Abstract
Objectives:
This study was aimed to investigate the therapeutic potential of coenzyme Q10 and its combination with metformin on streptozotocin (STZ)-nicotinamide-induced diabetic nephropathy (DN).
Materials and Methods:
Type 2 diabetes in rats was induced with STZ-nicotinamide. The diabetic rats were treated with coenzyme Q10 (10 mg/kg, p.o.) alone or coenzyme Q10 + metformin. Various parameters of renal function tests such as serum creatinine, urea, uric acid, and markers of oxidative stress such as renal malondialdehyde (MDA) level, superoxide dismutase (SOD), and catalase (CAT) activities were measured. Tumor necrosis factor-α (TNF-α), myeloperoxidase (MPO) activity, transforming growth factor-β (TGF-β), and nitrite content were estimated in renal tissues. All treated animal were subjected to histopathological changes of kidney.
Result:
Diabetic rats showed a significant reduction in renal function, which was reflected with an increase in serum urea, serum creatinine, uric acid. In addition, STZ-nicotinamide caused renal tubular damage with a higher MDA level, depletion of SOD and CAT activity and glutathione (GSH) level. Moreover, TNF-α, MPO activity, TGF-β, and nitrite content were significantly increased in diabetic rats, while treatment with coenzyme Q10 or metformin or their combination ameliorate STZ-nicotinamide induced renal damage due to improvement in renal function, oxidative stress, suppression of TNF-α, MPO activity, TGF-β and nitrite content along with histopathological changes.
Conclusions:
This finding suggests that the treatment with coenzyme Q10 or metformin showed significant renoprotective effect against STZ-nicotinamide-induced DN. However, concomitant administration of both showed a better renoprotective effect than coenzyme Q10 or metformin alone treatment.
KEY WORDS: Coenzyme Q10, diabetic nephropathy, metformin, transforming growth factor-β, tumor necrosis factor-α
Introduction
Type 2 diabetes mellitus is one of the leading causes of morbidity and mortality due its complication like nephropathy and other cardiovascular diseases.[1] Diabetic nephropathy (DN) is the major cause of end-stage renal failure.[2] Proteinuria, an indicator of underlying DN, which usually worsens with progression of diabetic kidney disease. It is characterized by declining glomerular filtration rate and kidney structural changes, including thickening of the basement membranes and mesangial sclerosis. It has been found that hyperglycemia is a major cause of increased advanced glycation end products end which is responsible for the cause of renal dysfunction in diabetes.[3,4] It has also been found that oxidative stress is further responsible for the pathogenesis of DN.[5]
Angiotensin-converting enzyme inhibitors or angiotensin receptor antagonists are the main therapeutic agents presently seem to produce partial reduction in proteinuria and attenuate progression of chronic kidney disease to end-stage kidney disease. However, many patients do not respond to these agents, and they progress to end-stage renal disease at an early stage.[6] It was reported that there has been increasing evidence in the protective effect of dietary antioxidants as potential adjuvant therapy to prevent or delay diabetic complication.[7,8]
2,3 dimethoxy-5 methyl-6-decaprenyl benzoquinone, i.e. coenzyme Q10 or ubiquinone is a vitamin-like substance which is lipid soluble in nature and hydrophobic interior of the phospholipid bilayer of the cell membrane. It exists in a wide range of dietary items including meat, fish, vegetable oils, and nuts.[9] It has been previously reported that coenzyme Q10 has a potent anti-inflammatory activity,[10] antiulcer,[11] antioxidant,[12] and antidiabetic.[9]
It was also reported that the ability of metformin may play a role in its renoprotective effect by modulating several oxidative stress markers and pro-inflammatory cytokines at the biochemical and gene expression levels.[13]
Therefore, it was thought to combine antioxidant like coenzyme Q10 and metformin to study their renoprotective effect in experimentally induced nephropathy. Hence, the present study was aimed to investigate the protective effect of coenzyme Q10 alone, and its combination with metformin on streptozotocin (STZ)-nicotinamide-induced DN.
Materials and Methods
Drugs and Chemicals
Metformin and coenzyme Q10 were obtained from Zydus Cadila, Ahmedabad, India. STZ and nicotinamide were purchased from Himedia (Mumbai, India). Serum creatinine (cat no. 85MB100-75), serum urea (cat no. 81MB100-61), serum uric acid (cat no. 82 LS200-50), urinary protein (cat no. 86 LS100-25) kits used in the study were procured from SPAN Diagnostics, India. Tumor necrosis factor-α (TNF-α) (cat no. SEA133RA) and transforming growth factor-β (TGF-β) (cat no. SEA124RA) kits were procured from USCN Life Science Inc. All other chemicals and reagents used in the study were of analytical grade.
Experimental Animals
The experimental protocol was approved by the Institutional Animal Ethics Committee for the Purpose of Control and Supervision of Experiments on Animals. The experiment was carried out on healthy adult Wistar rats weighing 200–250 g of either sex. Rats were housed in polypropylene cages, maintained under standardized condition (12 h light/dark cycle, 24°C, 35–60% humidity) and allowed free access to diet (Nav Maharashtra Oil Mills Pvt. Ltd., Pune, India) and purified drinking water ad libitium.
Induction of Diabetic Nephropathy
Type 2 diabetes was induced in overnight fasted adult albino Wistar rats (200–250 g) by a single intraperitoneal (i.p.) injection of 65 mg/kg STZ (dissolved in citrate buffer, pH 4.5), followed by the i.p. administration of 110 mg/kg of nicotinamide (dissolved in normal saline).[14,15] Hyperglycemia was confirmed by elevated blood glucose levels at 72 h and then on day 7 after injection. Those animals with fasting blood glucose level greater than 200 mg/dl were considered as diabetic and were used for DN studies.
Experimental Design
Diabetic rats were randomly divided into five groups each consisting six animals.
Group I: Normal control rats (distilled water 10 ml/kg, p.o.).
Group II: Diabetic control rats.
Group III: Diabetic rats treated with 10 mg/kg coenzyme Q10 (1% aqueous solution of Tween 80, p.o.).[16]
Group IV: Diabetic rats treated with metformin (500 mg/kg, p.o).[17]
Group V: Diabetic rats treated with a combination of coenzyme Q10 (10 mg/kg) and metformin (500 mg/kg).
All the aforementioned treatments were started 1 week (7 days) after injection of STZ-nicotinamide. All the treatments were given daily to the respective group of animals for 42 days.
At the end of the experiments, blood samples were collected from the retro orbital plexus of rats under light ether anesthesia, using glass capillaries and stored with or without disodium ethylene diamine tetra-acetate for estimation of biochemical parameter. For the separation of serum, blood was allowed to clot for 15 min, and it was then centrifuged at 5000 rpm for 20 min. The serum was stored at −20°C until further biochemical estimation.
Glycated hemoglobin (HbA1C) was estimated using whole blood. Creatinine, urea, and uric acid were estimated from serum using standard diagnostic kit (SPAN Diagnostics, India). Rats were kept in metabolic cages for 24 h for urine collection. Urine samples were centrifuged at 1400 rpm for 5 min after proper dilution, and the supernatant was collected to determine urinary micro protein level using standard kits.
Estimation of Biomarkers of Oxidative Stress
Kidney was removed and kept on precooked (autoclaved) inverted Petridis in cold conditions with ice cubes. The tissues were cross chopped with a surgical scalpel into fine slices in chilled 0.25 M sucrose, quickly blotted on filter paper. They were minced and homogenized in 10 mM Tris-HCl buffer, pH 7.4 (10%w/v) with 25 strokes of tight Teflon pestle of glass homogenizer at a speed of 10,000 × g at 0°C using the Remi cooling centrifuge. The clear supernatant obtained was used for the assay of lipid peroxidation (malondialdehyde [MDA] content), endogenous antiperoxidative enzymes such as superoxide dismutase (SOD), catalase (CAT) and glutathione (GSH). Lipid peroxidation or MDA formation was estimated by the method of Slater and Sawyer.[18] SOD was determined by the method of Mishra and Fridovich.[19] CAT was estimated by the method given by Aebi[20] and GSH was determined by the method of Moron et al.[21]
Estimation of Myeloperoxidation Assay
Myeloperoxidation (MPO) activity in kidney tissue was determined as described.[22] The kidney tissue was homogenized in 0.5% hexadecyltrimethylammonium bromide containing 50 mM potassium phosphate buffer (pH 6) using a polytron tissue homogenizer. After freeze-thawing 3 times, the samples were centrifuged at 20,000 × g for 15 min at 40°C, and the resulting supernatant was assayed spectrophotometrically for MPO activity. In brief, 0.1 ml of sample was mixed with 2.9 ml of 50 mM potassium phosphate buffer (pH 6) containing 0.167 mg/ml O-dianisidine dihydrochlorde and 0.0005% hydrogen peroxide. The change in absorbance at 460 nm was then measured for 5 min using spectrophotometer. MPO activity data are presented as U/g tissue.
Determination of Tumor Necrosis Factor-α and Transforming Growth Factor-β by Enzyme-linked Immunosorbent Assay
The TNF-α and TGF-β levels in homogenized kidney tissues were determined by quantitative enzyme-linked immunosorbent assay kits according to the manufacturer's instructions (Rat TNF-α and TGF-β kits).
Estimation of Tissue Nitrite Content
Nitrite was estimated calorimetrically with the Griess reagent in protein-free supernatant of kidney homogenate.[23] Equal volumes of protein-free supernatant of kidney homogenate and Griess reagent (sulfanilamide 1%w/v, naphthylenediamine dihydrochlorde 0.1% w/v and orthophosphoric acid 2.5% v/v) were mixed and incubated at room temperature for 10 min and the absorbance was determined at 540 nm wavelength and compared to those of known concentrations of sodium nitrite.
Histopathology
After sacrifice, kidney tissues of each group was rapidly dissected out and washed immediately with saline and fixed in 10% phosphate-buffered formalin. Paraffin-embedded specimens were cut into 5 μm-thick sections and stained with hematoxylin and eosin (H and E). The sections were examined under a light microscope (Olympus B × 10, Tokyo, Japan) for the presence of histopathological changes and photomicrographs (Olympus DP12 camera, Japan) were taken. The observer performing histopathological evaluation was blinded to the animal treatment group.
Statistical Analysis
All of the data are expressed as mean ± standard error of mean statistical significance between more than two groups was tested using one-way ANOVA followed by the Bonferroni multiple comparisons test as appropriate using computer-based fitting program (Prism, GraphPad version 5, GraphPad Software, Inc). The significance level was set at P < 0.05 for all tests.
Results
Effect of Coenzyme Q10, Metformin or Combination of Both on Body Weight and Kidney Weight
The body weight of the diabetic rats showed a significant (P < 0.001) decrease after the administration of STZ-nicotinamide. The treatment with coenzyme Q10 or metformin or coenzyme Q10 + metformin did not show any reduction in the body weight as compared with diabetic control rats [Table 1].
Table 1.
Effect of coenzyme Q10, metformin or combination of both on body weight, kidney weight, urine volume and urinary protein
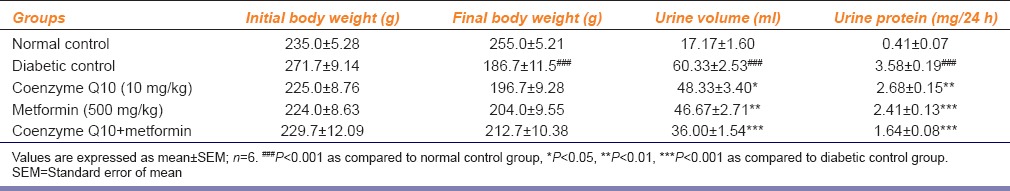
There was a significant (P < 0.001) increase in kidney weight after 6th week in diabetic control rats as compared to normal control rats, while the treatment with coenzyme Q10 or metformin or coenzyme Q10 + metformin showed a significant (P < 0.001) reduction in kidney weight as compared to diabetic control rats [Table 1].
Effect of Coenzyme Q10, Metformin or Combination of Both on Urine Volume and Urinary Protein
In diabetic control group, urine volume was significantly (P < 0.001) increased when compared to the normal control rats. When diabetic rats treated with coenzyme Q10, metformin or coenzyme Q10 + metformin showed a significant (P < 0.05; P < 0.01 and P < 0.001) reduction in urine volume as compared to diabetic control rats [Table 1].
Six week post STZ-nicotinamide injection caused a significant (P < 0.001) increase urinary protein of diabetic rats as compared to normal control rats. The treatment with coenzyme Q10, metformin or coenzyme Q10 + metformin showed a significant (P < 0.01; P < 0.001 and P < 0.001) reduction in urinary protein when compared to diabetic control rats [Table 1].
Effect of Coenzyme Q10, Metformin or Combination of Both on Glycated Hemoglobin Level
In the diabetic control rats, HbA1C level was significantly (P < 0.001) increased when compared to normal control rats. The diabetic rats treated with coenzyme Q10 showed a significant (P < 0.05) reduction in HbA1C level as compared to diabetic control rats. However, the treatment with metformin or coenzyme Q10 + metformin showed more significant (P < 0.001) reduction in HbA1C levels as compared to diabetic control rats [Table 2].
Table 2.
Effect of coenzyme Q10, metformin or combination of both on glycated hemoglobin level, serum creatinine, urea and uric acid
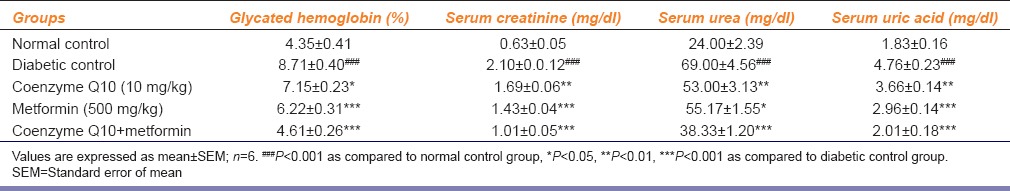
Effect of Coenzyme Q10, Metformin or Combination of Both on Serum Creatinine, Urea, and Uric Acid
Streptozotocin-nicotinamide injection caused a marked reduction in renal function, as characterized by significant (P < 0.001) increase in serum creatinine, urea, and uric acid levels as compared to normal control rats. Thus, these data indicate that a single i.p. injection of STZ-nicotinamide impairs kidney functions. Treatment with coenzyme Q10 (10 mg/kg), metformin (500 mg/kg) or coenzyme Q10 + metformin showed a significant (P < 0.01; P < 0.001 and P < 0.001) reduction in serum creatinine levels as compared to diabetic control rats [Table 2].
The treatment with coenzyme Q10, metformin or coenzyme Q10 + metformin showed a significant (P < 0.01; P < 0.05 and P < 0.001) reduction in urea levels as compared to diabetic control rats. Treatment with coenzyme Q10, metformin or coenzyme Q10 + metformin showed a significant (P < 0.01; P < 0.001 and P < 0.001) decrease in uric acid levels as compared to diabetic control rats, respectively [Table 2].
Effect of Coenzyme Q10, Metformin or Combination of Both on Markers of Oxidative Stress in Renal Tissue
The content of MDA, end product of lipid peroxidation and marker of oxidative stress was significantly (P < 0.001) increased in renal tissue of diabetic control rats as compared to non-diabetic rats after 6 weeks of study. There was a significant (P < 0.001) decrease in the levels of GSH, an endogenous antioxidant and antiperoxidative enzymes (SOD and CAT) in renal tissue as compared to normal control group [Table 3].
Table 3.
Effect of coenzyme Q10, metformin or combination of both on markers of oxidative stress in renal tissue
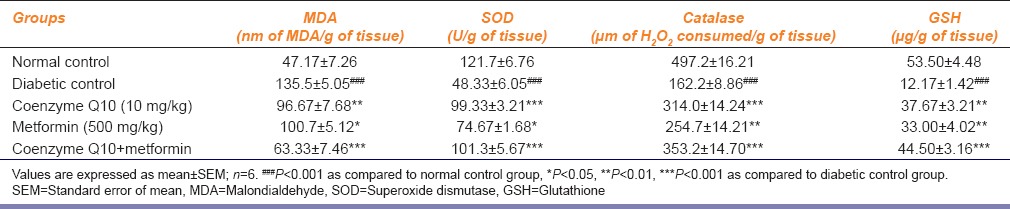
The treatment of diabetic rats with coenzyme Q10 or metformin or coenzyme Q10 + metformin showed a significant (P < 0.01; P < 0.05 and P < 0.001) decrease in the levels of MDA and GSH as compared to diabetic control rats. Coenzyme Q10 or coenzyme Q10 + metformin showed a significant (P < 0.001) increase in SOD and CAT activities, while the metformin treated rats showed a significant (P < 0.05; P < 0.01) increase in SOD and CAT activities [Table 3].
Effect of Coenzyme Q10, Metformin or Combination of Both on Tumor Necrosis Factor-α, Myeloperoxidase Activity, Transforming Growth Factor-β and Nitrite Content in Renal Tissue
Diabetic control rats showed a significant (P < 0.001) increase in inflammatory markers such as renal TNF-α level, MPO activity, and TGF-β as compared to normal control rats. The treatment with coenzyme Q10 in STZ-nicotinamide treated rats showed a significant reduction in TNF-α (P < 0.001) level, MPO activity (P < 0.05) and TGF-β (P < 0.01) in renal tissue when compared to diabetic control rats. However, treatment with coenzyme Q10 + metformin showed more significant (P < 0.001) decrease in TNF-α level, MPO activity and TGF-β in renal tissue as compared to diabetic rats treated with metformin or coenzyme Q10 alone [Table 4].
Table 4.
Effect of coenzyme Q10, metformin or combination of both on TNF-α, MPO activity, TGF-β and nitrite content in renal tissue
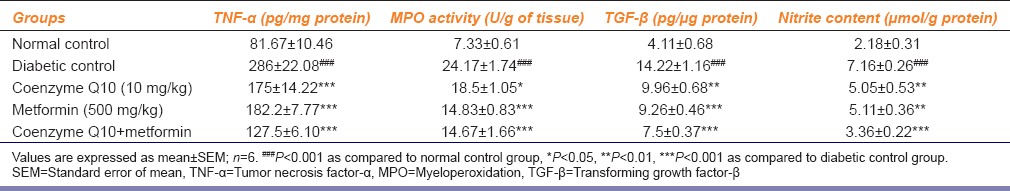
Nitrite content was significantly (P < 0.001) increasing in renal tissue of diabetic rats as compared to normal control group. The coenzyme Q10 + metformin treatment group showed more significant (P < 0.001) decrease renal nitrite content as compared to diabetic untreated group, while coenzyme Q10 alone or metformin alone caused a significant (P < 0.01) decrease nitrite content in renal tissue as compared to diabetic rats, but this effect was much lesser than combination therapy [Table 4].
Histopathological Studies
The architecture of the kidney was disturbed with diabetic control rats as compared to normal structural features of control animal. In the normal control group, the histopathological examination of kidney tissue showed normal appearance of glomeruli and tubules. Renal tissue section of diabetic rats showed glomerulosclerosis, tubular vacuolization, interstitial fibrosis, and thickening of glomerular basement membrane. The treatment with coenzyme Q10 or metformin showed moderate glomerular necrosis, interstitial fibrosis, moderate tubular vacuolization, and thickening of glomerular basement membrane. However, the treatment with coenzyme Q10 and metformin showed a mild tubular swelling, interstitial fibrosis, and thickening of glomerular basement membrane with absence of glomerulosclerosis [Figure 1a–e].
Figure 1.
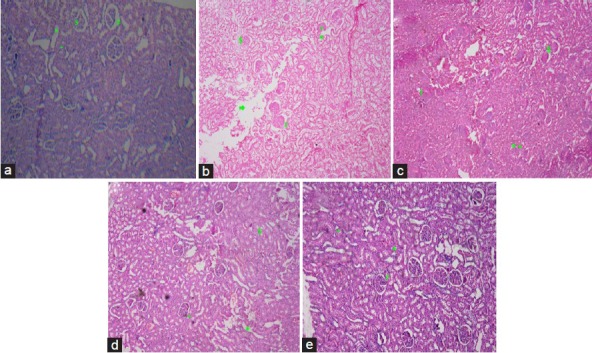
Light microscopy of kidney tissues from rats (H and E stained kidney sections). (a) Control group, (b) Diabetic control group, (c) Coenzyme Q10 (d) Metformin (e) Coenzyme Q10 + metformin
Discussion
Diabetic nephropathy in type 2 diabetes is the most common cause of end-stage renal disease and one of the leading causes of morbidity and mortality worldwide.[24] Preventing the progression of DN has been a challenge in biomedical research. Increased levels of serum glucose, serum creatinine, urea, and uric acid are the markers of DN.[25] Recent studies have shown that coenzyme Q10 has a beneficial effect in decreasing the elevated HbA1C, urea, and creatinine in alloxan-induced diabetic rats.[26] In the present study, co-administration of coenzyme Q10 and metformin has a significant reduction in elevated HbA1C, urea, creatinine, and uric acid as compared to diabetic rats. These results are in accordance with an earlier study in which it was shown that metformin alone produced a beneficial effect on DN.[27] In this study metformin when administered along with an antioxidant like coenzyme Q10 produced a synergic effect in reducing the development of DN.
Several studies have shown that oxidative stress plays a major role in the pathogenic pathway of diabetic injuries. Free radicals such as superoxide and lipid peroxidation product like MDA can induce cell and tissue damage.[28,29] Antioxidants such as vitamin E, coenzyme Q10, and antiperoxidative like SOD, CAT, GSH protect the cells and tissue against oxidative stress mediated injuries.[30] Results in the present study also indicate that there is an increase in the oxidative stress after STZ-nicotinamide induced DN. It has been further shown that lipid peroxidation was significantly reduced after the treatment with coenzyme Q10 or metformin or coenzyme Q10 + metformin. However, co-administration of coenzyme and metformin has more beneficial effect than when administered singly.
DN occurs as a result of the effects of both metabolic and hemodynamic insults, which at cellular level lead to the activation of intracellular signaling pathway and transcription factors. This effect is due to the release of TNF-α, MPO and TGF-β.[31,32] In the present study, administration of coenzyme Q10 or metformin or coenzyme Q10 + metformin resulted in a decrease in renal TNF-α, TGF-β and MPO levels as compared to diabetic control rats. However, treatment with coenzyme Q10 + metformin showed more renoprotective effect by virtue of a significant reduction of TNF-α, TGF-β, and MPO levels in renal tissues. It was stated that level of nitrite, an oxidized end product of NO, which might be attributed to the formation of peroxynitrite by reaction of NO with generated superoxide radicals.[33] In the present study, diabetic rats showed a significant increase in tissue nitrite content as compared to normal control rats, while the treatment with coenzyme Q10 or metformin or coenzyme Q10 + metformin restored the levels of renal nitrite.
Conclusion
These results indicate that the treatment with coenzyme Q10 or metformin showed significant renoprotective effect against STZ-nicotinamide induced DN. However, concomitant administration of both showed a better renoprotective effect than coenzyme Q10 or metformin alone treatment by virtue of amelioration of lipid peroxidation as well as due to improvement of renal function and suppression of TNF-α, MPO activity, TGF-β, and nitrite content in renal tissue. Finally, it was concluded that adjuvant therapy of coenzyme Q10 with antidiabetic drug might prevent or delay the DN.
Footnotes
Source of Support: Nil.
Conflict of Interest: No.
References
- 1.Yan HD, Li XZ, Xie JM, Li M. Effects of advanced glycation end products on renal fibrosis and oxidative stress in cultured NRK-49F cells. Chin Med J (Engl) 2007;120:787–93. [PubMed] [Google Scholar]
- 2.Barnett A. Prevention of loss of renal function over time in patients with diabetic nephropathy. Am J Med. 2006;119:S40–7. doi: 10.1016/j.amjmed.2006.01.013. [DOI] [PubMed] [Google Scholar]
- 3.Lee SH, Heo SJ, Hwang JY, Han JS, Jeon YJ. Protective effects of enzymatic digest from Ecklonia cava against high glucose-induced oxidative stress in human umbilical vein endothelial cells. J Sci Food Agric. 2010;90:349–56. doi: 10.1002/jsfa.3833. [DOI] [PubMed] [Google Scholar]
- 4.Aljofan M, Ding H. High glucose increases expression of cyclooxygenase-2, increases oxidative stress and decreases the generation of nitric oxide in mouse microvessel endothelial cells. J Cell Physiol. 2010;222:669–75. doi: 10.1002/jcp.21986. [DOI] [PubMed] [Google Scholar]
- 5.Baynes JW, Thorpe SR. Role of oxidative stress in diabetic complications: A new perspective on an old paradigm. Diabetes. 1999;48:1–9. doi: 10.2337/diabetes.48.1.1. [DOI] [PubMed] [Google Scholar]
- 6.Bos H, Andersen S, Rossing P, De Zeeuw D, Parving HH, De Jong PE, et al. Role of patient factors in therapy resistance to antiproteinuric intervention in nondiabetic and diabetic nephropathy. Kidney Int Suppl. 2000;75:S32–7. [PubMed] [Google Scholar]
- 7.Makni M, Sefi M, Fetoui H, Garoui el M, Gargouri NK, Boudawara T, et al. Flax and Pumpkin seeds mixture ameliorates diabetic nephropathy in rats. Food Chem Toxicol. 2010;48:2407–12. doi: 10.1016/j.fct.2010.05.079. [DOI] [PubMed] [Google Scholar]
- 8.Punithavathi VR, Prince PS, Kumar R, Selvakumari J. Antihyperglycaemic, antilipid peroxidative and antioxidant effects of gallic acid on streptozotocin induced diabetic Wistar rats. Eur J Pharmacol. 2011;650:465–71. doi: 10.1016/j.ejphar.2010.08.059. [DOI] [PubMed] [Google Scholar]
- 9.Kamei M, Fujita T, Kanbe T, Sasaki K, Oshiba K, Otani S, et al. The distribution and content of ubiquinone in foods. Int J Vitam Nutr Res. 1986;56:57–63. [PubMed] [Google Scholar]
- 10.Schmelzer C, Lindner I, Rimbach G, Niklowitz P, Menke T, Döring F. Functions of coenzyme Q10 in inflammation and gene expression. Biofactors. 2008;32:179–83. doi: 10.1002/biof.5520320121. [DOI] [PubMed] [Google Scholar]
- 11.Kohli Y, Suto Y, Kodama T. Effect of hypoxia on acetic acid ulcer of the stomach in rats with or without coenzyme Q10. Jpn J Exp Med. 1981;51:105–8. [PubMed] [Google Scholar]
- 12.Lenaz G, Fato R, Formiggini G, Genova ML. The role of coenzyme Q10 in mitochondrial electron transport. Mitochondrion. 2007;7(Suppl):S8–33. doi: 10.1016/j.mito.2007.03.009. [DOI] [PubMed] [Google Scholar]
- 13.Alhaider AA, Korashy HM, Sayed-Ahmed MM, Mobark M, Kfoury H, Mansour MA. Metformin attenuates streptozotocin-induced diabetic nephropathy in rats through modulation of oxidative stress genes expression. Chem Biol Interact. 2011;192:233–42. doi: 10.1016/j.cbi.2011.03.014. [DOI] [PubMed] [Google Scholar]
- 14.Kamble HV, Bodhankar SL. Trigonelline and sitagliptin attenuates nicotinamide-streptozotocin induced diabetic nephropathy in Wistar rats. Int J Pharm Pharm Sci. 2013;5:583–9. [Google Scholar]
- 15.Maheshwari RA, Khatri K, Sailor GU, Balaraman R. Antidiabetic activity of Dibolin (a polyherbal formulation) in streptozotocin-nicotinamide induced type 2 diabetic rats. Int J Pharm Pharm Sci. 2014;2:893–7. [Google Scholar]
- 16.Garjani A, Andalib S, Biabani S, Soraya H, Doustar Y, Garjani A, et al. Combined atorvastatin and coenzyme Q10 improve the left ventricular function in isoproterenol-induced heart failure in rat. Eur J Pharmacol. 2011;666:135–41. doi: 10.1016/j.ejphar.2011.04.061. [DOI] [PubMed] [Google Scholar]
- 17.Waisundara VY, Hsu A, Tan BK, Huang D. Baicalin reduces mitochondrial damage in streptozotocin-induced diabetic Wistar rats. Diabetes Metab Res Rev. 2009;25:671–7. doi: 10.1002/dmrr.1005. [DOI] [PubMed] [Google Scholar]
- 18.Slater TF, Sawyer BC. The stimulatory effects of carbon tetrachloride and other halogenoalkanes on peroxidative reactions in rat liver fractions in vitro. General features of the systems used. Biochem J. 1971;123:805–14. doi: 10.1042/bj1230805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Misra HP, Fridovich I. The role of superoxide anion in the autoxidation of epinephrine and a simple assay for superoxide dismutase. J Biol Chem. 1972;247:3170–5. [PubMed] [Google Scholar]
- 20.Aebi H. Catalase in vitro. Methods Enzymol. 1984;105:121–6. doi: 10.1016/s0076-6879(84)05016-3. [DOI] [PubMed] [Google Scholar]
- 21.Moron MS, Depierre JW, Mannervik B. Levels of glutathione, glutathione reductase and glutathione S-transferase activities in rat lung and liver. Biochim Biophys Acta. 1979;582:67–78. doi: 10.1016/0304-4165(79)90289-7. [DOI] [PubMed] [Google Scholar]
- 22.Krawisz JE, Sharon P, Stenson WF. Quantitative assay for acute intestinal inflammation based on myeloperoxidase activity. Assessment of inflammation in rat and hamster models. Gastroenterology. 1984;87:1344–50. [PubMed] [Google Scholar]
- 23.Guevara I, Iwanejko J, Dembinska-Kiec A, Pankiewicz J, Wanat A, Anna P, et al. Determination of nitrite/nitrate in human biological material by the simple Griess reaction. Clin Chim Acta. 1998;274:177–88. doi: 10.1016/s0009-8981(98)00060-6. [DOI] [PubMed] [Google Scholar]
- 24.Ntemka A, Iliadis F, Papanikolaou N, Grekas D. Network-centric analysis of genetic predisposition in diabetic nephropathy. Hippokratia. 2011;15:232–7. [PMC free article] [PubMed] [Google Scholar]
- 25.Idonije BO, Festus O, Oluba OM. Plasma glucose, creatinine and urea levels in type 2 diabetic patient attending a Nigerian Teaching Hospital. Res J Med Sci. 2011;5:1–3. [Google Scholar]
- 26.Ahmadvand H. Effects of coenzyme Q10 on hemoglobin A1C, serum urea and creatinine in alloxan-induced Type 1 diabetic rats. Iran J Pharmacol Ther. 2012;11:64–7. [Google Scholar]
- 27.Morales AI, Detaille D, Prieto M, Puente A, Briones E, Arévalo M, et al. Metformin prevents experimental gentamicin-induced nephropathy by a mitochondria-dependent pathway. Kidney Int. 2010;77:861–9. doi: 10.1038/ki.2010.11. [DOI] [PubMed] [Google Scholar]
- 28.Ahmadvand H, Tavafi M, Khosrowbeygi A. Amelioration of altered antioxidant enzymes activity and glomerulosclerosis by coenzyme Q10 in alloxan-induced diabetic rats. J Diabetes Complications. 2012;26:476–82. doi: 10.1016/j.jdiacomp.2012.06.004. [DOI] [PubMed] [Google Scholar]
- 29.Maheshwari RA, Sailor GU, Patel L, Balaraman R. Amelioration of cisplatin-induced nephrotoxicity by statins. Indian J Pharmacol. 2013;45:354–8. doi: 10.4103/0253-7613.115016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cornejo-Garcia JA, Mayorga C, Torres MJ, Fernandez TD, R-Pena R, Bravo I, et al. Anti-oxidant enzyme activities and expression and oxidative damage in patients with non-immediate reactions to drugs. Clin Exp Immunol. 2006;145:287–95. doi: 10.1111/j.1365-2249.2006.03149.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.David S, Peter M. Understanding Diabetic Nephropathy–Is There a Genetic Susceptibility? Eur Endocrinol. 2008;4:66–9. [Google Scholar]
- 32.Kettle AJ, van Dalen CJ, Winterbourn CC. Peroxynitrite and myeloperoxidase leave the same footprint in protein nitration. Redox Rep. 1997;3:257–8. doi: 10.1080/13510002.1997.11747120. [DOI] [PubMed] [Google Scholar]
- 33.Stamler JS, Singel DJ, Loscalzo J. Biochemistry of nitric oxide and its redox-activated forms. Science. 1992;258:1898–902. doi: 10.1126/science.1281928. [DOI] [PubMed] [Google Scholar]


