Abstract
Introduction:
L-arginine has a protective effect on gentamicin-induced renal failure and it may decrease the tubular reabsorption of another cationic substance, gentamicin due to its cationic structure. The aim of this study is to compare the possible protective effects of L-arginine and its inactive isomer D-arginine on gentamicin-induced nephrotoxicity in rats.
Materials and Methods:
Wistar albino rats were housed in metabolic cages and assigned to six groups as: control group, gentamicin (100 mg/kg), gentamicin + L-arginine (2 g/l), gentamicin + D-arginine (2 g/l), gentamicin + L-arginine + Nv-nitro-L-arginine methyl ester (L-NAME) (100 mg/l) and gentamicin + D-arginine + L-NAME. Gentamicin was administered by subcutaneous injections and the other drugs were added in drinking water for seven consecutive days. The animals were killed by decapitation and intracardiac blood and urine samples were obtained on the seventh day. Blood urea nitrogen, serum creatinine, sodium, potassium, urine gamma glutamyl transferase, creatinine, sodium, potassium and gentamicin levels were measured using High Performance Liquid Chromatography (HPLC) technique.
Results:
Gentamicin treated group had significant increase in blood urea nitrogen, serum creatinine, fractional Na excretion and urine gamma glutamyl transferase levels, and significant decrease in creatinine clearance compared to the control group. L-arginine and D-arginine reversed these findings. L-NAME abolished the nephroprotective effect of L-arginine. The urinary levels of gentamicin were significantly increased in rats treated with L-arginine or D-arginine compared to those treated with gentamicin. L-arginine and D-arginine reversed the advanced degenerative changes due to gentamicin administration in histopathological examination.
Conclusion:
Our study revealed the protective effect of L-arginine on gentamicin-induced nephrotoxicity, the contribution of the cationic feature of L-arginine, and the major role of NO in this protective effect.
KEY WORDS: D-arginine, gentamicin, HPLC, L-arginine, nephrotoxicity
Introduction
Aminoglycoside antibiotics are the most commonly used worldwide in the treatment of gram (-)negative bacterial infections. However, aminoglycosides induce nephrotoxicity in observed 10-20% of therapeutic courses.[1] The widespread therapeutic use of the gentamicin is limited because of its nephrotoxic side effect and oxidative damage which can lead to acute renal failure.[1,2,3]
Although the mechanism underlying gentamicin-induced renal cellular damage has not been entirely elucidated, generation of superoxide anion, hydrogen peroxide (H2O2) and hydroxyl radicals has been attributed to its deleterious effect on the kidney.[4,5] An association between nephrotoxicity and oxidative stress has been confirmed in many experimental models.[6,7] The possible involvement of the L-arginine-Nitric oxide (NO) pathway in gentamicin-induced nephrotoxicity has been suggested by Rivas-Cabanero et al. These investigators reported increased glomerular synthesis of NO in rats with gentamicin-induced renal failure.[8]
Arginine, an essential amino acid, has a positively charged guanidine group. Arginine is well-designed to bind the phosphate anion, and is often found in the active centers of proteins that bind phosphorylated substrates. As a cation, arginine plays an important role in maintaining the overall charge balance of a protein. L-Arginine has been intensively investigated, mainly since it is a precursor of NO that is a highly reactive and multifunctional molecule involved in many physiological processes, such as cardiovascular system, nervous system and immunological reactions.[9] On the other hand, D-arginine is frequently used in studies on L-arginine/NO pathway is an inactive form of L-arginine, because the D-enantiomer is not a substrate for the stereospecific NO synthase.[10,11,12,13,14]
Data from recent studies showed that the cationic proteins and peptides, inhibit the uptake of a nephrotoxic drug, gentamicin, which is highly accumulated in the kidneys.[15,16] Some authors report that L-arginine might have protected tubular function simply by decreasing gentamicin reabsorption in the proximal tubules.[17]
It is known that gentamicin has an organic polycationic structure. L-arginine consists of a functional cationic group of guanidin and due this cationic structural trait it can reduce gentamicin tubular reabsorption and increase gentamicin urinary excretion via competition. Therefore, in the present study, we aimed to investigate the possible protective effects other than NO synthesis of L-arginine and its inactive isomere D-arginine on gentamicin-induced nephrotoxicity in rats due to their structural similarities using High Performance Liquid Chromatography (HPLC) technique.
Materials and Methods
Experimental Animals
In this study, 42 male Wistar albino rats (appoximately 250–300 g weight) were procured from the Medical Science Experimental Research Center at Cukurova University. Rats were sheltered in metabolic cages at room temperature (24 ± 2°C) under 12 hours daylight, 12 hours dark conditions. The study was approved by the Medical Science Experimental Research Center Ethics Committee.
Experimental Protocol
The rats were divided into six groups and each group consisted of seven rats. The groups were as follows:
Group 1(Control group): This group was treated with normal food and tap water
Group 2(Gentamicin Group): This group was treated with (100 mg/kg gentamicin) subcutaneous (s.c.) injection for seven days
Group 3(Gentamicin + L-arginine Group): This group was treated with gentamicin injection and L-arginine (2 g/l) was added to their drinking water
Group 4(Gentamicin + D- arginine Group): This group was treated with gentamicin injection and D-arginine (2 g/l) was added to their drinking water
Group 5(Gentamicin + L-arginine + L-NAME Group): This group was treated with gentamicin injection and L-arginine (2 g/l) and L-NAME (100 mg/l) were added to their drinking water
Group 6 (Gentamicin + D-arginine + L- NAME Group): This group was treated with gentamicin injection and D-arginine (2 g/l) and L-NAME (100 mg/l) was added to in their drinking water.
During seven days water intake and urine volume were followed.
After the gentamicin injection, urine samples were collected on the first (initial), fourth (middle) and eighth day (final) to compare gentamicin levels. Ketamine (40 mg/kg) was given for anesthesia 24 hours after the final injection and intracardiac blood was obtained and animals were killed by decapitation. Providing the aseptic conditions, left kidney was removed by middle abdominal line incision for histopathological examination.
Biochemical Analysis
Blood urea nitrogen (BUN), serum creatinine, sodium (Na+), potassium (K+) levels were investigated from the blood of experimental animals. In addition, gama glutamyl transferase (GGT), creatinine, Na+ K+ levels in their urine were measured at Central Laboratory of Cukurova University. The clearance of creatinine (CCr) and fractional Na excretion (FENa) were calculated using the standard methods as below.
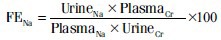
Histopathology of Kidney
The kidneys were treated with paraffine after the formaline (10% solution) fixation and approximately 3-5 μm cross-sections were dyed with hematoxylin–eosin. Tubular necrosis, degenerative variations and tubular regeneration were found under fluorescence microscope.
Measurement of Gentamicin Levels
Gentamicin levels of urine samples were evaluated according to the Kafkas et al., (2008) with major modifications.[18] The samples were stored at −20°C until analysis. HPLC analysis was performed in the Subtropical Fruits Research and Experimental Center Laboratory of Cukurova University. Identification and quantification were conducted using gentamicin (Sigma) standards. HPLC technique was used to identification and quantification of urine gentamicin levels.
Fort this purpose, Hewlett Packard 1100 series, HP-1100 UW dedector (210 nm) and Zorbax SB-C18 (10 cm × 4.6 mm, 1.8 μm, Beckman) column were used and flow speed was applied 1 ml/min.
Statistical analysis
Data was presented as means ± Standard error of mean (S.E.M) for the groups. A one-way variance analysis (One-Way ANOVA) method was used for comparison of results. Comparison of groups were performed using Bonferroni test. The level of significance was set as P < 0.05.
Experimental Drugs
In this study, L-Arginine monohydrochloride, N-nitro-L-arginine methyl ester monohydrochloride, D-Arginine monohydrochloride were procured from Sigma whereas gentamicin sulphate was procured from Schering-Plough.
Results
Control Group
The results of the blood and urine samples on the eighth day were found normally [Figures 1–4 and Table 1]. The renal particles and kidney tubules with normal structure were seen in the histopathological examination of the kidney tissues (data not shown).
Figure 1.
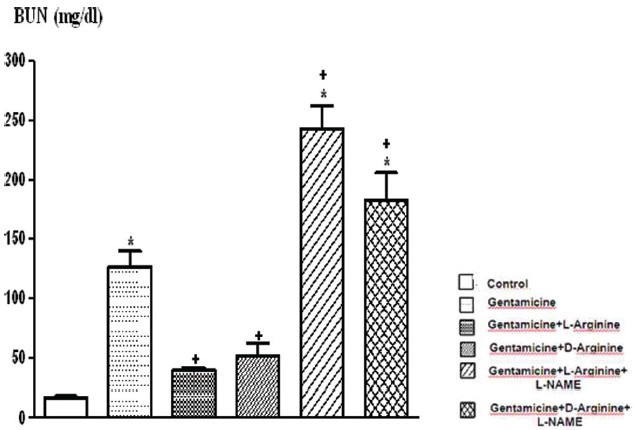
The comparison of blood urea nitrogen levels of the groups. Data are expressed as means ± S.E.M. for the groups; n = 7 in each group. Statistical analysis was performed by one-way analysis of variance (ANOVA) and comparison of groups were performed using Bonferroni test. *(P < 0.05) vs control group, +(P < 0.05) vs gentamicin group
Figure 4.
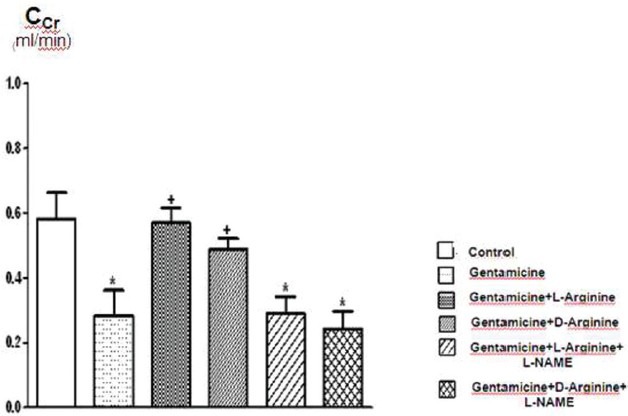
The comparison of Ccr (creatinine clearance) values of the groups. Data are expressed as means ± S.E.M. for the groups; n = 7 in each group. Statistical analysis was performed by one-way analysis of variance (ANOVA) and comparison of groups were performed using Bonferroni test. *(P < 0.05) vs control group, +(P < 0.05) vs gentamicin group
Table 1.
Plasma Na+, plasma K+ and urine GGT (gama glutamyl transferase) values of the groups. Data are expressed as means±S.E.M. for the groups; n=7 in each group. Statistical analysis was performed by one-way analysis of variance (ANOVA) and comparison of groups were performed using Bonferroni test
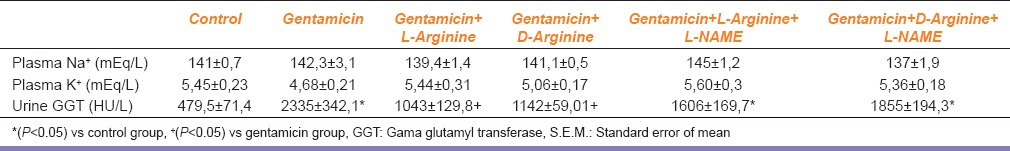
Figure 2.
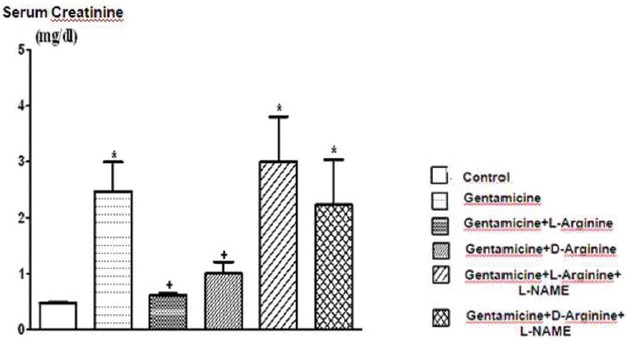
The comparison of serum creatinine levels of the groups. Data are expressed asmeans ± S.E.M. for the groups; n = 7 in each group. Statistical analysis was performed by one-way analysis of variance (ANOVA) and comparison of groups were performed using Bonferroni test. *(P < 0.05) vs control group, +(P < 0.05) vs gentamicin group
Gentamicin Group
Serum creatinine, BUN, FENa and urine GGT levels increased significantly after 8 days of gentamicin administration compared to the controls P < 0.05 [Figures 1–3 and Table 1]. This group was also characterized by a significantly lower creatinine clearance. However, there is no significant differences in other parameters. Degenerative changes were observed in the epitelial tissue of tubular epithelial tissue in the group treated with gentamicin. The epithelial cells covering the tubulus showed an increased acidophilic epithelial cell cytoplasm and nuclei couldn’t be identified, epithelial cells spilled renal particles abnormal capsular space was determined and also became narrow in some sites. The results of urine gentamicin levels were detected as shown in Figure 5.
Figure 3.
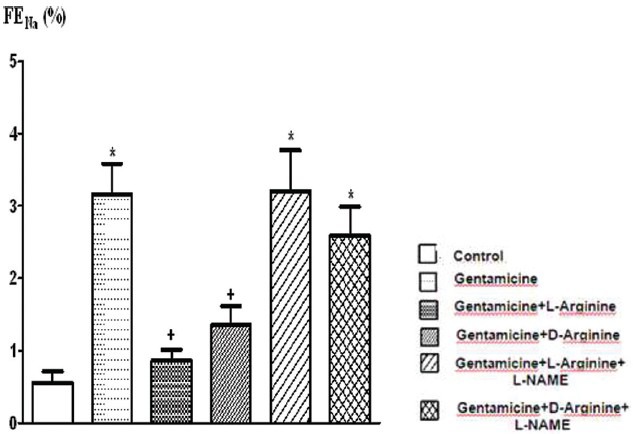
The comparison of FENa % (fractional Na excreation) levels of the groups. Data are expressed as means ± S.E.M. for the groups; n = 7 in each group. Statistical analysis was performed by one-way analysis of variance (ANOVA) and comparison of groups were performed using Bonferroni test. *(P < 0.05) vs control group, +(P < 0.05) vs gentamicin group
Figure 5.
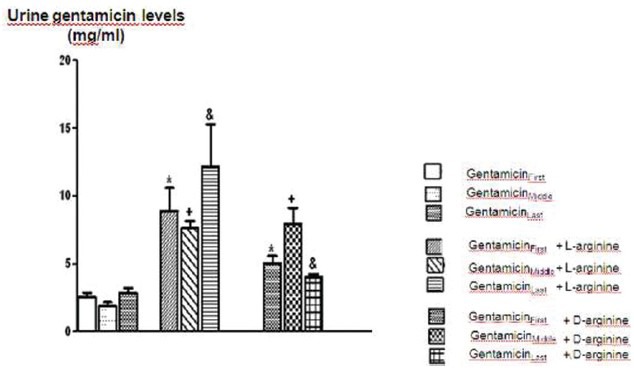
The comparison of urine gentamicin levels of Gentamicin, Gentamicin+L-arginine and Gentamicin+D-arginine applied groups. Data are expressed as means ± S.E.M. for the groups; n = 7 in each group. Statistical analysis was performed by one-way analysis of variance (ANOVA) and comparison of groups were performed using Bonferroni test. *(P < 0.05) vs gentamicin first group, +(P < 0.05) vs gentamicin in middle, and (P < 0.05) vs gentamicin in last group
Gentamicin + L-arginine Group
L-arginine administration provided marked protection manifested as significantly decreased serum creatinine, BUN, FENa and urine GGT levels and significantly increased creatinine clearance compared to the gentamicin group P < 0.05 [Figures 1–4 and Table 1]. However, other parameters detected were insignificant.
In this group, renal particles were observed as normal structure, the fallen of tubular epithelium and slight grade degenerative changes were also detected. The group which was applied only gentamicin was compared with the group gentamicin with L-argininine; the urine gentamicine levels were increased and this enhancement found to be statistically significant [Figure 5].
Gentamicin + D-arginine Group
Compared to the gentamicin group, D-arginine administration significantly decreased serum creatinine, BUN, FENa and urine GGT levels and significantly increased creatinine clearance P < 0.05 [Figures 1–4 and Table 1]. Besides this, other parameters detected were insignificant. In histopathological evaluation, slight grade degenerative changes and intracellular edema, necrosis and intermediate dropping were observed in tubular epithelium, as well.
The group which was applied only gentamicin was compared with the group gentamicin with D-arginine; the urine gentamicin levels were increased significantly [Figure 5].
Gentamicin + L-arginine + L-NAME Group
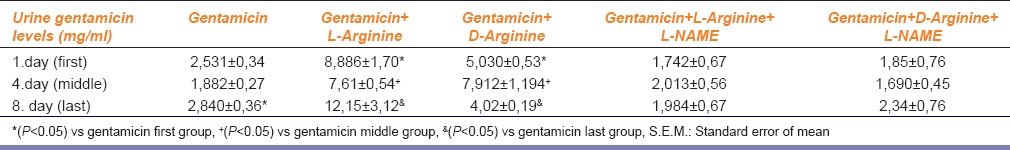
Comparison of gentamicin group, BUN levels increased significantly while other parameters were not found statistically significant [Figures 1–4 and Table 1]. According to the histopathological evaluation, enlargement of the renal corpuscle space, disappearance of cell details were observed, as well as fallen epithelium and advanced grade structural alternation were observed. As for the comparison with only gentamicin-applied to be group, the levels of gentamicin in urine were not significant [Table 2].
Table 2.
The urine gentamicin levels of experimental groups. Data are expressed as means±S.E.M. for the groups; n=7 in each group. Statistical analysis was performed by one-way analysis of variance (ANOVA) and comparison of groups were performed using Bonferroni test
Gentamicin + D-arginine + L-NAME Group
Compared with the gentamicin group, BUN, serum creatinine, FENa, CCr, urine GGT, plasma Na+ and plasma K+ values were not significant [Figures 1–4 and Table 1]. According to the histopathological evaulation, deformation observed in kidney tissue and tubular necrosis was high. Among the urine samples, compared with the only gentamicin applied group, gentamicin levels were not significant [Table 2].
Discussion
The aim of the present study was to investigate the possible protective effects of L-arginine and its inactive isomere D-arginine on gentamicin-induced nephrotoxicity in rats due to their structural similarities.
Aminoglycosides are totally eliminated by glomerular filtration and reabsorbed by the proximal tubules. Even therapeutic doses of these antibiotics commonly lead to proximal tubular cell necrosis in humans. This pathology was known due to the accumulation of gentamicin in renal cortex.[19,20] Similar results were obtained in this study and identification of nephrotoxicity criterias were found to be similar. The administration of gentamicin in rats induced a reduction in glomerular filtration rate as shown by a reduced creatinine clearance and increased serum creatinine. This impairment in glomerular function was accompanied by increased fractional excretion of Na+. This indicates proximal tubular dysfunction. The presence of tubular damage was further confirmed by the increased urinary excretion of the GGT and it indicates direct toxic injury. These findings correlated well with the renal morphological examination, which revealed tubular necrosis.
In rats treated with gentamicin and L-arginine together; biochemical and histopathological changes were found to be reversed. These results are similar to the previous reports.[21,22] However, L-arginine diminished the acute tubular necrosis as well. This effect can be due to the other mechanisms in addition to L-arginine-NO pathway. L-arginine is known as precursor of NO and can be used for nitric oxide synthesis. This amino acid is present and synthesized as endogenous. This includes cationic two functional guanidine groups.[23] This aminoacid can inhibit tubular reabsorbtion similar to gentamicin which has a cationic character. According to this information, L-Arginine increases the gentamicin excretion in urine. For this purpose, in this study we aimed to identify and quantify gentamicin levels after the injection of gentamicin first day (first), fourth day (middle) and eighth day (last) in urine samples by HPLC technique. As for the L-arginine given groups; urine gentamicin levels were increased statistically compared to only gentamicin given group. This result supports our hypothesis that L-arginin might have protected tubular function simply by decreasing gentamicin reabsorption. But, this increase can be explained as the increase of NO production which is known as a strong vasodilator and this can provide the improvement of hemodynamics. On the other hand, D-arginine has a similar and cationic structure but does not play a role in NO synthesis. We also detected an increase in urine gentamicin levels in D-arginine group. This result showed that the nephroprotective effect of D-arginine also can be explained as cationic characteristic.
The effect of nitric oxide synthase inhibition on renal failure has been investigated. Recent datas provided evidence that endothelial NOS leads to restoration of renal function after injury, while activation of inducible NOS, leading to excessive NO production, causes tubular cytotoxicity and aggravates renal failure. In our study, nephroprotective effects of L-arginine are reversed by L-NAME which is a non-selective inhibitor. It causes tubular deformation by inhibition of eNOS while positive effects of NOS inhibition may be prevented. This result shows that, NO has a major important role on protective effect of L-arginine. Nitric oxide is the most important paracrine modulator and mediator of controlling of renal functions such as renal blood flow, renal autoregulation, glomerular filtration, renin excretion and Na excretion. Nitric oxide plays an important role in diabetic nephropathy, inflammatory glomerular abnormality, acute and chronic kidney failure, effects of nephrotoxicity of medicine and several kidney defective states.[24,25]
In conclusion, the results of the present study demonstrate that administration of L-arginine and D-arginine has beneficial effects in rats with gentamicin-induced renal failure and that these effects are reversed by the NO-synthase inhibitor L-NAME. In addition, administration of L-arginine and its inactive form, D-arginine increases urine gentamicin levels. Althought it is known that NO has a major role on this protective effect, in our study L-arginine has also protected tubular function simply by decreasing gentamicin reabsorption in the proximal tubules due to having cationic structure. This protective mechanism other than NO synthesis can further be investigated.
Footnotes
Source of Support: Nil.
Conflict of Interest: No.
References
- 1.Balakumar P, Rohilla A, Thangathirupathi A. Gentamicin-induced nephrotoxicity: Do we have a promising therapeutic approach to blunt it? Pharmacol Res. 2010;62:179–86. doi: 10.1016/j.phrs.2010.04.004. [DOI] [PubMed] [Google Scholar]
- 2.Martinez-Salgado C, Lopez-Hernandez FJ, Lopez-Novoa JM. Glomerular nephrotoxicity of aminoglycosides. Toxicol Appl Pharmacol. 2007;223:86–98. doi: 10.1016/j.taap.2007.05.004. [DOI] [PubMed] [Google Scholar]
- 3.Stojiljkovic N, Mihailovic D, Veljkovic S, Stoiljkovic M, Jovanovic I. Glomerular basement membrane alterations induced by gentamicin administration in rats. Exp Toxicol Pathol. 2008;60:69–75. doi: 10.1016/j.etp.2008.02.007. [DOI] [PubMed] [Google Scholar]
- 4.Baliga R, Ueda N, Walker PD, Shah SV. Oxidant mechanisms in toxic acute renal failure. Am J Kidney Dis. 1997;29:465–77. doi: 10.1016/s0272-6386(97)90212-2. [DOI] [PubMed] [Google Scholar]
- 5.Walker PD, Barri Y, Shah SV. Oxidant mechanisms in gentamicin nephrotoxicity. Ren Fail. 1999;21:433–42. doi: 10.3109/08860229909085109. [DOI] [PubMed] [Google Scholar]
- 6.Ghaznavi R, Faghihi M, Kadkhodaee M, Shams S, Khastar H. Effects of nitric oxide on gentamicin toxicity in isolated perfused rat kidneys. J Nephrol. 2005;18:548–52. [PubMed] [Google Scholar]
- 7.Ozbek E, Turkoz Y, Sahna E, Ozugurlu F, Mizrak B, Ozbek M. Melatonin administration prevents the nephrotoxicity induced by gentamicin. BJU Int. 2000;85:742–6. doi: 10.1046/j.1464-410x.2000.00531.x. [DOI] [PubMed] [Google Scholar]
- 8.Rivas-Cabanero L, Montero A, Lopez-Novoa JM. Increased glomerular nitric oxide synthesis in gentamicin-induced renal failure. Eur J Pharmacol. 1994;270:119–21. doi: 10.1016/0926-6917(94)90088-4. [DOI] [PubMed] [Google Scholar]
- 9.Saavedra-Molina A, Pina E. Stimulation of L-ornithine uptake and L-citrulline and urea biosynthesis by D-arginine. Biochem Int. 1991;24:349–57. [PubMed] [Google Scholar]
- 10.Noda Y, Yamada K, Furukawa H, Nabeshima T. Involvement of nitric oxide in phencyclidine-induced hyperlocomotion in mice. Eur J Pharmacol. 1995;286:291–7. doi: 10.1016/0014-2999(95)00464-x. [DOI] [PubMed] [Google Scholar]
- 11.Calver A, Collier J, Vallance P. Dilator actions of arginine in human peripheral vasculature. Clin Sci (Lond) 1991;81:695–700. doi: 10.1042/cs0810695. [DOI] [PubMed] [Google Scholar]
- 12.Sander M, Chavoshan B, Victor RG. A large blood pressure-raising effect of nitric oxide synthase inhibition in humans. Hypertension. 1999;33:937–42. doi: 10.1161/01.hyp.33.4.937. [DOI] [PubMed] [Google Scholar]
- 13.Chambers DC, Ayres JG. Effect of nebulised L- and D-arginine on exhaled nitric oxide in steroid naive asthma. Thora×. 2001;56:602–6. doi: 10.1136/thorax.56.8.602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McVeigh GE, Allen PB, Morgan DR, Hanratty CG, Silke B. Nitric oxide modulation of blood vessel tone identifed by arterial waveform analysis. Clin Sci (Lond) 2001;100:387–93. [PubMed] [Google Scholar]
- 15.Nagai J, Komeda T, Yumoto R, Takano M. Effect of protamine on the accumulation of gentamicin in opossum kidney epithelial cells. J Pharm Pharmacol. 2013;65:441–6. doi: 10.1111/jphp.12005. [DOI] [PubMed] [Google Scholar]
- 16.Nagai J, Komeda T, Katagiri Y, Yumoto R, Takano M. Characterization of protamine uptake by opossum kidney epithelial cells. Biol Pharm Bull. 2013;36:1942–9. doi: 10.1248/bpb.b13-00553. [DOI] [PubMed] [Google Scholar]
- 17.Can C, Sen S, Boztok N, Tuglular I. Protective effect of oral L-arginine administration on gentamicin-induced renal failure in rats. Eur J Pharmacol. 2000;390:327–34. doi: 10.1016/s0014-2999(00)00025-x. [DOI] [PubMed] [Google Scholar]
- 18.Kafkas E, Ozgen M, Ozogul Y, Türemis N. Phytochemical and fatty acid profile of selected red raspberry cultivars: A comparative study. J Food Qual. 2008;1:67–78. [Google Scholar]
- 19.Mingeot-Leclercq MP, Tulkens PM. Aminoglycosides: Nephrotoxicity. Antimicrob Agents Chemother. 1999;43:1003–12. doi: 10.1128/aac.43.5.1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zaske DE. Aminoglycosides. In: Ewans WE, Schentag JJ, Jusko WJ, editors. Applied Pharmocokinetics. Principles of Therapeutic Drug Monitoring. 3rd ed. Vancouver: WA; 1992. pp. 141–7. [Google Scholar]
- 21.Karatas Y, Secilmis MA, Karayaylalı İ, Doran F, Buyukafsar K, Singirik E, et al. Effect of tempol (4-hydroxy tempo) on gentamicin-induced nephrotoxicity in rats. Fundam Clin Pharmacol. 2004;18:79–83. doi: 10.1046/j.0767-3981.2003.00211.x. [DOI] [PubMed] [Google Scholar]
- 22.Secilmis MA, Karatas Y, Yorulmaz O, Buyukafsar K, Singirik E, Doran F, et al. Protective effect of L-arginine intake on the impaired renal vascular responses in the gentamicin-treated rats. Nephron Physiol. 2005;100:p13–20. doi: 10.1159/000084657. [DOI] [PubMed] [Google Scholar]
- 23.Balsam L, Nikbakht N. L-Arginine inhibits vasopressin-stimulated mesangial cell Ca2+ Am J Physiol. 1998;275:C352–7. doi: 10.1152/ajpcell.1998.275.2.C352. [DOI] [PubMed] [Google Scholar]
- 24.Matsamura Y, Nishiura M, Deuchi S, Hashimato N, Ogawa T, Seo R. Protective effect of FK409, a spontenous nitric oxide releaser, on ischemic acute renal failure in rats. J Pharmacol Exp Ther. 1998;287:1084–91. [PubMed] [Google Scholar]
- 25.Noiri E, Peresieni T, Miller F, Goligorsky MS. In vivo targeting of inducible NO synthase with oligodeoxynucleotides protects rat kidney against ischemia. J Clin Invest. 1996;97:2377–83. doi: 10.1172/JCI118681. [DOI] [PMC free article] [PubMed] [Google Scholar]


