Abstract
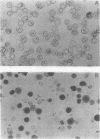
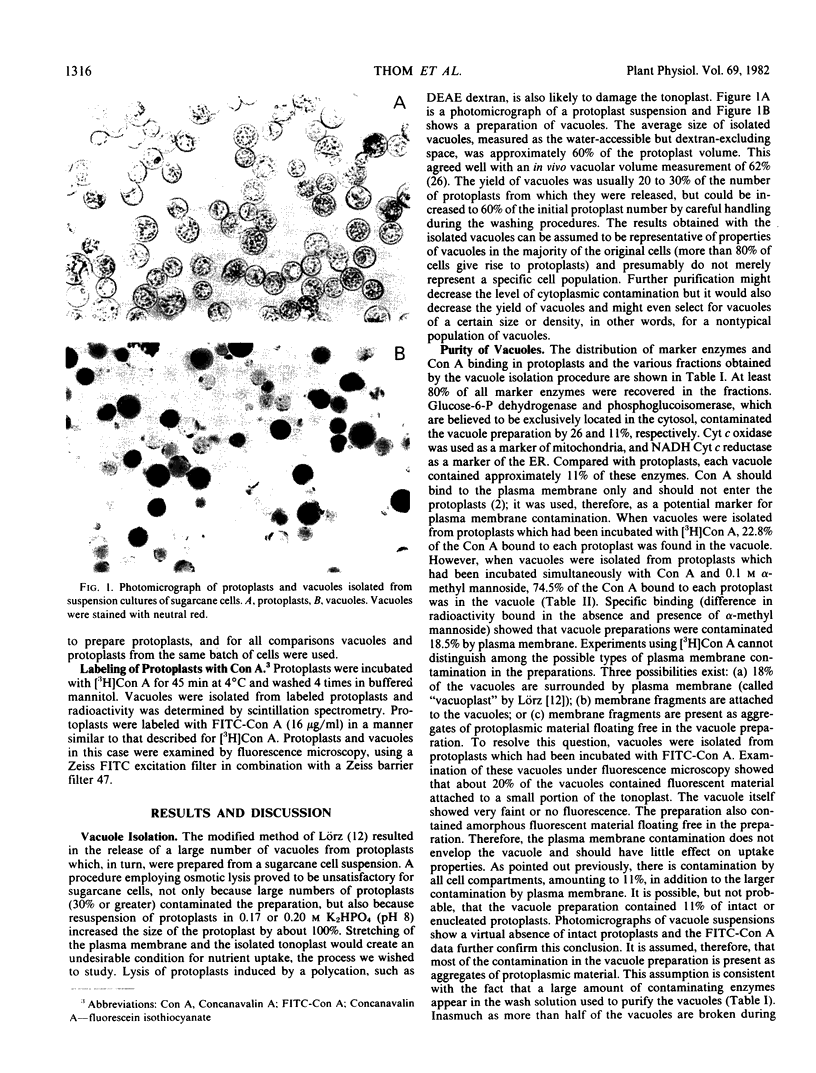
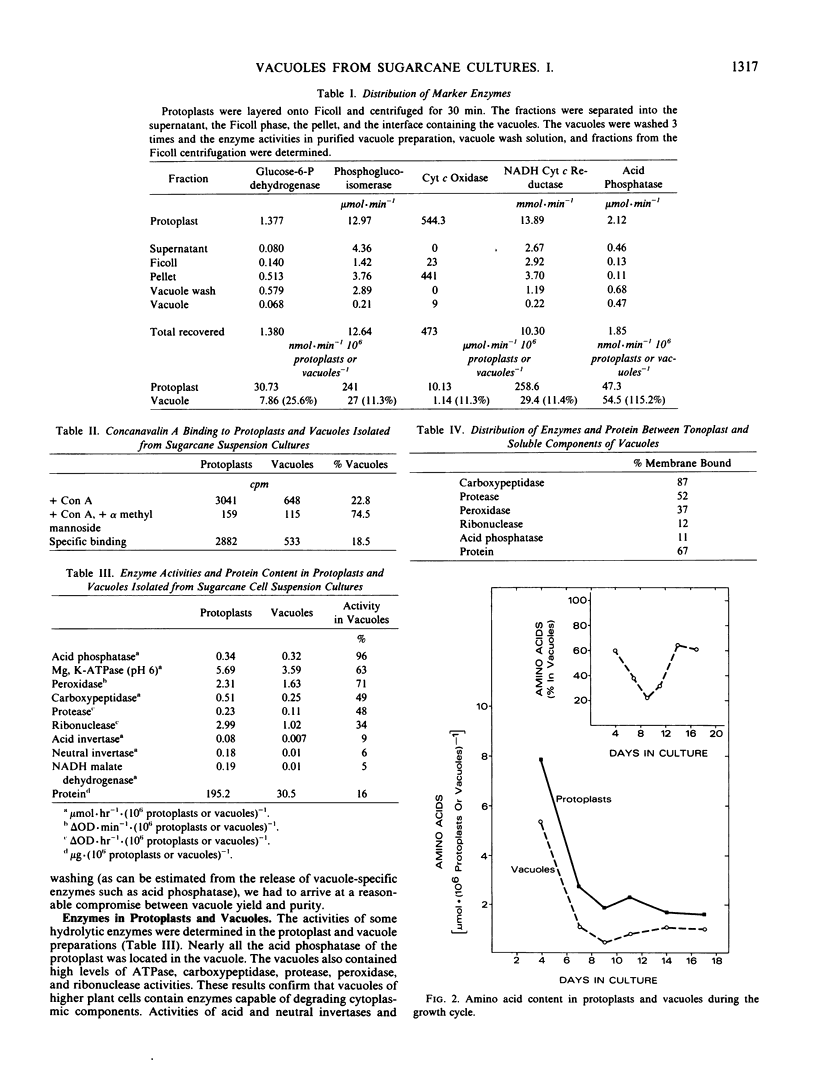
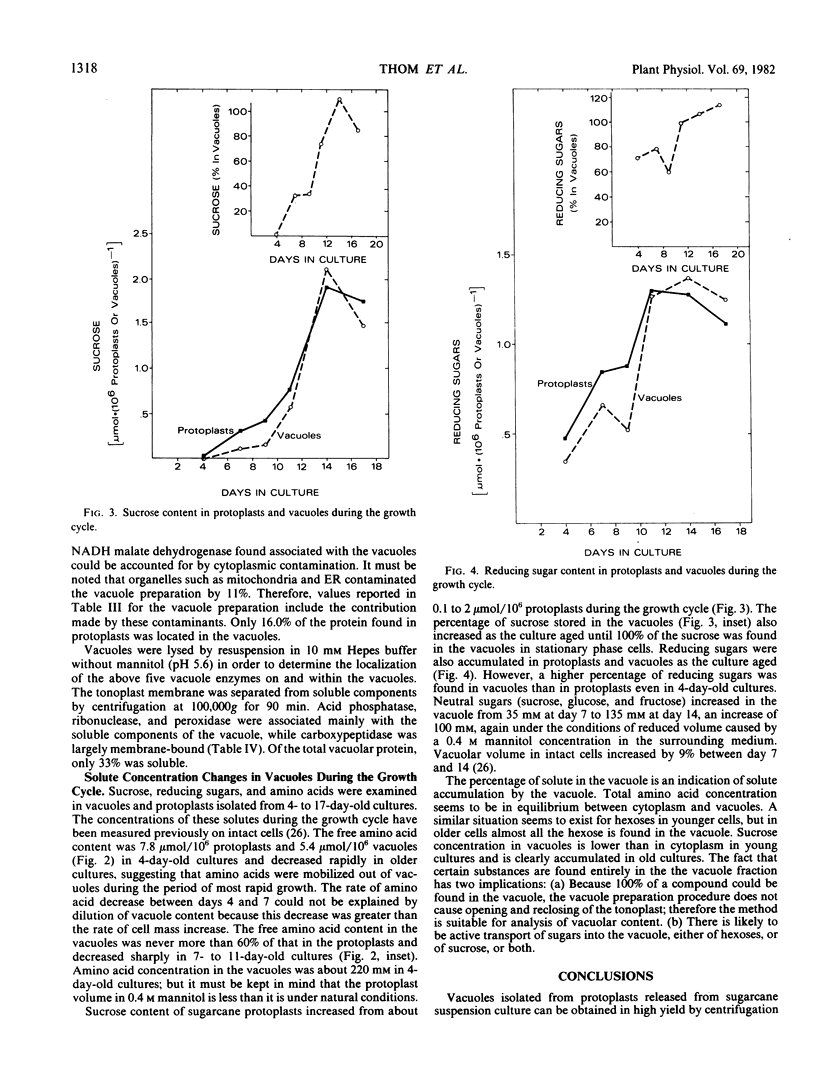
Vacuoles were isolated from suspension cultures of sugarcane (Saccharum sp.) cells by centrifugation of protoplasts at high g force against a 12% (w/v) Ficoll solution. Distribution of marker enzymes and Concanavalin A binding showed an 11% contamination of the vacuole preparation by cytoplasmic components, mitochondria, and endoplasmic reticulum, and 18% contamination by plasma membrane. Acid phosphatase, carboxypeptidase, protease, peroxidase, and ribonuclease activities were enriched in isolated vacuoles. Carboxypeptidase was tonoplast-bound, whereas the other enzymes were soluble. Sucrose, reducing sugars, and free amino acids were measured in protoplasts and vacuoles during growth of cells in suspension culture. Sucrose and reducing sugar content of vacuoles increased as the culture aged, while free amino acids decreased sharply.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berkowitz R. L., Travis R. L. Characterization and quantitation of concanavalin a binding by plasma membrane enriched fractions from soybean root. Plant Physiol. 1981 Nov;68(5):1014–1019. doi: 10.1104/pp.68.5.1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boller T., Kende H. Hydrolytic enzymes in the central vacuole of plant cells. Plant Physiol. 1979 Jun;63(6):1123–1132. doi: 10.1104/pp.63.6.1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boss W. F., Ruesink A. W. Isolation and Characterization of Concanavalin A-labeled Plasma Membranes of Carrot Protoplasts. Plant Physiol. 1979 Dec;64(6):1005–1011. doi: 10.1104/pp.64.6.1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dürr M., Boller T., Wiemken A. Polybase induced lysis of yeast spheroplasts. A new gentle method for preparation of vacuoles. Arch Microbiol. 1975 Nov 7;105(3):319–327. doi: 10.1007/BF00447152. [DOI] [PubMed] [Google Scholar]
- Guy M., Reinhold L., Michaeli D. Direct evidence for a sugar transport mechanism in isolated vacuoles. Plant Physiol. 1979 Jul;64(1):61–64. doi: 10.1104/pp.64.1.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodges T. K., Leonard R. T. Purification of a plasma membrane-bound adenosine triphosphatase from plant roots. Methods Enzymol. 1974;32:392–406. doi: 10.1016/0076-6879(74)32039-3. [DOI] [PubMed] [Google Scholar]
- Kringstad R., Kenyon W. H., Black C. C. The rapid isolation of vacuoles from leaves of crassulacean Acid metabolism plants. Plant Physiol. 1980 Sep;66(3):379–382. doi: 10.1104/pp.66.3.379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Leigh R. A., Branton D. Isolation of Vacuoles from Root Storage Tissue of Beta vulgaris L. Plant Physiol. 1976 Nov;58(5):656–662. doi: 10.1104/pp.58.5.656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MOORE S., STEIN W. H. A modified ninhydrin reagent for the photometric determination of amino acids and related compounds. J Biol Chem. 1954 Dec;211(2):907–913. [PubMed] [Google Scholar]
- Maretzki A., Thom M. Membrane transport of sugars in cell suspensions of sugarcane: I. Evidence for sites and specificity. Plant Physiol. 1972 Feb;49(2):177–182. doi: 10.1104/pp.49.2.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maretzki A., Thom M. The existence of two membrane transport systems for glucose in suspensions of sugarcane cells. Biochem Biophys Res Commun. 1972 Apr 14;47(1):44–50. doi: 10.1016/s0006-291x(72)80007-x. [DOI] [PubMed] [Google Scholar]
- Mettler I. J., Leonard R. T. Isolation and partial characterization of vacuoles from tobacco protoplasts. Plant Physiol. 1979 Dec;64(6):1114–1120. doi: 10.1104/pp.64.6.1114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saunders J. A. Investigations of vacuoles isolated from tobacco: I. Quantitation of nicotine. Plant Physiol. 1979 Jul;64(1):74–78. doi: 10.1104/pp.64.1.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang W. J., Maretzki A. Purification and properties of leaf ribonuclease from sugar cane. Biochim Biophys Acta. 1970 Aug 15;212(2):300–307. doi: 10.1016/0005-2744(70)90210-x. [DOI] [PubMed] [Google Scholar]
- Van Handel E. Direct microdetermination of sucrose. Anal Biochem. 1968 Feb;22(2):280–283. doi: 10.1016/0003-2697(68)90317-5. [DOI] [PubMed] [Google Scholar]
- Walker-Simmons M., Ryan C. A. Immunological Identification of Proteinase Inhibitors I and II in Isolated Tomato Leaf Vacuoles. Plant Physiol. 1977 Jul;60(1):61–63. doi: 10.1104/pp.60.1.61. [DOI] [PMC free article] [PubMed] [Google Scholar]