Abstract
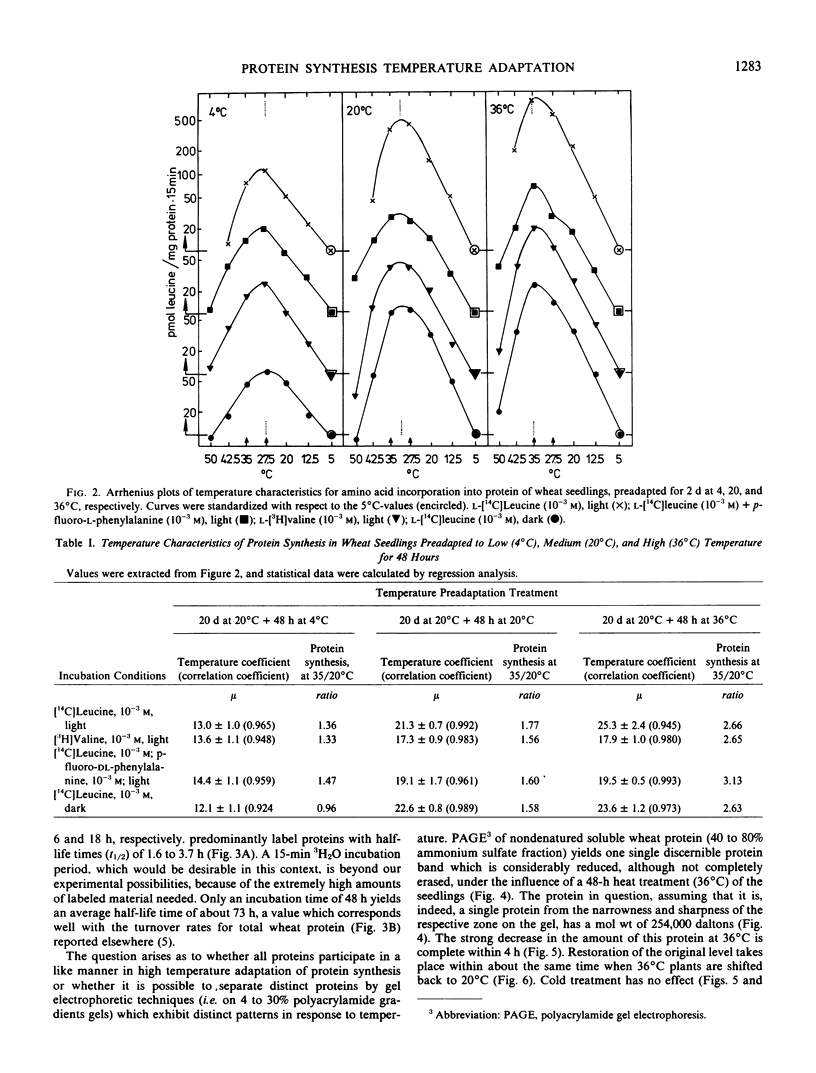
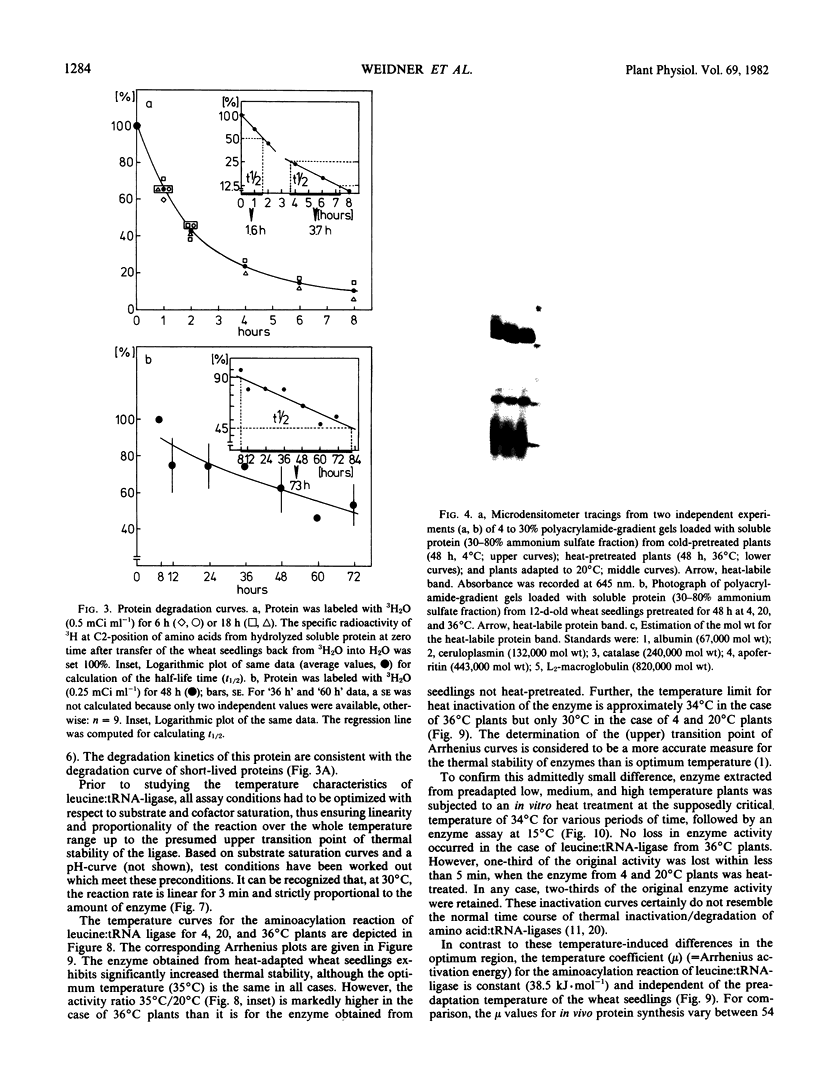
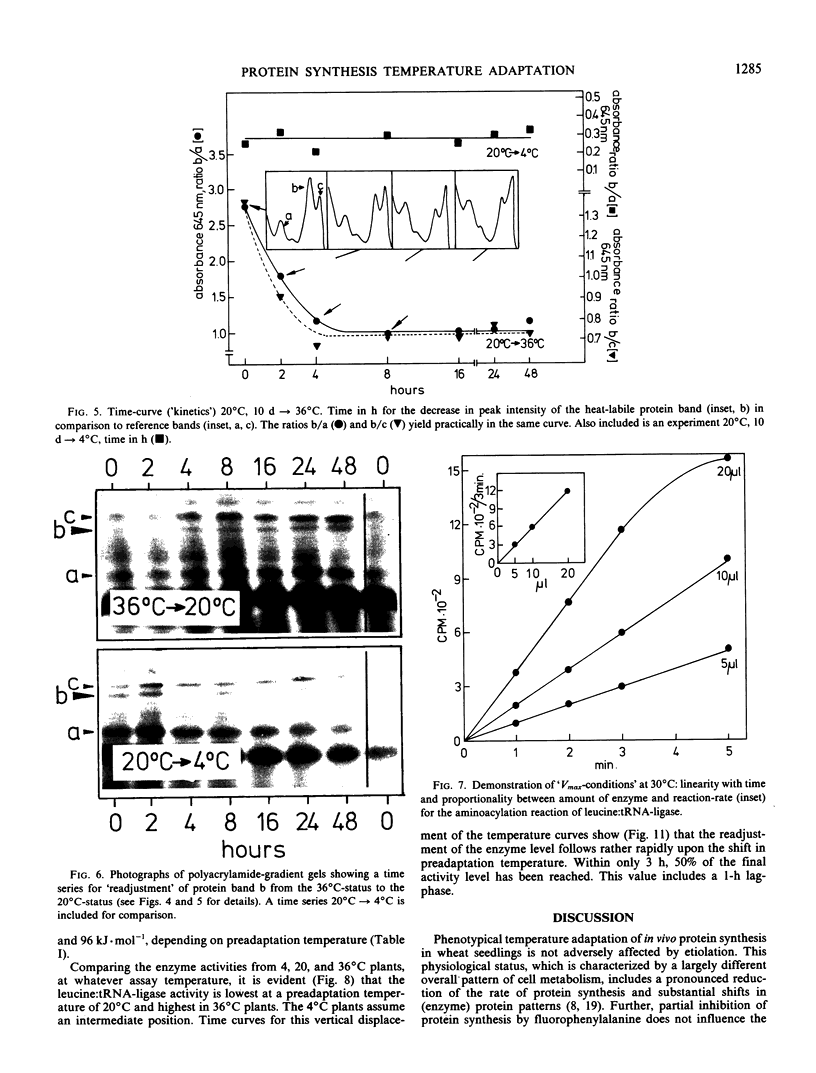
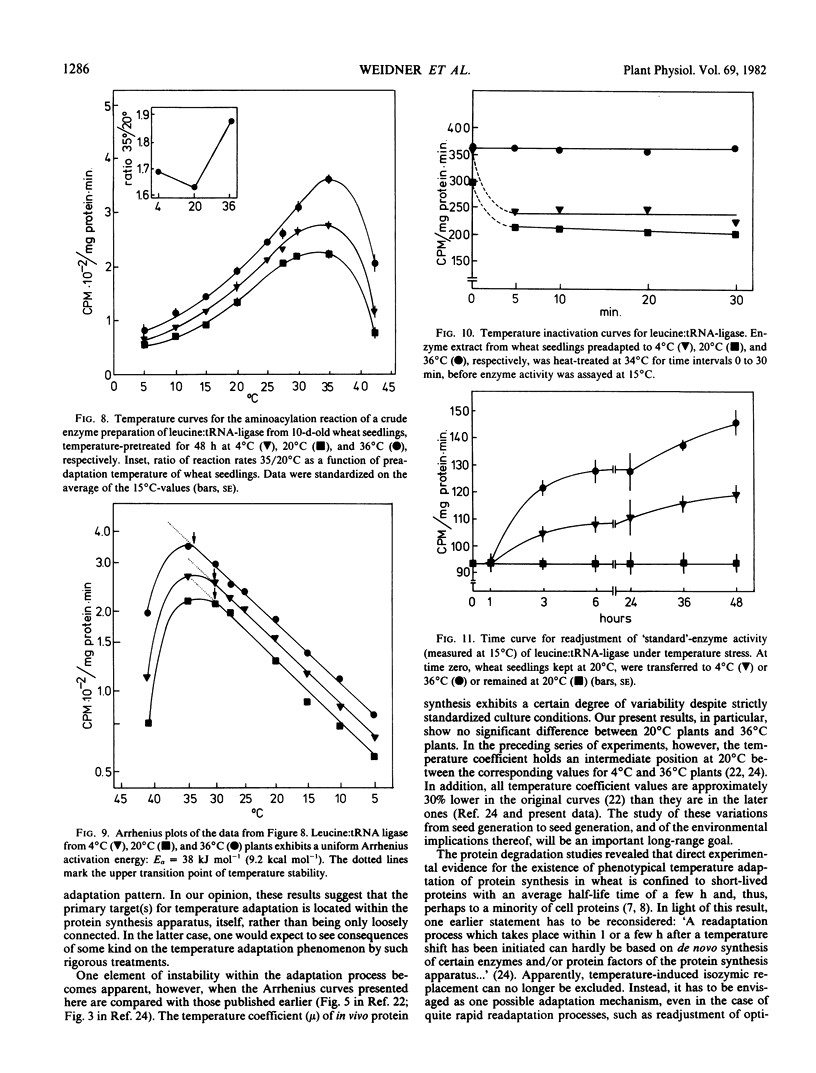
Phenotypical temperature adaptation of protein synthesis in wheat (Triticum aestivum L.) seedlings is not affected by darkness (etiolation), by partial inhibition of protein biosynthesis (10−3m fluorophenylalanine), or by changing the amino acid precursor and the radioisotope ([3H]valine instead of [14C]leucine). The temperature coefficient (μ), as well as the optimum temperature of in vivo protein synthesis, increases with rising preadaptation temperature, as normally observed. Protein turnover studies revealed that only proteins with a short half-life time (t½ = 2 to 4 hours) are labeled to a measurable extent during the temperature adaptation experiments. A heat-labile protein has been detected and partially characterized by means of polyacrylamide gradient gels. Leucine:tRNA-ligase (EC 6.1 1.4) from heat-pretreated wheat seedlings exhibits enhanced thermal stability. In Arrhenius curves, the upper transition point shifts from 30 to 34°C, depending on preadaptation temperature. Only the leucine:tRNA-ligase extracted from heat-adapted plants is stable when the enzyme extracts are subjected to a 34°C heat treatment.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- De Jong D. W., Olson A. C., Hawker K. M., Jansen E. F. Effect of cultivation temperature on peroxidase isozymes of plant cells grown in suspension. Plant Physiol. 1968 May;43(5):841–844. doi: 10.1104/pp.43.5.841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faw W. F., Jung G. A. Electrophoretic protein patterns in relation to low temperature tolerance and growth regulation of alfalfa. Cryobiology. 1972 Dec;9(6):548–555. doi: 10.1016/0011-2240(72)90177-0. [DOI] [PubMed] [Google Scholar]
- Hazel J. R., Prosser C. L. Molecular mechanisms of temperature compensation in poikilotherms. Physiol Rev. 1974 Jul;54(3):620–677. doi: 10.1152/physrev.1974.54.3.620. [DOI] [PubMed] [Google Scholar]
- Humphrey T. J., Davies D. D. A sensitive method for measuring protein turnover based on the measurement of 2-3H-labelled amino acids in protein. Biochem J. 1976 Jun 15;156(3):561–568. doi: 10.1042/bj1560561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanabus J., Cherry J. H. Isolation of an organ-specific leucyl-tRNA synthetase from soybean seedling. Proc Natl Acad Sci U S A. 1971 May;68(5):873–876. doi: 10.1073/pnas.68.5.873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Neihardt F. C., Parker J., McKeever W. G. Function and regulation of aminoacyl-tRNA synthetases in prokaryotic and eukaryotic cells. Annu Rev Microbiol. 1975;29:215–250. doi: 10.1146/annurev.mi.29.100175.001243. [DOI] [PubMed] [Google Scholar]
- Norris D., Fowden L. Substrate protection during selective heat inactivation of aminoacyl-tRNA synthetases and its use in enzyme studies. Biochim Biophys Acta. 1973 Jul 27;312(4):695–707. doi: 10.1016/0005-2787(73)90073-7. [DOI] [PubMed] [Google Scholar]
- Tao K. L., Hall T. C. Factors controlling aminoacyl-transfer-ribonucleic acid synthesis in vitro by a plant system. Biochem J. 1971 Feb;121(3):495–501. doi: 10.1042/bj1210495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weidner M., Combrink G. Phenotypical temperature adaptation of protein synthesis in wheat seedlings: time curves for readaptation. Plant Physiol. 1979 Jul;64(1):144–149. doi: 10.1104/pp.64.1.144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weidner M., Ziemens C. Preadaptation of protein synthesis in wheat seedlings to high temperature. Plant Physiol. 1975 Nov;56(5):590–594. doi: 10.1104/pp.56.5.590. [DOI] [PMC free article] [PubMed] [Google Scholar]