Abstract
Objectives:
Brain stroke is a leading cause of death without effective treatment. Feronia limonia have potent antioxidant activity and can be proved as neuroprotective against ischemia-reperfusion induced brain injury.
Materials and Methods:
We studied the effect of methanolic extract of F. limonia fruit (250 mg/kg, 500 mg/kg body weight, p.o.) and Vitamin E as reference standard drug on 30 min induced ischemia, followed by reperfusion by testing the neurobehavioral tests such as neurodeficit score, rota rod test, hanging wire test, beam walk test and elevated plus maze. The biochemical parameters, which were measured in animals brain were catalase, superoxide dismutase (SOD), malondialdehyde and nitric oxide in control and treated rats.
Results:
The methanolic extract of F. limonia fruit (250 mg/kg, 500 mg/kg body weight, p.o.) treated groups showed a statistically significant improvement in the neurobehavioral parameters such as motor performance (neurological status, significant increase in grasping ability, forelimb strength improvement in balance and co-ordination). The biochemical parameters in the brains of rats showed a significant reduction in the total nitrite (P < 0.01) and lipid peroxidation (P < 0.01), also a significant enhanced activity of enzymatic antioxidants such as catalase (P < 0.01) and SOD (P < 0.05).
Conclusion:
These observations suggest the neuroprotective and antioxidant activity of F. limonia and Vitamin E on ischemia reperfusion induced brain injury and may require further evaluation.
KEY WORDS: Brain ischemia-reperfusion, Feronia limonia, neuroprotection, oxidative stress
Introduction
Stroke is one of the leading causes of death worldwide.[1] Deficiency of blood in a part, usually due to functional constriction or actual obstruction of blood vessel is called ischemia. Ischemia contributes to the pathophysiology of many conditions faced by anesthesiologists, including myocardial infarction, peripheral vascular insufficiency, stroke, and hypovolemic shock. Although the restoration of blood flow to an ischemic organ is essential to prevent irreversible cellular injury, reperfusion may augment tissue injury in excess of that produced by ischemia alone. For example, the histological changes of injury after 3 h of feline intestinal ischemia, followed by 1 h of reperfusion are far worse than the changes observed after 4 h of ischemia alone.[2]
During ischemia, cellular adenosine triphosphate is degraded to form the hypoxanthine. Normally, hypoxanthine is oxidized by xanthine dehydrogenase to xanthine. However, during ischemia, xanthine dehydrogenase is converted to xanthine oxidase. Unlike xanthine dehydrogenase, which uses nicotinamide adenine dinucleotide as its substrate, xanthine oxidase uses oxygen and therefore, during ischemia, is unable to catalyze the conversion of hypoxanthine to xanthine, resulting in a buildup of excess tissue levels of hypoxanthine. When oxygen is reintroduced during reperfusion, conversion of the excess hypoxanthine by xanthine oxidase results in the formation of toxic reactive oxygen species (ROS). Reperfusion of ischemic tissues results in the formation of toxic ROS, including superoxide anions (O2-), hydroxyl radicals (OH-), hypochlorous acid (HOCl), hydrogen peroxide (H2O2) and nitric oxide-derived peroxynitrite. ROS are potent oxidizing and reducing agents that directly damage cellular membranes by lipid peroxidation[3] Peroxynitrite[4] and hydroxyl radical[5] are reported to produce deoxyribonucleic acid (DNA) nicking. ROS are also documented to activate lysosomal enzymes, which may contribute to neuronal injury.[6] In addition, mitochondrial damages due to ROS release may contribute to delayed cell death after cerebral ischemia and reperfusion.[7]
Feronia limonia Swingle (wood apple) belongs to the family Rutaceae (syns. Feronia elephantum Correa; Limonia acidissima) The ripe fruit of which is rich in beta-carotene, a precursor of Vitamin A, it also contains significant quantities of the B vitamins thiamine and riboflavin, and citric acid, oxalic acid and malic acid. The wood-apple fruit is more popular as medicine than as food. The tannin in wood-apple had an astringent effect and was used as a general tonic and as a traditional cure for dysentery, diarrhea, liver ailments, chronic cough and indigestion. The root juice was once popular as a remedy for snakebites.[8]
Antioxidant phytoconstituents are present in F. limonia Swingle fruit. Therefore, in this study we investigated the effects of this fruit on ischemia reperfusion induced brain injury by studying the antioxidant effect in a relationship with behavioral and biochemical parameters.
Materials and Methods
Materials and Subjects
The F. limonia (woodapple) pulp powder was Soxhlet-extracted with methanol, and the extract yield was 23% w/w on dry weight basis. The methanolic extract was dissolved in distilled water to obtain the required dosage concentration. Eight weeks old Albino Wistar rats of either sex weighing 200–250 g were used. The animals were maintained under controlled laboratory conditions and were exposed to a 12-h light-dark cycles. The animals were acclimatized for 7 days before the study. They had free access to standard pellets as basal diet and water ad libitum. Animals were habituated to laboratory conditions for 48 h prior to the experimental protocol to minimize if any of nonspecific stress.
Acute Toxicity
Toxicity study up and down procedure was carried out as per the guidelines set by Organization for Economic Co-operation and Development (OECD) guidelines 423, 425–430. Two groups of Wistar rats (n = 3) were fasted overnight with water ad libitum and foods were withheld for 3–4 h after oral administration of the extracts. One group of animals were treated with starting dose of 2000 mg/kg body weight orally and the maximum dose of 5000 mg/kg body weight was administered to the second group. Another group was treated with normal saline and were observed individually. Clinical signs including changes in skin fur, eyes and mucous membranes were observed. The gross behaviors like body positions, locomotion, rearing, tremors, gait was observed. The effect of the extract on passivity, grip strength, pain response, stereotypy, vocalization, righting reflex, body weight and water intake were observed. No mortality was observed with this dose. As per OECD guidelines the substance might be considered to have an LD50 value above 2000 mg/kg and 5000 mg/kg body weight.
Preparation of Doses
From acute oral toxicity study, it was found that methanolic extract of F. limonia fruit was safe at limit dose 5000 mg/kg and 2000 mg/kg, therefore dose 1/10th of this dose, that is, 500 mg/kg and below were used in subsequent study for all extract of F. limonia fruit pulp.
Experimental Method
All experiments were carried out in accordance with the guidelines of Committee for the Purpose of Control and Supervision of Experiments on Animals (CPCSEA) and the Institutional Animal Ethics Committee (IAEC) the approval no. was CPCSEA/IAEC/P’col-10/2010–11/26, dated December 11, 2010. Sufficient measures were adopted to minimize pain and discomfort with animal experimental procedures. Rats were anesthetized with ketamine (50 mg/ml/kg) injected intra-peritoneally. A small cut was made on the neck region, and the neck muscles were retracted for isolation of the common carotid artery. The internal carotid artery was subsequently isolated, and 30 min of ischemia was given to the rat by blocking the left internal branch of the common carotid artery was occluded with micro-vascular clip. After the ischemic period, the neck muscle was stitched, and an antibiotic was applied. The test drug solution or suspension was administered to the respective group of rats described below for a period of 8 days. The animals were randomly divided into six groups, each group comprising of 05 animals. Group 1 (sham) animals served as normal control and received vehicle (saline 10 ml/kg orally) for 8 days. The ischemia reperfusion was produced in Groups 2–6. After the induction of 30 min ischemia and reperfusion, Group 2 rats were administered with vehicle (saline 10 ml/kg orally for 8 days). Vitamin E (50 mg/kg orally) was administered to Group 3 animals. Group 4 and 5 animals were administered with methanolic extract of F. limonia (250 mg/kg and 500 mg/kg orally) respectively for 8 days. The saline, methanolic extract of F. limonia and Vitamin E were administered orally with the help of oral gavage needle. Seven additional days of post ischemic survival time were provided. On the 7th and 8th days behavioral studies were carried out. Rats were sacrificed by an overdose of ketamine (75 mg/ml/kg) injected intra-peritoneally as an anesthetic on the 8th day after completion of behavioral tests. The isolated brains were frozen for biochemical tests.
Neurobehavioral Test
Neurodeficit score
The neurological status of the animals was evaluated using the methods described by Bederson et al.[9] Accordingly, four categories of neurological findings were observed and noted: 0 = no observed neurological deficit; 1 = contralateral forelimb flexion with wrist flexion and shoulder adduction; 2 = reduced resistance to lateral push; and 3 = circling movements towards the ipsilateral side.
Rota Rod Test
Sensorimotor performance was evaluated using a rota rod test. All animals were tested for their ability to remain on the rotating bar at a speed of 20 revolutions/min (rpm) on a Rotarod apparatus (Inco). Each animal was trained for a minimum of three trials. After 8 post ischemic days, the animals were tested for motor impairment after administration of test drugs. Latency to fall off from the rotating rod was noted for each trial with a 5 min maximum to termination of the trials.
Hanging Wire
The experimental animals were suspended by its forelimbs on a wire stretched between two posts, 45 cm above a foam sheet. The time in seconds (s), until the animal fell down, was recorded. 2 min of cut off time was designated. This task was used as a measure of grasping ability and forelimb strength.
Beam Walk Test
Beam walk test was employed to evaluate fore and hind limbs motor co-ordination. Each animal was individually placed on a beam walk apparatus (Inco) made up of a wooden bar 60 cm long and 1.5 cm wide, height 50 cm. The motor performance of rat scored on a scale ranging from 0 to 4. This is a special test for animals subjected to cerebral ischemia and reperfusion. For motor incoordination, Number of foot slip; Number of falls; distance travelled along beam was studied.
Memory Test
Elevated plus maze
The elevated plus-maze apparatus (Inco) for rat consists of a central platform connected to two open arms and two enclosed arms. The maze is elevated to a height of 50 cm from the floor. During training trials the animal was placed at the end of an open arm, facing away from the central platform of the maze. The time taken by the animal to move from open arm and cross the line marked in enclosed arm with all four paws was recorded as transfer latency (TL) time. In case the rats did not enter the enclosed arm within 90 s, it was gently pushed into the enclosed arm and a TL of 90 s was assigned to it. The animal was allowed to remain in the maze for the duration of 10 s. The TL measured on the plus maze on the 1st day serves as an index of acquisition, whereas the TL measured after 24 h of acquisition trial was taken as an index of retrieval.
Biochemical Estimation
In this study, six rats per group were used. After completion of the behavioral test, the brain was isolated. All six brains were used for biochemical tests. The pooling of brain was not done for biochemical tests. The small homogenate of individual brain was made in saline and further divided in tubes and used for various biochemical tests.
Preparation of Post Mitochondrial Supernatant
The tissues were homogenized in chilled potassium phosphate buffer (50 mM, potential of hydrogen 7.4) using a Potter-Elvehjem homogenizer. The homogenate was centrifuged in a refrigerated centrifuge at (10,500 rpm) for 20 min at 4°C to obtain the post mitochondrial supernatant, which was used for several of enzymes such as catalase, superoxide dismutase (SOD) and nitric oxide.
Catalase activity was assayed in the postmitochondrial supernatant by the method of Clairborne (1985).[10] Superoxide dismutase (SOD) level was measured by the method of Marklund (1985).[11] The total protein was estimated by Lowry's et al. (1959) method.[12] Nitric oxide was estimated by an indirect measurement of nitrite, nitrate and total nitrite in rat brain extract supernatants obtained after centrifugation. The total nitrite contents of the sample were measured from the calibration curve for nitrite. The total nitrite was estimated by the method of Green et al. (1982).[13] The lipid peroxidation, that is, malondialdehyde (MDA) in the homogenate was determined by the method of Ohkawa et al. (1979).[14] The results were expressed as nmol of MDA/mg protein.
Statistical Analyses
Behavioural data were analyzed by analysis of variance followed by post-Dunnett's test. It was carried out by using with Graph Pad Instat followed by Dunnett's test at level of significance P < 0.05 value. All data were shown as the mean ± standard error of the mean. Statistical analysis was performed using Graph Pad statistical software (Graph Pad Software, Inc. California, USA).
Results
Behavioral Result
The rat model of ischemia was established by temporary blocking of the internal carotid artery for 30 min (ischemia) followed by reperfusion and it leads to sudden loss of vision, balance, coordination, and memory. The cognitive and neurological status of the rat was assessed by behavioral test.
Feronia Limonia Attenuates Neurological Score and Motor Performance in Ischemic Rats
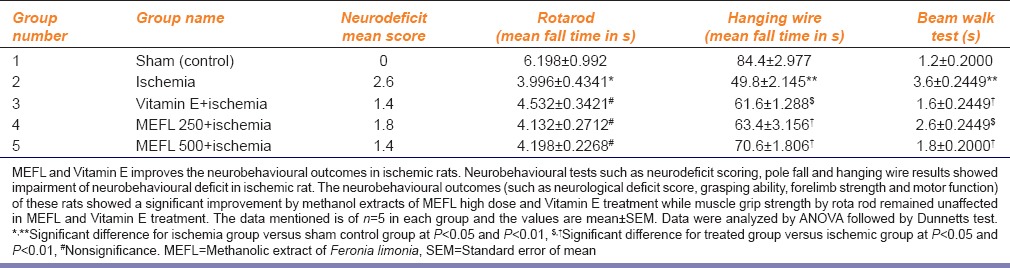
Neurobehavioral tests such as neurodeficit scoring, pole fall and hanging wire results showed impairment in neurobehavioral scale in Group 2 ischemic rats [Table 1]. The neurobehavioural outcomes (such as neurological deficit score, grasping ability, forelimb strength [P < 0.01] and [P < 0.05], and motor function [P < 0.01] and [P < 0.01]) were significantly improved by methanolic extracts of F. limonia (500 mg/kg) and Vitamin E (50 mg/kg) as compared to vehicle treated Group 2 animals, while methanolic extracts of F. limonia (250 mg/kg) does not significantly decreased the neurological deficit score. No significant improvement was observed in rotarod test by F. limonia and Vitamin E treatment as compared to Group 2 [Table 1].
Table 1.
Effect of MEFL and Vitamin E on neurobehavioral tests in ischemia-reperfusion injury in rats
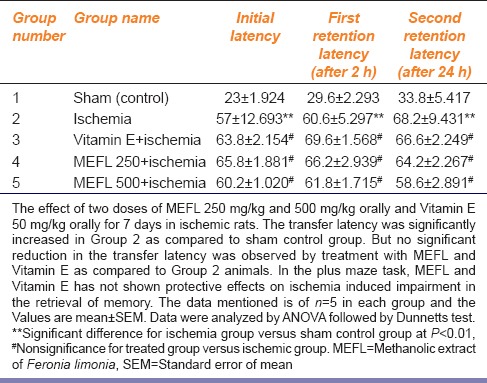
Feronia Limonia Does not Attenuate Ischemia Induced Memory Deficits
We tested the effect of two doses of F. limonia 250 mg/kg and 500 mg/kg orally and Vitamin E 50 mg/kg orally for 7 days in ischemic rats. The TL was significantly increased in Group 2 as compared to sham control group (P < 0.01). But no significant reduction in the TL was observed by treatment with F. limoniaand Vitamin E as compared to Group 2 animals. In the plus maze task, F. limonia and Vitamin E has not shown protective effects on ischemia induced impairment in the retrieval of memory [Table 2].
Table 2.
Effect of MEFL and Vitamin E on elevated plus maze in ischemia-reperfusion injury in rats
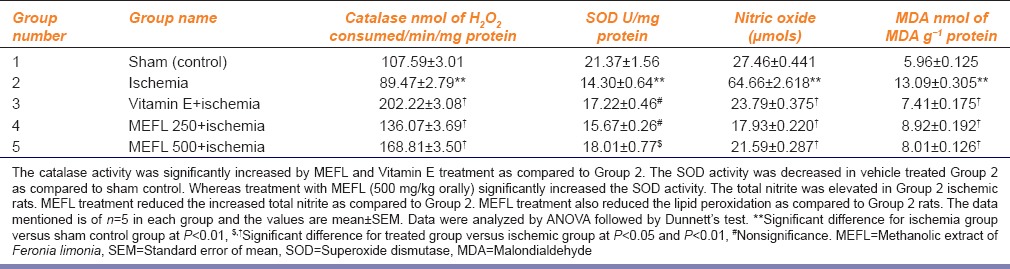
Feronia Limonia Exerts Antioxidant Effect in Ischemic Rats
The Catalase activity was significantly increased by F. limonia (P < 0.01) and Vitamin E (P < 0.01) treatment as compared to Group 2. The superoxide dismutase activity was decreased in vehicle treated Group 2 as compared to sham control (P < 0.01). Whereas treatment with F. limonia (500 mg/kg p.o.) significantly increased the superoxide dismutase activity (P < 0.05). The total nitrite was elevated in Group 2 ischemic rats. F. limonia treatment reduced the increased total nitrite as compared to Group 2. F. limonia treatment also reduced the lipid peroxidation as compared to Group 2 rats (P < 0.01) [Table 3].
Table 3.
Effects of MEFL and Vitamin E on catalase, SOD, nitric oxide and MDA in brain tissue of ischemia-reperfusion injury in rats
Discussion
Ischemia is the deficiency of blood in a part, usually due to functional constriction or actual obstruction of blood vessel and reperfusion injury refers to tissue damage caused when blood supply returns to the tissue after a period of ischemia. Ischemia-reperfusion associated with thrombolytic therapy, organ transplantation, coronary angioplasty, aortic cross-clamping, or cardiopulmonary bypass results in local and systemic inflammation. Brain stroke is a sudden loss of brain function usually caused by a blockade or leakage of a blood vessel. It develops from a complex cascade of cellular events that ultimately leads to cerebral infarction[15] and causes sudden loss of vision, balance, co-ordination, speech and memory.[16]
The present study was undertaken to evaluate the neuroprotective effect of F. limonia on ischemia reperfusion brain injury. The neurotoxicity was produced by blocking internal carotid artery with the help of microvascular clip followed by reperfusion. Reperfusion of ischemic tissue result in the formation of toxic ROS including, O2-, OH-, HOCl, H2O2 and nitric oxide-derived peroxynitrite radicals. These toxic ROS induces oxidative stress.[3] Chemically, oxidative stress is associated with increased production of oxidizing species or a significant decrease in the capability of antioxidant defenses, such as catalase and SOD enzymes. Severe oxidative stress can cause cell death and even moderate oxidation can trigger apoptosis, while more intense stresses may cause necrosis. ROS can cause cellular damage by oxidizing membrane lipids, essential cellular proteins and DNA.[17]
Feronia limonia (woodapple) is common fruit in India. The woodapple is rich in β carotene and citric acid. The earlier reports supports that the woodapple have potent antioxidant activity[18,19] they may prove as neuroprotective against ischemia-reperfusion induced brain injury, so this fruit was chosen for present study. In the absence of substantial results with single phytoconstituent direction, herbal extracts containing several phytoconstituents are being evaluated for neuroprotective and therapeutic effects. The neuroprotective effect of two oral doses of F. limonia namely, 250 mg/kg, 500 mg/kg and Vitamin E 50 mg/Kg as reference was tested in ischemic rats. In this study, we report that the two doses of F. limonia (250 mg/kg, 500 mg/kg) significantly improved the ischemia induced neurological status, forelimb strength, balance and co-ordination, that is, motor performance but no significant changes in memory in neurodeficit score, rotarod test, hanging wire test, beam walk test and elevated plus maze tasks. Though the solvent methanol alone can influence motor behavior of the animals, we used methanolic extract of F. limonia fruit pulp containing the phytoconstituents which are soluble in methanol and further the extract was evaporated at room temperature. Hence, the effect of alone methanol is totally reduced. This finding is supported by the previous studies where Bacopa monniera reversed these effects in ischemia reperfusion brain injury due to its antioxidant potential.[20]
Furthermore, it has been reported that B. monniera showed protective effect from phenytoin induced cognitive deficit due to the antioxidant activity.[21] These observations clearly indicate a protective role of F. limonia. Several other herbal drugs obtained from medicinal plants have also shown the potential neuroprotective effect in rodents.[22,23,24]
The biochemical investigations of the present study have shown that F. limonia significantly increased the catalase and superoxide dismutase enzyme activities, which suggests that F. limonia may reduce the formation of free radicals. It was also observed that F. limonia reduced the total nitrite and MDA which is the marker of lipid peroxidation, suggesting that the antioxidant potential of F. limonia may have reduced the formation of ROS and subsequently prevented the disastrous chain reaction. This finding is supported by the previous studies where curcumin, resveratrol, silymarin, green tea extract containing antioxidant potential have counteracted the ROS generation during ischemia-reperfusion induced brain injury. The phytoconstituents present in F. limonia are beta-carotene, B vitamins, thiamine and riboflavin, which have been already proved to be potent antioxidant. Hence, in this study the neuroprotective effects of F. limonia fruit were observed.
Therefore, we suggest that F. limonia fruit may be useful in stroke and may prove to be neuroprotective.
We conclude that F. limonia have attenuated the ischemia-reperfusion induced neurological deficit, decrease in motor performance on hanging wire and beam walk test and by exerting the antioxidant effects. Further investigations with the isolation of purified active phytoconstituents of F. limonia may also validate the neuroprotective and antioxidant effects.
Footnotes
Source of Support: Nil.
Conflict of Interest: No.
References
- 1.Rensink M, Schuurmans M, Lindeman E, Hafsteinsdóttir T. Task-oriented training in rehabilitation after stroke: Systematic review. J Adv Nurs. 2009;65:737–54. doi: 10.1111/j.1365-2648.2008.04925.x. [DOI] [PubMed] [Google Scholar]
- 2.Parks DA, Granger DN. Contributions of ischemia and reperfusion to mucosal lesion formation. Am J Physiol. 1986;250:G749–53. doi: 10.1152/ajpgi.1986.250.6.G749. [DOI] [PubMed] [Google Scholar]
- 3.Toyokuni S. Reactive oxygen species-induced molecular damage and its application in pathology. Pathol Int. 1999;49:91–102. doi: 10.1046/j.1440-1827.1999.00829.x. [DOI] [PubMed] [Google Scholar]
- 4.Epe B, Ballmaier D, Roussyn I, Briviba K, Sies H. DNA damage by peroxynitrite characterized with DNA repair enzymes. Nucleic Acids Res. 1996;24:4105–10. doi: 10.1093/nar/24.21.4105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Delaney CA, Green IC, Lowe JE, Cunningham JM, Butler AR, Renton L, et al. Use of the comet assay to investigate possible interactions of nitric oxide and reactive oxygen species in the induction of DNA damage and inhibition of function in an insulin-secreting cell line. Mutat Res. 1997;375:137–46. doi: 10.1016/s0027-5107(97)00009-2. [DOI] [PubMed] [Google Scholar]
- 6.Ollinger K, Brunk UT. Cellular injury induced by oxidative stress is mediated through lysosomal damage. Free Radic Biol Med. 1995;19:565–74. doi: 10.1016/0891-5849(95)00062-3. [DOI] [PubMed] [Google Scholar]
- 7.Fiskum G. Mitochondrial participation in ischemic and traumatic neural cell death. J Neurotrauma. 2000;17:843–55. doi: 10.1089/neu.2000.17.843. [DOI] [PubMed] [Google Scholar]
- 8.Phalale R, Thakur SM. Antioxidant activity and antimutagenic effect of phenolic compound in Feronia limonia (L) swingle fruit. Int J Pharm Sci. 2010;2:68–73. [Google Scholar]
- 9.Bederson JB, Pitts LH, Tsuji M, Nishimura MC, Davis RL, Bartkowski H. Rat middle cerebral artery occlusion: Evaluation of the model and development of a neurologic examination. Stroke. 1986;17:472–6. doi: 10.1161/01.str.17.3.472. [DOI] [PubMed] [Google Scholar]
- 10.Clairborne A. Catalase activity. In: Greenwald RA, editor. Handbook of Methods for Oxygen Radical Research. Boca Raton: CRC Press; 1985. pp. 283–4. [Google Scholar]
- 11.Marklund SL. Pyrogallol autooxidation. In: Greenwald RA, editor. Handbook of Methods for Oxygen Radical Research. Boca Raton: CRC Press; 1985. pp. 243–7. [Google Scholar]
- 12.Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951;193:265–75. [PubMed] [Google Scholar]
- 13.Green LC, Wagner DA, Glogowski J, Skipper PL, Wishnok JS, Tannenbaum SR. Analysis of nitrate, nitrite, and [15N] nitrate in biological fluids. Anal Biochem. 1982;126:131–8. doi: 10.1016/0003-2697(82)90118-x. [DOI] [PubMed] [Google Scholar]
- 14.Ohkawa H, Ohishi N, Yagi K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem. 1979;95:351–8. doi: 10.1016/0003-2697(79)90738-3. [DOI] [PubMed] [Google Scholar]
- 15.Hou ST, MacManus JP. Molecular mechanisms of cerebral ischemia-induced neuronal death. Int Rev Cytol. 2002;221:93–148. doi: 10.1016/s0074-7696(02)21011-6. [DOI] [PubMed] [Google Scholar]
- 16.O’Brien JT, Erkinjuntti T, Reisberg B, Roman G, Sawada T, Pantoni L, et al. Vascular cognitive impairment. Lancet Neurol. 2003;2:89–98. doi: 10.1016/s1474-4422(03)00305-3. [DOI] [PubMed] [Google Scholar]
- 17.Camhi SL, Lee P, Choi AM. The oxidative stress response. New Horiz. 1995;3:170–82. [PubMed] [Google Scholar]
- 18.Maxwell SR, Lip GY. Reperfusion injury: A review of the pathophysiology, clinical manifestations and therapeutic options. Int J Cardiol. 1997;58:95–117. doi: 10.1016/s0167-5273(96)02854-9. [DOI] [PubMed] [Google Scholar]
- 19.Yang J, Klaidman LK, Chang ML, Kem S, Sugawara T, Chan P, et al. Nicotinamide therapy protects against both necrosis and apoptosis in a stroke model. Pharmacol Biochem Behav. 2002;73:901–10. doi: 10.1016/s0091-3057(02)00939-5. [DOI] [PubMed] [Google Scholar]
- 20.Saraf MK, Prabhakar S, Anand A. Neuroprotective effect of Bacopa monniera on ischemia induced brain injury. Pharmacol Biochem Behav. 2010;97:192–7. doi: 10.1016/j.pbb.2010.07.017. [DOI] [PubMed] [Google Scholar]
- 21.Vohora D, Pal SN, Pillai KK. Protection from phenytoin-induced cognitive deficit by Bacopa monniera, a reputed Indian nootropic plant. J Ethnopharmacol. 2000;71:383–90. doi: 10.1016/s0378-8741(99)00213-5. [DOI] [PubMed] [Google Scholar]
- 22.Chen M, Wang Y, Liu Y, Hou XY, Zhang QG, Meng FJ, et al. Possible mechanisms underlying the protective effects of SY-21, an extract of a traditional Chinese herb, on transient brain ischemia/reperfusion-induced neuronal death in rat hippocampus. Brain Res. 2003;989:180–6. doi: 10.1016/s0006-8993(03)03331-6. [DOI] [PubMed] [Google Scholar]
- 23.Kim YT, Yi YJ, Kim MY, Bu Y, Jin ZH, Choi H, et al. Neuroprotection and enhancement of spatial memory by herbal mixture HT008-1 in rat global brain ischemia model. Am J Chin Med. 2008;36:287–99. doi: 10.1142/S0192415X08005771. [DOI] [PubMed] [Google Scholar]
- 24.Tian J, Li G, Liu Z, Zhang S, Qu G, Jiang W, et al. ND-309, a novel compound, ameliorates cerebral infarction in rats by antioxidant action. Neurosci Lett. 2008;442:279–83. doi: 10.1016/j.neulet.2008.07.033. [DOI] [PubMed] [Google Scholar]


