Abstract
Background: A large number of birth defects are related to nutrient deficiencies; concern that biotin deficiency is teratogenic in humans is reasonable. Surprisingly, studies indicate that increased urinary 3-hydroxyisovalerylcarnitine (3HIAc), a previously validated marker of biotin deficiency, is not a valid biomarker in pregnancy.
Objective: In this study we hypothesized that coexisting carnitine deficiency can prevent the increase in 3HIAc due to biotin deficiency.
Methods: We used a 2-factor nutrient depletion design to induce isolated and combined biotin and carnitine deficiency in HepG2 cells and then repleted cells with carnitine. To elucidate the metabolic pathogenesis, we quantitated intracellular and extracellular free carnitine, acylcarnitines, and acylcarnitine ratios using liquid chromatography–tandem mass spectrometry.
Results: Relative to biotin-sufficient, carnitine-sufficient cells, intracellular acetylcarnitine increased by 90%, propionylcarnitine more than doubled, and 3HIAc increased by >10-fold in biotin-deficient, carnitine-sufficient (BDCS) cells, consistent with a defensive mechanism in which biotin-deficient cells transesterify the acyl-coenzyme A (acyl-CoA) substrates of the biotin-dependent carboxylases to the related acylcarnitines. Likewise, in BDCS cells, the ratio of acetylcarnitine to malonylcarnitine and the ratio of propionylcarnitine to methylmalonylcarnitine both more than tripled, and the ratio of 3HIAc to 3-methylglutarylcarnitine (MGc) increased by >10-fold. In biotin-deficient, carnitine-deficient (BDCD) cells, the 3 substrate-derived acylcarnitines changed little, but the substrate:product ratios were masked to a lesser extent. Moreover, carnitine repletion unmasked biotin deficiency in BDCD cells as shown by increases in acetylcarnitine, propionylcarnitine, and 3HIAc (each increased by >50-fold). Likewise, ratios of acetylcarnitine:malonylcarnitine, propionylcarnitine:methylmalonylcarnitine, and 3HIAc:MGc all increased by >8-fold.
Conclusions: Our findings provide strong evidence that coexisting carnitine deficiency masks some indicators of biotin deficiency and support the potential importance of the ratios of acylcarnitines arising from the acyl-CoA substrates and products for biotin-dependent carboxylases in detecting the biotin deficiency that is masked by coexisting carnitine deficiency.
Keywords: biotin, carnitine, intermediary metabolism, mass spectrometry, biomarkers
Introduction
Biotin deficiency is teratogenic in several species (1). Of particular concern are 2 observations. First, maternal biotin deficiency during gestation is highly teratogenic in mice; furthermore, the murine teratogenic events occur at very high rates even at marginal degrees of biotin deficiency that produce no signs or symptoms in the mouse dam (2). Second, several studies documented that a marginal degree of biotin deficiency develops spontaneously in a substantial proportion of normal pregnancies and that the deficiency develops during the first trimester when teratogenic events are most common (3). Taken together, these observations raise concern that biotin deficiency may be teratogenic in humans (3, 4).
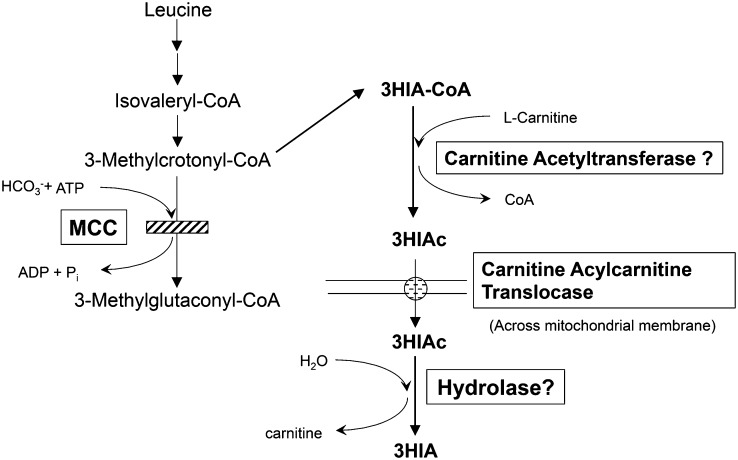
Evidence that supports biotin deficiency in pregnancy includes the following: 1) reduced activity of the biotin-dependent enzyme propionyl-CoA carboxylase (PCC)6 in peripheral blood lymphocytes and 2) increased urinary excretion of 3-hydroxyisovaleric acid (3HIA) (3). However, urinary 3-hydroxyisovalerylcarnitine (3HIAc) is not increased in pregnant biotin-deficient women (5). This observation is surprising because during biotin deficiency induced experimentally by our group (6), urinary 3HIA and 3HIAc increased in parallel, reflecting reduced activity of the biotin-dependent enzyme methylcrotonyl-CoA carboxylase (MCC) (Figure 1).
FIGURE 1.
Metabolic consequences of biotin deficiency. 3HIAc and 3HIA arise from accumulation of 3-methylcrotonyl-CoA due to decreased activity of the biotin-dependent enzyme MCC. MCC, methylcrotonyl-CoA carboxylase; Pi, inorganic phosphate; 3HIA, 3-hydroxyisovaleric acid; 3HIAc, 3-hydroxyisovalerylcarnitine.
The conflict between 3HIA and 3HIAc in pregnancy could be the result of a coexisting carnitine deficiency. Indeed, marginal carnitine deficiency has been repeatedly observed in pregnancy (7–9). For those women who are deficient in carnitine, 3HIAc excretion might be not a suitable biomarker/indicator of biotin status. Specifically, we offer here the novel hypothesis that carnitine deficiency either impairs carnitine transesterification of 3-methylcrotonyl-CoA to 3-methylcrotonylcarnitine in the mitochondrion or accelerates the conversion of 3HIAc (arising from 3-methylcrotonylcarnitine) to 3HIA in the cytosol, thereby facilitating reclamation of carnitine and reducing 3HIAc excretion, or both. Reported here is our initial test of this hypothesis. Using a 2 × 2 nutrient depletion design in hepatocyte cell cultures, we examined the isolated and combined effects of biotin and carnitine deficiency. We investigated whether carnitine deficiency prevents an increase of intracellular 3HIAc despite the presence of coexisting biotin deficiency, and in subsequent carnitine repletion studies, we investigated whether carnitine repletion unmasks the coexisting biotin deficiency as judged by intracellular 3HIAc. In a broader characterization of the metabolic perturbations caused by isolated and combined biotin and carnitine deficiency, we examined whether 3 additional previously validated indicators of marginal biotin deficiency (10)—the ratios of the acylcarnitines arising from the substrates and products of reactions catalyzed by 4 biotin-dependent carboxylases—were more robust in detecting the masked biotin deficiency. We calculated only 3, rather than 4, ratios because our method quantitates total intracellular acetylcarnitine and total malonylcarnitine. The 2 acetyl-CoA carboxylase (ACC) isoenzymes, ACC-1 and ACC-2, which are coded by distinct genes, are located in 2 different subcellular compartments and serve very distinct metabolic roles (Supplemental Figure 1). However, both convert acetyl-CoA to malonyl-CoA; the accumulating CoAs are then transesterified to acetylcarnitine and malonylcarnitine.
Methods
Materials
HepG2 (HB-8065), a human hepatocellular carcinoma cell line, was obtained from the American Type Culture Collection. Reagents and sources were as follows: MEM with Earle’s salts and l-glutamine (Cellgro); FBS (Atlas Biologicals), dialyzed FBS (Gibco), and penicillin/streptomycin (Cellgro); Optima LC/MS-grade water and formic acid (99% pure; Acros Organics, via Fisher Scientific); acetyl-dl-carnitine hydrochloride (Sigma-Aldrich); malonylcarnitine (99% pure), methylmalonylcarnitine (85% pure), and 3-methylglutarylcarnitine [(MGc); 95% pure; Chemische Laboratorien). The 3HIAc (98% pure), [N-methyl-D3]-3HIAc ([2H3]-3HIAc; 98% pure), propionylcarnitine (98% pure), and [N-methyl-D3]-propionylcarnitine ([2H3]-propionylcarnitine; 98% pure) were generous gifts from Cambridge Isotope Laboratories. All other chemicals and solvents were purchased from Fisher Scientific or Sigma Aldrich. All solvents used were HPLC grade or better.
General conditions used for cell culture
The general conditions for culture were as follows: HepG2 cells were initially cultured in complete growth medium containing biotin-free MEM, 10% FBS, and 1% penicillin/streptomycin at 37°C in a humidified 5% carbon dioxide atmosphere. Culture medium were replaced with fresh medium at 2- to 3-d intervals. Cells were subdivided before they reached confluence (every 4 or 5 d) during initial cell culture expansion and during the entire study. During the biotin depletion and carnitine depletion phases (see Experimental Design), complete medium was replaced by biotin-deficient and carnitine-deficient medium (MEM with 10% dialyzed FBS). For biotin-sufficient cells, this basal medium was supplemented with 10 nmol/L biotin; for carnitine-sufficient cells, this basal medium was supplemented with 60 μmol/L l-carnitine. Cells and culture media were harvested at various times for analysis of free carnitine and acylcarnitines (see Experimental Design).
Approach to nutrient depletion and repletion
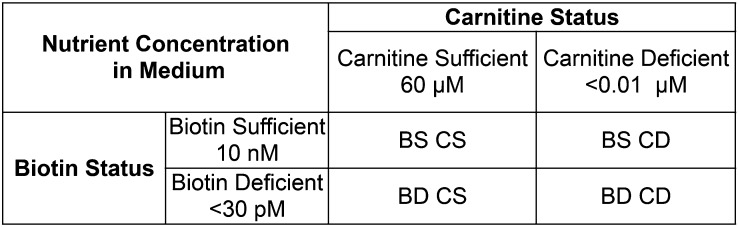
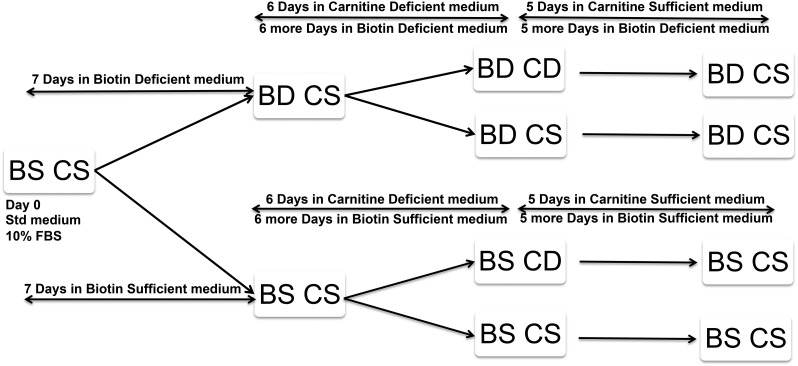
We used a 2-factor nutrient depletion design to produce a predetermined degree of depletion or sufficiency simultaneously in 4 experimental groups (Figure 2). For clarity, we refer hereafter to the cells using 4-letter abbreviations [e.g., biotin-deficient, carnitine-deficient (BDCD) as per Figure 3] and refer to the media with unabbreviated descriptions (e.g., biotin-deficient/carnitine-deficient medium). In 2 pilot studies, we determined that the duration of culture medium exposure needed to produce the desired degree of carnitine deficiency was not the same as that required to produce the desired degree of biotin deficiency. Accordingly, a staggered start design was used for these 2 × 2 studies as depicted in Figure 3.
FIGURE 2.
Four experimental groups of HepG2 cells, group designations, and media concentrations of biotin and carnitine. BD CD, biotin-deficient, carnitine-deficient; BD CS, biotin-deficient, carnitine-sufficient; BS CD, biotin-sufficient, carnitine-deficient; BS CS, biotin-sufficient, carnitine-sufficient.
FIGURE 3.
A staggered implementation of deficient media was used to achieve simultaneous biotin deficiency and carnitine deficiency in HepG2 cells. BD CD, biotin-deficient, carnitine-deficient; BD CS, biotin-deficient, carnitine-sufficient; BS CD, biotin-sufficient, carnitine-deficient; BS CS, biotin-sufficient, carnitine-sufficient; FBS, fetal bovine serum; Std, standard.
Pilot carnitine depletion study
Based on our literature search, culture conditions to deplete carnitine in HepG2 cells have not been reported. Animal studies using the administration of d-carnitine required 10 d (11, 12); animal studies using diets that were poor in carnitine combined with carnitine synthesis inhibitors (13, 14) required ∼21 d to deplete the liver of carnitine and short-chain acylcarnitines to ∼30% of that in controls. In this pilot study, we used a carnitine-deficient MEM medium to deplete the content of free carnitine in HepG2 cells by at least 60%. This target was chosen because, during pregnancy, plasma concentrations of free and total carnitine decrease by ∼60% from the normal mean of 40 μmol/L to 15 μmol/L (7, 9, 15). To assess the time course, we harvested cells at 5 and 10 d. These pilot data indicated that 5 d was sufficient to induce 99% depletion of free carnitine relative to control cells (control = biotin-sufficient/carnitine-sufficient medium as described above). This unexpectedly rapid depletion compared with in vivo rat studies was accompanied by a reduction in the intracellular content of the acylcarnitines derived from the substrates of the biotin-dependent carboxylases acetylcarnitine, propionylcarnitine, and 3HIAc to 1%, 3%, and 19%, respectively, of control values. In the carnitine-deficient cells, the pool size calculated as the sum of the 6 measured acylcarnitines was depleted by 98% relative to control carnitine-sufficient cells.
Depletion of biotin, carnitine, and their combination
Overview.
Because biotin depletion requires approximately twice as long as carnitine depletion, a staggered start design (Figure 3) was used as follows:
1) Biotin-deficient groups: Culture in the biotin-deficient/carnitine-sufficient medium was initiated on day 0, and carnitine-deficient medium was initiated at day 7 by subdividing the HepG2 cells cultured in biotin-deficient/carnitine-sufficient medium into 2 groups. One group (the BDCD group) was cultured in biotin-deficient/carnitine-deficient medium; the second group [the biotin-deficient, carnitine-sufficient (BDCS) group] was cultured in biotin-deficient/carnitine-sufficient medium as before.
2) Biotin-sufficient groups: In parallel, cells were cultured in biotin-sufficient/carnitine-sufficient medium for 7 d. The cells were then divided into 2 groups. One group [the biotin-sufficient, carnitine-deficient (BSCD) group] was cultured in biotin-sufficient/carnitine-deficient medium; the second group [the biotin-sufficient, carnitine-sufficient (BSCS) group] was cultured in the biotin-sufficient/carnitine-sufficient medium as before.
Aliquots of each of the 4 groups of cells (and their respective cell culture media) were concurrently harvested after culture for 6 more days. At this point (13 d total), cell morphology remained normal in all 4 groups.
Method for inducing biotin deficiency.
After cell culture expansion in biotin-sufficient/carnitine-sufficient medium, we initiated HepG2 culture in biotin-deficient/carnitine-sufficient medium on day 0. To attain our target degree of biotin depletion as reflected in reduced carboxylase activities and reduced mRNA content in a reasonable period of time, we used a previously described method (16). This choice was made on the basis of 2 studies that determined the time courses (9–15 d) for inducing biotin deficiency as reflected in biotinylated protein abundance of pyruvate carboxylase, PCC, MCC in HepG2 cells grown in 0.025 nmol/L biotin (17), mRNA abundance of holocarboxylase synthetase, isoform α of PCC (PCCA), and isoform α of ACC-1 in HepG2 cells grown in biotin-free medium (16). These conditions achieved a 50–90% reduction in most of these variables. We chose 13 d of culture in the biotin-free media to simulate the degree of reduced carboxylase activities (∼50% in dams and ∼90% in fetuses) that cause high rates of teratogenesis in mice (18). This degree resembles the marginal biotin deficiency that occurs spontaneously in human pregnancy as reflected in a 60% reduction in PCC activity in lymphocytes (3). In brief, HepG2 cells were depleted in biotin-free MEM medium supplemented with 10% dialyzed FBS. Biotin depletion of the biotin-deficient media was confirmed by using an avidin-binding assay as described previously (19); biotin concentrations in biotin-deficient media were below the assay detection limit (<0.030 nmol/L).
Method for ensuring carnitine sufficiency.
To ensure carnitine sufficiency, both the biotin-deficient/carnitine-sufficient medium and the biotin-sufficient/carnitine-sufficient medium were supplemented with 60 μmol/L l-carnitine.
Method for ensuring biotin sufficiency.
To ensure biotin sufficiency, beginning on day 0, both the biotin-sufficient/carnitine-sufficient medium and the biotin-sufficient/carnitine-deficient medium were supplemented with 10.5 nmol/L of biotin, as confirmed by avidin-binding assay and described previously (19). On day 7, we initiated HepG2 culture in biotin-sufficient/carnitine-deficient medium and biotin-deficient/carnitine-deficient medium as described above.
Carnitine repletion
To further test our hypothesis concerning the pathogenesis of the metabolic abnormalities described in Results, we repleted the carnitine in each of the 2 carnitine-deficient cell groups (BDCD and BSCD). We also continued culture of the 2 carnitine-sufficient cell groups (BSCS and BDCS) in carnitine-sufficient media as controls for the effects of culture duration. After 5 d, each of the 4 cell groups (and their media) were harvested. At that point (18 d of total culture), cell morphology remained normal by light microscopy for all 4 groups.
Extraction of carnitine and acylcarnitines from HepG2 cells and culture media
Harvested HepG2 cells and culture media were processed for LC-MS/MS as described previously (20, 21). Briefly, the media were transferred from a T-75 culture flask to a 15-mL tube and kept on ice until further processing. The cells were washed twice with ice-cold PBS, scraped into ice-cold PBS by using rubber scraper, and collected by centrifugation at 4°C at 250 × g for 7 min. Supernatants were discarded; cell pellet suspensions were disrupted by addition of 0.25 mL of ice-cold 0.2% formic acid in water, followed by ice-cold 0.2% formic acid in acetonitrile (1.05 mL). Internal standards were added during the resuspension. Samples were incubated at 4°C for 30 min; precipitates were centrifuged for 10 min at 14,000 × g at 4°C. The precipitates were collected and frozen at −70°C for later protein quantitation. The supernatants (containing free carnitine and acylcarnitines) were evaporated to dryness by using a SpeedVac (model SVC200H; Savant) and then resuspended in water. Any remaining cell particulates were separated by centrifugation for 5 min at 14,000 × g and discarded. Supernatants were transferred to a LC vial and stored at −70°C until LC-MS/MS analysis for acylcarnitines as described below. Results were normalized by cellular protein.
To assess the extent to which the intracellular perturbations of acylcarnitines are reflected in the extracellular environment, 200 μL medium was mixed with 1mL of ice-cold 0.2% formic acid in water, 4.2 mL of ice-cold 0.2% formic acid in acetonitrile, and internal standards as described below. The mixtures were then processed and assayed for free carnitine and acylcarnitine concentrations as described for the cell pellets; results were expressed in concentrations rather than being normalized by cellular protein.
Intracellular and extracellular acylcarnitine analysis
Intracellular and extracellular free carnitine, acylcarnitines, and acylcarnitine ratios were quantitated by LC-MS/MS assay as described previously (10). Free carnitine content and content of the individual acylcarnitines of interest were normalized to the recovery of internal standards as previously described (10).
Cellular protein quantitation
Total cellular protein content was measured by using the bicinchoninic acid protein assay kit (Pierce). To account for differences in the number of cell harvested, intracellular free carnitine content and content of individual acylcarnitines were also normalized by cellular protein mass (pmol/mg protein).
Statistical analysis
The data are presented as means ± 1 SDs from 4 replicate culture flasks. A 2-factor ANOVA model was used to evaluate the effects of group and time in various media. This model also included a group-by-day interaction term to determine whether profile across days was similar among the groups. If the interaction term was found to be significant, preplanned contrasts examining the effect of biotin status on metabolites at each of the 3 time points were applied. A Bonferroni adjustment was applied to the P values that resulted from these comparisons. In experiments in which we sought to evaluate the effect of a single nutrient change (e.g., biotin deficiency in carnitine-sufficient cells or carnitine repletion in carnitine-deficient cells), differences in the mean intracellular content (or extracellular concentration) of free carnitine, the acylcarnitines, or the ratios were tested for significant differences by using unpaired, 2-tailed Student’s t tests. Differences were considered significant if P < 0.05 (after Bonferroni correction if multiple t tests were used). All tests were performed by using either KaleidaGraph (Synergy Software) or SAS software.
Results
Biotin depletion.
To assess progressive biotin deficiency, we chose the cell group cultured in biotin-sufficient/carnitine-sufficient medium as the control. For example, we compared BDCS to BSCS cells after equal culture times. By day 3, no significant differences in the content of any of the acylcarnitines were detectable in BDCS cells relative to BSCS cells (Figure 4A, B). We infer that the BDCS cells were not yet biotin deficient and chose day 3 as the reference point to gauge the development of biotin deficiency.
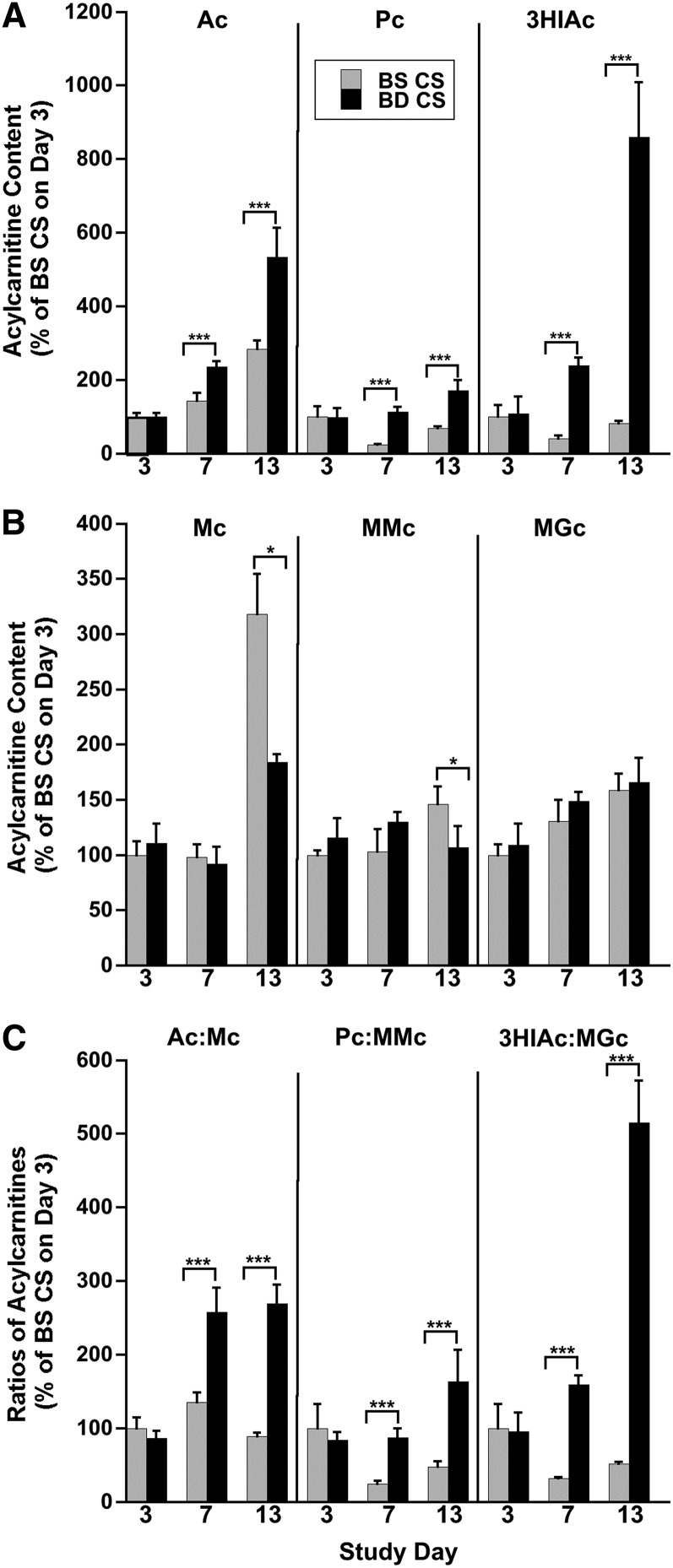
FIGURE 4.
Metabolic blocks caused by progressive biotin deficiency in HepG2 cells are reflected in changing intracellular acylcarnitine content and acylcarnitines ratios. (A) Content of acylcarnitines arising from the substrate CoAs: Ac, Pc, and 3HIAc. (B) Content of acylcarnitines arising from the product CoAs: Mc, MMc, and MGc. (C) Substrate:product ratios of the acylcarnitines (Ac:Mc, Pc:MMc, and 3HIAc:MGc). Results are presented as percentage increases relative to BSCS cells on day 3. Values are means ± SDs, n = 4. For all 3 substrate-derived acylcarnitines and the 3 ratios, 2-factor ANOVA revealed highly significant interactions (P < 0.0001) between biotin status and time. Pairwise contrasts between BSCS and BDCS cells at each time point revealed a significant effect of biotin status at both days 7 and 13. For product-derived acylcarnitines, interactions were also significant (P < 0.01) for Mc and MMc but not for MGc. Pairwise comparisons between 2 groups by time point showed significant decreases (rather than increases with biotin deficiency) on day 13 for Mc and MMc. *P < 0.05, ***P < 0.0001. Ac, acetylcarnitine; BD CS, biotin-deficient, carnitine-sufficient; BS CS, biotin-sufficient, carnitine-sufficient; Mc, malonylcarnitine; MGc, 3-methylglutarylcarnitine; MMc, methylmalonylcarnitine; Pc, propionylcarnitine; 3HIAc, 3-hydroxyisovalerylcarnitine.
For acetylcarnitine, propionylcarnitine, and 3HIAc, 2-factor ANOVA revealed significant effects of biotin status and time and the interaction between these 2 variables. These findings are consistent with our central hypothesis. Acetylcarnitine accumulates to a greater extent and faster in biotin-deficient cells than in biotin-sufficient cells. Similar effects were seen for propionylcarnitine and 3HIAc in the biotin-deficient cells. In contrast, the product-derived acylcarnitines did not accumulate in biotin-deficient cells.
By day 13, intracellular acetylcarnitine (which arises from acetyl-CoA, the substrate of both ACC-1 and ACC-2) increased by 90% (Figure 4A), propionylcarnitine (which arises from propionyl-CoA, the substrate of PCC) more than doubled, and 3HIAc (which arises from methylcrotonyl-CoA, the substrate of MCC) increased by >10 fold.
As predictors of what might be seen in plasma or urine in vivo, we measured changes in acylcarnitines in the extracellular media (Supplemental Figure 2A). Our results indicate that perturbations of these intracellular acylcarnitines are mirrored in the extracellular media.
Consistent with our hypothesized mechanism, the intracellular contents of the acylcarnitines derived from the products of the biotin-dependent carboxylases did not increase. Relative to the BSCS cells, the intracellular contents of malonylcarnitine (which arises from malonyl-CoA, the product of both ACC-1 and ACC-2), methylmalonylcarnitine (which arises from methylmalonyl-CoA, the product of PCC), and MGc (which arises after an additional metabolic conversion step from methylglutaconyl-CoA, the product of MCC) decreased by day 13 in BDCS cells (Figure 4B). Moreover, extracellular medium concentrations of the product-derived acylcarnitines changed little in the BDCS cells (Supplemental Figure 2B). Of note, the medium concentration of malonylcarnitine was too small for accurate quantitation.
Because the acylcarnitines arising from the substrate CoAs increased and the acylcarnitines arising from the product CoAs changed relatively little, the intracellular substrate:product ratios for the acylcarnitines increased with biotin depletion, exhibiting significant effects of biotin status, time, and the interaction between the 2 (Figure 4C). Relative to BSCS cells, acetylcarnitine:malonylcarnitine and propionylcarnitine:methylmalonylcarnitine ratios more than tripled, and the 3HIAc:MGc ratio increased >10 fold in BDCS cells by day 13 (Figure 4C). Extracellular changes in acylcarnitine substrate:product ratios increased and mirrored intracellular changes (Supplemental Figure 2C).
The intracellular and extracellular changes that resulted from biotin deficiency were not the result of inadvertent induction of difference in carnitine status. No differences in the intracellular and extracellular content of free carnitine were detectable in BDCS cells relative to BS CS cells.
Carnitine depletion.
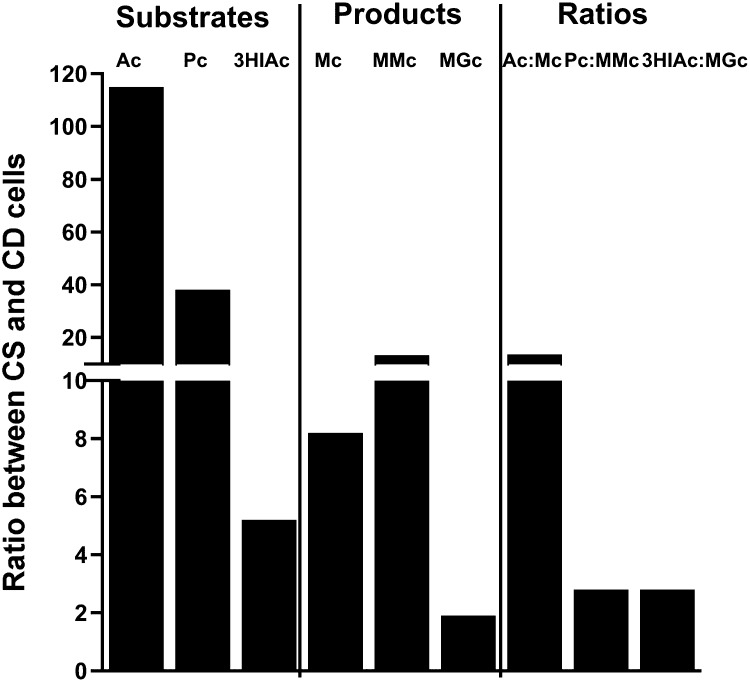
Carnitine depletion was assessed by intracellular and extracellular free carnitine and by the content of the relevant acylcarnitines. Changes in these compounds clearly showed that HepG2 cells grown for 6 d in biotin-sufficient/carnitine-deficient medium (day 13 of overall experiment) were depleted in carnitine. By day 13, the intracellular content of free carnitine in the BSCD cells was reduced to ∼1% of that in the BSCS cells (5.50 ± 0.864 vs. 464 ± 41.6 pmol/mg protein). Moreover, the absolute content of all 6 acylcarnitines decreased drastically (for absolute values see Supplemental Table 1). The ratios of the intracellular contents of acetylcarnitine, propionylcarnitine, 3HIAc, malonylcarnitine, methylmalonylcarnitine, and MGc in BSCD cells to BSCS cells were 114, 37, 5.3, 7.9, 13, and 1.9, respectively (Figure 5). As expected, extracellular free carnitine was much less than that in biotin-sufficient/carnitine-sufficient medium (16.5 ± 0.75 vs. 6630 ± 552 nmol/L). Importantly, extracellular concentrations of each of the 5 acylcarnitines that could be quantitated decreased significantly, mirroring the intracellular changes (Supplemental Figure 3, Supplemental Table 1).
FIGURE 5.
Effect of carnitine depletion on acylcarnitines arising from the substrate CoAs, product CoAs, and the ratios. Intracellular changes in HepG2 cells are presented as ratios between CS and CD cells. Ac, acetylcarnitine; CD, carnitine-deficient; CS, carnitine-sufficient; Mc, malonylcarnitine; MGc, 3-methylglutarylcarnitine; MMc, methylmalonylcarnitine; Pc, propionylcarnitine; 3HIAc, 3-hydroxyisovalerylcarnitine.
The ratio of intracellular acetylcarnitine:malonylcarnitine in BSCS cells to BSCD cells was 14. For propionylcarnitine:methylmalonylcarnitine, the ratio was 2.8, and for 3HIAc:MGc, the ratio was 2.8 (Figure 5). Extracellular changes in acylcarnitine substrate:product ratios mirrored intracellular changes (Supplemental Figure 3).
Combined biotin and carnitine depletion.
We hypothesized that the increase in the substrate-derived acylcarnitines due to biotin deficiency would be masked by carnitine deficiency. Consistent with that hypothesis, we observed that the 3 substrate-derived acylcarnitines did not increase in BDCD cells as much as they did in the BDCS cells; on day 13, acetylcarnitine was 109-fold greater, propionylcarnitine was 48-fold greater, and 3HIAc was 14-fold greater in the BDCS cells than in the BDCD cells (Figure 6A). Extracellular changes in acylcarnitines mirrored intracellular changes (Supplemental Figure 4A). In summary, these observations provide evidence that in hepatocytes with a combined deficiency of biotin and carnitine, the carnitine deficiency masks biotin deficiency, as shown by substrate-derived acylcarnitines, by preventing the increases of intracellular content and extracellular concentration of the substrate-derived acylcarnitines.
FIGURE 6.
Effect of combined biotin deficiency and carnitine deficiency on intracellular acylcarnitine content (A, B) and acylcarnitine ratios (C) at the point of greatest depletion of biotin, carnitine, or both in HepG2 cells. (A) Acylcarnitines Ac, Pc, and 3HIAc that arise from the acyl-CoAs that are substrates of the biotin-dependent carboxylases. (B) Acylcarnitines Mc, MMc, and MGc that arise from the acyl-CoAs that are products of the biotin-dependent carboxylases. (C) Substrate:product ratios of the acylcarnitines. Values are means ± SDs, n = 4. *,**,***Student’s t test: *P < 0.05, **P < 0.01, ***P < 0.001. Ac, acetylcarnitine; BD CD, biotin-deficient, carnitine-deficient; BD CS, biotin-deficient, carnitine-sufficient; BS CD, biotin-sufficient, carnitine-deficient; BS CS, biotin-sufficient, carnitine-sufficient; Mc, malonylcarnitine; MGc, 3-methylglutarylcarnitine; MMc, methylmalonylcarnitine; Pc, propionylcarnitine; 3HIAc, 3-hydroxyisovalerylcarnitine.
In our original hypothesis, we proposed that the use of acylcarnitine substrate:product ratios (rather than absolute content and concentrations) would at least attenuate the masking effect of carnitine deficiency. Indeed, on day 13, the 3 intracellular acylcarnitine substrate:product ratios were masked to a lesser extent in BDCD cells than was the absolute substrate-derived acylcarnitine content. Specifically, the acetylcarnitine:malonylcarnitine ratio was not as great in BDCD cells as in BDCS cells (19.3-fold decrease); hence, the masking effect was ∼5-fold weaker in comparison to that seen for acetylcarnitine (which exhibited a 109-fold decrease). Likewise, propionylcarnitine:methylmalonylcarnitine decreased by only 3.7-fold; thus, the masking effect was reduced by ∼13 fold in comparison to propionylcarnitine (which was 48-fold). Also, 3HIAc:MGc decreased by only 6-fold; thus, the masking effect was reduced by ∼2-fold in comparison to 3HIAc (14-fold) (Figure 6C). Furthermore, all 3 intracellular acylcarnitine ratios were greater in the BDCD cells relative to BSCD cells, providing additional evidence in support of the proposed biochemical pathogenesis and suggesting that acylcarnitine ratios might partially expose biotin deficiency in vivo despite coexisting carnitine deficiency (Figure 6C). For example, the 3HIAc:MGc ratio was 1.7-fold greater in BDCD cells than in BSCS cells (P < 0.05) despite coexisting carnitine deficiency, suggesting that this ratio might be more robust diagnostically. In contrast, the other 2 ratios (acetylcarnitine:malonylcarnitine and propionylcarnitine:methylmalonylcarnitine) were not greater in the BSCS cells. We infer that the assessment of diagnostic utility awaits evaluation in an animal model and then in appropriate clinical situations.
Extracellular changes in acylcarnitine substrate:product ratios mirrored intracellular changes (Supplemental Figure 4C). Similarly to intracellular increases, extracellular acylcarnitine ratios were greater in the biotin-deficient/carnitine-deficient medium relative to the biotin-sufficient/carnitine-deficient medium.
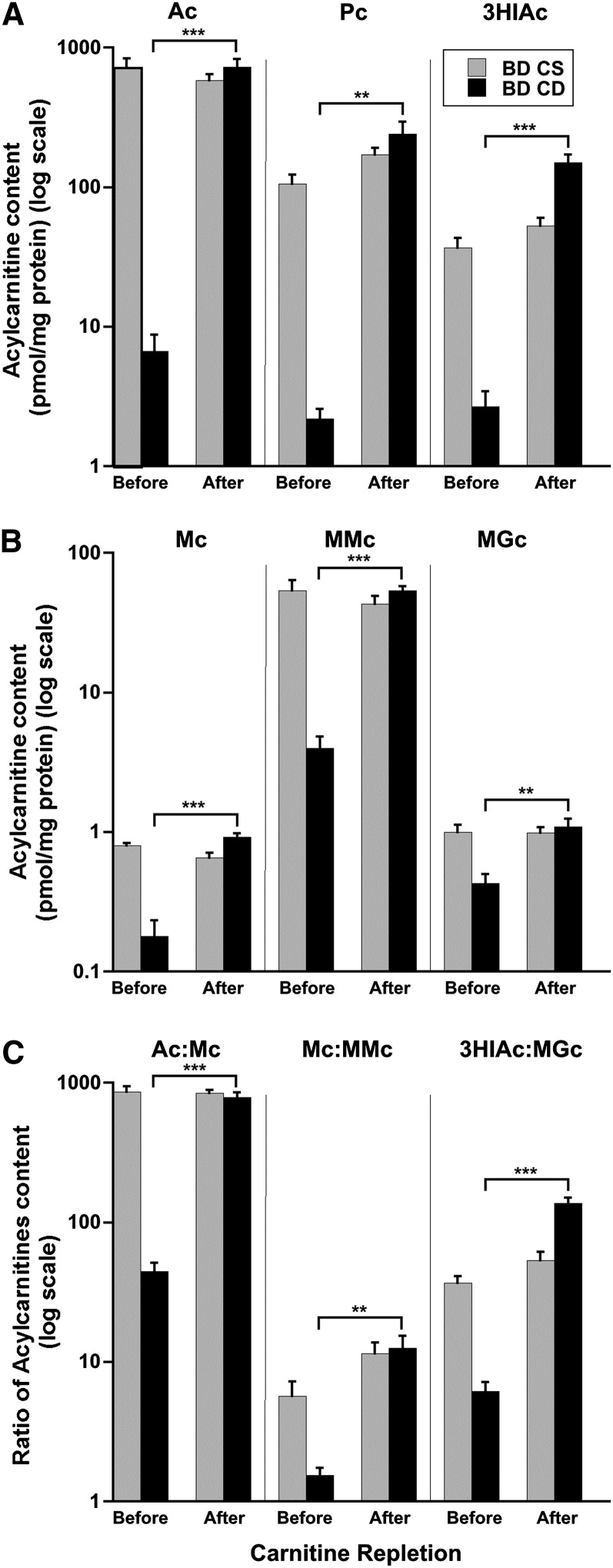
Carnitine repletion.
In BDCD cells, supplementation with carnitine for 5 d (day 13 to day 18 of total culture time) increased intracellular free carnitine content from 7.36 ± 2.31 pmol/mg protein to 413 ± 96.6 pmol/mg protein (P < 0.0001, unpaired t test). Repletion of carnitine in BDCD cells for 5 d completely unmasked the biotin deficiency as follows: 1) the intracellular content of acetylcarnitine increased by 109-fold over the 5 d and was not different than acetylcarnitine in the BDCS cells, 2) intracellular propionylcarnitine increased by 110-fold and was not different than propionylcarnitine in the BDCS cells, and 3) intracellular 3HIAc increased by >56-fold (Figure 7A) and was greater than 3HIAc in the BDCS cells. With carnitine repletion, the intracellular content of the 3 product-derived acylcarnitines increased much less: malonylcarnitine increased by >5-fold, methylmalonylcarnitine increased by 14-fold, and MGc increased by >2-fold (Figure 7B). As a consequence of the smaller increase in the content of the product acylcarnitines, the increases in the 3 substrate:product ratios with carnitine repletion were substantial, but not as great, as the increases in the absolute content of the 3 substrate-derived acylcarnitines. Acetylcarnitine:malonylcarnitine increased by 18-fold, propionylcarnitine:methylmalonylcarnitine increased by 8.2-fold, and 3HIAc:MGc increased by 22-fold (Figure 7C).
FIGURE 7.
Effect of carnitine repletion on intracellular acylcarnitine contents (A, B) and acylcarnitine ratios (C) in HepG2 cells before and after 5 d in carnitine-sufficient medium. (A) Acylcarnitines arising from the substrate CoAs: Ac, Pc, and 3HIAc. (B) Acylcarnitines arising from the product CoAs: Mc, MMc, and MGc. (C) Substrate:product ratios for Ac:Mc, Pc:MMc, and 3HIAc:MGc. Values are means ± SDs, n = 4. **,***Student’s t test: **P < 0.001, ***P < 0.0001. Ac, acetylcarnitine; BD CD, biotin-deficient, carnitine-deficient; BD CS, biotin-deficient, carnitine-sufficient; Mc, malonylcarnitine; MGc, 3-methylglutarylcarnitine; MMc, methylmalonylcarnitine; Pc, propionylcarnitine; 3HIAc, 3-hydroxyisovalerylcarnitine.
With carnitine repletion, extracellular changes in acylcarnitines mirrored intracellular changes; extracellular free carnitine concentrations increased from 21.4 ± 2.31 nmol/L at day 13 to 4950 ± 262 nmol/L (P < 0.0001). Extracellular concentrations of substrate-derived acylcarnitines increased substantially (Supplemental Figure 5A). Extracellular concentrations of product-derived acylcarnitines also increased (Supplemental Figure 5B). Furthermore, the substrate:product ratios for the measurable acylcarnitines increased substantially (Supplemental Figure 5C).
Discussion
Accurately diagnosing biotin deficiency is important because substantial evidence indicates that marginal biotin deficiency is common during pregnancy. Biotin deficiency develops during the first trimester (3) and was shown to be teratogenic in some mammals (3, 22). Consequently there is legitimate concern that biotin deficiency is a human teratogen. Although increased urinary 3HIA and 3HIAc are both valid indicators of marginal biotin deficiency (6, 23, 24), studies suggest that the increase in 3HIAc is not a useful indicator of biotin status during pregnancy (5). We hypothesized the following mechanism for the lack of validity for 3HIAc in pregnancy: the increase of 3HIAc (but not that of 3HIA) is attenuated or prevented entirely by coexisting carnitine deficiency.
Plasma concentrations of free carnitine are reduced in pregnancy, and this observation supports the possibility that carnitine can become limiting during pregnancy (7–9, 15, 25). However, carnitine depletion is organ specific; the liver is more susceptible to carnitine depletion than skeletal muscle, which is the primary carnitine reservoir (26, 27). Because plasma carnitine concentrations reflect hepatic biotin status, we inferred that carnitine-dependent hepatic processes such as FA transport and control of mitochondrial acyl-CoA concentrations might be compromised. However, the health implications are not yet clear because adverse clinical effects of putative carnitine depletion have not yet been demonstrated.
To our knowledge, no studies have reported the independent and combined effects of biotin and carnitine deficiency. To avoid the species-related applicability issues that arise when using a rodent model for in vivo experiments and to achieve well-controlled conditions for a specific degree of the depletion of each nutrient, we chose cell culture as the model for testing our hypotheses and for investigating the metabolic mechanisms that might underlie published observations in pregnancy. Given the organ-specific susceptibility to carnitine depletion, we chose HepG2 cells as a human cell line likely to become rapidly biotin and carnitine deficient (28, 29). In these cells, we investigated the mechanisms underlying our hypotheses: 1) carnitine deficiency disrupts an important cellular detoxification mechanism, 2) carnitine deficiency reduces the intracellular accumulation of 3HIAc and increases excretion of 3HIAc (but not intracellular 3HIA and excretion of 3HIA) that would have occurred with isolated biotin deficiency, and 3) carnitine deficiency thereby masks this important indicator of biotin deficiency. To further confirm the mechanism for the putative masking effect, we also repleted cells with carnitine.
Our study demonstrates that, in HepG2 cells, biotin deficiency leads to the accumulation of the acylcarnitines acetylcarnitine, propionylcarnitine, and 3HIAc, which arise from the cognate acyl-CoAs that are substrates for the biotin-dependent carboxylases (Supplemental Figure 1). In addition, the 3 intracellular and extracellular acetylcarnitine:malonylcarnitine, propionylcarnitine:methylmalonylcarnitine, and 3HIAc:MGc ratios of the acylcarnitines arising from the acyl-CoAs that are the substrates and products of reactions catalyzed by the biotin-dependent carboxylases significantly increased, consistent with the development of a metabolic block, accumulation of the acyl-CoA substrate at the block, and detoxification of the acyl-CoA via the acylcarnitine pathway. The metabolic disturbances reported here are consistent with current understanding of the metabolic pathogenesis of biotin deficiency (10, 30, 31) and of isolated genetic deficiency of individual or multiple biotin-dependent carboxylases. The metabolic disturbances reported here are also consistent with studies of isolated biotin deficiency in rats; Shigematsu et al. (32) reported increases in specific acylcarnitines including propionylcarnitine and 3HIAc in plasma, liver, and urine of biotin-deficient rats. These authors even demonstrated that biotin-deficient rats develop a secondary carnitine deficiency due to carnitine loss as urinary acylcarnitines.
This study contributes novel information with regard to the duration of culture in a carnitine-deficient medium needed to induce carnitine deficiency. The observed 6-d period contrasts strikingly with data from in vivo models; in rats, 6 wk of a carnitine-deficient diet are required for ∼50% depletion of carnitine in serum, muscle, and liver (33). Even when a carnitine-deficient diet is combined with inhibition of carnitine biosynthesis, 3 wk are still required to induce carnitine deficiency in rats (34). In contrast to these in vivo observations, the current study in HepG2 cells demonstrates that removal of carnitine from the culture medium results in a dramatic decrease in intracellular content of free carnitine and each of the acylcarnitines we measured in only 6 d; concentrations of these intermediates decreased to between 1% and 54% of those in control HepG2 cells. Accordingly, a 6-d time interval was chosen for carnitine depletion.
The concept of measuring specific acylcarnitines to detect metabolic disturbances in the mitochondrial CoA detoxification process is not novel. In inborn errors of both amino acid and FA metabolism, this detoxification pathway generates a demand for carnitine that can exceed dietary intake and endogenous synthesis, leading to secondary carnitine deficiency (28, 29, 35); clinical benefit from carnitine supplementation has been reported and was attributed to amelioration of the accumulation of acyl-CoAs or their metabolites that are toxic (1, 36). The novelty of the current study arises from the investigation of separate and combined deficiencies of biotin and carnitine, complemented by carnitine repletion studies in both BDCD cells and in BDCS cells. The determination of substrate:product ratios of the related acylcarnitines across reactions catalyzed by at least 3 biotin-dependent carboxylases also permitted testing of the novel hypothesis that the functional consequences of marginal biotin deficiency could be detected despite differences in the carnitine status of HepG2 cells.
Our study confirmed the hypothesis that, in HepG2 cells, carnitine deficiency masks biotin deficiency and prevents an increase in intracellular and extracellular substrate-derived acylcarnitines, including 3HIAc. Our observations in this cell culture model also support our hypothesis that an increase in 3HIAc caused by biotin deficiency during pregnancy is attenuated or prevented entirely by coexisting carnitine deficiency. The ratios of the acylcarnitines partially unmasked the biotin deficiency despite coexisting carnitine deficiency. Accordingly, we speculate that the ratios may prove to be more sensitive indicators of marginal biotin deficiency during pregnancy than urinary 3HIAc.
The hypothesis of a masking effect was further confirmed by the metabolic response to carnitine repletion. Carnitine repletion was effective based on restoration of the intracellular content of free carnitine to control concentrations by 5 d; carnitine repletion unmasked the coexisting biotin deficiency as shown by striking increases in intracellular and extracellular substrate-derived acylcarnitines and substrate:product ratios.
This study has limitations inherent to most cell culture models of in vivo metabolism. Intracellular increases in these acylcarnitines were mirrored extracellularly. However, acylcarnitine concentrations in extracellular media may not accurately predict absolute or even relative concentrations of acylcarnitines and free carnitine in plasma or the excretion rates of these metabolites in urine. Notwithstanding, data from this study support an important proof-of-principle that carnitine depletion can directly affect extracellular concentrations of several indicators of biotin deficiency. Furthermore, the observations presented here are consistent with 2 in vivo observations: 1) in biotin-deficient rats, substantial increases in hepatic acylcarnitines including propionylcarnitine and 3HIAc are mirrored in plasma (32), and 2) the surprising lack of 3HIAc excretion to identify biotin deficiency in pregnancy (5).
An additional limitation arises from the severity of the carnitine deficiency induced in these HepG2 cells. Relative to cells cultured in carnitine-supplemented media, intracellular and extracellular free carnitine and acylcarnitines were decreased to a greater extent than the plasma concentrations reported in pregnancy (7, 9, 15). Accordingly, confidence in our speculation concerning the diagnostic potential of the ratios (relative to urinary 3HIAc) remains to be evaluated in animal models of the separate and combined deficiencies and in pregnancy.
In conclusion, a reduction in hepatic carnitine leads to impaired carnitine transesterification of substrate-derived acyl-CoAs in the mitochondrion and prevents an increase in intracellular substrate-derived acylcarnitines, including 3HIAc. Thus, even though biotin is deficient, this indicator of biotin deficiency is masked. This cell culture study provides proof-of-principle concerning this central metabolic hypothesis. We infer that in populations who might be carnitine deficient, carnitine status of the participants should be assessed to prevent false negatives if plasma concentrations of acylcarnitines or urinary excretion of acylcarnitines are used to assess biotin status. The value of substrate:product acylcarnitine ratios in detecting biotin deficiency in the presence of mild carnitine deficiency remains to be determined. Furthermore, the possibility that this cellular defense mechanism is impaired in some pregnant women should be further explored, given the apparent safety and low cost of carnitine supplementation.
Supplementary Material
Acknowledgments
We thank Joel Bradley, Ron Trolard, and Rosemarie Bachand of Cambridge Isotope Laboratories for providing the 3HIA-carnitine and D3-3HIA-carnitine standards used in the performance of the work described herein. We also thank Horace J Spencer for statistical analysis. AB and DMM designed the research and wrote the manuscript; AB conducted the research, analyzed data, and prepared the figures; GB helped with early work on the development of the method for quantitation of the 3 ratios (propionylcarnitine:methylmalonylcarnitine, 3HIAc:MGc, and acetylcarnitine:malonylcarnitine) and provided equipment for analyses; and DMM is responsible for the final version of the manuscript. All authors participated in review and revision of the manuscript and approved the final manuscript.
Footnotes
Abbreviations used: ACC, acetyl-CoA carboxylase; BDCD, biotin-deficient, carnitine-deficient; BDCS, biotin-deficient, carnitine-sufficient; BSCD, biotin-sufficient, carnitine-deficient; BSCS, biotin-sufficient, carnitine-sufficient; MCC, methylcrotonyl-CoA carboxylase; MGc, 3-methylglutarylcarnitine; PCC, propionyl-CoA carboxylase; 3HIA, 3-hydroxyisovaleric acid; 3HIAc, 3-hydroxyisovalerylcarnitine.
References
- 1.Mock DM, Matthews NI. Biotin and pantothenic acid. In: Stipanuk MH, Caudill MA, editors. Biochemical, physiological and molecular aspects of human nutrition: 3rd ed. St. Louis (MO): Saunders/Elsevier; 2012. [Google Scholar]
- 2.Mock DM, Mock NI, Stewart CW, LaBorde JB, Hansen DK. Marginal biotin deficiency is teratogenic in ICR mice. J Nutr 2003;133:2519–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mock DM. Marginal biotin deficiency is common in normal human pregnancy and is highly teratogenic in the mouse. J Nutr 2009;139:154–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Said HM. Biotin bioavailability and estimated average requirement: why bother? Am J Clin Nutr 1999;69:352–3. [DOI] [PubMed] [Google Scholar]
- 5. Perry CW, West AA, Gayle AA, Lucas LK, Yan J, Jiang X, Malysheva O, Caudill MA. Pregnancy and lactation alter biomarkers of biotin metabolism in women consuming a controlled diet. J Nutr 2014;144:1977–84. [DOI] [PMC free article] [PubMed]
- 6.Stratton SL, Horvath TD, Bogusiewicz A, Matthews NI, Henrich CL, Spencer HJ, Moran JH, Mock DM. Urinary excretion of 3-hydroxyisovaleryl carnitine is an early and sensitive indicator of marginal biotin deficiency in humans. J Nutr 2011;141:353–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cho S, Cha Y. Pregnancy increases urinary loss of carnitine and reduces plasma carnitine in Korean women. Br J Nutr 2005;93(5):685–91. [DOI] [PubMed]
- 8.Keller U, van der Wal C, Seliger G, Scheler C, Ropke F, Eder K. Carnitine status of pregnant women: effect of carnitine supplementation and correlation between iron status and plasma carnitine concentration. Eur J Clin Nutr 2009;63:1098–105. [DOI] [PubMed] [Google Scholar]
- 9.Ringseis R, Hanisch N, Seliger G, Eder K. Low availability of carnitine precursors as a possible reason for the diminished plasma carnitine concentrations in pregnant women. BMC Pregnancy Childbirth 2010;10:10–7. [DOI] [PMC free article] [PubMed]
- 10.Bogusiewicz A, Horvath TD, Stratton SL, Mock DM, Boysen G. Measurement of acylcarnitine substrate to product ratios specific to biotin-dependent carboxylases offers a combination of indicators of biotin status in humans. J Nutr 2012;142:1621–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Arafa HM. Carnitine deficiency: a possible risk factor in paracetamol hepatotoxicity. Arch Toxicol 2009;83:139–50. [DOI] [PubMed] [Google Scholar]
- 12.Al-Majed AA, Sayed-Ahmed MM, Al-Yahya AA, Aleisa AM, Al-Rejaie SS, Al-Shabanah OA. Propionyl-L-carnitine prevents the progression of cisplatin-induced cardiomyopathy in a carnitine-depleted rat model. Pharmacol Res 2006;53(3):278–86. [DOI] [PubMed]
- 13.Spaniol M, Kaufmann P, Beier K, Wuthrich J, Torok M, Scharnagl H, Marz W, Krahenbuhl S. Mechanisms of liver steatosis in rats with systemic carnitine deficiency due to treatment with trimethylhydraziniumpropionate. J Lipid Res 2003;44:144–53. [DOI] [PubMed] [Google Scholar]
- 14.Zaugg CE, Spaniol M, Kaufmann P, Bellahcene M, Barbosa V, Tolnay M, Buser PT, Krahenbuhl S. Myocardial function and energy metabolism in carnitine-deficient rats. Cell Mol Life Sci 2003;60:767–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cederblad G, Fahraeus L, Lindgren K. Plasma carnitine and renal-carnitine clearance during pregnancy. Am J Clin Nutr 1986;44:379–83. [DOI] [PubMed] [Google Scholar]
- 16.Solórzano-Vargas RS, Pacheco-Alvarez D, Leon-Del-Rio A. Holocarboxylase synthetase is an obligate participant in biotin-mediated regulation of its own expression and of biotin-dependent carboxylases mRNA levels in human cells. Proc Natl Acad Sci USA 2002;99:5325–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mall GK, Chew YC, Zempleni J. Biotin requirements are lower in human Jurkat lymphoid cells but homeostatic mechanisms are similar to those of HepG2 liver cells. J Nutr 2010;140:1086–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sealey WM, Stratton SL, Mock DM, Hansen DK. Marginal maternal biotin deficiency in CD-1 mice reduces fetal mass of biotin-dependent carboxylases. J Nutr 2005;135:973–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mock DM. Determinations of biotin in biological fluids. In: McCormick DB, Suttie JW, Wagner C, editors. Methods in enzymology. New York: Academic Press; 1997. p. 265–75. [DOI] [PubMed] [Google Scholar]
- 20.Wikoff WR, Anfora AT, Liu J, Schultz PG, Lesley SA, Peters EC, Siuzdak G. Metabolomics analysis reveals large effects of gut microflora on mammalian blood metabolites. Proc Natl Acad Sci USA 2009;106:3698–703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jones DR, Boysen G, Miller GP. Novel multi-mode ultra performance liquid chromatography-tandem mass spectrometry assay for profiling enantiomeric hydroxywarfarins and warfarin in human plasma. J Chromatogr B Analyt Technol Biomed Life Sci 2011;879:1056–62. [DOI] [PubMed] [Google Scholar]
- 22.Zempleni J, Mock D. Marginal biotin deficiency is teratogenic. Proc Soc Exp Biol Med 2000;223:14–21. [DOI] [PubMed] [Google Scholar]
- 23.Mock NI, Malik MI, Stumbo PJ, Bishop WP, Mock DM. Increased urinary excretion of 3-hydroxyisovaleric acid and decreased urinary excretion of biotin are sensitive early indicators of decreased status in experimental biotin deficiency. Am J Clin Nutr 1997;65:951–8. [DOI] [PubMed] [Google Scholar]
- 24.Mock DM, Henrich CL, Carnell N, Mock NI. Indicators of marginal biotin deficiency and repletion in humans: validation of 3-hydroxyisovaleric acid excretion and a leucine challenge. Am J Clin Nutr 2002;76:1061–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Talián GC, Komlosi K, Decsi T, Koletzko B, Melegh B. Determination of carnitine ester patterns during the second half of pregnancy, at delivery, and in neonatal cord blood by tandem mass spectrometry: complex and dynamic involvement of carnitine in the intermediary metabolism. Pediatr Res 2007;62:88–92. [DOI] [PubMed] [Google Scholar]
- 26.Rebouche CJ. Carnitine function and requirements during the life cycle. FASEB J 1992;6(15):3379–86. [PubMed]
- 27.Evans AM, Fornasini G. Pharmacokinetics of L-carnitine. Clin Pharmacokinet 2003;42:941–67. [DOI] [PubMed] [Google Scholar]
- 28.Zammit VA, Ramsay RR, Bonomini M, Arduini A. Carnitine, mitochondrial function and therapy. Adv Drug Deliv Rev 2009;61:1353–62. [DOI] [PubMed] [Google Scholar]
- 29.Rebouche CJ, Engel AG. Kinetic compartmental analysis of carnitine metabolism in the human carnitine deficiency syndromes: evidence for alterations in tissue carnitine transport. J Clin Invest 1984;73:857–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Horvath TD, Stratton SL, Bogusiewicz A, Pack L, Moran J, Mock DM. Quantitative measurement of plasma 3-hydroxyisovaleryl carnitine by LC-MS/MS as a novel biomarker of biotin status in humans. Anal Chem 2010;82:4140–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mock DM, Stratton S, Horvath T, Bogusiewicz A, Matthews N, Henrich CL, Dawson AM, Spencer HJ, Owen SN, Boysen G, et al. Urinary excretion of 3-hydroxyisovaleric acid and 3-hydroxyisovaleryl carnitine increases in response to a leucine challenge in marginally biotin-deficient humans. J Nutr 2011;141:1925–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shigematsu Y, Bykov IL, Liu YY, Nakai A, Kikawa Y, Sudo M, Fujioka M. Acylcarnitine profile in tissues and body fluids of biotin-deficient rats with and without L-carnitine supplementation. J Inherit Metab Dis 1994;17:678–90. [DOI] [PubMed] [Google Scholar]
- 33.Heinonen OJ, Takala J. Moderate carnitine depletion and long-chain fatty acid oxidation, exercise capacity, and nitrogen balance in the rat. Pediatr Res 1994;36:288–92. [DOI] [PubMed] [Google Scholar]
- 34.Spaniol M, Brooks H, Auer L, Zimmermann A, Solioz M, Stieger B, Krahenbuhl S. Development and characterization of an animal model of carnitine deficiency. Eur J Biochem 2001;268(6):1876–87. [PubMed]
- 35.Chalmers RA, Roe CR, Stacey TE, Hoppel CL. Urinary excretion of l-carnitine and acylcarnitines by patients with disorders of organic acid metabolism: evidence for secondary insufficiency of l-carnitine. Pediatr Res 1984;18:1325–8. [DOI] [PubMed] [Google Scholar]
- 36.Matsuishi T, Stumpf DA, Seliem M, Eguren LA, Chrislip K. Propionate mitochondrial toxicity in liver and skeletal muscle: acyl CoA levels. Biochem Med Metab Biol 1991;45:244–53. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.