Abstract
Background: Human milk oligosaccharides (HMOs) have multiple immunomodulatory functions that influence child health.
Objective: In this study we investigated whether HMO composition influences survival to 2 y of age in HIV-infected and HIV-exposed, uninfected (HEU) children during and after breastfeeding.
Methods: In the context of an early weaning trial in 958 HIV-infected women in Lusaka, Zambia, we conducted a nested case-cohort analysis of mortality to 2 y of age among 103 HIV-infected and 143 HEU children. Breast-milk samples collected at 1 mo postpartum were analyzed for HMO content. Samples were selected to include mothers of all HIV-infected children detected by 6 wk of age, of whom 63 died at <2 y of age; mothers of all HEU children who died at <2 y of age (n = 66); and a random sample of 77 HEU survivors. Associations before and after weaning in HIV-infected and HEU infants separately were investigated by using Cox models.
Results: Among HEU children, higher maternal breast-milk concentrations of 2-linked fucosylated HMOs [2′-fucosyllactose and lacto-N-fucopentaose (LNFP) I] (HR: 0.33; 95% CI: 0.14, 0.74) as well as non–2-linked fucosylated HMOs (3-fucosyllactose and LNFP II/III; HR: 0.28; 95% CI: 0.13, 0.67) were significantly associated with reduced mortality during, but not after, breastfeeding after adjustment for confounders. Breastfeeding was protective against mortality only in HEU children with high concentrations of fucosylated HMOs. Among HIV-infected children, no consistent associations between HMOs and mortality were observed, but breastfeeding was protective against mortality.
Conclusions: The oligosaccharide composition of breast milk may explain some of the benefits of breastfeeding in HEU children. HIV infection may modulate some of the consequences of HMOs on child survival.
Keywords: mother-to-child HIV transmission, breastfeeding, human milk oligosaccharides, child survival, HIV-exposed uninfected
Introduction
In the absence of effective antiretroviral interventions, ∼20% of children born to HIV-infected mothers will acquire infection during the intrauterine or intrapartum period and an additional 15% will acquire infection during the postnatal period if breastfed for a long duration (1). The prognosis of intrauterine/intrapartum-infected children is poor; although survival after postnatally acquired infection is slightly better (2, 3). Although their survival is considerably more favorable than HIV-infected children, HIV-exposed uninfected (HEU)8 children (i.e., those children born to HIV-infected mothers who escape HIV infection) have higher mortality rates than children born to uninfected mothers in the same communities (4, 5). The increased risk of death in HEU children is exacerbated when their mothers have more advanced disease, but the association is not explained by maternal mortality (6, 7). Whether these risks in HEU children will be mitigated by maternal antiretroviral therapy remains unknown. Likewise, the biological basis of this phenomenon is also unknown (5).
We observed in the context of our clinical trial of early weaning in Lusaka, Zambia, that associations between more advanced maternal HIV disease, including low maternal CD4 counts, and higher HEU mortality were confined to breastfed children (8). One of the serious risks to the survival of HEU children is early weaning—a practice encouraged for some time to reduce the risks of postnatal HIV transmission (9). If associations between maternal status and HEU mortality have no true biological basis, then correlations between these 2 variables should be detectable regardless of infant feeding practice. Our data showing stronger effects of maternal status on HEU outcomes if breastfed led us to hypothesize that characteristics of breast milk may serve as the biological basis of some of the adverse consequences for HEU children who have a mother with more advanced disease.
One mucosal variable that may play a role in HEU mortality is human milk oligosaccharides (HMOs). HMOs are intriguing because they are the third most abundant component of breast milk and reach the mucosal surfaces of the infant’s gastrointestinal tract at high concentrations (10). HMOs act as prebiotics to promote the growth of desired bacterial communities in the infant gut as well as having several other immunomodulatory effects (10). We previously reported an association between higher overall HMO concentration and reduced risk of postnatal transmission via breastfeeding (11). We also reported that the proportion of 3′-sialyllactose (3′SL) per total HMOs was higher among transmitting than among nontransmitting women (11). A higher 3′SL concentration also correlated with higher plasma and breast-milk HIV RNA and lower maternal CD4 counts (11).
Here we investigated the role of HMOs on mortality by 2 y of age in children born to HIV-infected mothers. We distinguished between effects on HIV-infected vs. HEU children and considered possible consequences of HMO composition on child mortality both during and after breastfeeding cessation.
Methods
Study design.
We conducted a nested case-cohort analysis of mortality in 103 HIV-infected and 143 HEU children recruited during an early weaning trial in HIV-infected women and their children in Lusaka, Zambia (12). For the early weaning trial, 958 HIV-infected women were recruited during pregnancy at 2 antenatal clinics between May 2001 and September 2004 and followed to delivery and through 2 y postpartum with their children. Half of the women were randomly assigned to stop all breastfeeding at 4 mo and the other half to continue to breastfeed for a duration of their own choice. Randomization created a cohort with greater heterogeneity in duration of breastfeeding than found in usual observational studies, allowing for analyses stratified by breastfeeding status. Only single-dose nevirapine was given as a prevention of mother-to-child transmission intervention. Antiretroviral therapy only became available once the cohort was almost entirely enrolled. Written informed consent was obtained from all women. The study was approved by the institutional review boards of the participating investigators.
To establish HIV status in the children, heel-stick blood samples were collected from all children at birth, at 1 wk, monthly to age 6 mo, and every 3 mo to 24 mo of age and tested for HIV DNA by PCR. Maternal blood was collected at enrollment during pregnancy and tested for CD4 and CD8 cell counts (FACSCount system; BD Immunocytometry Systems), hemoglobin (HemoCue system) and plasma viral load (Roche Amplicor 1.5). Details of infant feeding practices were collected at each visit. Causes of all deaths were ascertained via verbal autopsy and review of medical records. For all child deaths, breastfeeding patterns up to the death were reviewed. If breastfeeding had only ended because of a preceding illness, the death was classified as occurring while breastfeeding. Only if breastfeeding had ended independent of events preceding the death (e.g., compliance with the early weaning protocol) was the death classified as occurring after weaning.
Selection of subjects.
For the HIV-infected cohort, we included 103 of 117 HIV-infected children identified in the parent trial with positive PCR tests by 42 d of age [43 with presumed intrauterine infection (positive PCR <3 d of birth) and 59 with presumed intrapartum/early postnatal infection (positive PCR at 3–42 d)]. The 143 HEU children were selected by using a case-cohort design. The HEU group included all HEU children who died before 2 y of age whose mothers had the necessary samples in the repository (cases) and 86 randomly selected HEU children chosen by using incidence-density sampling (11) for comparison. We also included breast milk from 36 HIV uninfected women who were recruited and followed on the same protocol at the same sites over the same time period.
Laboratory measurements.
We selected for this analysis breast milk that had been collected at 1 mo postpartum in order to have a consistent time point for predicting outcomes. Breast milk was manually expressed. Samples were centrifuged, and the lipid and aqueous fractions stored at −70°C before testing. HMOs were analyzed by using HPLC as previously described (11, 13, 14). The total concentration of HMOs was calculated as the sum of the specific oligosaccharides detected. The following HMOs were detected: 2′-fucosyllactose (2′FL), 3-fucosyllactose (3FL), 3′SL, lacto-N-tetraose (LNT), lacto-N-neotetraose (LNnT), lacto-N-fucopentaose (LNFP) I, LNFP II, and LNFP III. The latter 2 could not be distinguished in most samples and were reported out as the sum of LNFP II and LNFP III. The proportion of each HMO per total HMO concentration was calculated. Secretor status was defined by the presence of 2′FL or LNFP I. Lewis positive status was defined by the presence of LNFP II.
Statistical methods.
HMO distributions were compared across groups, and a principal components analysis was conducted to identify the main dimensions of HMO composition. Wilcoxon tests were used to compare the distribution of HMOs among groups. An α level of 0.05 was considered statistically significant. Associations between 2 continuous variables were described with Spearman rank order correlation coefficients. Comparisons between groups in categorical variables were performed by using chi-square tests.
HMO concentrations were categorized as being either above or below the median in the HEU group. Cox proportional hazards models of mortality were conducted in HIV-infected and HEU infants separately. Breastfeeding duration was included as a time-dependent covariate with an interaction term to define HMO main effects during or after breastfeeding. Multivariable models included all covariates that, when included together, were associated with mortality (P < 0.05). Interaction terms were included if P < 0.05. For consistency, the interaction term between maternal CD4 count and breastfeeding was retained based on a significant interaction in the whole data set (P = 0.03) (8). HRs and 95% CIs were calculated. All statistical analyses were performed by using SAS (version 9.4).
Results
Two-year mortality.
Of 103 HIV-infected children surviving to 1 mo and included in this analysis, the probability of death by 1 y was 0.486 (n = 47) and by 2 y was 0.669 (n = 63). Among all HEU children in the trial (n = 748), mortality by 1 y was 0.098 (n = 65) and by 2 y was 0.140 (n = 91). Of the 66 HEU children who died before 2 y of age included in this analysis, 44 died before 1 y of age. Clinical and social characteristics are displayed in Table 1.
TABLE 1.
Child, maternal, and social characteristics of 103 HIV-infected and 143 HIV-exposed, uninfected children from Lusaka, Zambia, with HMO measurements1
| HIV-infected children |
HIV-exposed, uninfected children |
|||||
| Survived (n = 40) | Died (n = 63) | P | Survived (n = 77) | Died (n = 66) | P | |
| Child characteristics | ||||||
| Male sex, n (%) | 13 (32.5) | 39 (61.9) | 0.004 | 37 (48.1) | 33 (50.0) | >0.10 |
| Timing of infection, n (%) | ||||||
| Intrauterine | 18 (45.0) | 25 (39.7) | ||||
| Intrapartum/early postpartum | 22 (55.0) | 38 (60.3) | >0.10 | |||
| Child age at death, mo | — | 8.1 (4.6–12.4)2 | — | — | 9.5 (5.6–13.4) | |
| Birth weight <2500 g, n (%) | 3 (7.5) | 10 (16.1) | >0.10 | 7 (9.2) | 10 (15.4) | >0.10 |
| Maternal characteristics | ||||||
| Maternal death, n (%) | 1 (2.5) | 13 (20.6) | 0.009 | 1 (1.3) | 9 (13.6) | 0.004 |
| Maternal age, y | 25.0 (23.5–28.0) | 26.0 (22–30) | >0.10 | 25.0 (22–29) | 26.5 (23–29) | >0.10 |
| CD4 count <350 cells/μL, n (%) | 26 (65.0) | 51 (81.0) | 0.07 | 35 (45.5) | 39 (59.1) | 0.10 |
| HIV RNA >100,000 copies/mL, n (%) | 22 (55.0) | 40 (63.5) | >0.10 | 16 (20.8) | 19 (28.8) | >0.10 |
| Symptomatic, n (%) | 16 (40.0) | 40 (63.5) | 0.02 | 33 (42.9) | 25 (37.9) | >0.10 |
| Social characteristics, n (%) | ||||||
| Food insecurity | 5 (12.5) | 16 (25.4) | >0.10 | 18 (23.4) | 23 (34.9) | >0.10 |
| More than 2 children aged <5 y in household | 4 (10.0) | 12 (19.1) | >0.10 | 8 (10.4) | 16 (24.2) | 0.03 |
HMO, human milk oligosaccharide.
Median; 25th–75th percentiles in parentheses (all such values).
Breastfeeding cessation and maternal death were associated with increased risk of death among both HIV-infected and HEU children. In HIV-infected children, symptomatic maternal disease and being male were associated with child mortality. In HEU children, low maternal CD4 counts and having >2 other children <5 y of age in the household also increased the risk of mortality. Among HEU children, low maternal CD4 counts had a stronger association with mortality among breastfed children (Table 2).
TABLE 2.
Risk factors for mortality in 103 HIV-infected and 143 HIV-exposed, uninfected children from Lusaka, Zambia1
| HIV-infected children | HIV-exposed, uninfected children | |
| Female vs. male | 0.52 (0.31, 0.89) | — |
| Breastfeeding cessation | 2.18 (1.24, 3.83) | 2.48 (1.08, 5.70)2 |
| Maternal CD4 <350 cells/μL | — | 1.99 (0.88, 4.51)2 |
| Maternal death | 2.73 (1.37, 5.47) | 2.25 (1.04, 4.86) |
| Mother symptomatic | 2.30 (1.32, 4.01) | |
| More than 2 other children aged <5 y in the household | — | 2.06 (1.14, 3.72) |
Values are HRs (95% CIs) derived by using a multivariable Cox proportional hazards model adjusting simultaneously for the covariates shown.
The model includes an interaction term between maternal CD4 and breastfeeding cessation. The HR shown for maternal CD4 <350 cells/μL refers to the association during breastfeeding. The HR shown for breastfeeding cessation refers to the association between infants whose mothers had CD4 >350 cells/μL.
Profile of HMOs.
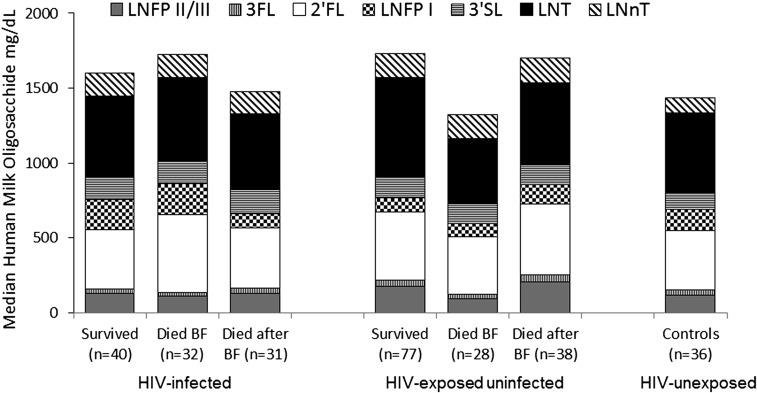
A principal components analysis identified 2 factors: one that was most strongly loaded by 3′SL, LNT, and LNnT (factor loadings of 0.47, 0.39, and 0.42, respectively) and the second that was positively loaded by 2′FL and LNFP I (loadings of 0.52 and 0.46, respectively) and negatively loaded by 3FL and LNFP II/III (factor loadings of −0.53 and −0.45, respectively). There were significantly lower concentrations of 3′SL in controls (median: 114 mg/L; IQR: 78–160 mg/L) than in HIV-infected women (median: 141 mg/L; IQR: 101–199 mg/L) (P = 0.006). There were also significantly lower concentrations of LNnT in controls (median: 99 mg/L; IQR: 67–168 mg/L) than in HIV-infected women (median: 155 mg/L; IQR: 92–221 mg/L) (P = 0.02) (Figure 1). Among HIV-infected women, lower CD4 counts were associated with a higher percentage of 3′SL per total HMOs (coefficient = −0.18, P = 0.005). There were no other correlations between HMOs and maternal CD4 count or viral load.
FIGURE 1.
Median concentrations of each oligosaccharide in breast milk from HIV-infected women with HIV-infected or uninfected children stratified by whether their children survived, died during breastfeeding, or died after breastfeeding had ended and from HIV-uninfected women. There were significantly lower concentrations of 3′SL and LNnT in HIV-uninfected controls than in HIV-infected women (P = 0.006 and 0.02, respectively). The groups shown (from left to right) are mothers of 1) HIV-infected children who survived to 2 y of age, 2) HIV-infected children who died while breastfeeding before 2 y of age, 3) HIV-infected children who died after weaning before 2 y of age, 4) HIV-exposed uninfected children who survived to 2 y of age, 5) HIV-exposed uninfected children who died while breastfeeding before 2 y of age, 6) HIV-exposed uninfected children who died after weaning before 2 y of age, and 7) HIV-unexposed, uninfected children (mother HIV-uninfected). BF, breastfeeding; LNFP, lacto-N-fucopentaose; LNnT, lacto-N-neotetraose; LNT, lacto-N-tetraose; 2′FL, 2′-fucosyllactose; 3FL, 3-fucosyllactose; 3′SL, 3′-sialyllactose.
Among HIV-infected children, there were no significant differences in HMO concentrations by survival status. Among the HEU children, concentrations of LNFP II/III tended to be lower (P = 0.05) among the mothers of HEU children who died while breastfeeding (median: 96 mg/L; IQR: 58–209 mg/L) than among mothers of survivors (median: 177 mg/L; IQR: 99–280 mg/L). Concentrations of LNT were significantly lower (P = 0.047) in the mothers of HEU children who died during breastfeeding (median: 442 mg/L; IQR: 336–742 mg/L) than among mothers of survivors (median: 663 mg/L; IQR: 400–1068 mg/L) (Figure 1).
HMOs and mortality.
In HIV-infected children, there were no consistent associations between HMOs and mortality during breastfeeding. In HEU children, LNFP II/III and non–2-linked fucosylated HMOs (i.e., 3FL + LNFP II/III combined) were associated with reduced mortality during breastfeeding. In contrast, there were no associations between HMOs and HEU mortality after breastfeeding (Table 3).
TABLE 3.
Associations between maternal breast-milk oligosaccharide composition and child mortality by 2 y of age stratified by child HIV and breastfeeding status1
| HIV-infected children |
HIV-exposed, uninfected children |
|||
| During breastfeeding | After breastfeeding | During breastfeeding | After breastfeeding | |
| Total HMOs ≥1.8 g/L | 1.07 (0.53, 2.14) | 0.66 (0.32, 1.34) | 0.53 (0.25, 1.12) | 1.02 (0.54, 1.94) |
| LNFP II/III >167 mg/L | 0.70 (0.32, 1.52) | 1.15 (0.56, 2.37) | 0.41 (0.19, 0.91) | 1.19 (0.63, 2.26) |
| 3FL >41 mg/L | 1.02 (0.50, 2.10) | 1.40 (0.67, 2.90) | 0.53 (0.25, 1.16) | 1.23 (0.64, 2.34) |
| Non–2-linked fucosylated HMOs (LNFP II/III + 3FL) >200 mg/L | 0.64 (0.29, 1.37) | 1.22 (0.60, 2.51) | 0.33 (0.15, 0.76) | 1.18 (0.62, 2.24) |
| 2′FL >419 mg/L | 1.88 (0.92, 3.85) | 0.74 (0.36, 1.50) | 0.62 (0.29, 1.32) | 1.05 (0.55, 1.98) |
| LNFP I >117 mg/L | 1.36 (0.64, 2.87) | 0.46 (0.22, 0.93) | 0.74 (0.35, 1.56) | 1.33 (0.70, 2.53) |
| 2′FL + LNFP I >550 mg/L | 1.62 (0.77, 3.42) | 0.67 (0.33, 1.37) | 0.59 (0.28, 1.24) | 1.30 (0.68, 2.46) |
| Secretor positive | 2.41 (0.33, 17.71) | 1.70 (0.23, 12.63) | 0.65 (0.27, 1.61) | 0.90 (0.32, 2.55) |
| LNT >585 mg/L | 0.85 (0.42, 1.72) | 0.96 (0.47, 1.97) | 0.50 (0.23, 1.09) | 0.65 (0.35, 1.24) |
| LNnT >157 mg/L | 1.07 (0.53, 2.14) | 0.57 (0.27, 1.20) | 0.74 (0.35, 1.57) | 1.07 (0.56, 2.02) |
| 3′SL >138 mg/L | 1.24 (0.61, 2.52) | 0.67 (0.33, 1.39) | 0.53 (0.25, 1.13) | 0.98 (0.52, 1.85) |
| 3′SL% of total HMOs >7.1% | 1.37 (0.65, 2.91) | 1.11 (0.51, 2.42) | 1.26 (0.60, 2.65) | 1.23 (0.65, 2.33) |
| 3′SL + LNnT + LNT >921 mg/L | 0.66 (0.32, 1.37) | 0.75 (0.36, 1.56) | 0.69 (0.33, 1.46) | 0.71 (0.37, 1.34) |
| Lewis-antigen positive | 0.79 (0.28, 2.26) | 1.73 (0.52, 5.71) | 0.83 (0.25, 2.77) | 1.22 (0.47, 3.13) |
Values are HRs (95% CIs). All HMO variables were classified as above or below the median concentration in the HIV-exposed, uninfected group. HMO, human milk oligosaccharide; LNFP, lacto-N-fucopentaose; LNnT, lacto-N-neotetraose; LNT, lacto-N-tetraose; 2′FL, 2′-fucosyllactose; 3FL, 3-fucosyllactose; 3′SL, 3′-sialyllactose.
In multivariable analysis, the best-fitting model for mortality in HEU infants is shown in Table 4. Higher concentrations of non–2-linked fucosylated HMOs (3FL + LNFP II/III) and higher concentrations of 2′FL + LNFP I were associated with reduced mortality during breastfeeding, adjusting for known confounders. The inclusion of 2′FL, rather than the combined category, produced similar results, but summing it with LNFP I (as suggested by the principal components analysis) produced slightly stronger associations. Neither of these variables was associated with mortality occurring after breastfeeding had ended regardless of adjustments. LNT had the strongest association if deaths during and after breastfeeding were not differentiated; hence, it was retained without an interaction term. Further adjustment for other markers of more advanced maternal disease (e.g., plasma viral load, hemoglobin, being symptomatic), low birth weight, or child sex did not improve the model.
TABLE 4.
Multivariable analysis of associations between breast-milk oligosaccharide composition and child mortality by 2 y of age stratified by child HIV and breastfeeding status1
| HIV-infected children | HIV-exposed, uninfected children | |
| Non–2-linked fucosylated HMOs (LNFP II/III + 3FL) >200 mg/L | 0.89 (0.38, 2.08) | 0.28 (0.13, 0.67)2 |
| 2′FL + LNFP I >550 mg/L | 1.44 (0.64, 3.21) | 0.33 (0.14, 0.74)3 |
| LNT >585 mg/L | 1.43 (0.77, 2.67) | 0.58 (0.34, 0.98) |
| Maternal CD4 <350 cells/μL | 2.66 (1.14, 6.22)4 | |
| Mother symptomatic | 2.42 (1.35, 4.35) | |
| Maternal death | 2.88 (1.42, 5.86) | 2.16 (0.97, 4.82) |
| More than 2 other children aged <5 y in household | 2.12 (1.12, 4.01) | |
| Female sex | 0.52 (0.30, 0.90) | |
| Stopping breastfeeding | 3.32 (1.18, 9.35) | 0.59 (0.19, 1.82) |
Values are HRs (95% CIs). All human milk oligosaccharide variables were classified as above or below the median concentration in the HIV-exposed, uninfected group. LNFP, lacto-N-fucopentaose; LNT, lacto-N-tetraose; 2′FL, 2′-fucosyllactose; 3FL, 3-fucosyllactose.
Included in the model with an interaction term with breastfeeding: P-interaction = 20.002, 30.004, and 40.14.
The comparable HMO effects in HIV-infected children, adjusted for factors associated with mortality in this group, are shown in Table 4. None of the HMO variables were associated with mortality, and adjustment for several potential confounders did not alter this. Of interest in HIV-infected children, breastfeeding cessation continued to confer a significantly increased risk of mortality, even after adjustment for HMOs. In contrast, breastfeeding was protective against mortality only in HEU children with higher concentrations of fucosylated HMOs.
Among 28 HEU deaths occurring during breastfeeding, 18 (64%) were attributed to pneumonia, 13 to diarrhea (7 had both), and 4 were because of other causes. Among 38 HEU deaths occurring after breastfeeding, 12 were attributed to pneumonia, 21 to diarrhea (4 children had both), and 9 were because of other causes. In those children whose mothers had low concentrations of non–2-linked fucosylated HMOs in breast milk, 15 of 20 cases were attributed to pneumonia compared with 3 of 8 children whose mothers had concentrations of non–2-linked fucosylated HMOs in breast milk above the median (P = 0.09, Fisher's exact test). Diarrhea was attributed as the cause of death in 9 of 20 and 4 of 8 children whose mothers had low and high non–2-linked fucosylated HMOs in breast milk, respectively.
Discussion
HMOs were first discovered in the 1950s as part of research to characterize the “bifidus factor” thought at the time to explain the benefits of breastfeeding for infant health (10, 15). This discovery fueled a productive period of inquiry that established HMOs as part of innate immune protection and as a substrate promoting growth of beneficial bacteria in the infant gut (10, 15). More recently, not only have the prebiotic benefits of HMOs been further elaborated (16) but other immune protective properties have been identified, including as antiadhesive antimicrobials, decoy molecules, and modulators of epithelial cell responses (10, 17).
Despite the richness of the experimental and biochemical research in this area, in vivo, clinical research linking the profile of HMOs to child health outcomes has been surprisingly limited. An important exception are studies conducted in Mexico that demonstrated significantly reduced diarrheal morbidity, especially due to Campylobacter jejuni, in infants whose mothers had high concentrations of 2-linked fucosylated oligosaccharides in breast milk, especially 2′FL (18, 19). Thus, our findings of significantly lower rates of mortality during breastfeeding for HEU infants with higher maternal concentrations of 2′FL and LNFP I is a confirmation of this previous observation. We also showed significantly lower rates of death during breastfeeding with higher concentrations of non–2-linked fucosylated HMOs, including 3FL and LNFP II/III.
Fucosylated HMOs were shown to block binding of C. jejuni in in vitro and mouse experiments (17–19). Deaths in HEU children were overwhelmingly because of infectious causes, although the specific pathogens are unknown. Deaths during breastfeeding, which were the deaths associated with fucosylated HMOs, were primarily because of respiratory illness. Given the likely diversity of pathogens, it seems most probable that the benefit of fucosylated HMOs may have been through a non–pathogen-specific mechanism. Like the clinical studies in Mexico, we observed HMO benefits during but not after breastfeeding, observations supporting the role of HMOs in inhibiting pathogen binding (17–19). Theoretically, HMOs may also have effects after weaning via their priming of the intestinal microbiota. Bifidobacterial counts were found to remain high after cessation of breastfeeding (20). For LNT, one of the core HMOs that is neither fucosylated nor sialylated, we detected a benefit that extended across both periods, suggesting that, if this proves to be a robust association, then the mechanisms responsible may involve longer lasting alterations in the intestinal microbiota.
We did not observe any benefit for HEU mortality of the concentration of 3′SL in mothers’ breast milk. In our previous analysis, a higher percentage of 3′SL per total HMOs was associated with increased risk of postnatal HIV transmission (11). Experimental studies identified sialic acid as important in brain development and sialylated HMOs, including 3′SL, are the major source of sialic acid in breast milk (10). Sialylated HMOs were shown to reduce neutrophil activation and leukocyte adhesion and rolling in in vitro models (21, 22). Our data suggest that it is the non-sialylated HMOs that are most relevant for protection against infectious disease mortality in this population.
The influence of HMOs on child mortality appeared to be further modulated by the child’s HIV status. In contrast to observations among HEU children, there were no consistent associations between HMOs and mortality in HIV-infected children. The interpretation that HIV-related disease progression simply overwhelmed and masked the benefits of HMOs may be too simplistic, because strong protective associations of breastfeeding were still observed among infected children. The benefit of breastfeeding for infected children has been shown in other sub-Saharan African populations and even among HIV-infected children in Europe (12, 23, 24). Immunologic mechanisms underlying the benefits of breastfeeding are undoubtedly multifactorial. We speculate that although HMO pathways may be disrupted in HIV-infected infants, other pathways may be unaffected, allowing breastfeeding benefits to persist.
The profile of HMOs differed between HIV-infected women and uninfected control women; specifically, nonfucosylated HMOs, including LNT and 3′SL, occurred in higher concentrations among HIV-infected women. The percentage of total HMOs due to 3′SL was higher in HIV-infected women with lower CD4 counts. Few environmental factors have been found to influence HMO composition, which is determined by genetic characteristics, namely Lewis and secretor status blood groups (10, 25). Nonetheless, preterm milk differs from term milk (26) and there appears to be some geographic variation. In our sub-Saharan African population, total HMO concentrations were lower in both infected and uninfected women relative to studies conducted in the United States and Europe, although the percentage due to specific types was similar and within-type correlations followed expected patterns (25). It is provocative that we observed increases in sialyated HMOs in HIV-infected relative to uninfected women and higher percentages of these in infected women with low CD4 counts, whereas it is the higher concentrations of nonsialyated, fucosylated HMOs that we found to be protective against mortality during breastfeeding in HEU children. Taken together, these findings raise the intriguing possibility that changes in the HMO profile of HIV-infected women may be part of the puzzle to explain the increased risks of morbidity and mortality observed in HEU children (5, 27).
Strengths of our study include the large cohort, which allowed us to select a sufficient number of child deaths as well as prospectively followed HIV-infected children for the case-cohort analysis. Consistent patterns of risk factors in the case-cohort study relative to the full cohort confirm that biases were not introduced in the selection of the samples for HMO analysis. The heterogeneity of feeding practices in the cohort due to the trial design and rigorous ascertainment of endpoints and confounders add further strengths. Limitations include a lack of information about specific pathogens responsible for deaths, a single measurement of HMOs because of cost, and the sample size, which was too small to have confidence in cause-specific analyses. Methodologies for measuring HMOs are not well standardized and although we used a well-validated and accepted method, we may have had limited sensitivity to capture the full diversity of HMO forms and could not make fine distinctions within some forms (e.g., between LNFPs II and III).
Antiretroviral interventions, if adequately implemented, now have the potential to reduce the risks of mother-to-child transmission to exceptionally low levels (28). Antiretroviral treatment can prevent morbidity and mortality almost entirely in HIV-infected children. Yet, no intervention specifically for HEU children, who are known to be at higher risk than the general population, has been shown to be effective. There is hope that antiretroviral drugs given to prevent transmission will have spillover benefits for this group, but this has not been demonstrated. Our data suggest the possibility that HMO-related interventions may be beneficial for HEU children. The use of manufactured HMOs is difficult given the high costs of producing them (10). Oligosaccharides currently used as prebiotics in trials are structurally and functionally different from HMOs (29). Probiotic interventions may be useful, but the identification of the appropriate bacteria and manufacturing adequate-quality products remain concerns (30). Despite these challenges, our results suggest that HMO-mediated pathways may provide a fruitful new line of investigation to improve outcomes among this neglected population of high-risk children who, although they have been saved from HIV, still face a significant burden of morbidity and mortality.
Acknowledgments
We acknowledge the significant contributions of the late Moses Sinkala who was one of the principal investigators of the Zambia Exclusive Breastfeeding Study (ZEBS). We also acknowledge the valuable contributions to the study by Drs. Prisca Kasonde, Cheswa Vwalika, Katherine Semrau, Erin Shutes, and Nancy Scott. We thank Stephanie Shiau for review of the manuscript. LK, CK, DMT, and GMA designed the research; CK and MM conducted the clinical research; LK and H-YK analyzed data; GMA conducted virologic assays; LB, LH, and CN conducted human milk oligosaccharide assays; and LK wrote the manuscript and had primary responsibility for final content. All authors read and approved the final manuscript.
Footnotes
Abbreviations used: HEU, HIV-exposed, uninfected; HMO, human milk oligosaccharide; LNFP, lacto-N-fucopentaose; LNnT, lacto-N-neotetraose; LNT, lacto-N-tetraose; 2′FL, 2′-fucosyllactose; 3FL, 3-fucosyllactose; 3′SL, 3′-sialyllactose.
References
- 1.Kourtis AP, Lee FK, Abrams EJ, Jamieson DJ, Bulterys M. Mother-to-child transmission of HIV-1: timing and implications for prevention. Lancet Infect Dis 2006;6:726–32. [DOI] [PubMed] [Google Scholar]
- 2.Fox MP, Brooks D, Kuhn L, Aldrovandi G, Sinkala M, Kankasa C, Mwiya M, Horsburgh R, Thea DM. Reduced mortality associated with breast-feeding-acquired HIV infection and breast-feeding among HIV-infected children in Zambia. J Acquir Immune Defic Syndr 2008;48:90–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Becquet R, Marston M, Dabis F, Moulton LH, Gray G, Coovadia HM, Essex M, Ekouevi DK, Jackson D, Coutsoudis A, et al. Children who acquire HIV infection perinatally are at higher risk of early death than those acquiring infection through breastmilk: a meta-analysis. PLoS ONE 2012;7:e28510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Marinda E, Humphrey JH, Iliff PJ, Mutasa K, Nathoo KJ, Piwoz EG, Moulton LH, Salama P, Ward BJ. Child mortality according to maternal and infant HIV status in Zimbabwe. Pediatr Infect Dis J 2007;26:519–26. [DOI] [PubMed] [Google Scholar]
- 5.Filteau S. The HIV-exposed, uninfected African child. Trop Med Int Health 2009;14:276–87. [DOI] [PubMed] [Google Scholar]
- 6.Kuhn L, Kasonde P, Sinkala M, Kankasa C, Semrau K, Scott N, Tsai WY, Vermund SH, Aldrovandi GM, Thea DM. Does severity of HIV disease in HIV-infected mothers affect mortality and morbidity among their uninfected infants? Clin Infect Dis 2005;41:1654–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Newell ML, Coovadia H, Cortina-Borja M, Rollins N, Gaillard P, Dabis F. Mortality of infected and uninfected infants born to HIV-infected mothers in Africa: a pooled analysis. Lancet 2004;364:1236–43. [DOI] [PubMed] [Google Scholar]
- 8.Kuhn L, Sinkala M, Semrau K, Kankasa C, Kasonde P, Mwiya M, Hu CC, Tsai WY, Thea DM, Aldrovandi GM. Elevations in mortality associated with weaning persist into the second year of life among uninfected children born to HIV-infected mothers. Clin Infect Dis 2010;50:437–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Coutsoudis A, Coovadia HM, Wilfert CM. HIV, infant feeding and more perils for poor people: new WHO guidelines encourage review of formula milk policies. Bull World Health Organ 2008;86:210–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bode L. Human milk oligosaccharides: every baby needs a sugar mama. Glycobiology 2012;22:1147–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bode L, Kuhn L, Kim HY, Hsiao L, Nissan C, Sinkala M, Kankasa C, Mwiya M, Thea DM, Aldrovandi GM. Human milk oligosaccharide concentration and risk of postnatal transmission of HIV through breastfeeding. Am J Clin Nutr 2012;96:831–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kuhn L, Aldrovandi GM, Sinkala M, Kankasa C, Semrau K, Mwiya M, Kasonde P, Scott N, Vwalika C, Walter J, et al. Effects of early, abrupt weaning on HIV-free survival of children in Zambia. N Engl J Med 2008;359:130–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jantscher-Krenn E, Zherebtsov M, Nissan C, Goth K, Guner YS, Naidu N, Choudhury B, Grishin AV, Ford HR, Bode L. The human milk oligosaccharide disialyllacto-N-tetraose prevents necrotising enterocolitis in neonatal rats. Gut 2012;61:1417–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jantscher-Krenn E, Lauwaet T, Bliss LA, Reed SL, Gillin FD, Bode L. Human milk oligosaccharides reduce Entamoeba histolytica attachment and cytotoxicity in vitro. Br J Nutr 2012;108:1839–46. [DOI] [PubMed] [Google Scholar]
- 15.Kunz C. Historical aspects of human milk oligosaccharides. Adv Nutr 2012;3(Suppl):430S–9S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zivkovic AM, German JB, Lebrilla CB, Mills DA. Human milk glycobiome and its impact on the infant gastrointestinal microbiota. Proc Natl Acad Sci USA 2011;108(Suppl 1):4653–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Newburg DS. Glycobiology of human milk. Biochemistry (Mosc) 2013;78:771–85. [DOI] [PubMed] [Google Scholar]
- 18.Morrow AL, Ruiz-Palacios GM, Altaye M, Jiang X, Guerrero ML, Meinzen-Derr JK, Farkas T, Chaturvedi P, Pickering LK, Newburg DS. Human milk oligosaccharides are associated with protection against diarrhea in breast-fed infants. J Pediatr 2004;145:297–303. [DOI] [PubMed] [Google Scholar]
- 19.Newburg DS, Ruiz-Palacios GM, Altaye M, Chaturvedi P, Meinzen-Derr J, Guerrero Mde L, Morrow AL. Innate protection conferred by fucosylated oligosaccharides of human milk against diarrhea in breastfed infants. Glycobiology 2004;14:253–63. [DOI] [PubMed] [Google Scholar]
- 20.Magne F, Hachelaf W, Suau A, Boudraa G, Mangin I, Touhami M, Bouziane-Nedjadi K, Pochart P. A longitudinal study of infant faecal microbiota during weaning. FEMS Microbiol Ecol 2006;58:563–71. [DOI] [PubMed] [Google Scholar]
- 21.Bode L, Kunz C, Muhly-Reinholz M, Mayer K, Seeger W, Rudloff S. Inhibition of monocyte, lymphocyte, and neutrophil adhesion to endothelial cells by human milk oligosaccharides. Thromb Haemost 2004;92:1402–10. [DOI] [PubMed] [Google Scholar]
- 22.Bode L, Rudloff S, Kunz C, Strobel S, Klein N. Human milk oligosaccharides reduce platelet-neutrophil complex formation leading to a decrease in neutrophil beta 2 integrin expression. J Leukoc Biol 2004;76:820–6. [DOI] [PubMed] [Google Scholar]
- 23.Tozzi AE, Pezzotti P, Greco D. Does breast-feeding delay progression to AIDS in HIV-infected children? AIDS 1990;4:1293–4. [DOI] [PubMed] [Google Scholar]
- 24.Taha TE, Kumwenda NI, Hoover DR, Kafulafula G, Fiscus SA, Nkhoma C, Chen S, Broadhead RL. The impact of breastfeeding on the health of HIV-positive mothers and their children in sub-Saharan Africa. Bull World Health Organ 2006;84:546–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Smilowitz JT, O'Sullivan A, Barile D, German JB, Lonnerdal B, Slupsky CM. The human milk metabolome reveals diverse oligosaccharide profiles. J Nutr 2013;143:1709–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.De Leoz ML, Gaerlan SC, Strum JS, Dimapasoc LM, Mirmiran M, Tancredi DJ, Smilowitz JT, Kalanetra KM, Mills DA, German JB, et al. Lacto-N-tetraose, fucosylation, and secretor status are highly variable in human milk oligosaccharides from women delivering preterm. J Proteome Res 2012;11:4662–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kuhn L, Thea DM, Aldrovandi GM. Bystander effects: children who escape infection but not harm. J Acquir Immune Defic Syndr 2007;46:517–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Abrams EJ, Myer L. Can we achieve an AIDS-free generation? Perspectives on the global campaign to eliminate new pediatric HIV infections. J Acquir Immune Defic Syndr 2013;63(Suppl 2):S208–12. [DOI] [PubMed] [Google Scholar]
- 29.Arslanoglu S, Moro GE, Schmitt J, Tandoi L, Rizzardi S, Boehm G. Early dietary intervention with a mixture of prebiotic oligosaccharides reduces the incidence of allergic manifestations and infections during the first two years of life. J Nutr 2008;138:1091–5. [DOI] [PubMed] [Google Scholar]
- 30.Deshpande GC, Rao SC, Keil AD, Patole SK. Evidence-based guidelines for use of probiotics in preterm neonates. BMC Med 2011;9:92. [DOI] [PMC free article] [PubMed] [Google Scholar]