Abstract
Background: The use of oral contraceptives (OCs) has been associated with low plasma pyridoxal 5′-phosphate (PLP). The functional consequences are unclear.
Objectives: To determine whether functional vitamin B-6 insufficiency occurs in OC users and is attributable to OCs, we investigated the associations of PLP with metabolites of one-carbon metabolism, tryptophan catabolism, and inflammation in OC users, and evaluated the effects of OCs on these metabolites.
Methods: Plasma metabolite concentrations were measured in 157 OC users (20–40 y of age). Associations between PLP and the metabolites were analyzed through use of generalized additive models and partial least squares–discriminant analysis (PLS-DA). Additionally, data from 111 of the 157 OC users were compared to previously reported data from 11 nonusers, at adequate and low vitamin B-6 status, with use of multivariate ANOVA.
Results: PLP showed significant (P < 0.05) negative nonlinear association with homocysteine, glutathione, and ratios of asymmetric dimethylarginine to arginine, 3-hydroxykynurenine to 3-hydroxyanthranilic acid, and 3-hydroxykynurenine to kynurenic acid. PLS-DA supported these conclusions and identified 3-hydroxykynurenine and the 3-hydroxykynurenine-to-kynurenine ratio as discriminating biomarkers in women with PLP ≤30 nmol/L. Among the many differences, OC users had significantly higher plasma pyridoxal (157% at adequate and 195% at low vitamin B-6 status), 4-pyridoxic acid (154% at adequate and 300% at low vitamin B-6 status), xanthurenic acid (218% at low vitamin B-6 status), 3-hydroxyanthranilic acid (176% at adequate and 166% at low vitamin B-6 status), quinolinic acid (127% at low vitamin B-6 status), and nicotinamide (197% at low vitamin B-6 status). Biomarkers of inflammation were not associated with PLP, and no differences were found between the 2 groups.
Conclusions: PLP is associated with biomarkers of one-carbon metabolism and tryptophan catabolism but not with biomarkers of inflammation in OC users. Independent of vitamin B-6 status, OCs have effects on metabolites and ratios of one-carbon metabolism and tryptophan catabolism but not on biomarkers of inflammation. This study was registered at clinicaltrials.gov as NCT01128244. The study from which data for nonusers was derived was registered as NCT00877812.
Keywords: metabolite profile, one-carbon, oral contraceptives, tryptophan, vitamin B-6
Introduction
Adequate vitamin B-6 status is important for health. Low vitamin B-6 status has been associated with increased risk of cardiovascular disease (1–3), venous thrombosis (4), and stroke (5–7). In 2012 the U.S. CDC (8) evaluated the NHANES 2005–2006 data and reported ∼11% of the U.S. population having plasma pyridoxal 5′-phosphate (PLP)9 <20 nmol/L, which is considered to be indicative of vitamin B-6 deficiency (9). A plasma PLP <20 nmol/L was more prevalent in females (13%) than in males (7%), and females 15–40 y of age had lower plasma PLP than other age groups (8). Oral contraceptives (OCs) are the leading contraception method in the United States and are used by ∼11 million women (15–44 y of age) (10). The use of OCs has been associated with low vitamin B-6 status for many years, although the implications are unclear. Relatively recent publications (11, 12) have suggested that current-generation OCs may continue as a factor aggravating the low vitamin B-6 status of women. Results derived from the analysis of NHANES 2003–2004 samples (12) indicated that 78% of women (21–44 y of age) taking OCs and not taking supplements had plasma PLP <20 nmol/L, and only 25% of females (21–44 y of age) who never used hormones had plasma PLP <20 nmol/L. Moreover, an Italian study showed that the prevalence of women having plasma PLP <21.7 nmol/L was higher in OC users (44%) than nonusers (25%) (11). Whether these differences in plasma PLP reflect an actual difference in functional vitamin B-6 status with impairment in PLP-dependent metabolic processes in OC users is unclear.
In one-carbon metabolism, PLP is a coenzyme for cytosolic and mitochondrial serine hydroxymethyltransferase as well as the glycine decarboxylase in the glycine cleavage system. PLP is also a coenzyme for cystathionine β-synthase and cystathionine γ-lyase in the trans-sulfuration pathway (13). In the tryptophan catabolic pathway, PLP is a cofactor for kynureninase and kynurenine aminotransferase. Kynureninase catalyzes the conversion of kynurenine to anthranilic acid and 3-hydroxykynurenine to 3-hydroxyanthranilic acid. Kynurenine aminotransferase catalyzes the conversion of kynurenine to kynurenic acid and 3-hydroxykynurenine to xanthurenic acid (14, 15).
Metabolic consequences of mild-to-moderate B-6 deficiency on one-carbon metabolism and tryptophan catabolism in controlled short-term vitamin B-6 restriction studies in men and women (nonusers) have been reported (16–21). Vitamin B-6 insufficiency had little effect on the in vivo rates of remethylation, transmethylation, and trans-sulfuration reactions of one-carbon metabolism (16–19, 21). However, this level of deficiency yielded increased plasma glycine, cystathionine, and serine, and decreased creatine, creatinine, and dimethylglycine concentrations (16, 20, 21). Moreover, an increase in plasma 3-hydroxykynurenine and a decrease in plasma kynurenic acid were reported after the restriction (20). Mathematic model predictions of changes in urinary tryptophan metabolite concentrations without previous tryptophan loading also demonstrated the increase of 3-hydroxykynurenine and the decrease of kynurenic acid in moderate vitamin B-6 deficiency. A more pronounced change was obtained at a more severe deficiency. Kynurenine and xanthurenic acid showed to respond (i.e., increase) at a more severe deficiency (22). The substrate-to-product ratios, 3-hydroxykynurenine to kynurenic acid, 3-hydroxykynurenine to 3-hydroxyanthranilic acid, and 3-hydroxykynurenine to xanthurenic acid, derived from metabolites involved in the PLP-dependent reactions have been shown to be strongly negatively associated with PLP and have been suggested as biomarkers of functional vitamin B-6 status (23).
The use of OCs has been associated with increased risk of cardiovascular disease (24), although the exact mechanisms are unclear. There is some evidence of increased homocysteine in women using OCs (25), but conflicting data have been reported (11, 24). Moreover, elevated C-reactive protein (CRP) has been reported in OC users when compared to nonusers (24, 25). Little is known about the effect of OCs on other aspects of one-carbon metabolism and other biomarkers of inflammation.
We report here studies conducted to more fully assess the nutritional status of OC users and the effects of OC usage on biomarkers of vitamin B-6 as well as one-carbon metabolism and tryptophan catabolism. This analysis evaluated the profiles of multiple biomarkers in OC users, followed by a comparison of these metabolite profiles with previously published results from nonuser controls.
Methods
Human participants and samples
For the OC users group, 157 women using common combined or single formulations of OCs containing 20–30 μg/d ethinyl estradiol and a progesterone were enrolled between August 2010 and August 2013 in Gainesville, FL. OC users meeting the following inclusion criteria were included: OC usage for >6 mo; aged between 20–40 y; normal total homocysteine concentration, no history of gastrointestinal surgery, abnormal kidney or thyroid function, or any other chronic disease; no smoking or chronic drug use or alcoholism; BMI <32 kg/m2, with the majority of OC users having a BMI <28 kg/m2; no vitamin, amino acid, or protein supplementation; no chronic consumption of a high-protein diet; and not pregnant. For the nonusers group, metabolite profile data were obtained from a previously published study (20). Metabolite concentrations were measured from fasting plasma samples of 11 healthy females not using OCs who participated in 2 previous identical dietary vitamin B-6 restriction studies (18, 21). The characteristics of the women and the complete restriction protocols have been reported elsewhere (18, 21). Briefly, the participants met the following inclusion criteria: no OC usage; aged between 20–40 y; normal total homocysteine concentration, no history of gastrointestinal surgery, abnormal kidney or thyroid function, or any other chronic disease; no smoking or chronic drug use or alcoholism; BMI <28 kg/m2; no vitamin, amino acid, or protein supplementation; no chronic consumption of a high-protein diet; not pregnant and with adequate nutritional status (18, 21). The restriction protocols both involved controlled feeding of a vitamin B-6 diet (mean ± SD: <0.37 ± 0.04 mg/d) for 28 d that induced a state of low vitamin B-6 status as indicated by PLP <30 nmol/L (18, 21). Metabolite profile analysis was performed on plasma samples from fasted participants before and after the restriction (20).
All OC users and participants from the previous restriction protocols signed an informed consent form. All procedures were reviewed and approved by the University of Florida Institutional Review Board and the University of Florida Clinical Research Center Scientific Advisory Committee. This study was registered at clinicaltrials.gov as NCT01128244. The restriction study, from which data for nonusers was derived, was registered as NCT00877812.
Experimental design
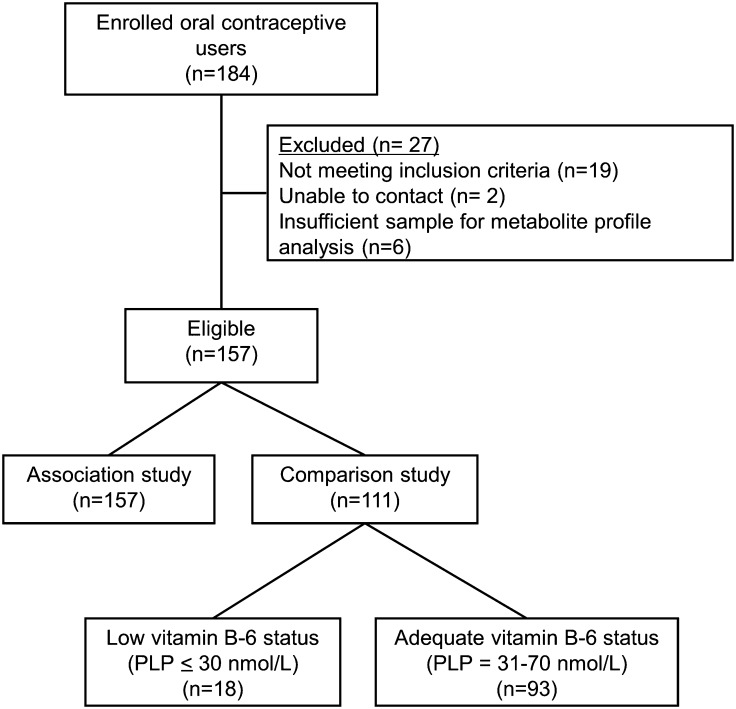
Data obtained from OC users (Figure 1) and nonusers were analyzed in 2 different ways, designated in the association study and comparison study. For the association study, metabolite data from all OC users were used to determine the association of plasma PLP with metabolites involved in one-carbon metabolism, tryptophan catabolism, and inflammation. For the comparison study, metabolite data from OC users were compared to previously published metabolite data from the nonuser subjects who participated in the vitamin B-6 restriction studies (18, 21). To investigate the effects of OCs on metabolites involved in these metabolic pathways, independent of vitamin B-6 status, the OC and nonuser groups were divided according to range of PLP concentration into adequate (PLP >30 nmol/L) and low (PLP ≤30 nmol/L) groups. For evaluation of OC effects on metabolite patterns in vitamin B-6–adequate subjects, we used data from 93 OC users and 11 nonusers (data from before restriction) having plasma PLP concentrations between 31 and 70 nmol/L. This range was selected to exclude OC users who had very high PLP concentrations possibly because of pyridoxine intake through consumption of highly fortified food or beverage products (26) or vitamin supplementation (27). Additionally, this range was related to the PLP concentration observed in the nonuser group, which did not use supplementation. For the evaluation of OC effects in subjects having low vitamin B-6 status, we used 18 OC users and 11 nonusers (data from after restriction) who had plasma PLP <30 nmol/L.
FIGURE 1.
CONSORT diagram of the oral contraceptive users included in the study. PLP, pyridoxal 5′-phosphate.
Measurements
Association study.
Fasting plasma PLP was determined by reversed-phase HPLC through use of the semicarbazide-derivative with fluorescence detection analysis (28). Plasma aminothiols, which include total homocysteine, total cysteine, cysteinylglycine, and total glutathione, were measured as the ammonium 7-fluorobenzo -2-oxa-1,3-diazole-4-sulfonate derivative by reversed-phase HPLC with fluorescence detection (29). A total of 29 metabolites including constituents of one-carbon and tryptophan metabolism were measured by LC-tandem MS (MS/MS) (30) at Bevital (Bergen, Norway). B-6 vitamers included pyridoxal, pyridoxine, and 4-pyridoxic acid. Plasma one-carbon related compounds included betaine, choline, dimethylglycine, creatine, creatinine, methionine, arginine, asymmetric dimethylarginine, symmetric dimethylarginine, homoarginine, trimethylysine, histidine, trimethyl N-oxide, riboflavin, and cystathionine. Tryptophan metabolites included tryptophan, kynurenine, 3-hydroxyanthranilic acid, kynurenic acid, anthranilic acid, xanthurenic acid, 3-hydroxyanthranilic acid, quinolinic acid, nicotinamide, and N1-methylnicotinamide. The following ratios were calculated: betaine to choline and dimethylglycine to betaine, which reflect reactions of betaine production and utilization; asymmetric dimethylarginine to arginine as a possible marker of endothelial dysfunction (20, 31); creatine to cystathionine, which was previously shown to be sensitive to vitamin B-6 restriction (20); and the ratios 3-hydroxykynurenine to kynurenic acid, 3-hydroxykynurenine to 3-hydroxyanthranilic acid, 3-hydroxykynurenine to xanthurenic acid, 3-hydroxykynurenine to anthranilic acid, and 3-hydroxykynurenine to kynurenine, which reflect PLP-dependent reactions in the tryptophan catabolic pathway. Biomarkers of inflammation included neopterin, measured by LC-MS/MS as above (30), plasma CRP [measured by ELISA (high-sensitivity CRP kit. no. CR120C; Calbiotech)], and the kynurenine-to-tryptophan ratio.
Comparison study.
Metabolite profile data for the nonusers were obtained from a previous study (20). These data consisted of 11 metabolites involved in one-carbon and related processes measured with use of LC-MS/MS at the Biomedical Mass Spectrometry Laboratory, Clinical and Translational Science Institute, University of Florida (20). These metabolites included betaine, choline, dimethylglycine, creatine, creatinine, methionine, arginine, asymmetric dimethylarginine, symmetric dimethylarginine, riboflavin, and cystathionine. The published 10 tryptophan metabolites and neopterin were measured by LC-MS/MS (30) at Bevital (Bergen, Norway), whereas plasma CRP was measured by ELISA (high-sensitivity CRP kit. no. CR120C; Calbiotech).
Statistical analysis
Association study.
Nonlinear associations between PLP, metabolites, and metabolite ratios were determined by fitting generalized additive models with use of SAS 13.1. The model helps bring to light nonlinear associations without making parametric assumptions and distributional restrictions. Statistical significance of an association was tested through use of the χ-square approximation and determined at the 0.05 level. Partial least squares–discriminant analysis (PLS-DA) (32, 33) was performed with use of pooled data for one-carbon, tryptophan metabolites and biomarkers of inflammation using SIMCA, version 13 (MKS Umetrics) (34). The B-6 vitamers, PLP, pyridoxal, 4-pyridoxic acid, and pyridoxine were omitted for this analysis to focus the analysis on the other metabolites. Score plots were used to evaluate overall differences in metabolite profiles according to vitamin B-6 concentration: low (PLP ≤30 nmol/L), medium (PLP = 31–99 nmol/L), and high (PLP ≥100 nmol/L). Concentrations ≥100 nmol/L were included to represent women who had very high PLP concentrations possibly because of supplementation (e.g., vitamin supplements) (27). A discriminant analysis of the contribution of each variable to group differences according to vitamin B-6 status was conducted through use of the variable influence on projection (VIP) method (33, 35). As stated previously (20, 36), the VIP values represent weighted sums of squares of the PLS weights of the various metabolites and take into account the proportion of Y-variance in each dimension (34). In each plot, each variable is provided with a VIP value and a 95% CI derived from jack-knifing (34, 35, 37). Variables for which the VIP value ± 95% CI exceeds 1 are designated as significant biomarkers in this analysis.
Comparison study.
All data are presented as means ± SDs. To meet the Gaussian assumption, logarithmic transformation was performed on the concentrations before comparing overall differences between OC users and nonusers. The multivariate ANOVA was used to assess overall significance, and if significant, was followed by stepdown tests with Sidak adjustment for multiple testing to compare individual metabolites and ratios responsible for the differences (38). SAS 13.1 was used for the analyses. Cystathionine, a sensitive biomarker of marginal vitamin B-6 deficiency, was specifically compared between OC users and nonusers of low vitamin B-6 status with use of Student’s 2-sample t test using SigmaPlot 11.0; data were log transformed before the Student’s t test. Statistical significance was determined at the 0.05 level.
Results
Participant characteristics
A total of 157 women with an age range between 20 and 34 y old were recruited. Only 18 (11.4%) of the 157 OC users had plasma PLP ≤30 nmol/L. Of the 18, only 3 women had plasma PLP <20 nmol/L (indicative of deficiency). The other 139 (89%) women had adequate plasma PLP concentrations ranging from 31 to 189 nmol/L. All data from the 157 OC users were used for the association study. The subset of 93 OC users used for the comparison study had plasma PLP concentrations between 31 and 70 nmol/L with a mean PLP of 45.9 ± 11.5 nmol/L (Table 1). For the low group, the18 OC users had a mean PLP of 24.7 ± 4.29 nmol/L (Table 1). The 11 nonusers, with an age range between 20 and 39 y old, who were used as the control group had normal plasma PLP concentrations before the restriction with a mean PLP of 45.7 ± 12.5 nmol/L (Table 1). After the restriction, the plasma PLP was lowered to 20.5 ± 3.75 nmol/L with plasma PLP values ranging from 14.8 to 25.3 nmol/L (18, 21) (Table 1).
TABLE 1.
Plasma concentrations of one-carbon metabolites and ratios in OC users vs. nonusers with adequate (PLP = 31–70 nmol/L) and low (PLP ≤30 nmol/L) vitamin B-6 status1
| Adequate vitamin B-6 status |
Low vitamin B-6 status |
|||
| Nonusers (n = 11) | OC users (n = 93) | Nonusers (n = 11) | OC users (n = 18) | |
| PLP,2 nmol/L | 45.7 ± 12.5 | 47.9 ± 11.5 | 20.5 ± 3.75 | 24.7 ± 4.29 |
| Pyridoxal, nmol/L | 7.71 ± 1.53 | 12.1 ± 4.46* | 4.06 ± 1.09 | 7.90 ± 2.61* |
| Pyridoxic acid, nmol/L | 12.2 ± 3.07 | 18.8 ± 7.47* | 4.47 ± 1.17 | 13.4 ± 4.30* |
| Pyridoxine, nmol/L | 0.003 ± 0.007 | 0.02 ± 0.09 | 0.012 ± 0.013 | 0.011 ± 0.032 |
| Cystathionine,3 μmol/L | 0.14 ± 0.03 | 0.12 ± 0.04 | 0.24 ± 0.11 | 0.14 ± 0.10† |
| Homocysteine, μmol/L | 6.22 ± 1.09 | 5.60 ± 1.47 | 6.05 ± 0.88 | 6.75 ± 2.15 |
| Cysteine, μmol/L | 241 ± 36.1 | 235 ± 26.9 | 233 ± 24.1 | 231 ± 31.0 |
| Glutathione, μmol/L | 6.43 ± 1.49 | 3.10 ± 0.83* | 7.29 ± 1.57 | 4.38 ± 1.45* |
| Chol, μmol/L | 7.84 ± 1.23 | 7.47 ± 1.54 | 8.75 ± 1.98 | 7.92 ± 1.73 |
| Bet, μmol/L | 37.8 ± 13.9 | 20.1 ± 5.92* | 40.1 ± 13.3 | 20.3 ± 5.03* |
| DMG, μmol/L | 2.53 ± 0.54 | 2.99 ± 0.93 | 2.38 ± 0.60 | 3.69 ± 3.87 |
| Creatinine, μmol/L | 72.9 ± 14.2 | 69.0 ± 7.59 | 71.2 ± 22.9 | 69.1 ± 10.8 |
| Methionine, μmol/L | 26.5 ± 3.74 | 26.2 ± 4.19 | 27.7 ± 6.95 | 25.5 ± 3.41 |
| Arg, μmol/L | 98.2 ± 21.6 | 74.5 ± 16.4* | 93.0 ± 26.5 | 73.5 ± 13.2 |
| ADMA, μmol/L | 0.75 ± 0.12 | 0.43 ± 0.07* | 0.71 ± 0.19 | 0.45 ± 0.04* |
| Symmetric dimethylarginine, μmol/L | 0.62 ± 0.05 | 0.48 ± 0.07* | 0.64 ± 0.15 | 0.52 ± 0.07 |
| Creatine, μmol/L | 35.0 ± 12.3 | 45.4 ± 21.5 | 18.0 ± 4.41 | 40.5 ± 21.3* |
| Riboflavin, nmol/L | 22.2 ± 22.2 | 18.3 ± 24.5 | 24.1 ± 25.1 | 17.5 ± 18.2 |
| Ratios | ||||
| Bet:Chol | 4.91 ± 1.86 | 2.77 ± 0.86 | 4.55 ± 0.86 | 2.62 ± 0.65* |
| DMG:Bet | 0.07 ± 0.02 | 0.16 ± 0.05* | 0.06 ± 0.01 | 0.18 ± 0.19 |
| ADMA:Arg | 0.008 ± 0.001 | 0.006 ± 0.001* | 0.008 ± 0.001 | 0.006 ± 0.001 |
Values are means ± SDs. Multivariate ANOVA was performed separately for adequate and low vitamin B-6 status. *Different from nonusers within vitamin B-6 status, adjusted P < 0.05. †Significant difference between groups of low vitamin B-6 status, P < 0.05. Adjustment for multiple testing was based on the Sidak method. ADMA, asymmetric dimethylarginine; Bet, betaine; Chol, choline; DMG, dimethylglycine; OC, oral contraceptive; PLP, pyridoxal 5′-phosphate.
PLP values for nonusers after vitamin B-6 restriction range from 14.8 to 25.3 nmol/L. PLP values for OC users of low vitamin B-6 status range from 14.4 to 29.4 nmol/L.
Student’s t test for cystathionine, a biomarker of marginal vitamin B-6 deficiency, at low vitamin B-6 status.
Associations of PLP with metabolites in OC users
One-carbon metabolism.
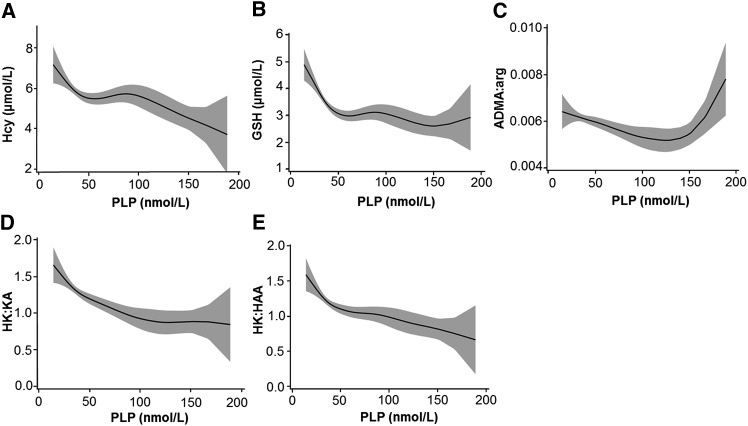
Significant nonlinear associations with PLP were obtained for total homocysteine, total glutathione, and the ratio of asymmetric dimethylarginine to arginine (Figure 2). Homocysteine and glutathione showed an overall negative relation with plasma PLP showing higher concentrations at PLP values lower than ∼50 nmol/L (Figure 2A, B). The ratio of asymmetric dimethylarginine to arginine gradually decreased as PLP concentration increased to a concentration of ∼130 nmol/L. However, the asymmetric dimethylarginine-to-arginine ratio increased for PLP >150 nmol/L (Figure 2C). Pyridoxal and 4-pyridoxic acid showed a significant general positive nonlinear association with plasma PLP reaching plateau at PLP concentrations of ∼150 nmol/L (data not shown). No significant associations were found between PLP and the other metabolites and ratios analyzed including cysteine, cysteinylglycine, cystathionine, creatine, creatinine, methionine, arginine, symmetric dimethylarginine, choline, betaine, dimethylglycine, trimethyllysine, trimethyl N-oxide, histidine, and riboflavin, as well as for the ratios betaine to choline, dimethylglycine to betaine, and creatinine to cystathionine.
FIGURE 2.
Associations (95%) of plasma PLP with one-carbon, tryptophan metabolites and ratios. Associations were modeled by GAMs with use of data from 157 oral contraceptive users. Shaded areas indicate 95% CIs. The significant metabolites, by χ-square test based on GAM, were (A) Hcy, (B) GSH, and the ratios (C) ADMA to Arg, (D) HK to KA, and (E) HK to HAA. ADMA:arg, asymmetric dimethylarginine-to-arginine ratio; GAM, generalized additive model; GSH, glutathione; Hcy, homocysteine; HK:HAA, 3-hydroxykynurenine–to–3-hydroxyanthranilic acid ratio; HK:KA, 3-hydroxykynurenine-to-kynurenic acid ratio; PLP, pyridoxal 5′-phosphate.
Tryptophan metabolism.
There was no significant association between PLP and tryptophan, kynurenine, 3-hydroxykynurenine, kynurenic acid, xanthurenic acid, anthranilic acid, 3-hydroxyanthranilic acid, quinolinic acid, nicotinamide or N1-methylnicotinamide. However, a significant overall negative nonlinear association of PLP with the ratios 3-hydroxykynurenine to kynurenic acid and 3-hydroxykynurenine to 3-hydroxyanthranilic acid was observed (Figure 2D, E). No significant associations were found for the ratios 3-hydroxykynurenine to xanthurenic acid, 3-hydroxykynurenine to anthanilic acid, and 3-hydroxykynurenine to kynurenine.
Biomarkers of inflammation.
There were no significant relations between neopterin, kynurenine-to-tryptophan ratio, or CRP with plasma PLP.
Multivariate analysis of pooled one-carbon, tryptophan metabolites and biomarkers of inflammation.
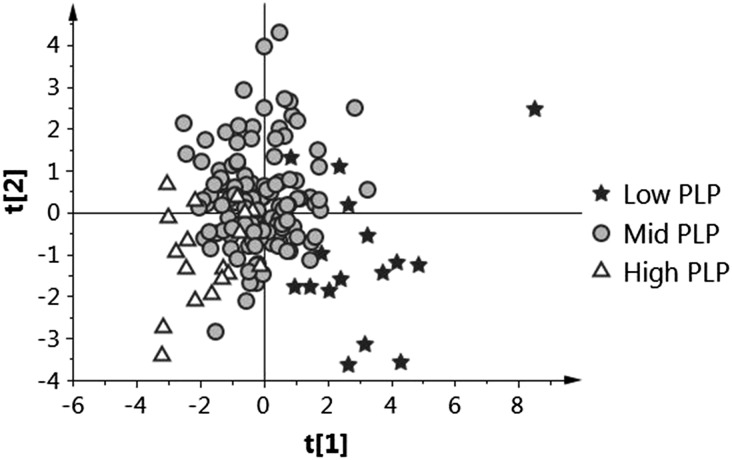
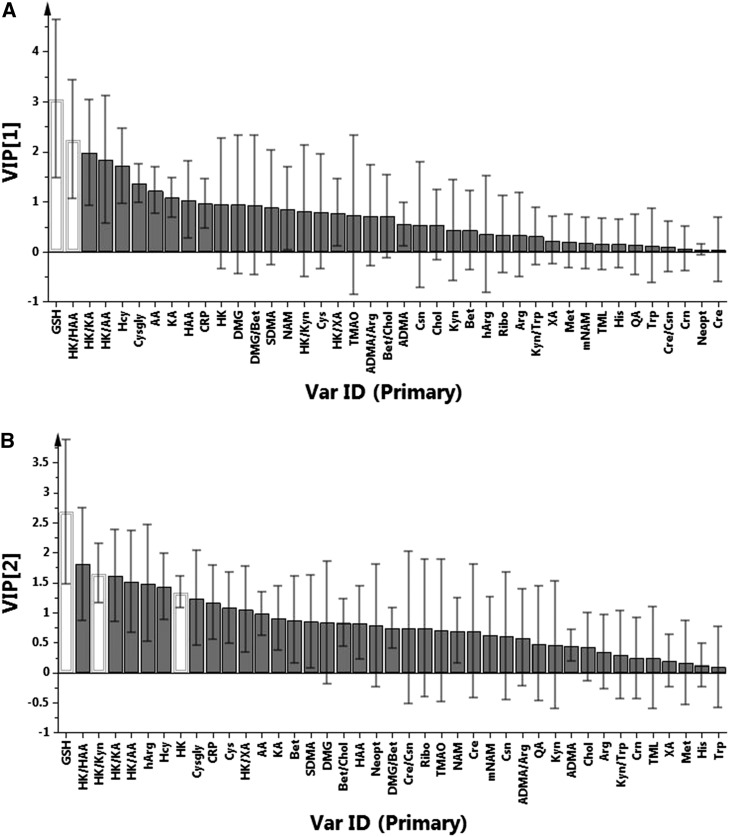
The overall effect of PLP concentration on the pooled analytes involved in one-carbon and tryptophan metabolism and biomarkers of inflammation was evaluated by PLS-DA (Figure 3). For this analysis, the data were categorized in 3 different classes based on plasma PLP concentrations: low, plasma PLP ≤30 nmol/L; medium, plasma PLP = 31–99 nmol/L; and high, plasma PLP >100 nmol/L. The results showed separation according to vitamin B-6 concentration indicating differences in the groups, with the main separation between PLP ≤30 nmol/L from the medium and high PLP groups. VIP plots were used to identify the metabolites and ratios that were significant differentiating factors among these groupings of plasma PLP concentration at the 95% confidence level (Figure 4). The VIP plot for component 1 of the analysis (Figure 4A) identified total glutathione and the ratio of 3-hydroxykynurenine to 3-hydroxyanthranilic acid as the significant discriminating biomarkers. The VIP plot for component 2 (Figure 4B) identified total glutathione, 3-hydroxykynurenine, and the ratio 3-hydroxykynurenine to kynurenine as the significant discriminating biomarkers. No significant discriminating effect was observed for the biomarkers of inflammation neopterin, CRP, and the kynurenine-to-tryptophan ratio. VIP analysis also showed trends toward significance (90% confidence level) for total glutathione, total homocysteine, cysteinylglycine, and the ratios 3-hydroxykynurenine to 3-hydroxyanthranilic acid and 3-hydroxykynurenine to kynurenic acid for PLS-DA component 1 and total glutathione, total homocysteine, 3-hydroxykynurenine, and the ratios 3-hydroxykynurenine to 3-hydroxyanthranilic acid, 3-hydroxykynurenine to kynurenine, and 3-hydroxykynurenine to kynurenic acid for component 2. Further analysis of these and related data will be reported elsewhere.
FIGURE 3.
Score plot from partial least squares–discriminant analysis of overall data for one-carbon, tryptophan metabolites, ratios, and biomarkers of inflammation in OC users according to PLP concentrations. Low PLP: ≤30 nmol/L; mid PLP: 31–99 nmol/L; high PLP: ≥100 nmol/L. Each data point represents a function of a pooled metabolite profile of each participant. Pyridoxal, 4-pyridoxic acid, and pyridoxine were omitted for this analysis to focus the analysis on the non–vitamin B-6 patterns of metabolites. Cumulative R2Y values for components 1 and 2 were 0.187 and 0.292, respectively. OC, oral contraceptive; PLP, pyridoxal 5′-phosphate; R2Y, variation of all Y explained by the model.
FIGURE 4.
VIP plots from partial least squares–discriminant analysis of overall data for one-carbon, tryptophan metabolites, ratios, and biomarkers of inflammation between low-, middle-, and high-plasma PLP concentration in OC users. (A) VIP [1], component 1; (B) VIP [2], component 2. The bars represent a weighted sum of squares with CIs. Variables in which the lower range of the CI exceed a VIP value of 1 are considered to be significant at the 95% level (white bars). AA, anthranilic acid; ADMA, asymmetric dimethylarginine; Bet, betaine; Chol, choline; Cre, creatine; Crn, creatinine; CRP, C-reactive protein; Csn, cystathionine; Cysgly, cysteinylglycine; DMG, dimethylglycine; GSH, glutathione; HAA, 3-hydroxyanthranilic acid; hArg, homoarginine; Hcy, homocysteine; HK, 3-hydroxykynurenine; KA, kynurenic acid; Kyn, kynurenine; mNAM, N1-methylnicotinamide; NAM, nicotinamide; Neopt, neopterin; OC, oral contraceptive; PLP, pyridoxal 5′-phosphate; QA, quinolinic acid; Ribo, riboflavin; SDMA, symmetric dimethylarginine; TMAO, trimethyl N-oxide; TML, trimethylysine; Var ID, variable identification; VIP, variable influence on projection; XA, xanthurenic acid.
Comparison study: OC users vs. nonusers
Targeted analysis of metabolites involved in one-carbon metabolism, tryptophan catabolism, and inflammation quantified a panel of 29 compounds (Tables 1–3). The results from the multivariate ANOVAs conducted separately for adequate and low vitamin B-6 status showed significant overall differences between OC users and nonusers for one-carbon metabolites at adequate (P < 0.0001) and low (P = 0.0019) vitamin B-6 status, and for tryptophan metabolites at adequate (P < 0.0001) and low (P = 0.0027) vitamin B-6 status. No overall differences between OC users and nonusers were found for biomarkers of inflammation at either adequate (P = 0.74) or low (P = 0.07) vitamin B-6 status.
TABLE 3.
Plasma concentrations of biomarkers of inflammation in OC users vs. nonusers with adequate (PLP = 31–70 nmol/L) and low (PLP ≤30 nmol/L) vitamin B-6 status1
| Adequate vitamin B-6 status |
Low vitamin B-6 status |
|||
| Nonusers (n = 11) | OC users (n = 93) | Nonusers (n = 11) | OC users (n = 18) | |
| Kyn/Trp | 0.022 ± 0.007 | 0.020 ± 0.005 | 0.019 ± 0.005 | 0.021 ± 0.005 |
| Neopterin, nmol/L | 6.57 ± 1.39 | 6.27 ± 2.58 | 6.81 ± 1.25 | 5.96 ± 1.42 |
| CRP, mg/L | 3.77 ± 4.02 | 3.77 ± 3.75 | 1.40 ± 2.39 | 1.34 ± 1.11 |
Values are means ± SDs. Multivariate ANOVA was performed separately for adequate and low vitamin B-6 status. CRP, C-reactive protein; Kyn, kynurenine; OC, oral contraceptive; PLP, pyridoxal 5′-phosphate.
Metabolites responsible for differences in one-carbon metabolite concentrations in OC users vs. nonusers.
We investigated 18 analytes involved in one-carbon metabolism and related processes in OC users and nonusers who had adequate or low vitamin B-6 status (Table 1). Independent of plasma PLP concentrations, pyridoxal and 4-pyridoxic acid were both higher in OC users (adjusted P = 0.0005, adjusted P = 0.0085, respectively). At adequate vitamin B-6 status, total glutathione (adjusted P = 0.00001), betaine (adjusted P = 0.0066), arginine (adjusted P = 0.030), asymmetric dimethylarginine (adjusted P < 0.00001), and symmetric dimethylarginine (adjusted P < 0.00001) were significantly lower in OC users than nonusers. At low vitamin B-6 status, total glutathione (adjusted P = 0.011), betaine (adjusted P = 0.012), and asymmetric dimethylarginine (adjusted P = 0.020) were significantly lower in OC users than nonusers, and creatine (adjusted P = 0.0088) was significantly higher in OC users than nonusers. Moreover, cystathionine, a sensitive biomarker of marginal vitamin B-6 deficiency, was significantly lower in OC users vs. nonusers of low vitamin B-6 status (P = 0.004, t test).
Investigation of selected metabolite ratios provided additional information about possible differences in reactions occurring in key metabolic processes. At adequate vitamin B-6 status, the ratio of asymmetric dimethylarginine to arginine (adjusted P = 0.0018) was significantly lower in OC users, whereas the ratio of dimethylglycine to betaine (adjusted P = 0.0004) was significantly higher in the OC group. At low vitamin B-6 status, the ratio of betaine to choline (adjusted P = 0.0002) was significantly lower in OC users.
Metabolites responsible for differences in tryptophan metabolism in OC users vs. nonusers.
We investigated 10 metabolites involved in tryptophan catabolism in OC users vs. nonusers at adequate and low vitamin B-6 status (Table 2). Significant overall differences were found at adequate and low vitamin B-6 status. The metabolites responsible included xanthurenic acid (adjusted P = 0.025), 3-hydroxyanthranilic acid (adjusted P = 0.009), quinolinic acid (adjusted P = 0.035), and nicotinamide (adjusted P = 0.025), which were higher in OC users than nonusers of low vitamin B-6 status. At adequate vitamin B-6 status, 3-hydroxyanthranilic acid (adjusted P = 0.0008) was significantly higher in OC users than nonusers. Xanthurenic acid (adjusted P = 0.067) and quinolinic acid (adjusted P = 0.006) showed a similar trend without reaching statistical significance. No significant differences were found for N1-methylnicotinamide and the other tryptophan metabolites. Additionally, the ratios 3-hydroxykynurenine to kynurenic acid, 3-hydroxykynurenine to xanthurenic acid, 3-hydroxykynurenine to anthranilic acid, and 3-hydroxykynurenine to kynurenine were not significantly different in OC users when compared to nonusers at adequate status. However, a trend toward a decrease for the ratios 3-hydroxykynurenine to xanthurenic acid, 3-hydroxykynurenine to 3-hydroxyanthranilic acid, and 3-hydroxykynurenine to kynurenine was observed at low vitamin B-6 status without reaching statistical significance.
TABLE 2.
Plasma concentrations of tryptophan metabolites and ratios in OC users vs. nonusers with adequate (PLP = 31–70 nmol/L) and low (PLP ≤30 nmol/L) vitamin B-6 status1
| Adequate vitamin B-6 status |
Low vitamin B-6 status |
|||
| Nonusers (n = 11) | OC users (n = 93) | Nonusers (n = 11) | OC users (n = 18) | |
| Tryptophan, μmol/L | 55.4 ± 9.13 | 63.6 ± 11.4 | 59.6 ± 8.13 | 64.3 ± 12.6 |
| Kyn, μmol/L | 1.18 ± 0.29 | 1.27 ± 0.24 | 1.08 ± 0.18 | 1.34 ± 0.26 |
| HK, nmol/L | 29.4 ± 10.9 | 33.8 ± 9.88 | 35.0 ± 15.0 | 35.5 ± 13.8 |
| KA, nmol/L | 26.7 ± 11.2 | 29.6 ± 10.0 | 19.7 ± 4.51 | 24.4 ± 6.99 |
| XA, nmol/L | 7.90 ± 4.56 | 14.2 ± 8.13 | 6.48 ± 2.08 | 14.1 ± 7.95* |
| AA, nmol/L | 12.8 ± 2.31 | 13.4 ± 12.1 | 12.8 ± 1.82 | 11.1 ± 3.77 |
| HAA, nmol/L | 18.4 ± 6.00 | 32.4 ± 12.1* | 15.8 ± 4.44 | 26.2 ± 10.1* |
| Quinolinic acid, nmol/L | 253 ± 70.5 | 346 ± 107 | 256 ± 43.6 | 326 ± 62.6* |
| Nicotinamide, nmol/L | 261 ± 217 | 387 ± 152 | 228 ± 111 | 449 ± 225* |
| N1-methylnicotinamide, nmol/L | 142 ± 92.1 | 150 ± 73.5 | 131 ± 96.2 | 174 ± 87.5 |
| Ratios | ||||
| HK:KA | 1.22 ± 0.64 | 1.21 ± 0.38 | 1.88 ± 0.94 | 1.54 ± 0.65 |
| HK:AA | 2.42 ± 1.34 | 2.65 ± 0.86 | 2.87 ± 1.56 | 3.68 ± 2.49 |
| HK:XA | 4.35 ± 2.38 | 3.08 ± 1.88 | 6.18 ± 4.18 | 3.33 ± 1.96 |
| HK:HAA | 1.63 ± 0.51 | 1.11 ± 0.32 | 2.35 ± 1.05 | 1.50 ± 0.73 |
| HK:Kyn | 25.4 ± 9.89 | 26.7 ± 5.97 | 31.7 ± 9.46 | 26.7 ± 8.62 |
Values are means ± SDs. Multivariate ANOVA was performed separately for adequate and low vitamin B-6 status. *Different from nonusers within vitamin B-6 status, adjusted P < 0.05. Adjustment for multiple testing was based on the Sidak method. AA, anthranilic acid; HAA, 3-hydroxyanthranilic acid; HK, 3-hydroxykynurenine; KA, kynurenic acid; Kyn, kynurenine; OC, oral contraceptive; PLP, pyridoxal 5′-phosphate; XA, xanthurenic acid.
Discussion
The use of OCs has been associated with low plasma PLP concentration (11, 12). The prevalence of low plasma PLP in OC users in our study was lower than expected based on 2003–2004 NHANES data (12). Only 3 (1.9%) of the women had plasma PLP <20 nmol/L (deficiency), and 15 (9.5%) women had plasma PLP between 20 and 30 nmol/L (marginal deficiency). These observations indicate that in this population, which mostly comprises college women who may have a generally healthy diet, the prevalence of vitamin B-6 deficiency in OC users is low. Additionally, the extremely large percentage of women with apparent vitamin B-6 deficiency reported in that study likely was attributable to an analytic issue. The enzymatic PLP assay used in that study later was reported to indicate almost double the number of low PLP results than the validated HPLC method (39).
Metabolic effects of inadequate vitamin B-6 nutritional status (PLP <30 nmol/L) on cellular metabolic processes including amino acid, lipid, organic acid, and one-carbon and tryptophan metabolism have been reported in men and women (nonusers) after short-term vitamin B-6 restriction (16, 18, 20, 21, 40, 41). Our association study further demonstrated relations between plasma PLP and metabolites involved in one-carbon metabolism. An expected inverse association between PLP and total homocysteine was observed. Homocysteine catabolism is a vitamin B-6–dependent process because of the role of PLP as a coenzyme for cystathionine β-synthase, involved in the formation of cystathionine, and cystathionine γ-lyase (CSE) yielding cysteine. No significant association between PLP and cysteine was observed in this study, which is in agreement with previous reports showing no effects of vitamin B-6 restriction on cysteine flux and concentration (16, 18). Cystathionine did not show significant association overall with vitamin B-6 status, although an increase at PLP <30 nmol/L has been previously reported (16, 18, 21). Activity of CSE is reduced during marginal vitamin B-6 deficiency, but the activity and in vivo flux of cystathionine β-synthase is maintained resulting in the accumulation of cystathionine (16, 42). However, the concentration of cystathionine in the OC group (0.14 ± 0.10 μmol/L) vs. nonusers (0.24 ± 0.11 μmol/L) appears to indicate that the level of deficiency in the OC users group was not sufficient to cause an effect on the activity of CSE. The inverse relation between PLP and total glutathione found in this study is supported by previous studies showing slightly increased glutathione concentrations after vitamin B-6 restriction (16, 42). In view of the fact that another vitamin B-6 restriction study showed no effect on plasma glutathione (43), the effects of vitamin B-6 on plasma glutathione appear to be weak and inconsistent. The lack of significant associations between PLP and creatine, creatinine, and dimethylglycine observed in this study was in contrast with the previously reported association of vitamin B-6 restriction with decreased plasma concentrations of these metabolites (20). Interestingly, the association between PLP and the asymmetric dimethylarginine-to-arginine ratio showed higher values at the lower and higher ends of the PLP concentration curve with the steepest slope observed at higher PLP concentrations (Figure 2C). A higher asymmetric dimethylarginine-to-arginine ratio might be associated with higher risk of cardiovascular disease because higher concentrations of asymmetric dimethylarginine are associated with endothelial dysfunction (31). Moreover, the lack of significant association between PLP and inflammatory biomarkers agrees with previous findings that induced vitamin B-6 deficiency does not cause inflammation (20).
Our study demonstrates and confirms the association between PLP and 3-hydroxykynurenine (Figure 4B) and the ratios 3-hydroxykynurenine to kynurenic acid and 3-hydroxykynurenine to 3-hydroxyanthranilic acid (Figure 2C, D). These results are in agreement with previous studies (20, 23, 44) and our mathematic modeling prediction of increased 3-hydroxykynurenine (22). PLP was not significantly associated with xanthurenic acid in contrast to earlier studies mainly involving urinary excretion after a tryptophan load (45–47). Kynureninase is more sensitive to vitamin B-6 deficiency than kynurenine aminotransferase (15), which explains the increase of 3-hydroxykynurenine during deficiency. Additionally, the use of tryptophan loads would cause an increase in tryptophan degradation, and therefore, greater xanthurenic acid concentrations are observed from the higher conversion of 3-hydroxykynurenine to xanthurenic acid in the vitamin B-6–deficient individuals (22).
Other important findings were obtained from the comparison study between OC users and nonusers. Pyridoxal and 4-pyridoxic acid were higher in the OC group than nonusers group. The higher 4-pyridoxic acid in OC users is in contrast to previous reports evaluating urinary 4-pyridoxic acid excretion (48, 49). Higher plasma 4-pyridoxic acid might suggest greater pyridoxal catabolism. The 4-pyridoxic acid-to-(pyridoxal + PLP) ratio, which has been reported as marker for vitamin B-6 catabolism (50), was higher in OC users than nonusers at adequate and low B-6 status (P = 0.03 and P < 0.001, respectively) (data not shown). However, the observed higher pyridoxal and 4-pyridoxic acid could also be the result of possibly higher pyridoxine intake by the OC users. Total glutathione was significantly lower in OC users. The association of low glutathione with sex hormones has been previously shown (51). Betaine was found to be lower in the OC users group. Whether the differences in betaine reflect differences in dietary intake or from the de novo biosynthesis from its precursor choline are unclear. There were no significant differences in choline and dimethylglycine between OC users and nonusers. There was, however, a significant lower betaine-to-choline ratio and a significant higher dimethylglycine-to-betaine ratio in OC users than nonusers, which suggests a possible effect of sex hormones on betaine production and utilization. Asymmetric dimethylarginine and symmetric dimethylarginine and the ratio asymmetric dimethylarginine to arginine were also lower in OC users than nonusers. Although asymmetric dimethylarginine is a direct inhibitor of NO production, symmetric dimethylarginine indirectly inhibits NO by competing with its precursor arginine for transport (52), suggesting that OC users may have greater protection than nonusers for endothelial dysfunction. The observation of lower arginine and creatine in OC users vs. nonusers of low vitamin B-6 status is unexpected and may reflect dietary differences between these groups; thus, the effects of OCs on these metabolites need further investigation. In contrast with previous reports (24, 25), there were not significant differences in plasma CRP between OC users and nonusers.
Our observations regarding tryptophan metabolites in OC users vs. nonusers are in accordance with previous studies that showed higher concentrations of these metabolites in OC users (53–55). Increased metabolite concentrations can be explained by the inductive effect of estrogen on tryptophan 2,3-dioxygenase, the first enzyme in the tryptophan catabolic pathway in the liver (56). None of the substrate-to-product ratios representing reactions catalyzed by PLP-dependent enzymes in the tryptophan catabolic pathway, and the nicotinamide product N1-methylnicotinamide, were significantly different between OC users and nonusers, which indicates no functional effect of OCs on these reactions.
This study showed the effects of OCs on metabolites involved in one-carbon metabolism that, to our knowledge, have not been previously reported. However, some limitations for the comparison study need to be noted. The sample size for the nonusers group was very small compared to the OC users group, which could have influenced the statistical power. In addition, some of the one-carbon metabolite concentrations were measured by different laboratories (i.e., betaine, choline, dimethylglycine, creatine, creatinine, methionine, arginine, asymmetric dimethylarginine, and symmetric dimethylarginine), and interlaboratory validation studies have not been conducted. Moreover, glycine, which has been shown to be affected by vitamin B-6 deficiency in previous reports, was not evaluated in this study. Finally, there is a lack of information regarding self-selected usual diets for the OC users, which precludes evaluation of dietary intakes between the 2 groups.
In summary, this observational study showed a low frequency of vitamin B-6 deficiency in the OC users we evaluated. Our results provided evidence of mild functional effects of low vitamin B-6 deficiency associated with OC usage on the metabolic processes investigated. It also extends our knowledge of the association of plasma PLP with metabolites involved in one-carbon metabolism, tryptophan catabolism, and inflammation in OC users and supports the findings from previous studies that proposed the use of 3-hydroxykynurenine, homocysteine, and the ratios 3-hydroxykynurenine to 3-hydroxyanthranilic acid and 3-hydroxykynurenine to kynurenic acid as reliable biomarkers for the diagnosis of vitamin B-6 insufficiency.
Acknowledgments
PWS and JFG designed the research; LR-A, BC, ØM, and PMU conducted the research; Y-YC and JFG analyzed the data; and LR-A and JFG wrote the paper. All authors read and approved the final manuscript.
Footnotes
Abbreviations used: CRP, C-reactive protein; CSE, cystathionine γ-lyase; MS/MS, tandem MS; OC, oral contraceptive; PLP, pyridoxal 5′-phosphate; PLS-DA, partial least squares–discriminant analysis; VIP, variable influence on projection.
References
- 1.Cheng CH, Lin PT, Liaw YP, Ho CC, Tsai TP, Chou MC, Huang YC. Plasma pyridoxal 5′-phosphate and high-sensitivity C-reactive protein are independently associated with an increased risk of coronary artery disease. Nutrition 2008;24:239–44. [DOI] [PubMed] [Google Scholar]
- 2.Page JH, Ma J, Chiuve SE, Stampfer MJ, Selhub J, Manson JE, Rimm EB. Plasma vitamin B(6) and risk of myocardial infarction in women. Circulation 2009;120:649–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rimm EB, Willett WC, Hu FB, Sampson L, Colditz GA, Manson JE, Hennekens C, Stampfer MJ. Folate and vitamin B6 from diet and supplements in relation to risk of coronary heart disease among women. JAMA 1998;279:359–64. [DOI] [PubMed] [Google Scholar]
- 4.Cattaneo M, Lombardi R, Lecchi A, Bucciarelli P, Mannucci PM. Low plasma levels of vitamin B(6) are independently associated with a heightened risk of deep-vein thrombosis. Circulation 2001;104:2442–6. [DOI] [PubMed] [Google Scholar]
- 5.Robinson K, Arheart K, Refsum H, Brattstrom L, Boers G, Ueland P, Rubba P, Palma-Reis R, Meleady R, Daly L, et al. Low circulating folate and vitamin B6 concentrations: risk factors for stroke, peripheral vascular disease, and coronary artery disease. European COMAC Group. Circulation 1998;97:437–43. [DOI] [PubMed] [Google Scholar]
- 6.Kelly PJ, Shih VE, Kistler JP, Barron M, Lee H, Mandell R, Furie KL. Low vitamin B6 but not homocyst(e)ine is associated with increased risk of stroke and transient ischemic attack in the era of folic acid grain fortification. Stroke 2003;34:e51–4. [DOI] [PubMed] [Google Scholar]
- 7.Kelly PJ, Kistler JP, Shih VE, Mandell R, Atassi N, Barron M, Lee H, Silveira S, Furie KL. Inflammation, homocysteine, and vitamin B6 status after ischemic stroke. Stroke 2004;35:12–5. [DOI] [PubMed] [Google Scholar]
- 8.CDC. Second national report on biochemical indicators of diet and nutrition in the U.S. population. Atlanta, GA: Department of Health and Human Services; 2012.
- 9.Institute of Medicine. Dietary reference intakes for thiamin, riboflavin, niacin, vitamin B6, folate, vitamin B12, pantothenic acid, biotin, and choline. Washington, DC: The National Academies Press; 1998. [PubMed]
- 10.Mosher WD, Martinez GM, Chandra A, Abma JC, Willson SJ. Use of contraception and use of family planning serviced in the United States: 1982–2002. Adv Data from Vital and Health Stat 2004;350:1–36. Available from: http://www.cdc.gov/nchs/data/ad/ad350.pdf. [PubMed]
- 11.Lussana F, Zighetti ML, Bucciarelli P, Cugno M, Cattaneo M. Blood levels of homocysteine, folate, vitamin B6 and B12 in women using oral contraceptives compared to non-users. Thromb Res 2003;112:37–41. [DOI] [PubMed] [Google Scholar]
- 12.Morris MS, Picciano MF, Jacques PF, Selhub J. Plasma pyridoxal 5′-phosphate in the US population: the National Health and Nutrition Examination Survey, 2003–2004. Am J Clin Nutr 2008;87:1446–54. [DOI] [PubMed] [Google Scholar]
- 13.Finkelstein JD. Methionine metabolism in mammals. J Nutr Biochem 1990;1:228–37. [DOI] [PubMed] [Google Scholar]
- 14.Dalgliesh CE, Knox WE, Neuberger A. Intermediary metabolism of tryptophan. Nature 1951;168:20–2. [DOI] [PubMed] [Google Scholar]
- 15.Ogasawara N, Hagino Y, Kotake Y. Kynurenine-transaminase, kynureninase and the increase of xanthurenic acid excretion. J Biochem 1962;52:162–6. [DOI] [PubMed] [Google Scholar]
- 16.Davis SR, Quinlivan EP, Stacpoole PW, Gregory JF. Plasma glutathione and cystathionine concentrations are elevated but cysteine flux is unchanged by dietary vitamin B-6 restriction in young men and women. J Nutr 2006;136:373–8. [DOI] [PubMed] [Google Scholar]
- 17.Davis SR, Scheer JB, Quinlivan EP, Coats BS, Stacpoole PW, Gregory JF. Dietary vitamin B-6 restriction does not alter rates of homocysteine remethylation or synthesis in healthy young women and men. Am J Clin Nutr 2005;81:648–55. [DOI] [PubMed] [Google Scholar]
- 18.Lamers Y, Coats B, Ralat M, Quinlivan EP, Stacpoole PW, Gregory JF. Moderate vitamin B-6 restriction does not alter postprandial methionine cycle rates of remethylation, transmethylation, and total transsulfuration but increases the fractional synthesis rate of cystathionine in healthy young men and women. J Nutr 2011;141:835–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nijhout HF, Gregory JF, Fitzpatrick C, Cho E, Lamers KY, Ulrich CM, Reed MC. A mathematical model gives insights into the effects of vitamin B-6 deficiency on 1-carbon and glutathione metabolism. J Nutr 2009;139:784–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.da Silva VR, Rios-Avila L, Lamers Y, Ralat MA, Midttun O, Quinlivan EP, Garrett TJ, Coats B, Shankar MN, Percival SS, et al. Metabolite profile analysis reveals functional effects of 28-day vitamin B-6 restriction on one-carbon metabolism and tryptophan catabolic pathways in healthy men and women. J Nutr 2013;143:1719–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lamers Y, Williamson J, Ralat M, Quinlivan EP, Gilbert LR, Keeling C, Stevens RD, Newgard CB, Ueland PM, Meyer K, et al. Moderate dietary vitamin B-6 restriction raises plasma glycine and cystathionine concentrations while minimally affecting the rates of glycine turnover and glycine cleavage in healthy men and women. J Nutr 2009;139:452–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rios-Avila L, Nijhout HF, Reed MC, Sitren HS, Gregory JF., 3rd A mathematical model of tryptophan metabolism via the kynurenine pathway provides insights into the effects of vitamin B-6 deficiency, tryptophan loading, and induction of tryptophan 2,3-dioxygenase on tryptophan metabolites. J Nutr 2013;143:1509–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ulvik A, Theofylaktopoulou D, Midttun O, Nygard O, Eussen SJ, Ueland PM. Substrate product ratios of enzymes in the kynurenine pathway measured in plasma as indicators of functional vitamin B-6 status. Am J Clin Nutr 2013;98:934–40. [DOI] [PubMed] [Google Scholar]
- 24.Cauci S, Di Santolo M, Culhane JF, Stel G, Gonano F, Guaschino S. Effects of third-generation oral contraceptives on high-sensitivity C-reactive protein and homocysteine in young women. Obstet Gynecol 2008;111:857–64. [DOI] [PubMed] [Google Scholar]
- 25.Norouzi V, Seifi M, Fallah S, Korani M, Samadikuchaksaraei A. Effect of oral contraceptive therapy on homocysteine and C-reactive protein levels in women: an observational study. Anadolu Kardiyol Derg 2011;11:698–702. [DOI] [PubMed] [Google Scholar]
- 26.Tucker KL, Olson B, Bakun P, Dallal GE, Selhub J, Rosenberg IH. Breakfast cereal fortified with folic acid, vitamin B-6, and vitamin B-12 increases vitamin concentrations and reduces homocysteine concentrations: a randomized trial. Am J Clin Nutr 2004;79:805–11. [DOI] [PubMed] [Google Scholar]
- 27.Bor MV, Refsum H, Bisp MR, Bleie O, Schneede J, Nordrehaug JE, Ueland PM, Nygard OK, Nexo E. Plasma vitamin B6 vitamers before and after oral vitamin B6 treatment: a randomized placebo-controlled study. Clin Chem 2003;49:155–61. [DOI] [PubMed] [Google Scholar]
- 28.Ubbink JB, Serfontein WJ, de Villiers LS. Stability of pyridoxal-5-phosphate semicarbazone: applications in plasma vitamin B6 analysis and population surveys of vitamin B6 nutritional status. J Chromatogr 1985;342:277–84. [DOI] [PubMed] [Google Scholar]
- 29.Pfeiffer CM, Huff DL, Gunter EW. Rapid and accurate HPLC assay for plasma total homocysteine and cysteine in a clinical laboratory setting. Clin Chem 1999;45:290–2. [PubMed] [Google Scholar]
- 30.Midttun Ø, Hustad S, Ueland PM. Quantitative profiling of biomarkers related to B-vitamin status, tryptophan metabolism and inflammation in human plasma by liquid chromatography/tandem mass spectrometry. Rapid Commun Mass Spectrom 2009;23:1371–9. [DOI] [PubMed] [Google Scholar]
- 31.Böger RH, Bode-Boger SM, Szuba A, Tsao PS, Chan JR, Tangphao O, Blaschke TF, Cooke JP. Asymmetric dimethylarginine (ADMA): a novel risk factor for endothelial dysfunction: its role in hypercholesterolemia. Circulation 1998;98:1842–7. [DOI] [PubMed] [Google Scholar]
- 32.Barker B, Rayens W. Partial least squares for discrimination. J Chemometrics 2003;17:166–73. [Google Scholar]
- 33.Wold S, Sjostrom M, Eriksson L. PLS-regression: a basic tool of chemometrics. Chemometr Intell Lab 2001;58:109–30.
- 34.Umetrics M. User guide to SIMCA. Malmo (Sweden): MKS Umetrics AB; 2013.
- 35.Wiklund S, Johansson E, Sjostrom L, Mellerowicz E, Edlund U, Shockcor J, Gottfries J, Moritz T, Trygg J. Visualization of GC/TOF-MS-based metabolomics data for identification of biochemically interesting compounds using OPLS class models. Anal Chem 2008;80:115–22. [DOI] [PubMed] [Google Scholar]
- 36.da Silva VR, Ralat MA, Quinlivan EP, DeRatt BN, Garrett TJ, Chi YY, Nijhout HF, Reed MC, Gregory JF. Targeted metabolomics and mathematical modeling demonstrate that vitamin B-6 restriction alters one-carbon metabolism in cultured HepG2 cells. Am J Physiol Endocrinol Metab 2014;307:E93–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Efron B, Gong G. A leisurely look at the bootstrap, the jacknife, and cross-validation. Am Statistician 1983;37:36–48.
- 38.Timm NH. Applied multivariate analysis. New York: Springer-Verlag; 2002.
- 39.National Center for Health Statistics. National Health and Nutrition Examination Survey (NHANES). Data documentation, codebook, and frequencies. Laboratory component. Vitamin B6. Survey years: 2005–2006. 2010 Sep. Available from: http://wwwn.cdc.gov/nchs/nhanes/2005-2006/VIT_B6_D.htm.
- 40.Gregory JF, Park Y, Lamers Y, Bandyopadhyay N, Chi YY, Lee K, Kim S, da Silva V, Hove N, Ranka S, et al. Metabolomic analysis reveals extended metabolic consequences of marginal vitamin B-6 deficiency in healthy human subjects. PLoS ONE 2013;8:e63544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhao M, Lamers Y, Ralat MA, Coats BS, Chi YY, Muller KE, Bain JR, Shankar MN, Newgard CB, Stacpoole PW, et al. Marginal vitamin B-6 deficiency decreases plasma (n-3) and (n-6) PUFA concentrations in healthy men and women. J Nutr 2012;142:1791–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lima CP, Davis SR, Mackey AD, Scheer JB, Williamson J, Gregory JFI. Vitamin B-6 deficiency suppresses the hepatic transsulfuration pathway but increases glutathione concentration in rats fed AIN-76A or AIN-93G diets. J Nutr 2006;136:2141–7. [DOI] [PubMed] [Google Scholar]
- 43.Lamers Y, O'Rourke B, Gilbert LR, Keeling C, Matthews DE, Stacpoole PW, Gregory JF. Vitamin B-6 restriction tends to reduce the red blood cell glutathione synthesis rate without affecting red blood cell or plasma glutathione concentrations in healthy men and women. Am J Clin Nutr 2009;90:336–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Midttun O, Ulvik A, Ringdal Pedersen E, Ebbing M, Bleie O, Schartum-Hansen H, Nilsen RM, Nygard O, Ueland PM. Low plasma vitamin B-6 status affects metabolism through the kynurenine pathway in cardiovascular patients with systemic inflammation. J Nutr 2011;141:611–7. [DOI] [PubMed] [Google Scholar]
- 45.Yeh JK, Brown RR. Effects of vitamin B-6 deficiency and tryptophan loading on urinary excretion of tryptophan metabolites in mammals. J Nutr 1977;107:261–71. [DOI] [PubMed] [Google Scholar]
- 46.Lepkovsky S, Roboz E, Haagen-Smit A. Xanthurenic acid and its role in the tryptophane metabolism of pyridoxine-deficient rats. J Biol Chem 1943;149:195–201. [DOI] [PubMed] [Google Scholar]
- 47.Yess N, Price JM, Brown RR, Swan PB, Linkswiler H. Vitamin B6 depletion in man: urinary excretion of tryptophan metabolites. J Nutr 1964;84:229–36. [DOI] [PubMed] [Google Scholar]
- 48.Leklem JE, Brown RR, Rose DP, Linkswiler HM. Vitamin B6 requirements of women using oral contraceptives. Am J Clin Nutr 1975;28:535–41. [DOI] [PubMed] [Google Scholar]
- 49.Brown RR, Rose DP, Leklem JE, Linkswiler H, Anand R. Urinary 4-pyridoxic acid, plasma pyridoxal phosphate, and erythrocyte aminotransferase levels in oral contraceptive users receiving controlled intakes of vitamin B6. Am J Clin Nutr 1975;28:10–9. [DOI] [PubMed] [Google Scholar]
- 50.Ulvik A, Midttun O, Pedersen ER, Eussen SJ, Nygard O, Ueland PM. Evidence for increased catabolism of vitamin B-6 during systemic inflammation. Am J Clin Nutr 2014;100:250–5. [DOI] [PubMed] [Google Scholar]
- 51.Flagg EW, Coates RJ, Jones DP, Eley JW, Gunter EW, Jackson B, Greenberg RS. Plasma total glutathione in humans and its association with demographic and health-related factors. Br J Nutr 1993;70:797–808. [DOI] [PubMed] [Google Scholar]
- 52.Bode-Böger SM, Scalera F, Kielstein JT, Martens-Lobenhoffer J, Breithardt G, Fobker M, Reinecke H. Symmetrical dimethylarginine: a new combined parameter for renal function and extent of coronary artery disease. J Am Soc Nephrol 2006;17:1128–34. [DOI] [PubMed] [Google Scholar]
- 53.Rose DP, Braidman IP. Excretion of tryptophan metabolites as affected by pregnancy, contraceptive steroids, and steroid hormones. Am J Clin Nutr 1971;24:673–83. [DOI] [PubMed] [Google Scholar]
- 54.Rose DP. The influence of oestrogens on tryptophan metabolism in man. Clin Sci 1966;31:265–72. [PubMed] [Google Scholar]
- 55.Price JM, Thornton MJ, Mueller LM. Tryptophan metabolism in women using steroid hormones for ovulation control. Am J Clin Nutr 1967;20:452–6. [DOI] [PubMed] [Google Scholar]
- 56.Braidman IP, Rose DP. Effects of sex hormones on three glucocorticoid-inducible enzymes concerned with amino acid metabolism in rat liver. Endocrinology 1971;89:1250–5. [DOI] [PubMed] [Google Scholar]