with greater performance on the incongruent trials, necessitating upregulation of cognitive control. Furthermore, overall diet quality was negatively related to response accuracy task interference, demonstrating that children with higher-quality diets had maintained accuracy despite an increase in task demands necessitated by the incongruent condition, reflecting superior cognitive flexibility. These data are among the first to indicate that higher diet quality, specifically fiber intake, may be important for upregulation of cognitive control when faced with higher cognitive demands in childhood.
Emerging literature concerning the relation between diet and cognitive function in childhood has begun to provide a better understanding of nutrient-brain interactions. Dietary lipids, in particular, have received considerable attention. Recently, performance on a hippocampus-dependent memory task was shown to negatively correlate with SFA intake and positively correlate with n–3 FA intake (19) among 8- to 9-y-olds. On the other hand, among a nationally representative sample of 6- to 16-y-olds, lower dietary cholesterol and higher PUFA intakes were cross-sectionally associated with higher scores on a digit span task (39). However, intake of SFAs—the primary dietary determinant of serum cholesterol—was not correlated with cognitive functioning. Among a large sample of fifth-grade students, a positive association of overall diet quality (assessed by using both the Diet Quality-International Index as well as the HEI-2005) with academic performance was found (20). Although data from the current study found a negative association between dietary cholesterol and incongruent response accuracy, this association was not significant after the adjustment of confounding variables. Furthermore, SFAs, n–3 FAs (including DHA), and total PUFAs were not related to any of the cognitive outcomes. However, we observed that total fat intake was associated with higher task interference, suggesting that children who consumed higher total fat were less accurate when cognitive demands were increased, reflecting poorer flexible modulation of cognitive control. Taken together, it is plausible that a differential association exists between dietary lipids and specific cognitive domains, i.e., SFAs may selectively influence hippocampus-dependent memory tasks whereas cholesterol and total fat may negatively affect prefrontal cognitive control processes. However, future studies with a much broader battery of cognitive tasks will be needed to further characterize these important relations.
The finding that dietary fiber was positively correlated with cognitive control among prepubertal children is a novel and important contribution to the current literature. Unlike dietary lipids, the relation between fiber intake and childhood cognitive function has received comparatively less attention. This is surprising given that dietary fiber is associated with superior glycemic control and insulin sensitivity (40), physiologic processes thought to contribute to not just physical but cognitive health as well. The effects of dietary fiber are likely based on the chemical structure of the fiber, which may alter function in the human gut and influence disease risk (41). Insoluble fibers, which remain undigested and exhibit limited fermentation in the gastrointestinal tract, are known for their ability to decrease transit time and constipation (40). On the other hand, increased intake of soluble fibers, which are resistant to enzymatic digestion but are highly fermentable, improves glycemic control and has hypocholesterolemic effects in both nondiabetic and diabetic individuals (42, 43). Although the effects of different dietary fibers on markers of cardiovascular health have been extensively studied, the effects of different fiber types on cognitive function are yet to be delineated. In the current study, total dietary fiber was positively associated with task accuracy, even after adjusting for demographic characteristics, fitness, and adiposity. This association may be generalized to other cognitive functions. In fact, our data support this notion because total and insoluble fiber intake was positively associated with accuracy in both congruent and incongruent trials. However, intake of pectin—a soluble and highly fermentable type of fiber—was found to be selectively and positively associated with accuracy in the incongruent trials, which require the upregulation of cognitive control. Therefore, there appears to be a generalized relation between total and insoluble dietary fiber and cognition, whereas the effect of pectins is selectively and disproportionately larger for tasks or task components that require greater amounts of cognitive (and, in this case, attentional inhibitory) control. As such, the effect may vary depending on the type of dietary fiber.
Dietary fiber may influence cognitive and brain health through immunomodulation and/or the gut-microbiota-brain system. In response to endotoxin-induced sickness behavior, mice fed pectins as the sole source of fiber had reduced brain IL-1β and TNF-α (10). Bacterial fermentation end products such as SCFAs were shown to downregulate proinflammatory cytokines ex vivo (44) and to increase brain-derived neurotrophic factor transcripts in the frontal cortex (24). In addition to SCFA production, soluble fibers—acting as prebiotics—have the potential to proliferate health-promoting bacteria (45). Recent advances in gnotobiotics/germ-free mouse models unequivocally show that the gut microbiota plays a significant role in brain development and function (46). Sudo et al. (47) showed that adult germ-free mice have exaggerated activation of the hypothalamic-pituitary-adrenal (HPA) axis compared with conventionalized mice (47). Interestingly, this stress response was completely reversed by inoculation with Bifidobacterium infantis, demonstrating a key role of the gut microbiota in brain function. The gastrointestinal tract provides the scaffold for the endocrine, immune, and neural pathways connecting the gut microbiome and the central nervous system (48). However, the influence of specific phylogenetic profiles or gut integrity on cognitive function has not been directly investigated in children. Future studies should investigate the functional significance of fiber-induced variation of the gastrointestinal microbiome and its implications for cognitive and brain health.
Although the current study provides support for the positive association between dietary fiber and cognitive control, there are several limitations worth considering. These results are based on cross-sectional analyses; therefore, we cannot make statements of causality. In the current interpretation it is assumed that cognitive control was driven by the diet measures; however, the opposite may be true, such that higher cognitive control may be implicated in better dietary choices. Future longitudinal and/or intervention studies examining the effects of fiber intake on cognitive function could provide definitive support for the directionality of the associations observed in the current study. Furthermore, the addition of fibers to foods during food production renders it difficult to accurately quantify total fiber intake. This is because functional fibers—fibers added to foods—are not currently included in nutrient composition databases. Another limitation is that diet quality was assessed by using the HEI-2005 and not the HEI-2010. Therefore, the diet quality outcomes reflect the degree of compliance with the 2005 rather than the 2010 dietary guidelines. However, there are currently no published procedures for calculating HEI-2010 scores using Nutrition Data Systems-Research output. In addition, there remains a large degree of consistency between the subcomponents used to calculate both indexes. Finally, without serum or plasmas markers of metabolism and inflammation or microbiome data, we can only speculate on the possible mechanisms that may underlie our observations. Nevertheless, the aforementioned limitations notwithstanding, the strengths of the current study included the adjustment of several key covariates known to influence childhood cognitive function (e.g., age, IQ, pubertal staging, aerobic fitness, and adiposity). Therefore, the finding that specific dietary components significantly correlated with attentional inhibition—after adjustment of key covariates—highlights the importance of assessing diet along with cognitive control outcomes in future studies.
In conclusion, the current study provides novel evidence relating dietary components to cognitive control among prepubertal children. Critically, we demonstrate that diet quality is positively related to the ability to flexibly modulate cognitive control when task demands are increased. In addition, a low intake of dietary fiber is associated with poorer performance on a task tapping into prefrontal cognitive control function. Given that dietary fiber is a chronically underconsumed nutrient, the finding that low dietary fiber intake is associated with poorer childhood cognitive function raises important public health concerns. On the other hand, these cross-sectional findings provide preliminary support for future intervention studies that may aim to optimize cognitive health by improving dietary fiber intake among pediatric populations.
Acknowledgments
We thank Bonnie Hemrick for her assistance in recruiting study participants. AFK and CHH designed the research; NAK, LBR, ESD, and MRS conducted the research; and NAK analyzed the data, wrote the first draft of the manuscript, and had primary responsibility for the final content. All authors read and approved the final manuscript.
Footnotes
Abbreviations used: HEI, Healthy Eating Index; IQ, intelligence quotient; SES, socioeconomic status; V̇O2max, maximal oxygen uptake.
References
- 1.Luna B. Developmental changes in cognitive control through adolescence. Adv Child Dev Behav 2009;37:233–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Krebs-Smith SM, Guenther PM, Subar AF, Kirkpatrick SI, Dodd KW. Americans do not meet federal dietary recommendations. J Nutr 2010;140:1832–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Johnson L, Mander AP, Jones LR, Emmett PM, Jebb SA. Energy-dense, low-fiber, high-fat dietary pattern is associated with increased fatness in childhood. Am J Clin Nutr 2008;87:846–54. [DOI] [PubMed] [Google Scholar]
- 4.Davis JN, Alexander KE, Ventura EE, Toledo-Corral CM, Goran MI. Inverse relation between dietary fiber intake and visceral adiposity in overweight Latino youth. Am J Clin Nutr 2009;90:1160–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tucker LA. Body fat percentage of children varies according to their diet composition. J Am Diet Assoc 1997;97:981. [DOI] [PubMed] [Google Scholar]
- 6.Ogden CL, Carroll MD, Kit BK, Flegal KM. Prevalence of childhood and adult obesity in the United States, 2011–2012. JAMA 2014;311:806–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Holloway CJ, Cochlin LE, Emmanuel Y, Murray A, Codreanu I, Edwards LM, Szmigielski C, Tyler DJ, Knight NS, Saxby BK, et al. A high-fat diet impairs cardiac high-energy phosphate metabolism and cognitive function in healthy human subjects. Am J Clin Nutr 2011;93:748–55. [DOI] [PubMed] [Google Scholar]
- 8.Kim DH, Grodstein F, Rosner B, Kang JH, Cook NR, Manson JE, Buring JE, Willett WC, Okereke OI. Seafood types and age-related cognitive decline in the women's health study. J Gerontol A Biol Sci Med Sci 2013;68:1255–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Georgieff MK. Nutrition and the developing brain: nutrient priorities and measurement. Am J Clin Nutr 2007;85(Suppl):614S–20S. [DOI] [PubMed] [Google Scholar]
- 10.Sherry CL, Kim SS, Dilger RN, Bauer LL, Moon ML, Tapping RI, Fahey GC, Jr, Tappenden KA, Freund GG. Sickness behavior induced by endotoxin can be mitigated by the dietary soluble fiber, pectin, through up-regulation of IL-4 and Th2 polarization. Brain Behav Immun 2010;24:631–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jang S, Dilger RN, Johnson RW. Luteolin inhibits microglia and alters hippocampal-dependent spatial working memory in aged mice. J Nutr 2010;140:1892–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hoyland A, Lawton CL, Dye L. Acute effects of macronutrient manipulations on cognitive test performance in healthy young adults: a systematic research review. Neurosci Biobehav Rev 2008;32:72–85. [DOI] [PubMed] [Google Scholar]
- 13.Miller EK, Cohen JD. An integrative theory of prefrontal cortex function. Annu Rev Neurosci 2001;24:167–202. [DOI] [PubMed] [Google Scholar]
- 14.Diamond A. Executive functions. Annu Rev Psychol 2013;64:135–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hillman CH, Kamijo K, Pontifex MB. The relation of ERP indices of exercise to brain health and cognition. In: Boecker H, Hillman CH, Scheef L, Struder H, editors. Functional neuroimaging in exercise and sport sciences. New York: Springer; 2012. p. 419–46. [Google Scholar]
- 16.Kamijo K, Khan NA, Pontifex MB, Scudder MR, Drollette ES, Raine LB, Evans EM, Castelli DM, Hillman CH. The relation of adiposity to cognitive control and scholastic achievement in preadolescent children. Obesity (Silver Spring) 2012;20:2406–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Molteni R, Barnard R, Ying Z, Roberts C, Gomez-Pinilla F. A high-fat, refined sugar diet reduces hippocampal brain-derived neurotrophic factor, neuronal plasticity, and learning. Neuroscience 2002;112:803–14. [DOI] [PubMed] [Google Scholar]
- 18.Kanoski SE, Zhang Y, Zheng W, Davidson TL. The effects of a high-energy diet on hippocampal function and blood-brain barrier integrity in the rat. J Alzheimers Dis 2010;21:207–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Baym CL, Khan NA, Monti JM, Raine LB, Drollette ES, Moore RD, Scudder MR, Kramer AF, Hillman CH, Cohen NJ. Dietary lipids are differentially associated with hippocampal-dependent relational memory in prepubescent children. Am J Clin Nutr 2014;99:1026–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Florence MD, Asbridge M, Veugelers PJ. Diet quality and academic performance. J Sch Health 2008;78:209–15. [DOI] [PubMed] [Google Scholar]
- 21.Cho SS, Qi L, Fahey GC, Jr, Klurfeld DM. Consumption of cereal fiber, mixtures of whole grains and bran, and whole grains and risk reduction in type 2 diabetes, obesity, and cardiovascular disease. Am J Clin Nutr 2013;98:594–619. [DOI] [PubMed] [Google Scholar]
- 22.Trock B, Lanza E, Greenwald P. Dietary fiber, vegetables, and colon cancer: critical review and meta-analyses of the epidemiologic evidence. J Natl Cancer Inst 1990;82:650–61. [DOI] [PubMed] [Google Scholar]
- 23.Moshfegh A, Goldman J, Cleveland L. What we eat in America, NHANES 2001–2002: usual nutrient intakes from food compared to dietary reference intakes. Beltsville (MD): USDA, Agricultural Research Service; 2005. [Google Scholar]
- 24.Schroeder FA, Lin CL, Crusio WE, Akbarian S. Antidepressant-like effects of the histone deacetylase inhibitor, sodium butyrate, in the mouse. Biol Psychiatry 2007;62:55–64. [DOI] [PubMed] [Google Scholar]
- 25.Pereira MA, Jacobs DR, Jr, Pins JJ, Raatz SK, Gross MD, Slavin JL, Seaquist ER. Effect of whole grains on insulin sensitivity in overweight hyperinsulinemic adults. Am J Clin Nutr 2002;75:848–55. [DOI] [PubMed] [Google Scholar]
- 26.Kant AK. Indexes of overall diet quality: a review. J Am Diet Assoc 1996;96:785–91. [DOI] [PubMed] [Google Scholar]
- 27.Kaufman ASK-BIT. Kaufman brief intelligence test. Circle Pines (MN): American Guidance Service; 1990. [Google Scholar]
- 28.Woodcock RW, McGrew K, Mather N. Woodcock-Johnson tests of achievement. Itasca (IL): Riverside Publishing; 2001. [Google Scholar]
- 29.Tanner JM. Growth at adolescence. Oxford (UK): Blackwell Scientific Publications; 1962. [Google Scholar]
- 30.Miller PE, Mitchell DC, Harala PL, Pettit JM, Smiciklas-Wright H, Hartman TJ. Development and evaluation of a method for calculating the Healthy Eating Index-2005 using the nutrition data system for research. Public Health Nutr 2011;14:306–13. [DOI] [PubMed] [Google Scholar]
- 31.Kuczmarski RJ, Ogden CL, Grummer Strawn LM, Flegal KM, Guo SS, Wei R, Mei Z, Curtin LR, Roche AF, Johnson CL. CDC growth charts: United States. Atlanta (GA): Advance data from Vital Health Statistics of the National Center for Health Statistics; 2000:1–27. [PubMed] [Google Scholar]
- 32.Armstrong L. ACSM's guidelines for exercise testing and prescription/American College of Sports Medicine. 7th ed. Philadelphia: Lippincott Williams & Wilkins; 2006. [Google Scholar]
- 33.Freedson PS, Goodman TL, editors. Measurement of oxygen consumption. Champaign (IL): Human Kinetics; 1993. [Google Scholar]
- 34.Bar-Or O. Pediatric sports medicine for the practitioner: from physiologic principles to clinical applications. New York: Springer; 1983. [Google Scholar]
- 35.Utter AC, Roberson RJ, Nieman DC, Kang J. Children's OMNI scale of perceived exertion: walking/running evaluation. Med Sci Sports Exerc 2002;34:139–44. [DOI] [PubMed] [Google Scholar]
- 36.Eriksen CW, Eriksen BA. Effects of noise letters upon the identification of a target letter in a non-search task. Percept Psychophys 1974;2:249–63. [Google Scholar]
- 37.Pontifex MB, Saliba BJ, Raine LB, Picchietti DL, Hillman CH. Exercise improves behavioral, neurocognitive, and scholastic performance in children with attention-deficit/hyperactivity disorder. J Pediatr 2013;162:543–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Faul F, Erdfelder E, Buchner A, Lang A. Statistical power analyses using G* power 3.1: tests for correlation and regression analyses. Behav Res Methods 2009;41:1149–60. [DOI] [PubMed] [Google Scholar]
- 39.Zhang J, Hebert JR, Muldoon MF. Dietary fat intake is associated with psychosocial and cognitive functioning of school-aged children in the united states. J Nutr 2005;135:1967–73. [DOI] [PubMed] [Google Scholar]
- 40.Papathanasopoulos A, Camilleri M. Dietary fiber supplements: effects in obesity and metabolic syndrome and relationship to gastrointestinal functions. Gastroenterology 2010;138:65–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gemen R, de Vries JF, Slavin JL. Relationship between molecular structure of cereal dietary fiber and health effects: Focus on glucose/insulin response and gut health. Nutr Rev 2011;69:22–33. [DOI] [PubMed] [Google Scholar]
- 42.Sierra M, Garcia JJ, Fernandez N, Diez MJ, Calle AP, Sahagun AM. Farmafibra Group. Effects of ispaghula husk and guar gum on postprandial glucose and insulin concentrations in healthy subjects. Eur J Clin Nutr 2001;55:235–43. [DOI] [PubMed] [Google Scholar]
- 43.Sierra M, Garcia J, Fernandez N, Diez M, Calle A. Therapeutic effects of psyllium in type 2 diabetic patients. Eur J Clin Nutr 2002;56:830–42. [DOI] [PubMed] [Google Scholar]
- 44.Cox MA, Jackson J, Stanton M, Rojas-Triana A, Bober L, Laverty M, Yang X, Zhu F, Liu J, Wang S, et al. Short-chain fatty acids act as antiinflammatory mediators by regulating prostaglandin E(2) and cytokines. World J Gastroenterol 2009;15:5549–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Quigley EM. Prebiotics and probiotics; modifying and mining the microbiota. Pharmacol Res 2010;61:213–8. [DOI] [PubMed] [Google Scholar]
- 46.Manco M. Gut microbiota and developmental programming of the brain: from evidence in behavioral endophenotypes to novel perspective in obesity. Front Cell Infect Microbiol 2012;2:109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sudo N, Chida Y, Aiba Y, Sonoda J, Oyama N, Yu X, Kubo C, Koga Y. Postnatal microbial colonization programs the hypothalamic–pituitary–adrenal system for stress response in mice. J Physiol 2004;558:263–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cryan JF, Dinan TG. Mind-altering microorganisms: the impact of the gut microbiota on brain and behaviour. Nat Rev Neurosci 2012;13:701–12. [DOI] [PubMed] [Google Scholar]